Abstract
Alcohol consumption has long been associated with a majority of liver diseases and has been found to influence both fetal and adult liver functions. In spite of being one of the major causes of morbidity and mortality in the world, currently, there are no effective strategies that can prevent or treat alcoholic liver disease (ALD), due to a lack of human-relevant research models. Recent success in generation of functionally active mature hepatocyte-like cells from human-induced pluripotent cells (iPSCs) enables us to better understand the effects of alcohol on liver functions. Here, we describe the method and effect of alcohol exposure on multistage hepatic cell types derived from human iPSCs, in an attempt to recapitulate the early stages of liver tissue injury associated with ALD. We exposed different stages of iPSC-induced hepatic cells to ethanol at a pathophysiological concentration. In addition to stage-specific molecular markers, we measured several key cellular parameters of hepatocyte injury, including apoptosis, proliferation, and lipid accumulation.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Keywords
- Induced pluripotent stem cells
- Alcoholic liver disease
- Hepatic differentiation
- Liver steatosis
- Apoptosis
1 Introduction
Alcoholic liver disease (ALD) is one of the major causes of morbidity and mortality in the world (1, 2). It includes a broad range of progressive disease stages: fatty liver, liver fibrosis, liver cirrhosis, and hepatocellular carcinoma (3). Approximately 80–90 % of individuals with excessive alcohol consumption develop liver steatosis, of which 20–40 % progress into liver fibrosis without abstinence (2). Heavy alcohol consumption during pregnancy can alter the development of multiple organs in the fetus, including the brain, heart, and liver, leading to fetal alcohol spectrum disorders (FASD) (4). The reported hepatic abnormalities in subjects with FASD include hyperbilirubinemia, elevated levels of liver enzymes, and hepatomegaly, suggesting that excessive alcohol intake can lead to structural liver defects. Liver biopsy from a child with FASD has demonstrated parenchymal fat with portal and perisinusoidal fibrosis, which resembles the changes in adult human ALD (5). Animal studies have also demonstrated a wide range of liver defects under prenatal alcohol exposure (6, 7). Thus, both animal and human studies provide compelling evidence on alcohol-induced liver injury and dysfunction.
Significant challenges remain for developing preventive or curative approaches targeting ALD (8–11). This is in part due to a lack of human-relevant model systems to study alcohol effect on liver development and regeneration (12). In recent years, human-induced pluripotent stem cells (iPSCs) have been generated from diverse human somatic cells (13–16), which can then be differentiated into a spectrum of mature human cell types including functional hepatocytes (17). This development enables us to access an unlimited supply of hepatocytes, which has been one of the major challenges in the past. Moreover, human iPSCs retain the same genetic information of the donor (i.e., patient) tissues, making iPSCs a promising resource to study human genetic or acquired diseases.
We have established human-induced pluripotent stem cell lines from healthy donors and multiple liver disease patients (13–15, 18). Using our stepwise hepatic differentiation protocol, iPSCs can be induced to definite endoderm (DE), hepatic progenitor cells (HP), and then mature hepatocyte-like cells (MH) under defined conditions (13–15, 17). This in vitro process has been designed to recapitulate human liver development. Here we exposed different stages of iPSC-induced hepatic cells to alcohol (ethanol) at a pathophysiological concentration (100 mM) (19). We observe that exposure to ethanol at the pathophysiological dosage significantly reduces the expression of AFP, an early hepatic cell marker, and induces cell apoptosis, during differentiation of iPSC-derived endoderm into hepatic progenitor cells. Proliferative activity of more mature stage hepatic cells is significantly lowered. Increased amounts of lipid droplets are detected in ethanol-treated iPSC-derived hepatocytes compared to controls.
2 Materials
This study was performed in accordance with the Johns Hopkins Intuitional Stem Cell Research Oversight regulations and followed approved protocols by the Johns Hopkins Institutional Review Board.
2.1 Human-Induced Pluripotent Stem Cell (iPSC) Lines
The human iPSC lines used in this study were previously generated from diverse healthy donor tissues (13–15) and cultured in a feeder-free condition (mTeSR1 medium and Matrigel-coated plates).
2.2 Human iPSC Culture Reagents
-
1.
Matrigel hESC qualified matrix (BD Biosciences, Cat. No. 354277), store at −20 °C.
-
2.
Human iPSC culture medium: mTeSR1 Medium Kit (STEMCELL Technologies, Cat. No. 05850).
-
3.
DMEM/F12 medium (Corning, Cat. No. 10-092-CV), store at 4 °C.
-
4.
Collagenase Type IV (Sigma, Cat. No. C5138-5 g), store at 4 °C. Prepare 1 mg/ml collagenase IV solution with DMEM/F12 and filter for sterilization. Store the collagenase solution at 4 °C.
-
5.
Accutase solution (Sigma, Cat. No. A6964-100 ml), store at −20 °C.
-
6.
Cell scraper 25 cm (Sarstedt, Cat. No. 83.1830).
-
7.
Y-27632 dihydrochloride (a ROCK inhibitor; Tocris Bioscience, Cat. No. 1254-50 mg). Make 20 μl stock aliquots of 100 mM in PBS and store at −20 °C. For use in experiments, thaw the frozen aliquot, dilute the 20 μl in 380 μl PBS to yield a 5 mM solution, and store at 4 °C. The desired final concentration to treat cells is 2–10 μM.
-
8.
Penicillin-streptomycin (Gibco, Cat. No. 15140-122), store at −20 °C.
-
9.
CryoStem Freezing Medium (Stemgent, Cat. No. 01-0013-50), store at 4 °C.
-
10.
Tissue-culture-treated 12-well plastic plates (Thermo Scientific, Cat. No. 130185).
2.3 Hepatic Differentiation Reagents
-
1.
RPMI 1640 Medium with GlutaMAX (Gibco, Cat. No. 61870-036), store at 4 °C.
-
2.
Recombinant human Activin A (R&D systems, Cat. No. 338-AC/CF). Make 25 μl stock aliquots of 1 mg/ml in PBS and store at −80 °C. For use in experiments, thaw the frozen aliquot, dilute the 25 μl in 225 μl RPMI1640 medium to yield a 100 μg/ml solution, and store at 4 °C. The desired concentration to treat cells is 100 ng/ml.
-
3.
B27 supplement (Gibco, Cat. No. 17504-044), aliquot, and store at −20 °C.
-
4.
CHIR 99021 (Tocris, Cat. No. 4423, GSK-3 inhibitor). Make 250 μl stock aliquots of 20 mM in DMSO and store at −20 °C. For use in experiments, thaw the frozen aliquot, dilute the 250 μl in 250 μl DMSO to yield a 10 mM solution, and store at 4 °C. The desired final concentration to treat cells is 1–2 μM.
-
5.
William’s Medium E, no glutamine (Gibco, Cat. No. 12551-032), store at 4 °C.
-
6.
Insulin-Transferrin-Selenium (ITS) (Corning Cellgro, Cat. No. 25-800-CR), store at 4 °C. This solution contains 1,000 mg/l human recombinant insulin, 550 mg/l human recombinant transferrin, and 0.67 mg/l selenious acid.
-
7.
HEPES solution (Sigma, Cat. No. H0887-100 ml), store at 4 °C.
-
8.
GlutaMAX Supplement (Gibco, Cat. No. 35050-061), store at 4 °C.
-
9.
Dexamethasone (Sigma, Cat. No. D8893). Make stock aliquots of 10 mM in DMSO and store at 4 °C.
-
10.
Gentamicin solution (Sigma, Cat. No. G1397-10 ml), store at 4 °C.
-
11.
Recombinant human FGF-4 (R&D systems, Cat. No. 235-F4/CF). Make 50 μl stock aliquots of 500 μg/ml in PBS and store at −80 °C. For use in experiments, thaw the frozen aliquot, dilute the 50 μl in 200 μl PBS to yield a 100 μg/ml solution, and store at −20 °C. The desired concentration to treat cells is 10 ng/ml.
-
12.
Recombinant human HGF (R&D systems, Cat. No. 294-HG/CF). Make 50 μl stock aliquots of 500 μg/ml in PBS and store at −80 °C. For use in experiments, thaw the frozen aliquot, dilute the 50 μl in 200 μl PBS to yield a 100 μg/ml solution, and store at −20 °C. The desired concentration to treat cells is 10 ng/ml.
-
13.
Recombinant Human Oncostatin M (OSM) (R&D systems, Cat. No. 295-OM/CF). Make 50 μl stock aliquots of 500 μg/ml in PBS and store at −80 °C. For use in experiments, thaw the frozen aliquot, dilute the 50 μl in 200 μl PBS to yield a 100 μg/ml solution, and store at −20 °C. The desired concentration to treat cells is 10 ng/ml.
-
14.
Fetal bovine serum (HyClone, Cat. No. SH30070.03). Make aliquots and store at −20 °C.
-
15.
Hepatocyte culture medium (HCM): The base HCM contains 15 mM of HEPES, 1 % of GlutaMAX, 1 % of ITS solution, 0.1 μM of dexamethasone, and 0.1 % of gentamicin in William’s Medium E. Filter this base medium and store at 4 °C. Prior to use in cell culture, supplement this medium with HGF, FGF-4, and OSM, each at final concentration of 10 ng/ml.
2.4 Antibodies Used in the Study (Table 1)
2.5 Primer Probes Used for Real-Time PCR (Table 2)
2.6 Other Reagents and Kits
-
1.
TRIzol Reagent (Life technologies, Cat. No. 10296-028).
-
2.
High Capacity cDNA Reverse Transcription Kit (Life technologies, Cat. No. 4368813).
-
3.
0.5 % Oil Red O solution (Sigma, Cat. No. O1391).
-
4.
Annexin V Apoptosis Detection Kit (BD Pharmingen, Cat. No. 559763).
3 Methods
3.1 Matrigel Coating of 12-Well Plates for Pluripotent Stem Cell Culture
The whole procedure should be performed under extra aseptic condition.
-
1.
Thaw frozen Matrigel overnight at 4 °C. Prepare 1:4 dilution of Matrigel using chilled DMEM/F12. Prepare 5 ml aliquots with chilled pipettes and freeze them at −20 °C.
-
2.
Thaw one 5 ml aliquot on ice.
-
3.
Transfer the thawed aliquot to cold 500 ml DMEM/F-12 medium, mix well, and keep on ice.
-
4.
Add 1 ml/well diluted Matrigel into 12-well plates using chilled pipettes. Wrap the coated plates using aluminum foil and incubate at room temperature for 1 h. Store in 4 °C (see Note 1 ).
-
5.
When the plate is ready for iPSC culture, bring the plate to room temperature.
-
6.
Remove the Matrigel solution. Ensure that the coated surface is not scratched by pipette.
-
7.
Immediately add 0.5 ml/well iPSC culture medium and then plate cells.
3.2 Human iPSC Culture and Hepatic Differentiation
3.2.1 Human iPSC Culture: Thaw iPSC Lines Onto Matrigel-Coated 12-Well Plates (See Note 2)
Culture the cells in mTeSR1 medium at 37 °C with 5 % CO2. Observe the morphology of the colonies under the microscope and change medium every day. When the colonies are large and ready to merge, passage the cells with Accutase or collagenase IV (see Note 3 ).
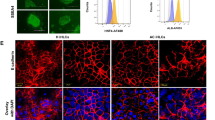
3.2.2 Differentiation of Human iPSCs to Hepatocytes (Fig. 1)
Differentiation of human iPSCs into multistage hepatic cells. (a) A schematic diagram of hepatic differentiation procedure and corresponding bright field images of hepatic cells at each stage. (b) A representative image showing the expression of Sox17 (red), a definitive endoderm marker, at differentiation day 4. (c) Flow cytometric analysis shows that ~99 % of cells express CXCR4, another definitive endoderm marker, at day 4. (d) Expression of AFP (green), a hepatocyte progenitor marker, at day 9 after initiation of hepatic differentiation. (e and f) At day 20, most of the cells express mature hepatocyte markers such as albumin (ALB, green) and alpha-1 antitrypsin (AAT, red). Scale bars, 100 μm
It is important that the iPSC colonies be evenly distributed and reach 40–80 % confluence before starting differentiation.
-
1.
Day 0: Replace iPSC culture medium with warm RPMI medium supplemented with 100 ng/ml Activin A and 1–2 μM CHIR99021 (see Note 4 ). Incubate the cells at 37 °C with 5 % CO2.
-
2.
Day 1–4/5: Continue replacing the previous culture medium with fresh RPMI supplemented with 100 ng/ml Activin A and 0.05–2 % B27 daily (see Note 5 ). After day 4, more than 90 % of the cells express endodermal markers like Sox17 and CXCR4 (Fig. 1b, c).
-
3.
Day 4–9/10: Change medium to RPMI supplemented with 100 ng/ml Activin A, 0.1–1 % B27, 10 ng/ml HGF, and 10 ng/ml FGF-4 every day. Following HGF/FGF-4 treatment, a majority of cells express hepatic progenitor marker AFP (Fig. 1d).
-
4.
Day 9/10–20: Change medium to HCM supplemented with 1–3 % FBS, 10 ng/ml HGF, 10 ng/ml FGF-4, and 10 ng/ml OSM daily (see Note 6 ). At day 20, 80–90 % of cells express characteristic mature hepatocyte markers including albumin (ALB) and alpha-1 antitrypsin (AAT) (Fig. 1e, f).
3.3 Alcohol Exposure Method and Effects of Alcohol on Hepatic Differentiation
Alcohol exposure in this model is achieved by directly adding ethanol into multistage hepatic cell culture at a final concentration of 100 mM. Constant alcohol atmosphere was maintained in the culture wells by using a microclimate chamber to prevent alcohol evaporation (20).
-
1.
Effects of alcohol on differentiation of iPSCs into definitive endoderm (DE): Treat the iPSCs with 100 mM ethanol from day 0 to 3 of endoderm differentiation. Prepare the medium containing ethanol freshly every day before use. At day 4, harvest the cells for real-time PCR, flow cytometry, and immune staining. Based upon cell morphology and Sox17 expression, endoderm differentiation is not significantly influenced by alcohol exposure (Fig. 2a). Flow cytometry analysis demonstrates increased Annexin V-positive apoptotic cells after alcohol treatment (Fig. 2a). Cell proliferation shows little to no change at this stage (Fig. 3a).
Fig. 2 Effects of alcohol on hepatic differentiation and cell viability. (a) Real-time PCR analysis of Sox17 expression (left panel) at day 4 definitive endoderm (DE) stage, following 100 mM ethanol treatment from day 0 to day 3. Percentage of Annexin V-positive cells was obtained from analysis of cell apoptosis by flow cytometry (right panel). Apoptotic cells were increased with alcohol exposure at DE stage. (b) Significant reduction in AFP expression (left panel) was observed in hepatic progenitor (HP) cells at day 9, on exposure to 100 mM ethanol from day 4 to day 8, and the quantity of Annexin V-positive apoptotic cells also increased significantly (right panel). (c) Alcohol treatment from day 11 to day 15, followed by analysis of albumin (ALB) expression (left panel) and cell viability (right panel) of the mature hepatocyte-like cells at day 16, showed no statistical difference compared with control. *: p < 0.05, #: p < 0.01
Fig. 3 Effects of alcohol on proliferation of iPSC-derived multistage hepatic cells. (a) Representative images of Ki67 (Red)-positive cells at day 4 DE stage, (b) Ki67 (green)-positive cells at day 9 HP stage, (c) ALB-positive cells (green), and Ki67 (red)-positive cells at day 16 MH stage in control and ethanol-treated cells. Scale bars, 100 μm
-
2.
Effects of alcohol on differentiation of iPSC-derived DE cells into hepatic progenitor (HP) stage cells: Differentiate iPSCs to HP stage cells, as described in Sect. 3.2. Treat the cells with 100 mM ethanol from day 4 to 8. At day 9, perform real-time PCR, apoptosis assay, and Ki67 staining (Figs. 2b and 3b). Hepatic progenitor marker, AFP, shows significant reduction after ethanol treatment. This is accompanied with significantly increased cell apoptosis at HP stage (Fig. 2b). Cell proliferation does not change significantly at this stage (Fig. 3b).
-
3.
Effects of alcohol on differentiation of iPSC-derived HP cells into hepatocyte-like cells: In this section, treat the iPSC-derived early hepatic cells with ethanol from day 11 to 15. Measure the expression of hepatocyte-specific marker albumin at day 16, by real-time PCR (Fig. 2c). Maturation and viability of iPSC-derived hepatocytes is not significantly affected by ethanol treatment at this stage. However, Ki67-positive cells are remarkably reduced (Fig. 3c), which suggests that alcohol influences cell proliferation of iPSC-derived hepatocytes.
3.4 Estimation of Fatty Changes in iPSC-Derived Mature Hepatocytes on Alcohol Exposure
Liver steatosis is the most common form of ALD, which can be found in more than 80 % patients with chronic alcohol consumption.
-
1.
Differentiate human iPSCs to mature hepatocyte-like cells (Section 3.2).
-
2.
At day 20 of differentiation, a majority of the cells express mature hepatocyte markers ALB and AAT (Fig. 1e, f). Treat these cells with 100 mM ethanol in HCM supplemented with 2 % FBS, 10 ng/ml HGF, 10 ng/ml FGF-4, and 10 ng/ml OSM, from day 20 to 24. Ethanol containing culture medium is prepared freshly prior to treatment. Seal the culture plates with parafilm to limit ethanol evaporation and maintain ethanol concentration (21). Change medium daily and culture at 37 °C and 5 % CO2.
-
3.
At day 25, harvest the cells for real-time PCR or fix them using 4 % PFA for measurement of lipid accumulation (Fig. 4).
Fig. 4 Ethanol exposure induces fat accumulation in differentiated hepatocytes from iPSCs. (a and b) Detection of lipid droplets by Oil Red O. (c) Quantification of lipid accumulation with Oil Red O staining. (d) Real-time PCR analysis of expression levels of fatty acid synthase (FASN) in control and 100 mM ethanol-treated groups. *: p < 0.05. Scale bars, 100 μm
-
4.
Staining of lipid droplet: Fix the cells with 4 % PFA at room temperature for 30 min. Wash the cells with PBS once and twice with ddH2O. Incubate the cells in 60 % isopropanol for 5 min, followed by 60 % Oil Red O solution (diluted in ddH2O) for 5 min. Finally, wash the cells with ddH2O until excess stain is no longer apparent. Keep cells covered with ddH2O at all times. Lipid droplets appear red under microscope (Fig. 4a, b).
-
5.
Quantification of lipid accumulation: After Oil Red O staining, wash the cells thrice with 60 % isopropanol. Extract the Oil Red O by treating the cells with 100 % isopropanol for 5 min with gentle rocking. Use 100 % isopropanol as background control when reading absorbance at 492 nm. Human iPSC-derived hepatocytes contain increased amount of lipid droplets after 100 mM alcohol treatment (Fig. 4c).
-
6.
To further confirm alcohol-induced lipid accumulation, analyze the expression of fatty acid synthase (FASN) using real-time PCR. Cells treated with 100 mM alcohol show increased FASN transcription, when compared with the untreated control (Fig. 4d).
4 Notes
-
1.
Use the cell culture plates within 1 week of coating with Matrigel. The plates should not be used for human iPSC/ESC culture, if not fully coated.
-
2.
Freeze cells from 6 wells of a 12-well plate (approximately two to four million cells) in one freezing vial with CryoStem Freezing medium. When thawing, thaw the cells from one freezing vial into an entire 12-well plate. Do not start the hepatic differentiation right after thawing the iPSCs when the cells are still quiescent.
-
3.
Pass the colonies as small cell aggregates. The split ratio varies with the growth condition of different cell lines (1:2–1:5). Y-27632 at 2–10 μM can be added to the culture medium to increase cell viability.
-
4.
There is difference in terms of ingredients among RPMI 1640 medium from different suppliers. The RPMI 1640 medium from Gibco works better for endoderm differentiation of most of the iPSC lines tested by us.
-
5.
Observe the cells under the microscope every day, and then determine an optimum concentration of B27 (0.02–2 %) based upon cell viability and differentiation status. Ideally, there should be cells with uniform DE morphology attached to the plate and less than 5 % of dead cells floating in the medium. When cell viability is compromised, increase B27 concentration to protect the cells. It is essential to keep an even distribution of the cells in the plates in order to obtain the best DE differentiation efficiency (>99 %).
-
6.
Adding FBS can increase viability but is not necessary for hepatic differentiation or hepatic functionality. Therefore, FBS can be omitted for certain purposes including xeno-free human liver cell generation or experiments requiring serum-free conditions.
References
Daldrup-Link HE, Nejadnik H (2014) MR imaging of stem cell transplants in arthritic joints. J Stem Cell Res Ther 4(2):165. doi:10.4172/2157-7633.1000165
Altamirano J, Bataller R (2011) Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol 8(9):491–501. doi:10.1038/nrgastro.2011.134
Tilg H, Day CP (2007) Management strategies in alcoholic liver disease. Nat Clin Pract Gastroenterol Hepatol 4(1):24–34. doi:10.1038/ncpgasthep0683
Hofer R, Burd L (2009) Review of published studies of kidney, liver, and gastrointestinal birth defects in fetal alcohol spectrum disorders. Birth Defects Res A Clin Mol Teratol 85(3):179–183. doi:10.1002/bdra.20562
Lefkowitch JH, Rushton AR, Feng-Chen KC (1983) Hepatic fibrosis in fetal alcohol syndrome. Pathologic similarities to adult alcoholic liver disease. Gastroenterology 85(4):951–957
Buts JP, Sokal EM, Van Hoof F (1992) Prenatal exposure to ethanol in rats: effects on postnatal maturation of the small intestine and liver. Pediatr Res 32(5):574–579. doi:10.1203/00006450-199211000-00018
Kockaya EA, Akay MT (2006) Histological changes in the liver of fetuses of alcohol-treated pregnant rats. Cell Biochem Funct 24(3):223–227. doi:10.1002/cbf.1215
Bataller R, North KE, Brenner DA (2003) Genetic polymorphisms and the progression of liver fibrosis: a critical appraisal. Hepatology 37(3):493–503. doi:10.1053/jhep.2003.50127
Gao B, Bataller R (2011) Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141(5):1572–1585. doi:10.1053/j.gastro.2011.09.002
Neff GW, Duncan CW, Schiff ER (2011) The current economic burden of cirrhosis. Gastroenterol Hepatol 7(10):661–671
Siegmund SV, Dooley S, Brenner DA (2005) Molecular mechanisms of alcohol-induced hepatic fibrosis. Dig Dis 23(3–4):264–274. doi:10.1159/000090174
Weber SN, Wasmuth HE (2010) Liver fibrosis: from animal models to mapping of human risk variants. Best Pract Res Clin Gastroenterol 24(5):635–646. doi:10.1016/j.bpg.2010.07.013
Choi SM, Liu H, Chaudhari P, Kim Y, Cheng L, Feng J, Sharkis S, Ye Z, Jang YY (2011) Reprogramming of EBV-immortalized B-lymphocyte cell lines into induced pluripotent stem cells. Blood 118(7):1801–1805. doi:10.1182/blood-2011-03-340620
Liu H, Kim Y, Sharkis S, Marchionni L, Jang YY (2011) In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med 3(82):82ra39. doi:10.1126/scitranslmed.3002376
Liu H, Ye Z, Kim Y, Sharkis S, Jang YY (2010) Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology 51(5):1810–1819. doi:10.1002/hep.23626
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318(5858):1917–1920. doi:10.1126/science.1151526
Choi SM, Kim Y, Shim JS, Park JT, Wang RH, Leach SD, Liu JO, Deng C, Ye Z, Jang YY (2013) Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology 57(6):2458–2468. doi:10.1002/hep.26237
Choi SM, Kim Y, Liu H, Chaudhari P, Ye Z, Jang YY (2011) Liver engraftment potential of hepatic cells derived from patient-specific induced pluripotent stem cells. Cell Cycle 10(15):2423–2427
Dolganiuc A, Szabo G (2009) In vitro and in vivo models of acute alcohol exposure. World J Gastroenterol 15(10):1168–1177
Szabo G, Mandrekar P (2008) Human monocytes, macrophages, and dendritic cells: alcohol treatment methods. Methods Mol Biol 447:113–124. doi:10.1007/978-1-59745-242-7_9
Miranda RC, Santillano DR, Camarillo C, Dohrman D (2008) Modeling the impact of alcohol on cortical development in a dish: strategies from mapping neural stem cell fate. Methods Mol Biol 447:151–168. doi:10.1007/978-1-59745-242-7_12
Acknowledgements
This work was supported in part by grants from Maryland Stem Cell Research Funds (2010-MSCRFII-0101 and 2013-MSCRFII-0170 and 2014-MSCRFF-0655) and by NIH (R43 ES023514, R21AA020020).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this protocol
Cite this protocol
Tian, L., Prasad, N., Jang, YY. (2014). In Vitro Modeling of Alcohol-Induced Liver Injury Using Human-Induced Pluripotent Stem Cells. In: Nagy, A., Turksen, K. (eds) Patient-Specific Induced Pluripotent Stem Cell Models. Methods in Molecular Biology, vol 1353. Humana Press, New York, NY. https://doi.org/10.1007/7651_2014_168
Download citation
DOI: https://doi.org/10.1007/7651_2014_168
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3033-3
Online ISBN: 978-1-4939-3034-0
eBook Packages: Springer Protocols