Abstract
Large-scale hypoxia is an inherent natural property of the Baltic Sea caused by geographically and climatically determined insufficiency of oxygen supply to the deep water layers. During 1961–2005, the hypoxic zone covered by waters with oxygen concentration less than 2 mL L–1 extended on average over a huge area of about 50,000 km2, albeit with large seasonal (a few thousand km2) and, especially inter-annual (dozens of thousand km2) variations, the later caused by an irregular ventilation with sporadic inflows of saline oxygen-enriched waters. The expansion of hypoxia induces a reduction of dissolved inorganic nitrogen pool due to denitrification and an increase of dissolved phosphate pool by internal loading, these changes reaching hundred thousand tonnes of N and P. The resulting excess of phosphate pool over the “Redfield” demand by phytoplankton is favourable for the dinitrogen fixation by cyanobacteria in amounts sufficient to compensate for denitrification and to counteract possible reductions of the nitrogen land loads.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anoxia
- Denitrification
- Eutrophication
- Hypoxic area
- Internal phosphorus loading
- Nitrogen fixation
- Oxygen deficiency
1 Introduction
Occurrence, duration, extension, and intensity of hypoxia (oxygen deficiency) in natural waters are determined by an imbalance between oxygen consumption and supply. Oxygen is consumed for oxidation of organic matter (OM) and other reductants wherever they are present in the water column and bottom sediments, and the intensity of consumption is ultimately dependent on the input of OM and ambient temperature. Because of these positive feedbacks with eutrophication and global warming and because low oxygen concentration is detrimental for aerobic organisms, hypoxia is increasingly considered an important indicator of the environmental change and “ecosystem health” (e.g. [1–4]). Mechanisms of oxygen supply vary in relation to a location within the aquatic system. In the surface water layers, where dissolved oxygen is produced by photosynthetic organisms and its concentration is regulated by air–water gas exchange and intensive vertical mixing, hypoxia may occur only when such aeration is greatly suppressed, for instance, under ice cover or beneath thick green algal mats. In the deeper layers, oxygen transports are governed by multi-scale water movements and, consequently, are hampered by any restrictions to water exchange, especially by the water stratification and geomorphologic obstacles. Oxygen conditions in the surface sediments are primarily determined by oxygen concentration in the near-bottom waters. At a certain depth deeper in the sediments, where slow downward oxygen diffusion in pore waters is not sufficient to meet oxidative demand, oxygen becomes completely exhausted and anoxia (absence of oxygen) inevitably sets in.
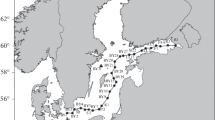
These basic mechanisms of oxygen depletion as well as related effects on nutrients have been described since long-time ago (e.g. [5] and references therein). A lot of such understanding has been acquired in the Baltic Sea (Fig. 1, [6, 7]), one of the best sampled marine systems in the world (e.g. [8, 9]). Especially hypoxia prone at a large scale is the Baltic Proper, where a combination of bathygraphic peculiarities, limited and variable water exchange with the ocean, and permanent halocline generated by estuarine circulation hampers aeration of deep water layers (Fig. 2). Deep waters of the Gulf of Bothnia have not suffered from hypoxia in the modern history both because of relatively low rates of primary production and subsequent degradation of OM and because of better ventilation due to lower salinity and weaker stratification. As in hundreds of other places [2], a local short-term, days to a few weeks, hypoxia sporadically or periodically occurs also in many shallow spots along the coasts, from the eastern Gulf of Finland to the Baltic Straits in the west (e.g. [10–12]). However important such events are for the local ecosystems and human communities, they are beyond the scope of this study.
The Baltic Sea and geographical objects mentioned in the text. GF the Gulf of Finland; GR the Gulf of Riga; BS the Baltic Straits; EGD the eastern Gotland Deep. Background map from (http://www.aquarius.geomar.de/omc/)
Geographical prerequisites of the large-scale Baltic Sea hypoxia. (a) Bathymetric scheme of the Baltic Proper with horizontal resolution of five nautical miles and a depth step of 60 m, showing a transect through international monitoring stations; (b) Salinity (psu) and (c) Oxygen (mL L–1) distributions averaged over September–October 1996–2005 (graphs were prepared from 3D fields reconstructed with DAS, see below)
Instead, this chapter focusses on the large-scale long-term oxygen dynamics and their biogeochemical consequences in the open Baltic Proper. Major aspects of these phenomena are well known for decades and have been abundantly covered in publications (e.g. [7, 13–16]). Here this knowledge is briefly reviewed and extended with estimates of such integral quantities as areas of the sediments and volumes of the water enveloped by hypoxia as well as magnitudes of the basin-wide nutrient pools, computed with special tools from big observational database. Some results obtained with this approach have already been presented in several papers (e.g. [14, 17–24]). Here these results are consistently updated and revised, including clarification of a few ambiguities and confusions that had crept into some of the above mentioned papers. One of the goals of this publication is to make computed time-series available to the readers in a numerical table form. Therefore, presentation and analysis of data is preceded by detailed description of techniques necessary for understanding the advantages and limitations of these data.
2 Data, Tools, and Methods
Data for this study are mostly taken from a marine segment of the Baltic Environment Database (BED) which contains the best part of nutrient and hydrographical measurements performed in the Baltic Sea since the end of the nineteenth century from over a hundred of research vessels belonging to all the riparian countries [25]. These data have been delivered to BED by many individuals, institutions, and agencies, whose contribution is very much appreciated (http://nest.su.se/bed/ACKNOWLE.shtml). Naturally, both the number of oceanographic stations sampled annually and the variety of chemical analyses have increased over time: from a few thousand hydrographic measurements per year in the 1900s to over 150–200 thousand measurements including finely resolved vertical profiling as well as over 20,000 nutrient samples in the 2000s. From the very beginning, the oxygen concentration has been measured by comparable modifications of the Winkler jodometric titration method, and during a century the number of measurements made annually increased by three orders of magnitude – from hundreds to amounts similar to hydrographic measurements. Following Baltic tradition [6], hydrogen sulfide concentrations, rather routinely measured in the Baltic Sea since the middle of 1960s, are stored in BED and will be presented and analyzed later as “negative oxygen” equivalents: 1 mL of H2S L–1 = –2 mL O2 L–1. Comparison to studies that used different units can be made with the following conversions: 1 mL O2 L–1 = 1.429 mg O2 L–1 = 44.6 µM; 1 mL H2S L–1 = 1.363 mg H2S L–1 = 42.6 µM.
Further on, in demonstration and discussion of the long-term dynamics I will use data from one of the major long-monitored stations situated in the centre of the Gotland Deep and often considered as a representative for typical conditions in the entire Baltic Proper. This station is widely known as BY15, a number designated to it during the international Baltic Year 1969–1970 that aimed at redox alterations in the Baltic deep layers as one of the major targets. Because the exact positions of oceanographic stations sampled in this area by different research vessels have been slightly shifting both over decades and between sampling occasions, the data were extracted from BED within the range of 57° 15'–57° 25' N and 19° 50'–20° 10' E, that is within an area of about 10 × 10 nautical miles well covering all these deviations.
A prominent feature of the Baltic Sea oceanographic data is their ample temporal and spatial coverage. The importance of regular observations on a few deep-water stations was recognized already in the 1890s, and long-term measurements have been maintained in the main Baltic deeps since then by international efforts (cf. [9]). Likewise, almost from the very beginning of measurements the oceanographic surveys performed by different countries during the year would jointly cover large parts of the Baltic Sea. The spatial data coverage has particularly improved since the 1970s due to a multi-lateral coordination of research cruises and international monitoring programme under the intergovernmental Helsinki Convention (e.g. [26, 27]). In correspondence to these particular features, two specific data analysis tools accessing BED via Internet have been developed [25, 28].
SwingStations tool has been built mainly for the analysis of temporal variations of oceanographic parameters [28]. It allows users to select vertically distributed data for a specified time interval from a geographical rectangle delimited by given coordinates of its corners. The extracted data are then pooled together as if being measured in the centre of a rectangle area and in the middle of a specific depth layer, and step-wise averaged within prescribed time window, for example within a month, or 90 days, or a year, etc. These average vertical profiles are further used to plot time-depth distribution of analyzed parameter, where intervals between the profiles are filled by interpolation. When such interpolated intervals are substantially longer than the characteristic scales of studied variations, there is a risk of misinterpretation of displayed dynamics. Therefore, user is allowed to consciously limit extent of the time interval that can be filled by interpolation. If this time interval is shorter than the gap between available measurements, the plot area is left blank. Nowadays, many of these functional features are integrated into the decision support system Nest and supplemented with some statistical analysis tools [29–31].
The Data Assimilation System [25, 32] is especially useful in the analyses of spatial aspects of the Baltic Sea hydrographic and trophic conditions. The hydrographic and nutrient data extracted for specified time intervals and regions of the Baltic Sea are processed to reconstruct three-dimensional fields with a user-determined resolution. The reconstruction starts from pooling together all the measurements found within every cell of the regular three-dimensional grid formed by columns of vertical layers with a rectangular base. The average of pooled measurements is prescribed to a centre of the corresponding cell. At the next step, the grid cells with no real measurements are filled in by linear interpolation. Since the result of 3D interpolation depends on the order of elementary one-dimensional interpolations along each of the three axes, an average of six possible permutations is used. To prevent unrealistic extrapolations, their extension should be limited by specified length interval, usually in the order of a few dozen miles, whereas concentration is kept constant further onward. After all the cells were filled in, the field is smoothed by a Tukey’s cosine-filter [33].
The reconstructed original three-dimensional gridded fields can further be subjected to any of the four arithmetic operations performed either on a single field or between two fields. The resulting field can be stored and used in a further consecutively chained algorithm, for example summing up the ammonium, nitrite, and nitrate fields to reconstruct the field of dissolved inorganic nitrogen (DIN), producing the N:P ratio field, performing stoichiometric calculations of nitrate or phosphate anomalies, etc.
Both original and derivative fields can be exploited in several ways. Most conventional are graphical presentations of spatial distribution along horizontal (maps) and vertical (transects) planes (cf. Fig. 2). Besides graphical demonstration and visual analysis of distribution, these plots are also very useful in finding questionable data indicated by peculiar “spots” in the distribution. The researcher can then analyze these data, for example comparing them to measurements made nearby and closely in time by other research vessels, and decide whether to exclude such data from the reconstruction. However, such plots can be produced by many graphical program tools. The distinctive power of DAS is a possibility to compute different characteristics from the interpolated field for a specified domain within a chosen region of the sea. The domain may be bounded by space coordinates (latitude, longitude and depth interval) and by specified range of interpolated variables. Computations produce integral quantities and volume-weighted averages of the gridded variable as well as estimates of the water volumes and bottom areas occurring within these boundaries. For instance, reconstructed time series of the salinity and oxygen fields can be used to estimate temporal dynamics of the so-called “cod reproductive volume” comprising in the brackish Baltic Sea waters with salinity over 11 psu and oxygen concentration higher than 2 mL L–1 (e.g. [34]).
There are several peculiar consequences of the described DAS implementation, which should be borne in mind while analyzing and interpreting the original and derivative gridded fields as well as the integral and average characteristics computed from them. Each original field constructed with DAS over a specific time interval represents one unique entity, characterizing spatial distribution averaged over this interval. However, a “winter” field reconstructed for a given 3-month interval may include measurements performed only in January in one part of the region and only in March in another part. Furthermore, the actual spatial and temporal distribution of oceanographic samples used for reconstruction may irregularly differ from field to field. For instance, in generation of time-series of the winter fields, the distribution and amount of measurements may differ between each of 3-month intervals representing consecutive winters. Individual ammonium and nitrate fields summed up for the reconstruction of the DIN field may each be based on different amount and distribution of actual samples. The annual average field would be affected by both seasonal variations in the surface layer and possible propagation of salt water inflow in the deep layers with related redox alterations.
In the following DAS computations, a threshold to hypoxic conditions is arbitrarily set at a bounding value of 2 mL O2 L–1 (2.86 mg O2 L–1 or 89 μM O2). Such thresholds are often inferred from the responses and vulnerability of pelagic and benthic animals, usually in the range of 1–4 mg O2 L–1 (e.g. [2, 35, 36]. Furthermore, most observational programs have routinely taken “near-bottom” samples from several meters above the bottom to both avoid contamination from the sediments and secure the equipment. As may be assumed from estimates of oxygen consumption and transfer in the Baltic deep layers [37, 38], when dissolved oxygen concentration in the near-bottom waters is below 2 mL O2 L–1, the sediment surface beneath viscous and diffusive sub-layers is actually anoxic. In other words, the sediments covered by hypoxic waters can be reasonably considered anoxic.
3 Long-Term Large-Scale Oxygen Dynamics
Actual observations of dissolved oxygen concentration are available in BED for the Gotland Deep since 1902 and are shown in Fig. 3 as time series of measurements made within certain layers. Already such, “traditional” manner of presentation can be used as a pretext for reminding on several important features of the long-term oxygen variations in the Baltic Sea.
Long-term variation of oxygen and hydrogen sulfide (shown as negative oxygen equivalents, mL L–1) in the layers 145–155 m (filled) and below 230 m (open) at oceanographic station BY15 in the Gotland Deep (57° 15′–57° 25′ N and 19° 50′–20° 10′E). For comparisons, 10 mL O2 L–1 = 446 µM of oxygen; –10 mL O2 L–1 correspond to 213 µM of hydrogen sulphide
As indicated by both direct measurements and paleoenvironmental reconstructions, the intermittent hypoxia appears to be an inherent property of the Baltic Sea. Available observations show occurrence of hypoxia already in the beginning of the previous century (cf. Fig. 3), while according to Fonselius [39], hydrogen sulfide was first found (smelled) in the samples of bottom water in 1931. Although we cannot truly quantify the intensity of anoxia in the past because of a lack of analytical measurements of hydrogen sulfide concentration, some proxies indicate sporadic occurrence of hypoxic conditions both at centennial and millennium scales. One of such proxies is the distribution of laminated sediments ([40] and references therein). Laminae are formed due to seasonal variations both in sedimentation rate and composition of sedimented material, and in anoxic conditions, in absence of benthic macrofauna, are buried undisturbed. In contrast, sediments formed and buried in oxic conditions are homogenized by different processes of bioturbation performed by active animals living in the surface sediments. Another indication of redox alterations can be found in distribution and mineral form of metals (e.g. Fe, Mn, Mo, U, V, Cu, and Zn) that would be differently affected by oxic vs. reduced conditions (e.g. [41–44]). As follows from the analysis of extensive lamination in the upper sediment layers, the deepest parts of the Baltic Proper have been anoxic for over 100–200 years [40, 45, 46]. Vertical distribution of “metallic indices” implies that alterations between oxic and anoxic marine environments have occurred in the Gotland Deep at least since the 1600s [7, 41]. Although with a lesser chronological precision and certainty, analyses of both lamination and metal’s distributions show that anoxic conditions can be traced back to the Littorina Sea stage (ca. 6,000 years BC) and occurred also in the Gulf of Bothnia, which then used to be saltier and sharper stratified, compared to the later and present stages (e.g. [46, 47]).
Occasions of “inverted” vertical distribution, with oxygen concentration in the bottom layer (deeper than 230 m) being at some moments higher than that in the above layer (145–155 m; cf. Fig. 3), clearly illustrate the main mechanism of the deep water ventilation – lateral advection of the saline waters that are heavy enough to penetrate into deep layers and displace (“push up and farther”) old stagnant waters. Since propagating water is continuously being diluted by ambient, less saline water, it is a combination of salinity and volume of the water arriving from the Baltic Straits, which determines how far and deep it can reach into the Baltic Sea. Investigations of this phenomenon, which is called “the major Baltic inflow”, date back to the end of the nineteenth century [48] and can be summarized after Schinke and Matthäus [49] and Matthäus et al. [16] in the following way.
In the first outflow phase the high atmospheric pressure over the Baltic region, augmented by easterly winds, forces water out of the Baltic Sea, where the sea level decreases below normal. In addition, this type of atmospheric circulation reduces both atmospheric precipitation and river runoff, thus further decreasing the total water volume of the sea. During the second, main phase the well-developed zonal atmospheric circulation between the North Atlantic and Europe generates strong westerly winds and increases the sea level in Kattegat. Consequently, large volumes of water are forced into the Baltic Sea through the Baltic Straits by a combined action of sea level difference, wind stress, and “sucking” action of low atmospheric pressure over the region. To produce the necessary effect, the first phase of elevated atmospheric pressure has to prevail over 1–2 months (usually in September–October), while the second phase of extremely strong and persistent westerly winds must last during several weeks. Neither condition is common for the Baltic region. In addition, the salinity in the Kattegat should stay high enough to provide for sufficient amount of salt [50]. Thus, the combination of consecutive events needed to create a continuous inflow of large amount of highly saline water (100–260 km3 with salinity higher than 17 psu; [16, 51]) happens rather seldom and is difficult to predict (Figs. 4 and 5).
Major Baltic inflows in 1880–2008 characterized by the salt transport with the inflow event (109 t). Thick columns indicate several events during the year, while no data are available during 1914–1920 and 1940–1946 because of two world wars. The estimates made by Fischer and Matthäus [51] and Matthäus [48] are borrowed from the Digital Supplement to Feistel et al. [8]
Further propagation of saline waters farther into the Baltic interior along the chain of depressions separated by sills depends on density of the “resident” waters, which, in turn, is defined by the recent hydrographic history. Inflowing oxygenated waters either penetrate into near-bottom layers and displace old waters (Fig. 6) or interleave into halocline and into intermediate, dynamically active layers just beneath it [50]. As estimated from the chronology of hydrographic measurements, the propagation of inflow from the Baltic Straits to the Gotland Deep, that is on a distance of about 650 km, usually takes 4–5 months (but, for instance, only 2 months in 1993 due to specific hydrographic situation; [16]. It is important also to remember that propagating waters are to a large extent composed of ambient Baltic waters, whose entrainment into the saline flow increases its volume by a factor of three [52].
In the bottom layers of the Gotland Deep, the deepest point en route of the high-salinity waters, the relation between salinity and oxygen concentration is straightforward: both are increased due to lateral advection of saltier waters and are decreased during the following stagnation. Salinity is decreased because of the slow diapycnic mixing across the density gradients, while oxygen is decreased because such mixing is too slow to override the biochemical consumption. In the deep active intermediate layers the relationship changes into the opposite – as illustrated by the 1980s/1990s dynamics, the decreasing salinity and weakening stratification result in improved vertical exchange and better aeration (see Fig. 5). Further to the north, at the entrances to the Gulf of Bothnia and Gulf of Finland, the advection brings in either oxygen-enriched waters propagated along the intermediate layers or oxygen-depleted waters pushed up from the “upstream” bottom layers, or often a mixture of both.
This atmospherically governed and topographically conditioned ventilation of the deep layers explains why hypoxia had occurred in the Baltic Sea already under an absent or negligible human influence. Therefore, in contrast to other regions [2, 3], a relative significance of natural vs. anthropogenic drivers of the basin scale hypoxia in the Baltic Sea has been a subject of long lasting discussion (e.g. [6, 23, 53–57]). Apparently, a measure of anthropogenic impact could be related to the oxygen consumption that should intensify along with the increasing primary production. Indeed, a long-term worsening of oxygen conditions since the 1900s, clearly seen in Fig. 3 until 1993, has been widely considered as an indicator of man-made eutrophication [6, 58]. Another indication of intensified oxygen consumption is often sought in the observation that, even though inflows of 1993/1994 and 2003 were able to aerate bottom waters in the Gotland Deep up to 3–4 mL O2 L–1, that is to the levels of the 1930s, their effects did not last long and anoxia set in faster and became “severer” than before (cf. Fig. 3).
However, as pointed out by Matthäus et al. [16], since the late nineteenth century and till the early 1980s, the episodic major inflows had occurred more or less regularly and in clusters, while only three strong inflows have happened during past quarter of the twentieth century (cf. Fig. 4). As can be estimated from the salt transport (original data by Matthäus and co-workers referred to in Fig. 4), the major inflows that occurred from 1960 to 1975 had integrally delivered through the Baltic Straits 24.1 × 109 t of salt, the weak inflows of 1976–1983 transported another 3.9 × 109 t and then, after a 10-year break, the latest three inflow brought in during 10 years (1993–2003) only 2.7 × 109 t. Considering these estimates as proxies of oxygen transport to the deep layers, the severe reduction in the natural ventilation potential is evident. On the other hand, a rate of the net decrease of oxygen concentration, which can roughly be estimated as a difference between local maximal and minimal values during several “stagnation” periods in the last 50 years, spans the range of 2–12 μL O2 L–1 d–1 and was actually higher in the 1960s (cf. Fig. 3). More sophisticated estimates of the integral oxygen consumption, which have also taken into account the oxygen transport supply, fell within the same order of magnitude and did not reveal any trends either [58, 59]. This drastic reduction of oxygen supply in combination with the relatively unchanged rate of oxygen consumption clearly indicates that the importance of anthropogenic contribution to the intensification of large-scale hypoxia should be revaluated.
In addition to site-specific data on concentration that are useful for the demonstration and analysis of the temporal oxygen dynamics, even more important at the entire ecosystem scale are the sediment areas and water volumes enveloped by hypoxia (Table 1 and Fig. 7). As was demonstrated by Andersin et al. [60] and ourselves ([14, 17]) and can be seen in Figs. 3 and 7, the hypoxic zone extension varies both seasonally and inter-annually. Being averaged over 1961–2005, the seasonal changes caused by intensification of oxygen consumption for oxidation of freshly produced and sedimented OM result in an increase of the hypoxic area from winter (January–March) extension of 43,363 ±12,567 km2 to autumn (August–September) extension of 49,124 ± 12,097 km2 (mean ± standard deviation), that is by about 13%.
Time-series in Table 1 are estimated from annually averaged fields, each of which has been reconstructed from all the measurements made during the year and pooled together. At the sites with regular observations, this DAS algorithm results in averaging of several measurements, while the inclusion of sites with occasional sampling during specialised cruises results in a kind of “cumulative” effect, that is concentration measured at such sites only once would represent the entire year. That is why the long-term (1961–2005) mean annual extension of the hypoxic area of 49,349 ± 11,077 km2 is practically equal to its maximal autumn extension presented above. In a certain sense, these annual estimates are more appropriate for studies of ecosystem effects of hypoxia, especially in the deeper layers less subject to seasonal variations. For instance, spatial expansion of hypoxia determines distribution and abundance of zoobenthos [60–62] and controls the cod reproductive volume [34]. Even a short-term anoxia could result in “dead bottoms” that would not be re-colonized fast, for example during the same year. Similarly, even a short-term occurrence of hypoxia over restricted sediment area and water volume would cause nutrient transformations and fluxes with long-lasting biogeochemical consequences.
Generally, our quantitative understanding of the main governing mechanisms has been sufficient enough to realistically simulate contemporary hypoxia and its biogeochemical consequences in mathematical models of the Baltic Sea (e.g. Fig. 8, [21, 63–68]).
4 Redox Alterations of Biogeochemical Cycles
Hypoxia-induced alterations of nitrogen and phosphorus biogeochemical cycles (Fig. 9, see also Fig. 6 above) constitute another important effect of oxygen spatial and temporal variations on ecosystem dynamics, particularly, on its eutrophication aspects.
Many evidences point at a strong dependence between redox conditions and phosphorus transformations in the water column and sediments of aquatic systems, including the Baltic Sea, although exact physical–chemical mechanisms and quantitative relationships found in different water bodies are still under debates (e.g. [6, 68–77]). Phenomenologically, in oxic conditions, dissolved inorganic phosphorus (DIP) is transformed into particulate state and removed from the water column and pore waters of sediments, while in anoxic conditions it is released back into the solution. Local manifestation of these processes is seen in Fig. 9c as accumulation of DIP in hypoxic and especially anoxic waters. A basin-wide relationship between hypoxia and phosphorus pool in the Baltic Sea was first demonstrated by us [17] on time-series estimated from three-dimensional oxygen and phosphate fields reconstructed with DAS for every winter (January–March) of 1970–2000. Here I update this large scale quantitative description of reversible phosphorus transformations and expand it over 1963–2005.
Within the larger Baltic Proper, including the Gulf of Finland and the Gulf of Riga, winter-to-winter changes of hypoxic area up to 20–30 thousand square kilometers induce the changes of integral DIP pool in the order of dozens and up to hundred thousand tonnes of phosphorus (Fig. 10). Being expressed on daily basis, the average exchange rate correspondent to empirical regression in Fig. 10b is about 0.2 mmol DIP-P m–2 day–1. Both integral and area-specific rates are quite comparable to other available estimates [17–19, 72, 78, 79]. The inter-annual changes of marine DIP pool are also comparable to and often larger than the annual net exchange with neighbouring basins [18], [19], but also, what is even more important, than the annual TP input from land to the entire Baltic Sea of 29.9 ± 4.9 × 103 t TP (mean ± SD, 1994–2006, [80]). The fact that the large scale variations of DIP pool caused by naturally occurring major Baltic inflows are up to two orders of magnitude larger than the variations of external phosphorus input has further fuelled the discussion about relative significance of climatic vs. anthropogenic drivers of the Baltic Sea eutrophication (e.g. [17, 23, 80]). However, as can be deduced from the long-term averages of winter-to-winter changes of 0.4 ± 11.2 × 103 km2 and 8 ± 45 × 103 t P, these large scale internal transformations are completely reversible, that is dozens of thousand tonnes of phosphorus are just going back and forth between the particulate and dissolved fractions. On the other hand, the total external input is unidirectional and, according to the recent reconstruction by Savchuk and Wulff [21], could have brought into the Baltic Sea about 1,800 × 103 t P over past 40 years. Apparently, this permanent addition has not been balanced by the net export to neighbouring basins and permanent sediment burial. The resulting accumulation caused by this misbalance is the reason of a 200–300 thousand tonnes difference in DIP pool between the 1960s and the 2000s; in spite of about the same extension and variation of the hypoxic area (cf. Fig. 10a).
Hypoxia and phosphate in the Baltic Proper, including the Gulf of Finland and the Gulf of Riga (cf. Fig 1). (a) Long-term dynamics of winter (January–March) extension of hypoxic area (line) and total DIP content (columns) and (b) Winter-to-winter changes of hypoxic area and DIP pool derived from data in (a)
Effects of hypoxia on the nitrogen biochemical cycle are less straightforward and cannot be reliably quantified from the site-specific observations (cf. Fig. 9a and b). On the one hand, variations of hypoxia directly determine the rate and integral amount of denitrification, especially evident in dynamics of oxidised nitrogen. Here we consider denitrification as a removal of nitrogen from the biotic cycling by any processes that lead to transformation of combined nitrogen into gaseous end products [81]. According to this biogeochemically relevant definition, denitrification comprises both canonical heterotrophic denitrification and autotrophic anammox metabolism regardless of their localization in space and time. On the other hand, in the absence of oxidized nitrogen in developed anoxic conditions, nitrogen in the form of ammonium is preserved in the system, becoming available for the biotic cycling after the redox conditions flop over. However, as we have already demonstrated [23], the resulting integral basin-wide effect of hypoxia on the nitrogen pelagic pool is quite definitely negative, that is opposite to the consequences for DIP pool (Fig. 11).
Note that being interested in simultaneous effect of hypoxia on both N and P cycles, here I use estimates that are slightly different from those used earlier in Vahtera et al. [23] and in Fig. 10. The annual deep-water nutrient pool computed only for the domain below 60 m (see Table 1) is used instead of the total basin-wide integral winter amount because the total DIP amount is weaker related to the extension of hypoxia, in contrast to their year-to-year changes (cf. Fig. 10). In an attempt to better account for a possible denitrification also in anoxic waters, the hypoxic volume comprising all the waters with oxygen concentration less than 2 mL L–1 is used instead of the denitrification volume with oxygen concentration ranging from 0 to 1 mL L–1. Although these replacements have not qualitatively affected the empirically justified theoretically casual relationships, quantitatively they resulted in slightly higher coefficients of determination.
Simultaneous nitrogen depletion and phosphorus enrichment of the pelagic nutrient pools result in a shift of marine ecosystem towards nitrogen limitation. Most importantly, the high linear correlation between the hypoxic volume and the inorganic N:P molar ratio is found not only in the deep water pools below 60 m in the Baltic Proper (coefficient of determination R 2 = 0.63) but also within the entire Baltic Proper from surface to bottom, including the Gulf of Finland and the Gulf of Riga (Fig. 12). With a 2-year lag between annual extension of hypoxia and basin-wide inorganic N:P ratio, the relationship becomes even stronger, rising up to R 2 = 0.80 in deep layers and R 2 = 0.75 in the entire system. For the time being, I have no quantitative explanation for this delay except of some vague qualitative guesses about the time needed for changes generated in the central deep layers to envelope the entire water body.
In the Baltic Proper, variations in a degree of nitrogen limitation affect not only the primary production of OM by the common phytoplankton, but also the occurrence and extension of spectacular and noxious cyanobacteria blooms (e.g. [82–85]) that are believed to be occurring here for millennia [86]. Even more important in the eutrophication perspective is that these cyanobacteria fix substantial amounts of molecular dinitrogen in the range of 200–500 × 103 t N per year [87, 88], which are quite comparable to the contemporary external input of total nitrogen from the land and atmosphere of 850 × 103 t N per year [80]. The significance of this nitrogen source, which is governed by the internal biogeochemical feedbacks, for counteracting the nitrogen load reductions is crucially important in discussions and decisions within the ecosystem approach to the environmental management [23, 80, 89].
The hypoxia-related perturbations occurring mostly in the Baltic Proper are also very important for the ecosystem dynamics in neighbouring basins. A good example is a well-documented fate of saline, oxygen-depleted and phosphate-enriched waters that were displaced in the Gotland Deep by the 1993/1994 deepwater inflows (cf. Fig. 6). Being pushed up and farther, these waters reached the Eastern Gulf of Finland by autumn 1995–spring 1996, where they significantly sharpened vertical stratification and reduced deep water oxygen concentrations [90]. Extensive autumn hypoxia, determined by these settings, not only adversely affected macrozoobenthos [91] but also induced massive internal loading of phosphate equivalent to about half of annual land loads that were added to the phosphorus exported from the west [92]. According to DAS computations, comparing to the early 1990s, this expansion of hypoxia resulted in 1997 in almost a doubling of the annual DIP pool up to 20 × 103 t P and decrease of the DIN pool from about 90 × 103 t N to 60 × 103 t N. Corresponding phosphorus surplus indicated by reduction of the N:P ratio from almost Redfield to cyanobacteria-favouring values of 6–7 [85, 93, 94] caused an expansion of cyanobacteria over the entire Gulf of Finland, in contrast to previous years when the bloom did not extend beyond its entrance [94].
5 Conclusions
The large-scale hypoxia is an inherent property of the Baltic Sea caused by geographically and climatically determined insufficiency of oxygen supply to the deep water layers. Occurrence of hypoxia are documented by direct oxygen measurements from the beginning of the twentieth century and inferred backwards over centuries and millennia from lamination and metallic indices in the dated sediment cores. Therefore, in contrast to local coastal areas, where the recent hypoxia is often related to man-made eutrophication, the anthropogenic contribution into extension and intensity of hypoxia in the deep offshore waters is still under debate. Apparently, the convincing quantitative estimates of such contribution should be obtained with the aid of mathematical models that are already now capable to realistically simulate the long-term variations of large scale hypoxia and its biogeochemical consequences.
The extension of hypoxia varies both seasonally and from year to year. The inter-annual variations reaching dozens of thousand square kilometres generate large scale effects in basin-wide nutrient pools. In the expansion phase, DIN pool is reduced by denitrification and DIP pool increases due to phosphate release from anoxic sediments, while in the shrinkage phase the changes are opposite. The expansion of hypoxia results in decreased N:P ratio that is favourable for the blooms of dinitrogen fixing cyanobacteria, another common feature of the Baltic Sea ecosystem. Nitrogen fixed by cyanobacteria becomes available for further biotic cycling, thus to a large degree compensating for the nitrogen removal by denitrification.
A historical misbalance between external input of phosphorus vs. its insufficient removal by advection and sediment burial resulted in “extra” 200–300 × 103 t of phosphorus, accumulated in the Baltic Proper since the 1960s, that in two ways counteract the environmental management measures aimed at reducing eutrophication. First, a longer time is needed to deplete the larger phosphorus pool even by the drastic reductions of the phosphorus land loads. Second, this excessive phosphorus stock supports the cyanobacterial nitrogen fixation that counteracts the nitrogen land load reductions. Therefore, it is the phosphorus load reduction that should be the priority managerial target in the Baltic Proper. The possibility to speed up the reduction of excessive phosphorus pool by such engineering methods as the forced ventilation of intermediate water layers [63, 96], or the artificial co-precipitation of phosphate should be studied in a greater quantitative detail.
Abbreviations
- BED:
-
Baltic environmental database
- DAS:
-
Data assimilation system
- DIN:
-
Dissolved inorganic nitrogen
- DIP:
-
Dissolved inorganic phosphorus
- HELCOM:
-
Helsinki commission
- OM:
-
Organic matter
References
Conley DJ, Carstensen J, Raquer-Sunyer R, Duarte CM (2009) Ecosystem thresholds with hypoxia. Hydrobiologia 629:21–29
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321:926–929
Rabalais NN, Turner RE, Diaz RJ, Justić D (2009) Global change and eutrophication of coastal waters. ICES J Mar Sci 66:1528–1537
Stramma L, Johson GC, Sprintall J, Mohrholz V (2008) Expanding oxygen-minimum zones in the tropical oceans. Science 320:655–658
Richards FA (1965) Anoxic basins and fjords. In: Riley JP, Skirrow G (eds) Chemical oceanography, Vol I. Acad Press, London
Fonselius SH (1969) Hydrography of the Baltic deep basins. III. Fish Bd Sweden, Ser Hydrogr 23
Grasshoff K, Voipio A (1981) Chemical oceanography. In: Voipio A (ed) The Baltic Sea. Elsevier, Amsterdam
Feistel R, Nausch G, Wasmund N (eds) (2008) State and evolution of the Baltic Sea, 1952–2005. Wiley, New Jersey
Fonselius S, Valderrama J (2003) One hundred years of hydrographic measurements in the Baltic Sea. J Sea Res 49:229–241
Conley DJ, Carstensen J, Ærtebjerg G, Christensen PB, Dalsgaard T, Hansen JLS, Josefson AB (2007) Long-term changes and impacts of hypoxia in Danish coastal waters. Ecol Appl 17(Suppl):S165–S184
Karlson K, Rosenberg R, Bonsdorff E (2002) Temporal and spatial large-scale effects of eutrophication and oxygen deficiency on benthic fauna in Scandinavian and Baltic waters – a review. Oceanogr Mar Biol Ann Rev 40:427–489
Maximov AA (2006) Causes of the bottom hypoxia in the eastern part of the Gulf of Finland in the Baltic Sea. Oceanology 46:185–191
Fonselius SH (1981) Oxygen and hydrogen sulphide conditions in the Baltic Sea. Mar Poll Bull 12:187–194
Conley DJ, Björck S, Bonsdorff E, Carstensen J, Destouni G, Gustafsson BG, Hietanen S, Kortekaas M, Kuosa H, Meier HEM, Müller-Karulis B, Nordberg K, Norkko A, Nürnberg G, Pitkänen H, Rabalais NN, Rosenberg R, Savchuk OP, Slomp CP, Voss M, Wulff F, Zillén L (2009) Hypoxia-related processes in the Baltic Sea. Environ Sci Technol 43:3412–3420
Kalejs M (1989) Oxygen. In: Davidan IN, Savchuk OP (eds) Problems of studies and mathematical modelling of the Baltic Sea ecosystem. 4. Main tendencies of the ecosystem’s evolution. Gidrometeoizdat, Leningrad (In Russian)
Matthäus W, Nehring D, Feistel R, Nausch G, Mohrholz LH-U (2008) The inflow of highly saline water into the Baltic. In: Feistel R, Nausch G, Wasmund N (eds) State and evolution of the Baltic Sea. Wiley, New Jersey, pp 1952–2005
Conley DJ, Humborg C, Rahm L, Savchuk OP, Wulff F (2002) Hypoxia in the Baltic Sea and basin-scale changes in phosphorus biogeochemistry. Environ Sci Technol 36:5315–5320
Savchuk OP (2005) Resolving the Baltic Sea into seven subbasins: N and P budgets for 1991–1999. J Mar Syst 56:1–15
Savchuk OP (2005) Studies of the Baltic Sea eutrophication. Proc Russ State Oceanogr Inst 209:272–285 (In Russian)
Savchuk OP, Wulff F (2007) Modeling the Baltic Sea eutrophication in a decision support system. Ambio 36:141–148
Savchuk OP, Wulff F (2009) Long-term modeling of large-scale nutrient cycles in the entire Baltic Sea. Hydrobiologia 629:209–224
Savchuk OP, Wulff F, Hille S, Humborg C, Pollehne F (2008) The Baltic Sea a century ago – a reconstruction from model simulations, verified by observations. J Mar Syst 74:485–494
Vahtera E, Conley DJ, Gustafsson BG, Kuosa H, Pitkänen H, Savchuk OP, Tamminen T, Viitasalo M, Voss M, Wasmund N, Wulff F (2007) Internal ecosystem feedbacks enhance nitrogen-fixing cyanobacteria blooms and complicate management in the Baltic Sea. Ambio 36:186–194
Witek Z, Humborg C, Savchuk O, Grelowski A, Lysiak-Pastuszak E (2003) Nitrogen and phosphorus budgets of the Gulf of Gdansk (Baltic Sea). Estuar Coast Shelf Sci 57:239–248
Sokolov A, Andrejev O, Wulff F, Rodriguez Medina M (1997) The data assimilation system for data analysis in the Baltic Sea. Systems Ecology Contributions 3, Stockholm University, Stockholm
HELCOM (1984) Guidelines for the Baltic Monitoring Programme for the second stage. Balt Sea Environ Proc 12:1–249
HELCOM (2008) Manual for marine monitoring in the COMBINE programme of HELCOM. http://www.helcom.fi/groups/monas/CombineManual/en_GB/main. Accessed 21 May 2009
Sokolov A, Wulff F (1999) SwingStations: a web-based client tool for the Baltic environmental database. Comput Geosci 25:863–871
Johansson S, Wulff F, Bonsdorff E (2007) The MARE Research Programme 1999–2006: reflections on program management. Ambio 36:119–122
Nest – an information environment for decision support system at the Baltic Nest Institute, Stockholm University. http://nest.su.se/nest. Accessed on 2 Dec 2009
Sokolov A (2002) Information environment and architecture of decision support system for nutrient reduction in the Baltic Sea. http://nest.su.se/nest. Accessed 22 May 2009
DAS – Data Assimilation System and Baltic Environment Database at the Baltic Nest Institute, Stockholm University. http://nest.su.se/das Accessed 2 Dec 2009
Tukey JW (1977) Exploratory data analysis. Addison-Wesley, Reading, Mass
MacKenzie BR, Hinrichsen H-H, Plikshs M, Wieland K, Zezera A (2000) Quantifying environmental heterogeneity: estimating the size of habitat for successful cod Gadus morhua egg development in the Baltic Sea. Mar Ecol Progr Ser 193:143–156
Diaz RJ (2001) Overview of hypoxia around the world. J Environ Qual 30:275–281
Vaquer-Sunyer R, Duarte CM (2008) Thresholds of hypoxia for marine biodiversity. Proc Nat Acad Sci 105:15452–15457
Rahm L (1987) Oxygen consumption in the Baltic proper. Limnol Oceanogr 32:973–978
Rahm L, Svensson U (1989) On the mass transfer properties of the benthic boundary layer with an application to oxygen fluxes. Neth J Sea Res 24:27–35
Fonselius S (1962) Hydrography of the Baltic Deep Basins. Fish Bd Sweden, Ser. Hydrogr 13
Jonsson P, Carman R, Wulff F (1990) Laminated sediments in the Baltic – a tool for evaluating nutrient mass balances. Ambio 19:152–157
Hallberg RO (1974) Paleoredox conditions in the Eastern Gotland Basin during the recent centuries. Merentuttkimuslait Julk 238:3–16
Sohlenius G, Emies KC, Andrén E, Andrén T, Kohly A (2001) Development of anoxia during the Holocene fresh – brackish water transition in the Baltic Sea. Mar Geol 177:221–242
Sternbeck J, Sohlenius G, Hallberg RO (2000) Sedimentary trace elements as proxies to depositional changes induced by a Holocene fresh-brackish transition. Aquat Geochem 6:325–345
Swarzenski PW, Campbell PL, Osterman LE, Poore RZ (2008) A 1000-year sediment record of recurring hypoxia off the Mississippi River: the potential role of terrestrially-derived organic matter inputs. Mar Chem 109:130–142
Hille S, Leipe T, Seifert T (2006) Spatial variability of recent sedimentation rates in the Eastern Gotland Basin (Baltic Sea). Oceanologia 48:297–317
Zillén L, Conley DJ, Andrén T, Andrén E, Björck S (2008) Past occurrences of hypoxia in the Baltic Sea and the role of climate variability, environmental change and human impact. Earth-Sci Rev 91:77–92
Ignatius H, Axberg S, Niemistö L, Winterhalter B (1981) Quaternary geology of the Baltic Sea. In: Voipio A (ed) The Baltic Sea. Elsevier, Amsterdam
Matthäus W (2006) The history of investigation of salt water inflows into the Baltic Sea – from the early beginning to recent results. Mar Sci Rep 65:1–73
Schinke H, Matthäus W (1998) On the causes of major Baltic inflows – an analysis of long time series. Cont Shelf Res 18:67–97
Stigebrandt A (2001) Physical Oceanography of the Baltic Sea. In: Wulff F, Rahm L, Larsson P (eds) A systems analysis of the changing Baltic Sea. Springer, Berlin, Heidelberg, New York
Fischer H, Matthäus W (1996) The importance of the Drogden Sill in the sound for major Baltic inflows. J Mar Res 9:137–157
Stigebrandt A, Gustafsson BG (2003) The response of the Baltic Sea to climate change – theory and observations. J Sea Res 49:243–256
Conley DJ, Bonsdorff E, Carstensen J, Destouni G, Gustafsson BG, Hansson L-A, Rabalais NA, Voss M, Zillén L (2009) Tackling hypoxia in the Baltic Sea: is engineering a solution? Environ Sci Technol 43:3407–3411
Elmgren R (2001) Understanding human impact on the Baltic ecosystem: changing views in recent decades. Ambio 30:222–231
Kuparinen J, Tuominen L (2001) Eutrophication and self-purufication: counteractions forced by large-scale cycles and hydrodynamic processes. Ambio 30:190–194
Nehring D, Matthäus W (1991) Current trends in hydrographic and chemical parameters and eutrophication in the Baltic Sea. Int Rev Gesamten Hydrobiol 76:297–316
Savchuk OP (1989) On relative significance of natural and anthropogenic factors of eutrophication. In: Davidan IN, Savchuk OP (eds) “Baltica” Project. Problems of research and modelling of the Baltic Sea ecosystem. Issue 4. Main tendencies of the ecosystem’s evolution. Hydrometeoizdat, Leningrad (In Russian)
Gustafsson BG, Stigebrandt A (2007) Dynamics of nutrients and oxygen/hydrogensulfide in the Baltic Sea deep water. J Geophys Res 112:G02023
Pers C, Rahm L (2000) Changes in apparent oxygen removal rate in the Baltic Proper deep water. J Mar Syst 25:421–429
Andersin A-B, Lassig J, Parkkonen L, Sandler H (1978) The decline of macrofauna in the deeper parts of the Baltic proper and the Gulf of Finland. Kieler Meeresforsch Sonderhft 4:23–52
Laine AO, Sandler H, Andersin A-B, Stigzelius J (1997) Long-term changes of macrozoobenthos in the Eastern Gotland Basin and the Gulf of Finland (Baltic Sea) in relation to the hydrographical regime. J Sea Res 38:135–159
Zmudzinski L (1975) The Baltic Sea pollution. Pol Arch Hydrobiol 22:601–614
Gustafsson BG, Meier HEM, Savchuk OP, Eilola OP, Axell L, Almroth E (2008) Simulation of some engineering measures aiming at reducing effects from eutrophication of the Baltic Sea. Earth Sci Rep Ser, C82, Göteborg University
Eilola K, Meier HEM, Almroth E (2009) On the dynamics of oxygen, phosphorus and cyanobacteria in the Baltic Sea; a model study. J Mar Syst 75:163–184
Kuznetsov I, Neumann T, Burchard H (2008) Model study on the ecosystem impact of a variable C:N:P ratio for cyanobacteria in the Baltic Proper. Ecol Mod 219:107–114
Savchuk O, Wulff F (1996) Biogeochemical transformations of nitrogen and phosphorus in the marine environment, Systems Ecology Contributions 2. Stockholm University, Stockholm, Sweden
Stigebrandt A, Wulff F (1987) A model for the dynamics of nutrients and oxygen in the Baltic Sea. J Mar Res 45:729–759
Yakushev EV, Pollehne F, Jost G, Kuznetsov I, Schneider B, Umlauf L (2007) Analysis of the water column oxic/anoxic interface in the Black and Baltic seas with a numerical model. Mar Chem 107:388–410
Blomqvist S, Gunnars A, Elmgren R (2004) Why the limiting nutrient differs between temperate coastal seas and freshwater lakes: a matter of salt. Limnol Oceanogr 49:2236–2241
Caraco N, Cole J, Likens GE (1990) A comparison of phosphorus immobilization in sediments of freshwater and coastal marine systems. Biogeochemistry 9:277–290
Golterman HL (2001) Phosphate release from anoxic sediments or ‘What did Mortimer really write?’. Hydrobiologia 450:99–106
Lehtoranta J, Ekholm P, Pitkänen H (2008) Eutrophication-driven sediment microbial processes can explain the regional variation in phosphorus concentrations between Baltic Sea sub-basins. J Mar Syst 74:495–504
Lukkari K, Leivuori VH, Kotilainen A (2009) The chemical character and burial of phosphorus in shallow coastal sediments in the northeastern Baltic Sea. Biogeochemistry 94:141–162
Mortimer CH (1971) Chemical exchanges between sediments and water in the Great Lakes – speculations on probable regulatory mechanisms. Limnol Oceanogr 16:387–404
Nehring D (1987) Temporal variations of phosphate and inorganic nitrogen compounds in central Baltic deep waters. Limnol Oceanogr 32:494–499
Santschi P, Hohener P, Benoit G, Buchholtz-ten Brink M (1990) Chemical processes at the sediment-water interface. Mar Chem 30:269–315
Sundby B, Gobeil C, Siverberg N, Mucci A (1992) The phosphorus cycle in coastal marine sediments. Limnol Oceanogr 37:1129–1145
Emeis K-C, Struck U, Leipe T, Pollehne F, Kunzendorf H, Christiansen C (2000) Changes in the burial rates and C:N:P ratios in the Baltic Sea sediments over the last 150 years. Mar Geol 167:43–59
Hille S, Nausch G, Leipe T (2005) Sedimentary deposition and reflux of phosphorus (P) in the Eastern Gotland Basin and their coupling with P concentrations in the water column. Oceanologia 17:663–679
HELCOM (2009) Eutrophication in the Baltic Sea – an intergrated thematic assessement of the effects of nutrient enrichment and eutrophication in the Baltic Sea region. Balt Sea Environ Proc 115B:1–152
Devol AH (2008) Denitrification including anammox. In: Capone DG, Bronk DA, Mulholland MR, Carpenter EJ (eds) Nitrogen in the marine environment, 2nd edn. Elsevier, Amsterdam
Finni T, Kononen K, Olsonen R, Wallström K (2001) The history of cyanobacterial blooms in the Baltic Sea. Ambio 30:172–178
Kahru M, Horstmann U, Rud O (1994) Satellite detection of increased cyanobacteria blooms in the Baltic Sea: natural fluctuation or ecosystem change? Ambio 23:469–472
Kahru M, Savchuk OP, Elmgren R (2007) Satellite measurements of cyanobacterial bloom frequency in the Baltic Sea: interannual and spatial variability. Mar Ecol Progr Ser 343:15–23
Niemi Å (1979) Blue-green algal blooms and N:P ratio in the Baltic Sea. Acta Bot Fenn 110:57–61
Bianchi TS, Engelhaupt E, Westman P, Andren T, Rolff C, Elmgren R (2000) Cyanobacterial blooms in the Baltic Sea: natural or human-induced? Limnol Oceanogr 45:716–726
Larsson U, Hajdu S, Walve J, Elmgren R (2001) Estimating Baltic nitrogen fixation from the summer increase in upper mixed layer total nitrogen. Limnol Oceanogr 46:811–820
Wasmund N, Nausch G, Schneider B, Nagel K, Voss M (2005) Comparison of nitrogen fixation rates determined with different methods: a study in the Baltic Proper. Mar Ecol Progr Ser 297:23–31
Savchuk O, Wulff F (1999) Modelling regional and large-scale response of Baltic Sea ecosystems to nutrient load reductions. Hydrobiologia 393:35–43
Lyakhin Ju A, Makarova SV, Maximov AA, Savchuk OP, Silina NI (1997) Ecological situation in the Eastern Gulf of Finland in July 1996. In: Davidan IN, Savchuk OP (eds) “Baltica” Project. Problems of research and modelling of the Baltic Sea ecosystem. Issue 5. Ecosystem models. Assessement of the modern state of the Gulf of Finland. Hydrometeoizdat, St Petersburg (In Russian)
Laine AO, Andersin A-B, Leiniö S, Zuur AF (2007) Stratification-induced hypoxia as a structuring factor of macrozoobenthos in the open Gulf of Finland (Baltic Sea). J Sea Res 57:65–77
Lehtoranta J (2003) Dynamics of sediment phosphorus in the brackish Gulf of Finland. Monogr Boreal Environ Res 24:1–58
Kononen K (1992) Dynamics of the toxic cyanobacterial blooms in the Baltic Sea. Finn Mar Res 261:3–36
Kononen K, Kuparinen J, Mäkelä K (1996) Initiation of cyanobacterial blooms in a frontal region at the entrance to the Gulf of Finland, Baltic Sea. Limnol Oceanogr 40:98–112
Kahru M, Leppänen J-M, Rud O, Savchuk OP (2000) Cyanobacteria blooms in the Gulf of Finland triggered by saltwater inflow into the Baltic Sea. Mar Ecol Progr Ser 207:13–18
Stigebrandt A, Gustafsson BG (2007) Improvement of Baltic Proper water quality using large-scale ecological engineering. Ambio 36:280–286
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Savchuk, O.P. (2010). Large-Scale Dynamics of Hypoxia in the Baltic Sea. In: Yakushev, E. (eds) Chemical Structure of Pelagic Redox Interfaces. The Handbook of Environmental Chemistry, vol 22. Springer, Berlin, Heidelberg. https://doi.org/10.1007/698_2010_53
Download citation
DOI: https://doi.org/10.1007/698_2010_53
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-32124-5
Online ISBN: 978-3-642-32125-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)