Abstract
Negative affective schema and associated biases in information processing have long been associated with clinical depression. Such an approach has guided the development of successful psychological therapies for this and other emotional disorders. However, until quite recently, there has been a large chasm between the practitioners and scientists working with this approach and those working on the neurobiological basis of depression and its treatment. Recent research, however, has started to bridge this gap and our understanding of the neural processes underpinning these cognitive processes has progressed markedly over the past decade. Moreover, rather than representing separate targets for psychological and biological treatments, novel findings suggest that pharmacological interventions for depression also modify these psychological maintaining factors early in treatment and may be involved in the later emergence of clinically relevant change. Such findings offer the possibility of greater integration between psychological and pharmacological conceptualisations of psychiatric illness and provide an experimental medicine model to generate and test specific predictions. Such a model could be applied to improve treatment development, stratification and combination approaches for patients with depression and provide a framework for considering and overcoming treatment nonresponse.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The first antidepressant drug treatments were discovered by chance over 50 years ago. Since then, intense research effort both in academic and industrial laboratories has explored the key processes underlying antidepressant drug action and in using this information to refine and develop more effective and targeted antidepressant agents. This characterisation has largely explored the neurophysiological actions of drug treatments on neurochemical, cellular and molecular processes. By contrast, there has been little work exploring how these neural changes act to reverse the psychological experiences seen in depression and little integration between pharmacological and psychological theories and treatment for depression (see Harmer et al. 2009a).
However, it is likely that these different approaches, rather than being mediated by opposing processes, simply reflect different levels of analysis. Indeed, recent work reveals that antidepressant drug treatments normalise key psychological processes in depression very early on in treatment and that these actions may mediate later therapeutic change (Harmer et al. 2009a, 2011a). This chapter will review the role of psychological emotional biases in depression and the evidence that antidepressant treatments may work via early correction of emotional biases.
2 Negative Schema and Emotional Processing Bias in Depression
The original cognitive model of depression was developed by Beck (1967) and provided a revolutionary framework for the empirical identification and characterisation of factors that lead to and maintain depression. This cognitive model has stimulated research and clinical practice since then and has served as the foundation for the development of cognitive therapy. In this model, risk for depression is associated with the existence of negative self-referent schema’s (such as the underlying belief that ‘I will never succeed’) which become activated by stressors. It is well documented in cognitive psychology that our own beliefs tend to shape the kind of information we receive and remember, since with limited capacity at all stages of cognitive processing and a world full of potential cues and sources of information, we preferentially process the stimuli which make most sense to us (Bartlett 1932). For the individual at risk of depression then, information processing becomes biased towards negative self-referent information at the expense of more positive cues, potentially reinforcing and maintaining negative self beliefs and low mood (Disner et al. 2011).
Since Beck’s original formulation, the existence of these kinds of negative biases in processing have been extensively researched and described. These kinds of biases affect processes of emotional perception, elements of attention, memory and response to feedback (Roiser et al. 2012; Elliott et al. 2011) and have been linked both to the risk of depression and risk of relapse. For example, depressed patients are more likely to label facial stimuli as showing sad expressions and are typically less able to detect mild facial expressions of happiness (Harmer et al. 2009b; Gur et al. 1992; Surguladze et al. 2005). This kind of bias has been found to predict depression levels 3 and 6 months later (Hale 1998) and subsequent relapse to depression (Bouhuys et al. 1999). Further support for the hypothesis that these biases play a causal role in depression comes from studies where emotional biases have been manipulated experimentally using implicit training paradigms (“cognitive bias modification”) and which have shown subsequent effects on mood responses to stressors. That is, artificially manufacturing negative biases in healthy people can mimic some of the symptoms seen in depression (Macleod et al. 2002).
Recent neurobiological formulations of the cognitive negative bias seen in depression emphasise the role of aberrant responses and interplay between limbic, frontal and striatal areas (Disner et al. 2011; Roiser et al. 2012; Elliott et al. 2011; Harmer et al. 2011a). An imbalance in processing stimuli across this network has been suggested to lead to increased salience of negative versus positive stimuli, inefficient attentional disengagement and control processes and reduced reward response. Evidence in favour of these formulations is provided by research findings, predominantly using functional magnetic resonance imaging in combination with the presentation of emotional stimuli. Results suggest that depression is associated with increased amygdala reactivity to negative stimuli that is both more intense and longer lasting (Fu et al. 2004; Sheline et al. 2001; Siegle et al. 2002). Such effects are seen, for example, during presentation of negative pictorial stimuli such as fearful or sad facial expressions and have been related (through interactions with the hippocampus) to enhanced memory for negative stimuli in depression (Hamilton and Gotlib 2008). It has also been suggested that the on-going perception and sustained attention towards negative stimuli may occur because of reduced inhibition from cognitive control networks including the dorsolateral prefrontal cortex, dorsal anterior cingulate and ventrolateral prefrontal cortex (Disner et al. 2011; Roiser et al. 2012). Indeed, altered responses in these key areas, along with altered connectivity with the amygdala, have been observed in depression during the processing of emotional stimuli (Disner et al. 2011; Roiser et al. 2012). Exaggerated responses to negative vs positive cues has also been reported in sensory areas such as the visual cortex, which receives backprojections from the amygdala, and may function to modify visual attention to stimuli depending on their salience or relevance to the individual (Vuilleumier and Driver 2007). The responses to anticipation and receipt of reinforcing stimuli (such as money or chocolate) in the nucleus accumbens and medial orbitofrontal cortex have also been reported to be blunted in depression (Knutson et al. 2008; Pizzagalli et al. 2009) even during remission (McCabe et al. 2009) and has been suggested to underpin the experience of anhedonia (Wacker et al. 2009).
Together, these results suggest that the preferential processing of negative affective information is a key process in depression which can be indexed using both behavioural and neuroimaging methods. It is likely that the root cause of these negative biases varies across individuals as a function of genetic and environmental factors. However, it is a key hypothesis that targeting these kinds of emotional biases may be an effective way of treating depression (Harmer et al. 2009b, 2011a; Pringle et al. 2011; Roiser et al. 2012).
3 Targeting Emotional Biases in the Treatment of Depression
Cognitive behavioural therapy (CBT) explicitly aims to reduce negative emotional biases in the treatment of depression and anxiety and the magnitude of these effects have been associated with therapeutic response (Mogg et al. 1995). However, it is only recently that the potential importance of antidepressant drug effects on these processes has begun to be recognised. These effects have largely been characterised using a healthy volunteer model to allow direct actions of the drug treatment on emotional processing to be assessed in a tightly controlled laboratory setting and independently from shifts in clinical symptoms. Such an approach allows the refinement of the design and hypotheses for subsequent clinical studies. Using this translational strategy, we have established that antidepressant administration affects emotional processing across a range of paradigms, early in treatment and independently from changes in mood (see Harmer et al. 2011a). For example, 7 days administration of the selective reuptake inhibitor (SSRI) antidepressant citalopram reduced the perception of facial expressions of fear, anger and disgust and decreased negative affective memory recall compared to placebo treatment in healthy volunteers (Harmer et al. 2004).
Such effects on emotional processing are characteristic of a number of different effective antidepressants across different pharmacological classes (including citalopram, reboxetine, duloxetine, agomelatine and mirtazapine) (Arnone et al. 2009; Harmer et al. 2003, 2008, 2011b). Each of these quite different antidepressants decreased interpretation and memory for negative compared to positive information in healthy volunteers early in treatment in double blind randomised controlled studies. Such effects would be expected to reverse negative biases seen in depression and reduce the influence of this important maintaining factor. Critically, a recent study suggests that depressed patients also show these early effects of antidepressants on emotional processing, before subjective changes are seen (Harmer et al. 2009b). Thus, a single dose of reboxetine (4 mg) was able to increase positive affective responses in facial expression recognition, emotional categorisation and emotional memory in depressed patients with a similar magnitude of effects to those seen in a sample of healthy controls. Such actions suggest that effects on emotional processing are apparent within the first few hours after the first antidepressant drug dose and could be an important mechanism for emergent therapeutic effects.
4 The Neural Correlates of Early Changes in Emotional Bias
The behavioural changes seen in emotional processing with antidepressant administration are associated with altered patterns of neural response across a network of areas including the amygdala, extra striate cortex and medial prefrontal cortex (see Harmer et al. 2009a, 2011a). The amygdala overactivity seen in depression to negative affective stimuli (Fu et al. 2004; Sheline et al. 2001; Siegle et al. 2002; Victor et al. 2010) is normalised following SSRI drug treatment after 1 week (Godlewska et al. 2012) and after longer term treatment (8 weeks or more) (Sheline et al. 2001; Fu et al. 2004; Victor et al. 2010). We have also shown similar effects in healthy volunteers early in treatment. Thus, administration of SSRIs (Harmer et al. 2006; Murphy et al. 2009a), noradrenaline reuptake inhibitors (Norbury et al. 2007) and receptor blocking antidepressants (Rawlings et al. 2010) decreased amygdala response to negative vs positive facial expressions. Similar effects have now been replicated in a number of independent research groups (Windischberger et al. 2010; Arce et al. 2008). In addition to these actions within the amygdala, emotionally specific drug effects are also seen within the medial prefrontal cortex (Di Simplicio et al. 2011) involved in self-referential processing and visual areas important for the translation of emotional salience into increased visual processing and attention (Norbury et al. 2007; Rawlings et al. 2010).
It is notable that the early effects of antidepressant drug treatments largely impact on “lower-level” responses to emotional information and there is a relative absence of reported effects on prefrontal and other higher order areas thought to play a role in regulation and control (see Harmer et al. 2011a). Although it is possible that this is an artefact of the kind of emotional paradigms used in these studies, it could indicate that pharmacological treatments are particularly working via this bottom-up route (i.e., decreasing automatic responses to emotional information rather than increasing more strategic control processes) (Harmer et al. 2011a; Roiser et al. 2012). Longer term antidepressant treatment has been reported to increase connectivity between frontal cortex and amygdala responses (Anand et al. 2005, 2007; Chen et al. 2008) though the direction of this action is not clear and may also occur through reduced amygdala responsively. These findings raise interesting questions about the initial locus of antidepressant drug action and whether efficacy could be improved by agents targeting the integrity and function of strategic control pathways (Roiser et al. 2012).
There is also increasing interest in the effect of drug treatment on resting-state connectivity patterns. Sheline et al. (2010) reported increased levels of connectivity between resting state networks involving the medial PFC (“Dorsal Nexus”) in depression, suggesting aberrant interconnection between cognitive and emotional processes. The effect of treatment on network function is currently being investigated, but an initial study found that a 1-week treatment of citalopram was able to reduce aberrant connectivity between the dorsal nexus and hippocampus in healthy controls (McCabe et al. 2011). Given the hypothesised role for emotional modulation of hippocampal memory encoding (Hamilton and Gotlib 2008), this may be an intriguing corollary of the reduced negative recall bias seen with antidepressant treatment (Harmer et al. 2009b, 2004). Again, further studies are needed to explore early effects in depression and in relation to early changes in emotional processing and to later therapeutic action.
Together, these results suggest that antidepressants can affect limbic areas underpinning emotional processing very early on in treatment. Such effects reinforce the findings seen in the behavioural models of cognitive bias and highlight a previously undiscovered action of antidepressants which appears to precede clinical response. Such results, however, raise the question of why the clinical therapeutic actions of antidepressants are not seen earlier in treatment.
5 Understanding the Delayed Onset of Clinical Therapeutic Antidepressant Effects
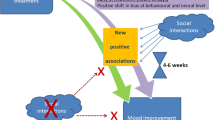
A key hypothesis of the cognitive neuropsychological model is that the time-lag in clinical therapeutic effects of antidepressant treatments occur because environmental and social interaction is needed before a change in emotional bias will be able to affect mood and behaviour (Harmer et al. 2009a). A similar explanation has been proposed to account for the delayed effects of other manipulations which target emotional bias on subjective mood and anxiety responses. In particular, cognitive bias modification, which experimentally manipulates emotional bias using a psychological training technique (such as repeatedly pairing a probe to which the volunteer has to respond quickly with the location of a threatening stimulus (Macleod et al. 2002) also affects mood and anxiety with a delayed onset of action. However, although subjective state is not immediately affected under baseline conditions, this cognitive bias modification does modulate emotional reactions to a stressful task situation. For example, volunteers who have been trained to attend to a threatening stimulus show an increased negative mood response to a laboratory stressor relative to those volunteers who had been trained to selectively attend away from threatening stimuli (MacLeod et al. 2002). These results reinforce the idea that environmental interactions are critically important in determining how changes in emotional bias will affect mood. In depressed patients receiving antidepressant treatment, such interactions are likely to happen naturally with every day challenges, social contact and life events. However, it is also possible to probe such environmental effects experimentally using a laboratory stressor.
To test the idea that antidepressant-induced changes in emotional bias impact on mood only in interaction with stress and emotional events, we investigated the effects of 7 days administration of citalopram on the response to a negative mood challenge in healthy volunteers. As expected, short-term citalopram administration did not affect mood or subjective state under baseline conditions, but was able to protect healthy volunteers against the mood lowering effects of a negative mood induction compared to double blind administration of placebo (Browning et al. 2011). Furthermore, as hypothesised by the cognitive neuropsychological model, the magnitude of this protective action on mood response was predicted by the effect of citalopram on emotional memory. That is, those volunteers showing the largest reduction in negative bias were least susceptible to the negative mood induction.
These findings support the idea that although changes in emotional bias may occur quickly with antidepressant administration, such treatment would still be expected to have a delayed effect on clinical symptoms such as depressed mood. As such, this approach can provide a psychological explanation for the clinical delay in antidepressant drug treatment efficacy and also suggests a plausible mechanism whereby changes in neurochemical function induced by antidepressant drug treatment become translated into clinical improvement. Consistent with this delayed onset of action, results from a clinical study suggested that the change in the recognition of happy facial expressions with antidepressant use at 2 weeks correlated with therapeutic change 4 weeks later in depressed patients (Tranter et al. 2009).
While other models have been proposed to account for the delay in clinical efficacy of antidepressant drug treatment, these hypotheses usually emphasise the importance of delayed neurobiological actions in treatment effects. For example, the neurotrophic theory suggests that antidepressant effects on brain plasticity are required for successful antidepressant response (Duman and Monteggia 2006). These delayed antidepressant effects on plasticity are believed to include increased elaboration of neurotrophic factors (such as BDNF), neurogenesis, neuronal maturation and synaptic plasticity and at a behavioural level have been linked to changes in learning (Nissen et al. 2010; Normann et al. 2007). In isolation, it is difficult to explain how these improvements in plasticity and learning are linked to reduction in the different clinical symptoms seen in depression. However, such effects may well be critical for the translation of increased positive emotional bias described above into sustained improvements in the affect and social function of depressed patients. Hence, it may be predicted that the therapeutic effects of antidepressant agents which positively bias emotional processing could benefit from additional effects on neuroplasticity, particularly in view of the known deleterious effects of depression on learning and memory performance (Elliott 1998).
6 Applications
The surprising observation that antidepressants have early effects on emotional processing both in depressed and healthy volunteers has a number of theoretical and practical applications. This approach provides an experimental medicine model which can be used to generate specific predictions regarding treatment development, nonresponse and combination therapy.
6.1 Drug Development
Despite innovations in the process of drug development, new treatments for central nervous disorder conditions often fail relatively late in development because of problems in efficacy (Kola and Landis 2004). Typically, these candidate treatments are assessed for safety in Phase 1 healthy volunteer studies before efficacy is tested in Phase 2 and 3 studies in depressed patients. Phase 2 and 3 clinical trials are expensive, time-consuming and expensive but are often embarked on in the absence of any indication of efficacy in humans. It is clear that the inclusion of efficacy markers in Phase 1 studies may provide information for decision making and refinement of the compound for development but until recently there has been the absence of these kinds of biomarker models of efficacy which could be used in healthy volunteers.
The data reviewed above, however, suggests that early effects of drug treatments on emotional processing may provide a possible biomarker. These measures of emotional processing are affected early on in treatment, in healthy volunteers as well as depressed patients and target a key psychological process known to be important in depression. It is also notable that these effects are seen across antidepressant classes and in the absence of more overt changes in mood. Such results suggest that characterising novel candidate treatments early in development may provide some key information concerning effective dose, clinical profile and mechanism of action. Consistent with this, we have recently found that these human models were maximally sensitive to the same dose of the novel antidepressant agomelatine identified as effective in RCTs (Harmer et al. 2011b). Furthermore, these measures of emotional processing were unaffected, or not consistently affected, by failed antidepressant candidates such as the NK1 antagonist aprepitant (Chandra et al. 2010) which were positive in preclinical animal models of depression. These preliminary findings support the use of this human experimental medicine approach for the characterisation and screening of new agents for the treatment of depression and anxiety. Further work is required to assess the ability of these models to predict wanted and unwanted actions of novel drug treatments and differentiate between different candidates at this early stage of development.
6.2 Prediction of Treatment Nonresponse
While around 30 % of patients achieve remission with a single course of an antidepressant, this figure can be effectively doubled by trying different treatment iterations (Rush et al. 2007). However, such a trial and error approach for the treatment of a patient, coupled with the slow onset of clinical antidepressant drug effects, can lead to significant delay in effective management and increase risk of long-term morbidity and disability. Recent data suggest that early change in emotional processing with antidepressant administration may predict therapeutic response seen 4 weeks later (Tranter et al. 2009).
We are currently investigating whether the initial emotional processing response with treatment has sufficient sensitivity and specificity to inform whether a change in drug or dose is necessary early in treatment. This framework also provides a model for understanding why a particular treatment may fail for an individual. Thus, a treatment would be predicted to fail if the drug was unsuccessful in shifting cognitive bias. However, a treatment may also be unsuccessful if, despite a shift in cognitive bias, the patient is not receiving sufficient exposure to emotional and social stimuli to allow the change in processing to affect mood. This may be a particular problem for depression which is often characterised by social isolation and withdrawal and would be predicted to limit the ability of the antidepressants to improve depressed mood. For other patients, cognitive biases may be too entrenched to shift with standard antidepressant treatment. It is an intriguing idea that it may be possible to boost the effects of pharmacological treatment with specific psychological intervention though further investigation is needed to assess the best methods for this combination. For example, it is interesting to consider whether boosting exposure to and interpretation of the environment and social situations early in the treatment with antidepressants could build on these early positive effects. Such hypotheses need to be explored systematically using these experimental medicine models but could have far reaching implications for clinical practice.
6.3 Treatment Stratification
While different antidepressants with different pharmacological effects have common actions on the processing of emotional information which may be important in their therapeutic actions, there also seems to be some specific differences between agents which may be relevant to their clinical profiles. For example, negative biases in emotional memory recall have been suggested to be particularly relevant to depression, with less clear-cut effects emerging in the anxiety disorders (Bradley et al. 1997; Williams et al. 1997). Drug treatments for depression also have very consistent effects on emotional memory. Thus, SSRIs like citalopram, novel antidepressants like agomelatine and receptor blocking antidepressants such as mirtazapine all decreased relative recall for negative compared to positive emotional information in healthy volunteers in double blind placebo controlled studies (see Harmer et al. 2011b). Such effects have also been described for non-drug treatments for depression such as vagus nerve stimulation (Critchley et al. 2007), negative ion administration (Malcolm et al. 2009; Harmer et al. 2012) and cognitive bias modification (Browning et al. 2011). These actions suggest that remediation of negatively biased memory processes may be important in different therapeutic strategies for the treatment of depression, perhaps representing a final common pathway important for therapeutic effects.
By contrast, anxiety and anxiolytic drug treatments have been particularly associated with changes in attentional bias and physiological reactivity to threatening stimuli (Grillon and Charney 2011; MacLeod et al. 1986). The visual dot-probe task is able to provide a snapshot of attention to threat across time by varying the duration of stimuli presentations (MacLeod et al. 1986). Anxiety disorders and high trait levels of anxiety have been associated with increased vigilance to threatening information which is most consistently evident with short stimulus durations of 150 ms or less i.e., reflecting increased early orienting to threat rather than increased maintenance of attention (see Mogg and Bradley 1998). While attentional biases in depression have also been reported, such effects are usually seen with longer exposure durations in the order of 1,000 ms, possibly reflecting increased sustained attention to negative information rather than fast attentional orienting (Bradley et al. 1997). Consistent with this, we have observed key effects of drug treatments used for anxiety on the early attentional processing of threat in healthy volunteers (Murphy et al. 2008, 2009b). Thus, both the SSRI citalopram (also used in the treatment of anxiety) and the benzodiazepine diazepam reduced attentional vigilance to fearful facial expressions at exposure durations of 100 ms or less in healthy volunteers. These effects also seem to be relatively specific since we found that diazepam did not affect emotional memory and an antidepressant without profound anxiolytic action, reboxetine, did not affect attentional dot-probe performance (Murphy et al. 2008, 2009b). Hence, these results suggest that correction of initial hypervigilance to threat may be a common effect of drug treatments for anxiety, whereas remediation of biases in emotional memory may be particularly relevant for antidepressant agents. These differences between drug treatments imply that it may be possible to predict who is likely to benefit from different antidepressants from particular patterns of emotional bias shown before treatment. In other words, a treatment may work best for an individual if drug action is matched to the particular emotional processing bias seen in that person. These speculations remain to be tested, however, in large-scale randomised clinical trials.
7 Conclusions
It has often been assumed that antidepressants have no clinically relevant effects until they have been given over days or weeks of treatment. However, recent evidence suggests that key psychological processes which are important in depression, are affected early in treatment and may over time and experience be an important mechanism of therapeutic change in depression. This approach refocuses research on key processes that are occurring shortly after treatment initiation and provides a model by which psychological and pharmacological processes in depression can be integrated and understood. This approach also provides an experimental medicine model for the generation of specific predictions which may overcome some limitations in drug development, treatment nonresponse and the application of psychological–pharmacological combination strategies. There remains the need for large-scale clinical translation of this work, where we examine to what extent these biases predict therapeutic response, whether this could be used to improve clinical practice and treatment allocation and provide a testbed for the development and screening of novel treatment strategies.
References
Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ (2005) Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry 57(10):1079–1088
Anand A, Li Y, Wang Y, Gardner K, Lowe MJ (2007) Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an FMRI study. J Neuropsychiatry Clin Neurosci 19(3):274–282
Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP (2008) Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology 196(4):661–672
Arnone D, Horder J, Cowen PJ, Harmer CJ (2009) Early effects of mirtazapine on emotional processing. Psychopharmacology 203(4):685–691
Bartlett FC (1932) Remembering: a study in experimental and social psychology. Cambridge University Press, Cambridge
Beck AT (1967) Depression: clinical, experimental and theoretical aspects. Harper & Row, New York
Bouhuys AL, Geerts E, Gordijn MC (1999) Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. J Nerv Ment Dis 187:595–602
Bradley BP, Mogg K, Lee SC (1997) Attentional biases for negative information in induced and naturally occurring dysphoria. Behav Res Ther 35:911–927
Browning M, Grol M, Ly V, Goodwin GM, Holmes EA, Harmer CJ (2011) Using an experimental medicine model to explore combination effects of pharmacological and cognitive interventions for depression and anxiety. Neuropsychopharmacology. doi:10.1038/npp.2011.15
Chandra P, Hafizi S, Massey-Chase RM, Goodwin GM, Cowen PJ, Harmer CJ (2010) NK1 receptor antagonism and emotional processing in healthy volunteers. J Psychopharmacol 24(4):481–487 (Epub 2009 Apr 7)
Chen CH, Suckling J, Ooi C, Fu CH, Williams SC, Walsh ND, Mitterschiffthaler MT, Pich EM, Bullmore E (2008) Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology 33(8):1909–1918
Critchley HD, Lewis PA, Orth M, Josephs O, Deichmann R, Trimble MR, Dolan RJ (2007) Vagus nerve stimulation for treatment-resistant depression: behavioral and neural effects on encoding negative material. Psychosom Med 69(1):17–22
Di Simplicio M, Norbury R, Harmer CJ (2011) Short-term antidepressant administration reduces negative self-referential processing in the medial prefrontal cortex in subjects at risk for depression. Mol Psychiatry. Mar 1 (Epub ahead of print)
Disner SG, Beevers CG, Haigh EA, Beck AT (2011) Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci 12(8):467–477. doi:10.1038/nrn3027
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59(12):1116–1127
Elliott R (1998) The neuropsychological profile in unipolar depression. Trends Cogn Sci 2(11):447–454
Elliott R, Zahn R, Deakin JF, Anderson IM (2011) Affective cognition and its disruption in mood disorders. Neuropsychopharmacology 36(1):153–182
Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET (2004) Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry 61(9):877–889
Godlewska BR, Norbury R, Selvaraj S, Cowen PJ, Harmer CJ (2012) Effect of short term SSRI treatment on amygdala hyperactivity in depressed patients: a placebo controlled study. Psychol Med (in press)
Grillon C, Charney DR (2011) In the face of fear: anxiety sensitizes defensive responses to fearful faces. Psychophysiology 48:1745–1752
Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC (1992) Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res 42:241–251
Hale WW III (1998) Judgment of facial expressions and depression persistence. Psychiatry Res 80:265–274
Hamilton JP, Gotlib IH (2008) Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry 63:1155–1162
Harmer CJ, Hill SA, Taylor MJ, Cowen PJ, Goodwin GM (2003) Toward a neuropsychological theory of antidepressant drug action: increase in positive emotional bias after potentiation of norepinephrine activity. Am J Psychiatry 160:990–992
Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM (2004) Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry 161:1256–1263
Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM (2006) Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 59:816–820
Harmer CJ, Heinzen J, O’Sullivan U, Ayres RA, Cowen PJ (2008) Dissociable effects of acute antidepressant drug administration on subjective and emotional processing measures in healthy volunteers. Psychopharmacology 199:495–502
Harmer CJ, Goodwin GM, Cowen PJ (2009a) Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry 195:102–108
Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ (2009b) Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry 166:1178–1184
Harmer CJ, Cowen PJ, Goodwin GM (2011a) Efficacy markers in depression. J Psychopharmacol 25:1148–1158
Harmer CJ, de Bodinat C, Dawson GR, Dourish CT, Waldenmaier L, Adams S, Cowen PJ, Goodwin GM (2011b) Agomelatine facilitates positive versus negative affective processing in healthy volunteer models. J Psychopharmacol 25:1159–1167
Harmer CJ, Charles M, McTavish S, Favaron E, Cowen PJ (2012) Negative ion treatment increases positive emotional processing in seasonal affective disorder. Psychol Med (Epub)
Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH (2008) Neural responses to monetary incentives in major depression. Biol Psychiatry 63(7):686–692
Kola I, Landis J (2004) Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 3(8):711–715
MacLeod C, Mathews A, Tata P (1986) Attentional bias in emotional disorders. J Abnorm Psychol 95(1):15–20
MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L (2002) Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. J Abnorm Psychol 111:107–123
Malcolm CP, Cowen PJ, Harmer CJ (2009) High-density negative ion treatment increases positive affective memory. Psychol Med 39(11):1930–1932
McCabe C, Cowen PJ, Harmer CJ (2009) Neural representation of reward in recovered depressed patients. Psychopharmacology 205(4):667–677
McCabe C, Mishor Z, Filippini N, Cowen PJ, Taylor MJ, Harmer CJ (2011) SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol Psychiatry 16(6):592–594
Mogg K, Bradley BP, Millar N, White J (1995) A follow-up study of cognitive bias in generalized anxiety disorder. Behav Res Ther 33(8):927–935
Mogg K, Bradley BP (1998) A cognitive-motivational analysis of anxiety. Behav Res Ther 36:809–848
Murphy SE, Downham C, Cowen PJ, Harmer CJ (2008) Direct effects of diazepam on emotional processing in healthy volunteers. Psychopharmacology 199:503–513
Murphy SE, Norbury R, O’Sullivan U, Cowen PJ, Harmer CJ (2009a) Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry 194:535–540
Murphy SE, Yiend J, Lester KJ, Cowen PJ, Harmer CJ (2009b) Short-term serotonergic but not noradrenergic antidepressant administration reduces attentional vigilance to threat in healthy volunteers. Int J Neuropsychopharmacol 12:169–179
Nissen C, Holz J, Blechert J, Feige B, Riemann D, Voderholzer U, Normann U (2010) Learning as a model for neural plasticity in major depression. Biol Psychiatry 68(6):544–552
Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ (2007) Short-term antidepressant treatment and facial processing. Functional magnetic resonance imaging study. Br J Psychiatry 190:531–532
Normann C, Schmitz D, Fürmaier A, Bach M, Döing C (2007) Long-term plasticity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry 62(5):373–380
Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Ilosifescu DV, Rauch SL, Fava M (2009) Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 166(6):702–710
Pringle A, Browning M, Cowen PJ, Harmer CJ (2011) A cognitive neuropsychological model of antidepressant drug action. Prog Neuropsychopharmacol Biol Psychiatry 35(7):1586–1592
Rawlings NB, Norbury R, Cowen PJ, Harmer CJ (2010) A single dose of mirtazapine modulates neural responses to emotional faces in healthy people. Psychopharmacology 212(4):625–634
Roiser JP, Elliott R, Sahakian BJ (2012) Cognitive mechanisms of treatment in depression. Neuropsychopharmacology 37(1):117–136
Rush AJ (2007) STAR*D: what have we learned? Am J Psychiatry 164:201–204
Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA (2001) Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry 50(9):651–658
Sheline YI, Price JL, Yan Z, Mintun MA (2010) Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A 107(24):11020–11025
Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS (2002) Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry 51(9):693–707
Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML (2005) A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 57(3):201–209
Tranter R, Bell D, Gutting P, Harmer C, Healy D, Anderson IM (2009) The effect of serotonergic and noradrenergic antidepressants on face emotion processing in depressed patients. J Affect Disord 118:87–93
Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC (2010) Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry 67(11):1128–1138
Vuilleumier P, Driver J (2007) Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos Trans R Soc Lond B Biol Sci 362(1481):837–855
Wacker J, Dillon DG, Pizzagalli DA (2009) The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage 46(1):327–333
Williams JMG, Watts FN, MacLeod C, Mathews A (1997) Cognitive psychology and emotional disorders, 2nd edn. Wiley, Chichester
Windischberger C, Lanzenberger R, Holik A, Spindelegger C, Stein P, Moser U, Gerstl F, Fink M, Moser E, Kasper S (2010) Area-specific modulation of neural activation comparing escitalopram and citalopram revealed by pharmaco-fMRI: a randomized cross-over study. Neuroimage 49(2):1161–1170 (Epub 2009 Oct 13)
Conflict of Interest
Catherine serves on the advisory board of P1vital Ltd and owns shares in the company. She is also a company director of Oxford Psychologists Ltd and has received consultancy fees over the last 3 years from Eli-Lilly, P1vital, GSK and Servier.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Harmer, C.J. (2012). Emotional Processing and Antidepressant Action. In: Cowen, P., Sharp, T., Lau, J. (eds) Behavioral Neurobiology of Depression and Its Treatment. Current Topics in Behavioral Neurosciences, vol 14. Springer, Berlin, Heidelberg. https://doi.org/10.1007/7854_2012_210
Download citation
DOI: https://doi.org/10.1007/7854_2012_210
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-35424-3
Online ISBN: 978-3-642-35425-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)