Abstract
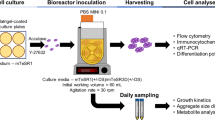
Human induced pluripotent stem cells (hiPSCs) have the potential to be used in a variety of biomedical applications, including drug discovery and Regenerative Medicine. The success of these approaches is, however, limited by the difficulty of generating the large quantities of cells required in a reproducible and controlled system. Bioreactors, widely used for industrial manufacture of biological products, constitute a viable strategy for large-scale production of stem cell derivatives. In this chapter, we describe the expansion of hiPSCs using the Vertical-Wheel™ bioreactor, a novel bioreactor configuration specifically designed for the culture of shear-sensitive cells. We provide protocols for the expansion of hiPSCs in suspension, both as floating aggregates and using microcarriers for cell adhesion. These methods may be important for the establishment of a scalable culture of hiPSCs, allowing the manufacturing of industrial- or clinical-scale cell numbers.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Shi Y, Inoue H, Wu JC, Yamanaka S (2017) Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov 16(2):115–130. https://doi.org/10.1038/nrd.2016.245
Rowe RG, Daley GQ (2019) Induced pluripotent stem cells in disease modelling and drug discovery. Nat Rev Genet. https://doi.org/10.1038/s41576-019-0100-z
Desgres M, Menasche P (2019) Clinical translation of pluripotent stem cell therapies: challenges and considerations. Cell Stem Cell 25(5):594–606. https://doi.org/10.1016/j.stem.2019.10.001
Zweigerdt R (2009) Large scale production of stem cells and their derivatives. Adv Biochem Eng Biotechnol 114:201–235. https://doi.org/10.1007/10_2008_27
Rodrigues CA, Fernandes TG, Diogo MM, da Silva CL, Cabral JM (2011) Stem cell cultivation in bioreactors. Biotechnol Adv 29(6):815–829. https://doi.org/10.1016/j.biotechadv.2011.06.009
Kropp C, Kempf H, Halloin C, Robles-Diaz D, Franke A, Scheper T, Kinast K, Knorpp T, Joos TO, Haverich A, Martin U, Zweigerdt R, Olmer R (2016) Impact of feeding strategies on the scalable expansion of human pluripotent stem cells in single-use stirred tank bioreactors. Stem Cells Transl Med 5(10):1289–1301. https://doi.org/10.5966/sctm.2015-0253
Chen VC, Couture SM, Ye J, Lin Z, Hua G, Huang HI, Wu J, Hsu D, Carpenter MK, Couture LA (2012) Scalable GMP compliant suspension culture system for human ES cells. Stem Cell Res 8(3):388–402. https://doi.org/10.1016/j.scr.2012.02.001
Abecasis B, Aguiar T, Arnault E, Costa R, Gomes-Alves P, Aspegren A, Serra M, Alves PM (2017) Expansion of 3D human induced pluripotent stem cell aggregates in bioreactors: bioprocess intensification and scaling-up approaches. J Biotechnol 246:81–93. https://doi.org/10.1016/j.jbiotec.2017.01.004
Badenes SM, Fernandes TG, Cordeiro CS, Boucher S, Kuninger D, Vemuri MC, Diogo MM, Cabral JM (2016) Defined essential 8 medium and Vitronectin efficiently support scalable Xeno-free expansion of human induced pluripotent stem cells in stirred microcarrier culture systems. PLoS One 11(3):e0151264. https://doi.org/10.1371/journal.pone.0151264
Lam AT, Li J, Chen AK, Birch WR, Reuveny S, Oh SK (2015) Improved human pluripotent stem cell attachment and spreading on Xeno-free Laminin-521-coated microcarriers results in efficient growth in agitated cultures. Biores Open Access 4(1):242–257. https://doi.org/10.1089/biores.2015.0010
Rodrigues AL, Rodrigues CAV, Gomes AR, Vieira SF, Badenes SM, Diogo MM, Cabral JMS (2019) Dissolvable microcarriers allow scalable expansion and harvesting of human induced pluripotent stem cells under Xeno-free conditions. Biotechnol J 14(4):e1800461. https://doi.org/10.1002/biot.201800461
Rodrigues CAV, Nogueira DES, Cabral JMS (2018) Next-generation stem cell expansion technologies. Cell Gene Therapy Insights 4(8):791–804. https://doi.org/10.18609/cgti.2018.076
Croughan MS, Giroux D, Fang D, Lee B (2016) Novel single-use bioreactors for scale-up of anchorage-dependent cell manufacturing for cell therapies. In: Silva CLD, Chase LG, Diogo MM (eds) Stem cell manufacturing. Elsevier, Cambridge, pp 105–139. https://doi.org/10.1016/B978-0-444-63265-4.00005-4
Rodrigues CAV, Silva TP, Nogueira DES, Fernandes TG, Hashimura Y, Wesselschmidt R, Diogo MM, Lee B, Cabral JMS (2018) Scalable culture of human induced pluripotent cells on microcarriers under xeno-free conditions using single-use Vertical-Wheel (TM) bioreactors. J Chem Technol Biotechnol 93(12):3597–3606. https://doi.org/10.1002/jctb.5738
Nogueira DES, Rodrigues CAV, Carvalho MS, Miranda CC, Hashimura Y, Jung S, Lee B, Cabral JMS (2019) Strategies for the expansion of human induced pluripotent stem cells as aggregates in single-use Vertical-Wheel bioreactors. J Biol Eng 13:74. https://doi.org/10.1186/s13036-019-0204-1
Lipsitz YY, Tonge PD, Zandstra PW (2018) Chemically controlled aggregation of pluripotent stem cells. Biotechnol Bioeng 115(8):2061–2066. https://doi.org/10.1002/bit.26719
Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW (2017) ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18(1):529. https://doi.org/10.1186/s12859-017-1934-z
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7):676–682. https://doi.org/10.1038/nmeth.2019
Acknowledgments
The authors acknowledge financial support from Fundação para a Ciência e a Tecnologia (FCT), Portugal and from Programa Operacional Regional de Lisboa 2020 (Project N. 007317) through iBB—Institute for Bioengineering and Biosciences (UID/BIO/04565/2019). The authors also acknowledge the funding received from the FCT project “CARDIOWHEEL” (PTDC/EQU-EQU/29653/2017) and Programa Operacional Regional de Lisboa 2020 through the project PRECISE—Accelerating progress toward the new era of precision medicine (PAC—PRECISE—LISBOA-01-0145-FEDER-016394, SAICTPAC/0021/2015). Diogo E.S. Nogueira thanks FCT for financial support (PD/BD/128376/2017).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Science+Business Media New York
About this protocol
Cite this protocol
Nogueira, D.E.S., Rodrigues, C.A.V., Hashimura, Y., Jung, S., Lee, B., Cabral, J.M.S. (2020). Suspension Culture of Human Induced Pluripotent Stem Cells in Single-Use Vertical-Wheel™ Bioreactors Using Aggregate and Microcarrier Culture Systems. In: Turksen, K. (eds) Stem Cells and Good Manufacturing Practices. Methods in Molecular Biology, vol 2286. Humana, New York, NY. https://doi.org/10.1007/7651_2020_287
Download citation
DOI: https://doi.org/10.1007/7651_2020_287
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1326-9
Online ISBN: 978-1-0716-1327-6
eBook Packages: Springer Protocols