Abstract
An enigmatic feature of behavioural state control is the rich diversity of wake-promoting neural systems. This diversity has been rationalized as ‘robustness via redundancy’, wherein wakefulness control is not critically dependent on one type of neuron or molecule. Studies of the brain orexin/hypocretin system challenge this view by demonstrating that wakefulness control fails upon loss of this neurotransmitter system. Since orexin neurons signal arousal need, and excite other wake-promoting neurons, their actions illuminate nonredundant principles of arousal control. Here, we suggest such principles by reviewing the orexin system from a collective viewpoint of biology, physics and engineering. Orexin peptides excite other arousal-promoting neurons (noradrenaline, histamine, serotonin, acetylcholine neurons), either by activating mixed-cation conductances or by inhibiting potassium conductances. Ohm’s law predicts that these opposite conductance changes will produce opposite effects on sensitivity of neuronal excitability to current inputs, thus enabling orexin to differentially control input-output gain of its target networks. Orexin neurons also produce other transmitters, including glutamate. When orexin cells fire, glutamate-mediated downstream excitation displays temporal decay, but orexin-mediated excitation escalates, as if orexin transmission enabled arousal controllers to compute a time integral of arousal need. Since the anatomical and functional architecture of the orexin system contains negative feedback loops (e.g. orexin ➔ histamine ➔ noradrenaline/serotonin—orexin), such computations may stabilize wakefulness via integral feedback, a basic engineering strategy for set point control in uncertain environments. Such dynamic behavioural control requires several distinct wake-promoting modules, which perform nonredundant transformations of arousal signals and are connected in feedback loops.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Postsynaptic Actions of Orexin/Hypocretin Mediate Arousal Control
Efficient behaviour requires optimal adjustment of behavioural state (i.e. arousal, wakefulness, activity, energy expenditure) to perturbations in the environment. The environmental perturbations come in many diverse types, which differ greatly in their speed and predictability, necessitating an evolution of behavioural control systems that can deal with this perturbation diversity. A fundamental example of a slow and predictable perturbation in the environment is the ca. 24 h day-night cycle on Earth. The hypothalamic suprachiasmatic nucleus (SCN) adjusts behaviour to this cycle by emitting sinusoidal (ca. 24 h period) neural signals that schedule behavioural activity for either the day (for diurnal animals) or the night (for nocturnal animals), depending on specific survival advantages afforded to different animals by being active during light or dark. These slow daily rhythms of behavioural activity collapse upon description of the SCN [1].
However, wakefulness also needs to be controlled on a much more rapid and unpredictable timescale than that controlled by the SCN. Most of us take this rapid wakefulness adjustment for granted, assuming that we will not fall asleep in the middle of laughing or talking. This is not so for patients suffering from the sleep-wake disorder narcolepsy, which affects about 1:2000 people and where sleep and paralysis suddenly and uncontrollably intrude into normal wakefulness [2, 3]. Most cases of human narcolepsy are associated with reduced levels of orexin/hypocretin peptides in the CSF and lack of central orexin/hypocretin-producing neurons in the brain [4–7]. Loss of orexin/hypocretin peptides in humans, dogs, mice and rats impairs arousal control, resulting in abnormally frequent and rapid loss of consciousness (‘sleep attacks’). It seems that without the orexin/hypocretin system, wakefulness is prone to instability in the face of rapid perturbations in the environment, and processes enabled by orexin/hypocretin cells keep this instability under control. Orexin/hypocretin and the SCN systems are thus two hypothalamic systems that are essential for appropriate matching of behavioural state to the environment on rapid and slow timescales, respectively.
The orexin/hypocretin cells act as controllers rather than critical generators of wakefulness: with them, the average amount of arousal (sleep and waking) does not change, but the ability to control arousal based on salient environmental set point is impaired [8, 9]. Coordination of many other behaviours, such as reward-seeking, also critically relies on orexin/hypocretin neurons [10–12], but since these behaviours are wakefulness dependent, it is often unclear to what extent these effects are secondary to wakefulness control. Since the discovery of orexin/hypocretin neurons, a key question has been why they are so critical for arousal control, considering the numerous other arousal-controlling neurons in the brain (e.g. noradrenaline, histamine, acetylcholine, serotonin cells). Here, we review the biological properties of brain orexin/hypocretin circuits related to wakefulness/arousal, with particular emphasis on postsynaptic actions of orexin/hypocretin peptides and from a viewpoint of basic principles of signal processing and dynamic set point control. For more comprehensive overviews of orexin/hypocretin physiology, we refer the reader elsewhere [10, 13].
In this article, we will take the view that orexin/hypocretin neurons signal arousal need (or, in control system language, arousal error – see below). We define arousal need as a need to counteract actual or potential dangers such as low energy levels, high CO2 levels, or potentially threatening sensory stimuli (e.g. sudden sounds, presence of another animal). Orexin neurons sense all these signals (Fig. 1), and thus their activity represents a sum of diverse ‘arousal demands’ (e.g. they are inhibited by glucose but excited by H3O−/CO2) [8, 14–18]. Orexin/hypocretin neurons are also inhibited by at least some of the other wakefulness-promoting transmitters such as serotonin and noradrenaline, i.e. transmitters that may represent the actual level of arousal [19–21] (discussed below). Thus, orexin/hypocretin cell output may represent an ‘arousal error’ (actual arousal minus required arousal), thereby signalling how much arousal should be increased. In the absence of these orexin/hypocretin signals, arousal is no longer appropriately coupled to internal and external environment, which is an alternative way of describing sleep-wake instability.
Input and outputs of orexin/hypocretin cells. Orexin/hypocretin neurons are activated during high vigilance states associated with high gamma EEG, requiring increased arousal such as exploratory behaviour or upon sensory or emotional stimulation. A multitude of excitatory and inhibitory substances modulates orexin/hypocretin cell activity. These include hormones, neuropeptides and small molecule transmitters as well as homeostatic signals. Orexin/hypocretin neurons receive direct inputs from brain areas involved in sleep/wake control, appetite control and reward. Orexin/hypocretin neurons integrate this information via the release of orexin peptides, affecting postsynaptic gain and synaptic drive in target neurons. Orexin/hypocretin activity may thereby gate relevant information based on environmental and homeostatic needs [14, 16, 124–129]
The wake-sleep instability seen upon loss of orexin/hypocretin-producing neurons is recapitulated by the loss of orexin/hypocretin type 2 receptors or of orexin/hypocretin peptides [22–24]. This suggests that postsynaptic actions of orexin/hypocretin peptides on orexin/hypocretin type 2 G-protein-coupled receptors are responsible for arousal control mediated by orexin/hypocretin cells. This also suggests that orexin type 2 receptor-independent actions of orexin/hypocretin cells, such as those mediated by their other transmitters (glutamate, dynorphin, Narp) or by orexin/hypocretin via type 1 receptors, are insufficient to achieve proper arousal control without orexin/hypocretin action of type 2 orexin/hypocretin receptors. The latter point may seem surprising, considering that some arousal-promoting neurons, such as noradrenaline neurons of the LC which are important for orexin/hypocretin-induced wakefulness [25], are excited by orexin/hypocretin via type 1 rather than type 2 OX receptors [26]. In contrast, other arousal-promoting neurons, such as histamine cells of the tuberomammillary hypothalamus, are excited by orexin/hypocretin via type 2 receptors [26, 27]. Below, we catalogue this biological complexity of postsynaptic actions of orexin/hypocretin cells in more detail and comment on some functional biophysical implications of this complexity. Then, we propose a framework that simplifies and generalizes the diversity of postsynaptic orexin/hypocretin actions into a control systems model that accounts for general dynamic features of orexin/hypocretin-dependent arousal and offers an organizing principle for the puzzling diversity of wakefulness-promoting neurons in the brain.
2 Sites and Biophysics of Postsynaptic Actions of Orexin/Hypocretin Neurons
From their location in the lateral hypothalamus, orexin/hypocretin cells project axons to the entire brain [28, 29] (Fig. 2). The anatomical distribution of these projections largely mirrors that of two G-protein-coupled receptors for orexin [30]. Increased firing rate of orexin/hypocretin neurons produces awakening [31], and most of the brain’s classical arousal-related systems are innervated by orexin/hypocretin axons and excited by orexin/hypocretin peptides (see Table 1, which lists many key findings alongside corresponding references [19, 26, 27, 32–106]). Orexin/hypocretin peptides also modulate neuronal activity in brain areas related to eating, emotion, autonomic function and motor control (Table 1). Here, we only list (Table 1) but do not discuss the latter actions of orexin/hypocretin in detail, since this has been covered extensively in recent publications (e.g. [10, 107]). We would just like to note that combined activation of arousal and reward systems may ensure that a heightened arousal accompanies reward-seeking, thereby increasing probability of reward discovery and of avoiding danger while exploring for rewards. Also, arousal and exploration may require motivational signals, since these behaviours are not intrinsically rewarding but are energy expending and potentially dangerous. Orexin/hypocretin may provide this motivation [10, 108], at least until reward consumption beings [109].
From a biophysical perspective on signal processing, the excitatory/depolarizing actions of orexin/hypocretin on central neurons (Table 1) can be divided into those increasing membrane conductance (e.g. activation of non-selective cation currents) and those decreasing it (e.g. inhibition of K+ currents). This has profound implications for input processing capabilities of orexin/hypocretin-modulated neurons. The ability of a current input (I) to change membrane potential (V) is inversely related to membrane conductance (g). This dependence is described by Ohm’s law, V = I/g. The neuronal firing output depends on the membrane potential (it is increased by depolarization, [110, 111]). Therefore, conductance-increasing actions of orexin/hypocretin will not only depolarize and electrically excite the target neuron but also reduce the sensitivity of the neuron’s firing to other inputs. This would effectively lock the neuron in a high-output state, which could be useful for overriding other inputs in times of danger. In turn, conductance-reducing actions of orexin/hypocretin will not only depolarize and excite the neuron but also increase its sensitivity to other inputs. This would enable the neuron to be readily modulated by other inputs (both stimulatory and inhibitory), thus allowing other inputs to either augment or cancel the orexin/hypocretin-induced excitation. Overall, these conductance-related actions of orexin/hypocretin can be viewed as not simply excitatory but also ‘gain modulating’. The ability to modulate the input-output gain is an important feature of neural computation [112]. Therefore, the functional/behavioural implications of gain-modulating postsynaptic actions of orexin/hypocretin are an important question for future investigations.
Although the direct postsynaptic actions of orexin/hypocretin are usually excitatory, there are some exceptions. Per1 neurons of the hypothalamic suprachiasmatic nucleus that function as the brain’s master circadian clock are inhibited by orexin/hypocretin via activation of leak-like K+ channels, as well as presynaptically by increasing GABA release [62]. Signal transduction pathways linking orexin/hypocretin receptors to the inhibitory channels remain undefined. The inhibition of mouse Per1 neurons by orexin/hypocretin may enable the circadian clock signals to be overridden by arousal need signalled by orexin/hypocretin cells.
The above summary and classification of postsynaptic actions of orexin/hypocretin highlight the diversity and some general themes of brain-wide orexin/hypocretin signalling. However, such descriptions do not reveal critical components of orexin/hypocretin actions nor why orexins/hypocretins are vital for brain function stability. To achieve the latter insights, it is necessary to understand the functionally critical modules mediating orexin/hypocretin action and the overall control system architecture implemented by these modules. We address this in the next section, continuing to focus on wakefulness control.
3 Which Orexin/Hypocretin-Regulated Sites Are Critical for Wake Stability in the Normal Brain?
The diversity of orexin/hypocretin-excited neurons throughout the brain raises the question of the relative roles of different orexin targets in preventing the narcoleptic instability of wakefulness. An optimal way to deconstruct these natural roles would be to examine the effects on wakefulness stability of targeted, specific and reversible inactivation of orexin receptors in molecularly defined neurons in adult mice. Such technically demanding experiments have not yet been accomplished. The relevant studies performed to date used other approaches, such as global receptor deletion followed by targeted receptor restoration [113, 114] or experimental stimulation of orexin/hypocretin cells concurrent with experimental silencing of specific downstream targets [25]. These approaches have caveats as far as the natural roles of orexin/hypocretin targets in wake stability are concerned. For example, given the feedback loops in wakefulness circuits (see below), the role of an orexin/hypocretin receptor site in wakefulness control when all other orexin/hypocretin sites are genetically deleted is not the same as its role in the natural brain. In turn, experimental stimulation of orexin/hypocretin cells does not reproduce their natural firing patterns, and the ability of orexin/hypocretin cells to stimulate awakening is not an assay of wakefulness stability. Nevertheless, the existing studies provide fundamental information about causal links between specific neurons and wakefulness, as well as proof-of-concept information relevant to narcolepsy treatment. Therefore, we briefly comment on some of them here (for more in-depth discussions of current literature on this topic, see [2, 115]).
Carter et al. examined the mechanism of orexin-mediated wakefulness by optogenetically stimulating orexin/hypocretin neurons while concurrently optogenetically silencing one of their downstream effectors, the orexin type 1 receptor expressing noradrenaline neurons of the locus coeruleus (LC) [25]. Note that this does not address the question of which orexin/hypocretin targets are critical for orexin/hypocretin-dependent wakefulness stability. They found that when the LC noradrenaline neurons were inactivated, stimulation of orexin/hypocretin neurons no longer produced awakening from sleep. This seminal finding establishes the noradrenaline neurons as critical generators for the orexin/hypocretin-dependent stimulation of wakefulness. However, it remains unclear how these generators are controlled in order to maintain stable wakefulness, especially since the orexin type 2 receptor (not the type 1 expressed by the LC noradrenaline neurons) is essential for the wake stability. In the next sections, we will propose a unifying framework that can reconcile the wake-generator function of the noradrenaline neurons with wake-controller functions of orexin type 2 receptor neurons.
Mochizuki et al. globally deleted orexin type 2 receptors in mice, producing a narcoleptic instability of wakefulness [113]. They then used a viral expression strategy to restore these receptors locally in the tuberomammillary hypothalamus, an area rich in histamine neurons that normally express high levels of orexin type 2 receptors. This local manipulation rescued the wakefulness instability (but interestingly, not sleep instability that also results from loss of orexin/hypocretin function). This suggests that the histamine neurons could be critical for wake-controllers that signal to wake-generators (such as noradrenaline cells) to adjust their signals properly.
Hasegawa et al. knocked out both types of orexin/hypocretin receptors in mice and then reintroduced both of them at specific brain sites by viral delivery under a non-specific promoter. They found that such receptor overexpression in the LC restored the normal duration and number of wakefulness episodes [114]. It is not clear whether this is the normal function of the orexin signals to the locus coeruleus or an outcome of overexpression of orexin receptors that are not normally there. In contrast, the dual orexin/hypocretin receptor overexpression in the tuberomammillary hypothalamus did not restore the normal duration and number of wakefulness episodes. This shows that orexin/hypocretin signalling in the tuberomammillary hypothalamus is insufficient for normal wakefulness when all orexin receptors are missing from the locus coeruleus. Together with the data of Mochizuki et al., this can be interpreted to suggest that the tuberomammillary hypothalamus requires an orexin-sensitive downstream wakefulness generator in order to control wakefulness. For effective wakefulness control, orexin/hypocretin may need to alter the activity of both wakefulness regulators and generators in the brain, with different kinetics and via different receptors (see Fig. 3, discussed below).
Brain arousal systems as control modules in a feedback loop. (a) A generalized control system architecture (integral feedback loop) for tracking a desired set point (D) despite unpredictable disturbances. After [120]. (b) Possible implementation of A by a diversity of wake-promoting neurons in the brain (from more detail, see [18])
4 Mapping Orexin/Hypocretin Biology onto Control Operations
The above-discussed biological measurements define functionally important components of orexin/hypocretin systems and the general signs (plus or minus) for interactions between these components. This knowledge is fundamental, but alone is insufficient to account for control operations performed by orexin/hypocretin to achieve stable wakefulness. To clarify what we mean by control operations, a brief formal definition of tracking and stability is warranted. From a general evolutionary perspective, a highly desirable attribute of arousal control is set point tracking, i.e. the ability to adjust a set point to relevant inputs while rejecting disturbances. A good tracking system will follow salient inputs while resisting disturbances. Disturbance resistance is the ability to protect a set point from irrelevant disturbance, e.g. noise in brain/body internal signals, external events not requiring arousal responses, etc. A system capable of disturbance-resistant tracking can be considered ‘robust yet flexible’. Note that this robust flexibility has to exist in the real world, i.e. where neither noise/disturbance nor important inputs are completely predictable, i.e. the control system has to be uncertainty proof. This need to deal with uncertainty imposes important requirements (and thus constraints) on system architecture (see below). For more detailed discussions of control principles as applied to orexin/hypocretin networks, see [18].
Can the actions of orexin/hypocretin be considered to implement such robust-yet-flexible arousal? We believe the answer is yes, since without orexin/hypocretin, arousal becomes both flexible and less robust. For example, when orexin/hypocretin is knocked out, mice cannot respond to potentially dangerous intrusions by properly increasing blood pressure [116], and they cannot properly adapt to a fall in their energy levels by increasing locomotion [8]. Thus, a vital flexibility of arousal is lost without orexin/hypocretin. In terms of robustness, it is well known that without orexin/hypocretin arousal can dip to inappropriately low levels (unconsciousness) upon disturbances such as laughter in humans or sight of delicious food in animals [2, 117, 118]. Without orexin/hypocretin, there is no appropriate tracking/adjustment of arousal state to internal and external state.
If orexin/hypocretin actions implement arousal tracking, the understanding of arousal control will increase by viewing orexin/hypocretin system from general perspectives of robust-yet-flexible control systems. Such systems generally must contain autocorrecting feedback loops, since neither the world nor system performance can be precisely predicted [119, 120]. As a minimum, such a feedback loop circuit must contain at least three operationally different elements in order to be robust yet flexible, which we here call a comparator, a controller and a generator (Fig. 3). This error-based feedback system is a canonical engineering strategy to track a set point despite noise [119, 120]. Note that although artificial, stimulation of each of these elements would increase the final output of the system (e.g. arousal). However, this ‘test’ does not mean that the elements are redundant: their functions and dynamics are fundamentally distinct.
These distinct functions of the three components in this autocorrecting system architecture (Fig. 3) have been discussed in detail in control engineering literature [119, 121] and recently in arousal control literature [18, 58]. A summary of the latter discussions is that orexin/hypocretin neurons display functional hallmarks of comparators, some orexin type 2 receptor neurons (histamine cells) exhibit functional signatures of controllers, while some orexin type 1 receptor neurons (noradrenaline cells) have operational features of generators (for detailed arguments, see [18]). A particularly curious feature of some orexin type 2 receptor cells is that they appear to transmit a signal resembling a temporal integral of orexin/hypocretin neuron activity (Fig. 4) [18, 58]. This integration may enable them to function as integral controllers, engineering signals that are theoretically necessary and sufficient for robust-and-flexible control mediated by orexin/hypocretin in general and its type 2 receptors in particular [18]. Therefore, from an operational perspective, integral feedback is an important candidate mechanism for how orexin/hypocretin maintains appropriate behavioural state.
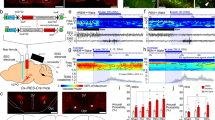
Orexin/hypocretin and glutamate co-transmission enables fast and sustained control of histamine neurons. (a) Targeted expression of light-sensitive ion channel ChR2 enables selective control of excitatory membrane currents in orexin/hypocretin cells. (b) Cre-dependent expression of virally delivered ChR2-eYFP in orexin-cre mice allows selective expression in lateral hypothalamic orexin-positive neurons. Data are from [61]. (c) Left: Light-activated action potential firing in ChR2-eYFP-expressing orexin/hypocretin cells recorded using whole-cell patch clamp. Right: Whole-cell recording of histamine neurons shows increased glutamate inputs (bottom) and action potential firing (top) upon light stimulation of adjacent orexin/hypocretin fibres. Adapted from [130], Fig. 2. (d) Top: Prolonged stimulation of orexin/hypocretin fibres for 30 s at 20 Hz produces fast and sustained increase in histamine cell firing. Bottom: Blockade of CNQX-sensitive glutamate currents blocks the fast rise in histamine firing, while a slow, long-lasting component remains. In contrary, orexin 2 receptor blockade (TCS) abolishes the slow component, leaving the fast component unaltered. Data from [58]. (e) Orexin-mediated increase in histamine firing integrates orexin/hypocretin activity (top), while glutamate-mediated increase in histamine firing does not (bottom). Data from [58]
5 Explanatory and Predictive Value of Viewing Orexin/Hypocretin Actions as Control Computations
What is the scientific value, for orexin/hypocretin biology and clinical applications, of control engineering theories such as those shown in Fig. 3?
First, an important corollary is that these control schemes assign a clear operational reason for the hitherto puzzling diversity of seemingly redundant wake-promoting neurons in the brain. If brain wakefulness control was operating via integral control or a related feedback scheme, there would have to be several operationally nonredundant neural types (comparators, controllers, generators) cooperating together. Note that these neurons are nonredundant in the sense of operations they perform, for example in this case, addition, integration and amplification, respectively. However, the comparator, controller and generator neurons are redundant in the sense that they all promote wakefulness if separately stimulated (this follows mathematically from the scheme in Fig. 3). The latter ‘redundancy’, however, is a by-product of experimental manipulation – it could be useful clinically for achieving rapid arousal, but it does not mean that the wakefulness control architecture is redundant. Such considerations are attractive because they settle a long-standing enigma in the field – the diversity of wake-promoting neurons – and emphasize that wakefulness control/stability/flexibility/robustness is a separate process from wakefulness stimulation, which allows the terms redundant and nonredundant to be applied more precisely. Thus, a control view adds clarity and explanatory power to understanding how the complex biology of arousal control relates to the need for the brain to operate flexibly yet robustly in uncertain environments.
Second, control schemes such as those shown in Fig. 3 are formal mathematical theories that produce precise experimentally testable predictions about the temporal dynamics of distinct neurons. Such predictions of dynamics can be directly compared with real biological dynamics (measuring of temporal patterns of neuronal activity) and are thus essential for falsifying any theories of dynamic brain function. For example, when mathematically simulated, comparator, regulator and generator neurons produce different temporal signatures of activity in response to an input [18], and this can be experimentally tested. Furthermore, a mathematical control scheme such as that in Fig. 3 allows a proof-of-concept examination of whether a particular experimentally discovered neural operation is necessary to account for wakefulness stability. For example, if integration by orexin type 2 receptor cells is taken out of the model and replaced by a different computation (amplification), it can be mathematically demonstrated that both robustness and flexibility of the system are lost [18]. In contrast, more conventional (in biology) descriptions of arousal-implicated orexin/hypocretin biology that we gave earlier in this chapter (Fig. 1 and Table 1) are not mathematical theories and do not produce useful predictions and wakefulness dynamics. Thus, control engineering theories are useful for biology because they generate clearer predictions to guide experiments aimed at brain dynamics.
6 Overview, Omissions and Future Perspectives
In summary, we have reviewed postsynaptic orexin/hypocretin actions relating to arousal and presented a control theoretical view of these actions. This view theoretically accounts both for how orexin/hypocretin generates robust-yet-flexible arousal and for why multiple nonredundant types of arousal-promoting neurons exist in the brain.
We have omitted from this brief article many publications on the topic that have potential bearing on our interpretations. However, to the best of our knowledge, there are currently no experimental observations that invalidate our general argument. For example, under some behavioural manipulations, the actions of noradrenaline on orexin/hypocretin cells in vitro have been reported to switch from inhibition to excitation [21, 122]. However, this does not mean that under such circumstances the negative feedback of arousal signals to orexin/hypocretin neurons does not exist; for example, it can be signalled by 5-HT. It would also be important to determine whether fast transmitters expressed by arousal-promoting neurons (e.g. GABA) affect orexin/hypocretin neurons. It has also been reported that some orexin/hypocretin neurons sense orexin/hypocretin themselves via orexin type 2 receptors [123], although this is controversial [19]. This opens up the possibility that orexin/hypocretin population may perform a dual function of regulators and comparators/error generators). Furthermore, we did not discuss the possible reasons for why different arousal-regulating neurons synthesize and use different transmitters, considering that activating or inhibiting downstream targets could be accomplished via glutamate, GABA and their many receptors. We speculate that transmitter diversity evolved to facilitate parallel signalling in a tight space [62], but it is beyond the scope of this review to discuss this in detail.
Overall, we feel that the results of our analysis provide some evidence that control-based logic is used by orexin/hypocretin system to dynamically control arousal. Furthermore, the integral feedback model provides a framework for studying wakefulness stability in both animal models and patients with narcolepsy. This model predicts transient responses of orexin/hypocretin neurons and more sustained responses of their downstream effector neurons to a change in arousal need [58]. These specific predictions can be tested in animal models with tools such as cell type-specific neural recordings, and this testing may aid the development of biomimetic medical robotics for patients with wakefulness disabilities.
References
Saper CB, Scammell TE, Lu J (2005) Hypothalamic regulation of sleep and circadian rhythms. Nature 437(7063):1257–1263
Scammell TE (2015) Narcolepsy. N Engl J Med 373(27):2654–2662
Dauvilliers Y, Arnulf I, Mignot E (2007) Narcolepsy with cataplexy. Lancet 369(9560):499–511
Peyron C et al (2000) A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 6(9):991–997
Ripley B et al (2001) CSF hypocretin/orexin levels in narcolepsy and other neurological conditions. Neurology 57(12):2253–2258
Nishino S et al (2001) Low cerebrospinal fluid hypocretin (orexin) and altered energy homeostasis in human narcolepsy. Ann Neurol 50(3):381–388
Thannickal TC et al (2000) Reduced number of hypocretin neurons in human narcolepsy. Neuron 27(3):469–474
Yamanaka A et al (2003) Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38(5):701–713
Hara J et al (2001) Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30(2):345–354
Mahler SV et al (2014) Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci 17(10):1298–1303
Harris GC, Wimmer M, Aston-Jones G (2005) A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437(7058):556–559
Boutrel B et al (2005) Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A 102(52):19168–19173
Sakurai T (2014) The role of orexin in motivated behaviours. Nat Rev Neurosci 15(11):719–731
Williams RH et al (2008) Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci U S A 105(33):11975–11980
Williams RH et al (2007) Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A 104(25):10685–10690
Karnani MM et al (2011) Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron 72(4):616–629
Mileykovskiy BY, Kiyashchenko LI, Siegel JM (2005) Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46(5):787–798
Kosse C, Burdakov D (2014) A unifying computational framework for stability and flexibility of arousal. Front Syst Neurosci 8:192
Li Y et al (2002) Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron 36(6):1169–1181
Yamanaka A et al (2003) Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun 303(1):120–129
Grivel J et al (2005) The wake-promoting hypocretin/orexin neurons change their response to noradrenaline after sleep deprivation. J Neurosci 25(16):4127–4130
Lin L et al (1999) The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98(3):365–376
Chemelli RM et al (1999) Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98(4):437–451
Willie JT et al (2003) Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron 38(5):715–730
Carter ME et al (2012) Mechanism for hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci U S A 109(39):E2635–E2644
Mieda M et al (2011) Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J Neurosci 31(17):6518–6526
Eriksson KS et al (2001) Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci 21(23):9273–9279
Peyron C et al (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18(23):9996–10015
Ciriello J, Caverson MM (2014) Hypothalamic orexin-A (hypocretin-1) neuronal projections to the vestibular complex and cerebellum in the rat. Brain Res 1579:20–34
Sakurai T (2007) The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 8(3):171–181
Adamantidis AR et al (2007) Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450(7168):420–424
Eggermann E et al (2001) Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience 108(2):177–181
Arrigoni E, Mochizuki T, Scammell TE (2010) Activation of the basal forebrain by the orexin/hypocretin neurones. Acta Physiol (Oxf) 198(3):223–235
Hoang QV et al (2014) Orexin (hypocretin) effects on constitutively active inward rectifier K+ channels in cultured nucleus basalis neurons. J Neurophysiol 92(6):3183–3191
Ferrari LL et al (2015) Dynorphin inhibits basal forebrain cholinergic neurons by pre- and post-synaptic mechanisms. J Physiol 594(4):1069–1085
Wu M (2004) Hypocretin/orexin innervation and excitation of identified septohippocampal cholinergic neurons. J Neurosci 24(14):3527–3536
Wu M et al (2002) Hypocretin increases impulse flow in the septohippocampal GABAergic pathway: implications for arousal via a mechanism of hippocampal disinhibition. J Neurosci 22(17):7754–7765
Mukai K et al (2009) Electrophysiological effects of orexin/hypocretin on nucleus accumbens shell neurons in rats: an in vitro study. Peptides 30(8):1487–1496
Martin G et al (2002) Interaction of the hypocretins with neurotransmitters in the nucleus accumbens. Regul Pept 104(1-3):111–117
Lambe EK, Aghajanian GK (2003) Hypocretin (orexin) induces calcium transients in single spines postsynaptic to identified thalamocortical boutons in prefrontal slice. Neuron 40(1):139–150
Lambe EK et al (2005) Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J Neurosci 25(21):5225–5229
Marcus JN et al (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435(1):6–25
Bayer L et al (2004) Exclusive postsynaptic action of hypocretin-orexin on sublayer 6b cortical neurons. J Neurosci 24(30):6760–6764
Hay YA et al (2015) Orexin-dependent activation of layer VIb enhances cortical network activity and integration of non-specific thalamocortical inputs. Brain Struct Funct 220:3497–3512
Akbari E et al (2011) Orexin-1 receptor mediates long-term potentiation in the dentate gyrus area of freely moving rats. Behav Brain Res 216(1):375–380
Wayner MJ et al (2004) Orexin-A (hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides 25(6):991–996
Selbach O et al (2010) Orexins/hypocretins control bistability of hippocampal long-term synaptic plasticity through co-activation of multiple kinases. Acta Physiol (Oxf) 198(3):277–285
Bayer L et al (2002) Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. J Neurosci 22(18):7835–7839
Lungwitz EA et al (2012) Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol Behav 107(5):726–732
Conrad KL et al (2012) Yohimbine depresses excitatory transmission in BNST and impairs extinction of cocaine place preference through orexin-dependent, norepinephrine-independent processes. Neuropsychopharmacology 37(10):2253–2266
Ishibashi M et al (2005) Effects of orexins/hypocretins on neuronal activity in the paraventricular nucleus of the thalamus in rats in vitro. Peptides 26(3):471–481
Huang H, Ghosh P, Pol AN (2006) Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol 95(3):1656–1668
Kolaj M et al (2007) Orexin-induced modulation of state-dependent intrinsic properties in thalamic paraventricular nucleus neurons attenuates action potential patterning and frequency. Neuroscience 147(4):1066–1075
Palus K, Chrobok L, Lewandowski MH (2015) Orexins/hypocretins modulate the activity of NPY-positive and -negative neurons in the rat intergeniculate leaflet via OX1 and OX2 receptors. Neuroscience 300:370–380
Chrobok L, Palus K, Lewandowski MH (2015) Orexins excite ventrolateral geniculate nucleus neurons predominantly via OX2 receptors. Neuropharmacology 103:236–246
Govindaiah G, Cox CL (2006) Modulation of thalamic neuron excitability by orexins. Neuropharmacology 51(3):414–425
Yamanaka A et al (2002) Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun 290(4):1237–1245
Schöne C et al (2014) Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep 7(3):697–704
Hoang QV et al (2003) Effects of orexin (hypocretin) on GIRK channels. J Neurophysiol 90(2):693–702
Eriksson KS et al (2004) Orexin (hypocretin)/dynorphin neurons control GABAergic inputs to tuberomammillary neurons. Eur J Neurosci 19(5):1278–1284
Schöne C et al (2012) Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. J Neurosci 32(36):12437–12443
Belle MD et al (2014) Acute suppressive and long-term phase modulation actions of orexin on the mammalian circadian clock. J Neurosci 34(10):3607–3621
Kohlmeier KA, Inoue T, Leonard CS (2004) Hypocretin/orexin peptide signaling in the ascending arousal system: elevation of intracellular calcium in the mouse dorsal raphe and laterodorsal tegmentum. J Neurophysiol 92(1):221–235
Burlet S, Tyler CJ, Leonard CS (2002) Direct and indirect excitation of laterodorsal tegmental neurons by hypocretin/orexin peptides: implications for wakefulness and narcolepsy. J Neurosci 22(7):2862–2872
Kim J et al (2009) Orexin-A and ghrelin depolarize the same pedunculopontine tegmental neurons in rats: an in vitro study. Peptides 30(7):1328–1335
Horvath TL et al (1999) Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol 415(2):145–159
Soffin EM et al (2002) SB-334867-A antagonises orexin mediated excitation in the locus coeruleus. Neuropharmacology 42(1):127–133
Soffin EM et al (2004) Pharmacological characterisation of the orexin receptor subtype mediating postsynaptic excitation in the rat dorsal raphe nucleus. Neuropharmacology 46(8):1168–1176
Murai Y, Akaike T (2005) Orexins cause depolarization via nonselective cationic and K+ channels in isolated locus coeruleus neurons. Neurosci Res 51(1):55–65
Kreibich A et al (2008) Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci 28(25):6516–6525
Henny P et al (2010) Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience 169(3):1150–1157
Brown RE et al (2002) Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline). J Neurosci 22(20):8850–8859
Liu RJ, Pol AN, Aghajanian GK (2002) Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci 22(21):9453–9464
Haj-Dahmane S, Shen RY (2005) The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J Neurosci 25(4):896–905
Bisetti A et al (2006) Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience 142(4):999–1004
Pol AN et al (2004) Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron 42(4):635–652
Li Y, Pol AN (2006) Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci 26(50):13037–13047
Burdakov D, Liss B, Ashcroft FM (2003) Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium--calcium exchanger. J Neurosci 23(12):4951–4957
Muroya S et al (2004) Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci 19(6):1524–1534
Top M et al (2004) Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci 7(5):493–494
Ma X et al (2007) Electrical inhibition of identified anorexigenic POMC neurons by orexin/hypocretin. J Neurosci 27(7):1529–1533
Shirasaka T et al (2001) Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Physiol Regul Integr Comp Physiol 281(4):R1114–R1118
Muschamp JW et al (2014) Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A 111(16):E1648–E1655
Uramura K et al (2001) Orexin-a activates phospholipase C- and protein kinase C-mediated Ca2+ signaling in dopamine neurons of the ventral tegmental area. Neuroreport 12(9):1885–1889
Borgland SL, Storm E, Bonci A (2008) Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci 28(8):1545–1556
Korotkova TM et al (2003) Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci 23(1):7–11
Li A, Nattie E (2014) Orexin, cardio-respiratory function, and hypertension. Front Neurosci 8:22–22
Kohlmeier KA et al (2008) Dual orexin actions on dorsal raphe and laterodorsal tegmentum neurons: noisy cation current activation and selective enhancement of Ca2+ transients mediated by L-type calcium channels. J Neurophysiol 100(4):2265–2281
Dias MB, Li A, Nattie EE (2009) Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol 587(Pt 9):2059–2067
Shahid IZ, Rahman AA, Pilowsky PM (2012) Orexin and central regulation of cardiorespiratory system. Vitam Horm 89:159–184
Lazarenko RM et al (2011) Orexin A activates retrotrapezoid neurons in mice. Respir Physiol Neurobiol 175(2):283–287
Young JK et al (2005) Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol (1985) 98(4):1387–1395
Smith BN et al (2002) Selective enhancement of excitatory synaptic activity in the rat nucleus tractus solitarius by hypocretin 2. Neuroscience 115(3):707–714
Yang B, Ferguson AV (2003) Orexin-A depolarizes nucleus tractus solitarius neurons through effects on nonselective cationic and K+ conductances. J Neurophysiol 89(4):2167–2175
Azhdari-Zarmehri H, Semnanian S, Fathollahi Y (2015) Orexin-a modulates firing of rat rostral ventromedial medulla neurons: an in vitro study. Cell J 17(1):163–170
Huang SC et al (2010) Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J Pharmacol Exp Ther 334(2):522–529
Antunes VR et al (2001) Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol 281(6):R1801–R1807
Top M et al (2003) Orexins induce increased excitability and synchronisation of rat sympathetic preganglionic neurones. J Physiol 549(3):809–821
Zhang GH et al (2014) Orexin A activates hypoglossal motoneurons and enhances genioglossus muscle activity in rats. Br J Pharmacol 171(18):4233–4246
Corcoran A, Richerson G, Harris M (2010) Modulation of respiratory activity by hypocretin-1 (orexin A) in situ and in vitro. Adv Exp Med Biol 669:109–113
Ivanov A, Aston-Jones G (2000) Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport 11(8):1755–1758
Peever JH, Lai Y-Y, Siegel JM (2003) Excitatory effects of hypocretin-1 (orexin-A) in the trigeminal motor nucleus are reversed by NMDA antagonism. J Neurophysiol 89(5):2591–2600
Yu L et al (2015) Orexin excites rat inferior vestibular nuclear neurons via co-activation of OX1 and OX 2 receptors. J Neural Transm (Vienna) 122(6):747–755
Korotkova TM et al (2002) Selective excitation of GABAergic neurons in the substantia nigra of the rat by orexin/hypocretin in vitro. Regul Pept 104(1-3):83–89
Yu L et al (2010) Orexins excite neurons of the rat cerebellar nucleus interpositus via orexin 2 receptors in vitro. Cerebellum 9(1):88–95
Dergacheva O et al (2012) Orexinergic modulation of GABAergic neurotransmission to cardiac vagal neurons in the brain stem nucleus ambiguus changes during development. Neuroscience 209:12–20
Lecea L et al (2006) Addiction and arousal: alternative roles of hypothalamic peptides. J Neurosci 26(41):10372–10375
Patyal R, Woo EY, Borgland SL (2012) Local hypocretin-1 modulates terminal dopamine concentration in the nucleus accumbens shell. Front Behav Neurosci 6:82
Gonzalez JA et al (2016) Inhibitory interplay between orexin neurons and eating. Curr Biol 26(18):2486–2491
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117(4):500–544
Koch C (1999) Biophysics of computation. Oxford Universtiy Press, New York
Salinas E, Thier P (2000) Gain modulation: a major computational principle of the central nervous system. Neuron 27(1):15–21
Mochizuki T et al (2011) Orexin receptor 2 expression in the posterior hypothalamus rescues sleepiness in narcoleptic mice. Proc Natl Acad Sci U S A 108(11):4471–4476
Hasegawa E et al (2014) Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J Clin Invest 124(2):604–616
Sakurai T (2013) Orexin deficiency and narcolepsy. Curr Opin Neurobiol 23(5):760–766
Kayaba Y et al (2003) Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol 285(3):R581–R593
Meletti S et al (2015) The brain correlates of laugh and cataplexy in childhood narcolepsy. J Neurosci 35(33):11583–11594
Burgess CR et al (2013) Amygdala lesions reduce cataplexy in orexin knock-out mice. J Neurosci 33(23):9734–9742
DiStefano J Stubberud A, Williams I (2012) Feedback and control systems, 2nd ed. McGrawHill
Csete ME, Doyle JC (2002) Reverse engineering of biological complexity. Science 295:1664–1669
Aström K, Hagglund T (1995) PID controllers: theory, design, and tuning. Intrument Society of America (ISA). ISBN-10: 1556175167
Uschakov A et al (2011) Sleep-deprivation regulates alpha-2 adrenergic responses of rat hypocretin/orexin neurons. PLoS One 6(2):e16672
Yamanaka A et al (2010) Orexin directly excites orexin neurons through orexin 2 receptor. J Neurosci 30(38):12642–12652
Ohno K, Sakurai T (2008) Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front Neuroendocrinol 29(1):70–87
Lu J et al (2006) A putative flip-flop switch for control of REM sleep. Nature 441(7093):589–594
Sakurai T et al (2005) Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron 46(2):297–308
Yoshida K et al (2006) Afferents to the orexin neurons of the rat brain. J Comp Neurol 494(5):845–861
Tsujino N et al (2005) Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci 25(32):7459–7469
Gonzalez JA et al (2016) Awake dynamics and brain-wide direct inputs of hypothalamic MCH and orexin networks. Nat Commun 7:11395
Schöne C, Burdakov D (2012) Glutamate and GABA as rapid effectors of hypothalamic “peptidergic” neurons. Front Behav Neurosci 6:81
Acknowledgements
This work was funded by The Francis Crick Institute, which receives its core funding from Cancer Research UK, the UK Medical Research Council, and the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing AG
About this chapter
Cite this chapter
Schöne, C., Burdakov, D. (2016). Orexin/Hypocretin and Organizing Principles for a Diversity of Wake-Promoting Neurons in the Brain. In: Lawrence, A.J., de Lecea, L. (eds) Behavioral Neuroscience of Orexin/Hypocretin. Current Topics in Behavioral Neurosciences, vol 33. Springer, Cham. https://doi.org/10.1007/7854_2016_45
Download citation
DOI: https://doi.org/10.1007/7854_2016_45
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57534-6
Online ISBN: 978-3-319-57535-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)