Abstract
Most antimicrobials currently used in the clinical practice are tested as growth inhibitors against free-floating microorganisms in a liquid suspension, rather than against sessile cells constituting biofilms. Hence, reliable, fast, and reproducible methods for assessing biofilm susceptibility to antimicrobials are strongly needed. Isothermal microcalorimetry (IMC) is a nondestructive sensitive technique that allows for the real-time monitoring of microbial viability in the presence or absence of antimicrobial compounds. Therefore, the efficacy of specific antimicrobials, alone or in combination, may be promptly validated supporting the development of new drugs and avoiding the administration of ineffective therapies. Furthermore, the susceptibility of both planktonic and biofilm cells to antimicrobials can be conveniently assessed without the need for elaborated staining procedures and under nontoxic working conditions. Quantitative data regarding the antimicrobial effect against different strains might be collected by monitoring the microbial cell replication, and, more importantly, a dose-dependent activity can be efficiently detected by measuring the delay and decrease in the heat flow peak of the treated samples. A limitation of IMC for anti-biofilm susceptibility test is the inability to directly quantify the non-replicating cells in the biofilm or the total biomass. However, as IMC is a nondestructive method, the samples can be also analyzed by using different techniques, acquiring more information complementary to calorimetric data. IMC finds application also for the investigation of antibiotic eluting kinetics from different biomaterials, as well as for studying bacteriophages activity against planktonic and biofilm bacteria. Thus, the wide applicability of this ultra-sensitive and automated technique provides a further advance in the field of clinical microbiology and biomedical sciences.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Antimicrobial susceptibility assay
- Biofilm
- Isothermal microcalorimetry
- Medical microbiology
- Real-time analysis
1 Introduction
The evaluation of antimicrobial susceptibility is a crucial procedure in the development of new drugs, as well as in the prediction of the therapeutic outcome during the treatment of an infection. Determining the minimum inhibiting concentrations (MICs) against planktonic microorganisms represents the starting point to estimate the efficacy of antimicrobial agents with the aim of successfully manage acute infections (Bjarnsholt 2013). However, since 65–80% of human infections is caused by pathogens in the form of biofilms (Coenye and Nelis 2010), the difficulty of employing conventional susceptibility tests raised the need for the development of biofilm susceptibility assays (Ciofu et al. 2017; Percival et al. 2015).
Biofilms consist of complex aggregations of microbial cells embedded within a self-produced matrix which adhere to each other and to living or abiotic surfaces (Bjarnsholt et al. 2013). Biofilm microorganisms are rather different from their planktonic counterparts in terms of metabolic status and display a significantly higher resistance to the host immune response and antibiotic treatment (Zimmerli et al. 2004), ultimately causing chronic persisting infections.
Although no “gold standard” is currently available to reveal the presence of microbial biofilm from samples collected within clinical settings, various techniques have been developed for the analysis of biofilm-embedded cells, such as crystal violet, alamar blue (Di Luca et al. 2017), resazurin (Dalecki et al. 2016), and confocal laser scanning microscopy (Di Luca et al. 2017), as well as methods based on biofilm dislodging, culture plating, and colony counting. Nevertheless, most of these methods either requires the use of toxic reagents or implies scarce reproducibility and time-consuming procedures. In addition, many of them do not allow to perform a real-time monitoring of the drug activity and to use the sample for further analysis using different methods. Thus, highly sensitive and accurate methods for the real-time analysis of biofilm are required.
IMC is a nondestructive method which allows for the monitoring in the microwatt range of any exothermic or endothermic reaction related to physical and chemical process in the tested sample. All chemical and biological processes either generate or consume heat, which can be measured by IMC as heat flow. Indeed, IMC enables a precise real-time monitoring of the heat flow related to the microbial metabolism, which might proportionally correlate to the growth rate of the tested microorganism (Braissant et al. 2010, 2013) at any time point. Recent literature also reported on the convenient combination of IMC with another noninvasive and automated technique for the investigation of metabolic profiles belonging to mature biofilms of fast- and slow-growing bacteria (Solokhina et al. 2017). Moreover, previous studies showed the suitability of this nonconventional technique as an analytical method to assess the antimicrobial activity of different compounds against several pathogens (Gonzalez Moreno et al. 2017; Bormann et al. 2017; Oliva et al. 2014) and parasites in both their planktonic and biofilm forms (Gonzalez Moreno et al. 2017; Furustrand Tafin et al. 2013; Wenzler et al. 2012). Then, the ability of resorbable and degradable biomaterials to prevent biofilm formation of various bacterial strains (Butini et al. 2018) and to treat an already established biofilm infection (Casadidio et al. 2018) was also investigated by IMC. In addition, further studies reviewed the use of this sensitive technique for biofilms research applied to various field (Buchholz et al. 2010a) and investigated the ability of chip calorimetry in evaluating the activity of antimicrobials on biofilms (Buchholz et al. 2010b). Of note are also the application of IMC for investigating the metabolism of biofilms grown on zirconia and titanium surfaces (Roehling et al. 2017) and for quantifying the antimicrobial efficacy of implant coatings (Braissant et al. 2015b).
Among others, Streptococcus pyogenes is one of the pathogens that might be isolated from hematogenous implant-associated infections due to its ability to spread and form biofilm (Gonzalez Moreno et al. 2017).
Here, we described the use of IMC to evaluate in real time the susceptibility of planktonic and biofilm S. pyogenes to levofloxacin. In addition, we reported the procedure to test the capability of antimicrobial agents to prevent biofilm growth on porous glass beads. We defined the minimum heat inhibiting concentration (MHIC) as the minimum concentration of antibiotic able to suppress the metabolic heat production of planktonic bacteria and the minimum biofilm bactericidal concentration (MBBC) as the lowest concentration that strongly reduced biofilm cells viability. As IMC is a noninvasive technique that allows to reuse the sample for further analysis, the minimal biofilm eradicating concentration (MBEC) was also evaluated by sonication of biofilms formed on the beads and plating of sonication fluids for colony counting.
2 Materials and Methods
2.1 Storage and Culture of Bacterial Strain
Stocks of Streptococcus pyogenes (strain ATCC 19615) were prepared and maintained in cryovial bead preservation system at −80 °C. The bacterial strain was cultivated on trypticase soy agar (TSA) supplemented with 5% defibrinated sheep blood for 18 h at 37 °C under 5% CO2 atmosphere.
2.2 IMC
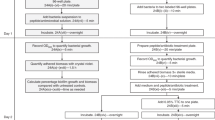
For isothermal microcalorimetric analysis, a TAM III-48 microcalorimeter (TA Instruments, New Castle, DE, USA) with a detection limit of heat production of 0.2 μW and equipped with 48 minicalorimeters was used. Sterile glass ampoules (4 ml volume) were sealed for air tightness and introduced into the minicalorimeters in the equilibration position. After 15 min, ampoules were lowered in the measuring position, and then heat flow (μW) and heat (J) were measured in real time.
2.3 Antimicrobial Assay Against Planktonic Bacteria by Real-Time IMC
An inoculum was prepared according to a McFarland standard turbidity of 0.5 (corresponding to ~108 Colony Forming Units (CFUs)/ml, λ = 565 ± 15 nm) and diluted in Cation Adjusted Müller Hinton Broth supplemented with 2.5% lysate horse blood (CAMHB+2.5% LHB) to a final concentration of ~106 CFUs/ml (T0). The exact CFUs/ml was determined by plating 100 μl of tenfold serial dilutions of the initial inoculum (T0) on TSA supplemented with 5% defibrinated sheep blood and counting colonies after 18-h incubation at 37 °C under 5% CO2 atmosphere. Then, twofold serial dilutions of 10× concentrations of levofloxacin (5 mg/ml, Sanofi) were prepared, and glass ampoules were filled with 2400 μl CAMHB+2.5% LHB, 300 μl 10× concentration of the diluted antibiotic, and 300 μl diluted bacterial suspension (T0) to a final concentration of ~1-5 × 105 CFUs/ml. One ampoule with 3000 μl CAMHB+2.5% LHB and another one with inoculated CAMHB+2.5% LHB (~1-5 × 105 CFUs/ml) were included as negative (sterility) and positive (growth) control, respectively. Ampoules were sealed for airtightness and inserted in the minicalorimeters, first in the equilibration position (15 min) and then in the measuring position. The analysis was carried out for 24 h at 37 °C, and the minimum heat inhibiting concentration (MHIC) was defined as the lowest antimicrobial concentration that inhibited the bacterial metabolic heat production during 24-h incubation in the microcalorimeter, thus resulting in an undetectable heat flow signal. Each experiment was performed in triplicate.
2.4 Antimicrobial Assay Against Biofilm Bacteria
2.4.1 Real-Time IMC
For biofilm formation on porous glass beads (diameter, 4 mm; pore size, 60 μm; porosity, 0.2 m2/g), a microbial inoculum was prepared according to a McFarland standard turbidity of 1.0 and subsequently diluted 1:10 in Tryptic Soy Broth supplemented with 2.5% lysate horse blood (TSB + 2.5% LHB). Then, sterile porous glass beads were incubated in the diluted bacterial suspension for 24 h at 37 °C. After incubation, beads with biofilm were carefully rinsed (3×) using sterile saline (0.9% NaCl) and exposed to twofold serial dilutions of antibiotic in glass ampoules filled with a final volume of 3000 μl fresh CAMHB+2.5% LHB (1 bead/1 ampoule). One ampoule containing 3000 μl CAMHB+2.5% LHB and one sterile bead and another one with 3000 μl CAMHB+2.5% LHB and a bead with untreated biofilm were included as negative (sterility) and positive (growth) control, respectively. IMC analysis was run for 24 h at 37 °C. The minimum heat inhibiting concentration for biofilm (MHICb) was defined as the lowest antimicrobial concentration that completely inhibited the heat production related to the viability of biofilm cells during 24-h incubation in the microcalorimeter, so resulting in an undetectable heat flow signal. Each experiment was performed in triplicate.
2.4.2 Sonication of Beads and Colony Counting
To determine the exact number of CFUs/ml on the glass bead after 24-h incubation, beads with biofilm were sonicated for colony counting. Briefly, washed beads were transferred to Eppendorf tubes filled with 1 ml phosphate buffered saline (PBS; pH 7.4, 10 mM) and vortexed for 30 s. Afterward, beads were sonicated for 1 min in a bath sonication instrument at 40 kHz and 0.2 W/cm2 and finally vortexed for 30 s. Fifty microliters of tenfold serial dilutions of the sonication fluid were plated on TSA supplemented with 5% defibrinated sheep blood, and colonies were counted after 18-h incubation at 37 °C under 5% CO2 atmosphere and expressed as CFUs/ml. Each experiment was performed in triplicate.
2.4.3 Evaluation of the Reduction/Eradication of Sessile Cells
2.4.3.1 By Sonication and Colony Counting
To evaluate the reduction/eradication of biofilm cells after IMC, ampoules containing biofilm showing no heat production and ampoules containing untreated biofilms (growth control) were opened, beads were carefully rinsed (3×) using sterile saline to remove any trace of antimicrobial agent, and sonication/colony counting was performed as described above (Sect. 2.4.2.). The minimum biofilm eradicating concentration (MBEC) was defined as the lowest antimicrobial concentration required to kill sessile cells (0 CFUs/bead on plate counts).
2.4.3.2 By IMC
Ampoules containing biofilm on beads showing no heat production and ampoules containing untreated biofilms (growth control) were opened, and beads were carefully rinsed (3×) using sterile saline to remove any trace of antimicrobial agent and incubated in ampoules filled with 3000 μl fresh CAMHB+2.5% LHB. One ampoule containing 3000 μl CAMHB+2.5% LHB and one sterile bead and another ampoule with 3000 μl CAMHB+2.5% LHB and a bead with untreated biofilm were included as negative control (sterility) and positive (growth) control, respectively. IMC analysis was carried out for 48 h at 37 °C. The minimum biofilm bactericidal concentration (MBBC) was defined as the lowest antimicrobial concentration that strongly reduced the number of viable bacterial cells within the biofilm, therefore leading to undetectable heat flow values. In this analysis, the heat monitored was related to the metabolic reactivation of cells within biofilm during 48-h incubation in fresh medium.
2.5 Biofilm Prevention Assay
An inoculum was prepared according to a McFarland standard turbidity of 0.5 and diluted in CAMHB+2.5% LHB to a final concentration of ~107 CFUs/ml (T0). The exact CFUs/ml was determined by plating tenfold serial dilutions of the initial inoculum (T0) and counting colonies after 18-h incubation at 37 °C under 5% CO2 atmosphere, as described above. Twofold serial dilutions of 10x concentration of levofloxacin were prepared, and test tubes were filled with 2400 μl CAMHB+2.5% LHB, 300 μl 10× concentration of the diluted antibiotic and 300 μl diluted bacterial suspension (T0) to a final concentration of ~1-5x106 CFUs/ml. Finally, one sterile porous glass bead was added to each tube. One tube with 3000 μl CAMHB+2.5% LHB and one sterile bead and another one with inoculated CAMHB+2.5% LHB (~1-5 × 106 CFUs/ml) and one sterile glass bead were included as negative (sterility) and positive (growth) control, respectively. After 24-h incubation, beads were carefully rinsed (3×) with sterile saline and incubated in sterile glass ampoules with 3000 μl CAMHB+2.5% LHB. One ampoule with 3000 μl CAMHB+2.5% LHB and one sterile bead and another one with 3000 μl CAMHB+2.5% LHB and a bead with untreated biofilm were included as negative control (sterility) as positive (growth) control, respectively. The IMC analysis was carried out at 37 °C for 48 h, defining then the minimum biofilm preventing concentration (MBPC) as the lowest antimicrobial concentration that prevented the formation of biofilm on the glass beads, thus leading to an undetectable heat flow signal during 48-h incubation in fresh medium.
2.6 Formulation of Levofloxacin-Loaded Physical Hydrogel, Drug Release by Agar Diffusion Assay, and Antibiotic Activity by IMC
Levofloxacin-loaded physical hydrogels, tested as antibiotic delivery reservoirs, were formulated in microcalorimetric glass ampoules dissolving hyaluronic acid (HA) (hyaluronic acid sodium salt, Sigma-Aldrich, Germany) in levofloxacin solution (5 mg/ml) to a final concentration of 15% w/v (final volume 300 μl). Upon mixing, ampoules containing hydrogels were incubated at 37 °C. As a control, HA physical hydrogels were formulated dissolving HA in PBS (pH 7.4, 10 mM) at a final concentration of 15% w/v.
Upon gelification, 1200 μl PBS buffer (pH 7.4, 10 mM) were added on top of the hydrogels, and ampoules were statically incubated at 37 °C. At different time points, 60 μl of release buffer were sampled and replenished. The concentration of active levofloxacin released was evaluated by agar diffusion assay against S. pyogenes (strain ATCC 19651), as previously reported (Butini et al. 2018; Casadidio et al. 2018). Briefly, a bacterial inoculum was prepared according to a McFarland standard turbidity of 0.5 (~1-5 × 108 CFUs/ml, λ = 565 ± 15 nm). Then, a sterile cotton swab was dipped into the bacterial suspension to evenly streak the surface of a CAMH agar plate supplemented with 5% defibrinated sheep blood. Next, a 6 mm hole was punched on the plate and filled with 60 μl of sampled release buffer. After 20 ± 4 h incubation, bacterial growth’s inhibition halos were measured, and the concentration of active levofloxacin was calculated according to a calibration curve. Each experiment was performed in triplicate.
The real-time microcalorimetric analysis of the antimicrobial activity of levofloxacin eluted by the physical hydrogel was monitored for 24 h at 37 °C. Briefly, ~1–5 × 105 CFUs/ml of S. pyogenes were inoculated in CAMHB+2.5% LHB and incubated in glass ampoules together with levofloxacin-loaded hydrogels (final volume bacteria+gel 1500 μl). As controls, bacterial cells in the same concentration were incubated in CAMHB+2.5% LHB with HA/PBS gel and without gel, whereas a negative control consisting in HA/PBS gel was incubated with sterile medium. Each experiment was performed in duplicate.
2.7 Data Analysis
IMC data analysis was accomplished using the manufacturer’s software (TAM Assistant; TA Instruments, New Castle, DE, USA). Resulted data were expressed as heat flow (μW) versus time (h) and as heat (J) versus time (h). Figures were plotted using GraphPad Prism 6.00 (GraphPad Software, La Jolla, CA, USA). IMC time to detection (TTD, h) was defined as the time between the insertion of the ampoule into the minicalorimeter and the exponentially increasing heat flow production exceeding the threshold of 10 μW (Trampuz et al. 2007). The maximum heat flow peak (Pmax, μW), the time of the maximum heat flow peak (Tmax, h), and the total heat produced (Htot, J) were defined as the highest value of the heat flow-time curve, the time at which the Pmax was detected and the cumulative amount of heat produced during the whole experiment, respectively. IMC data were converted into microbiologically relevant information such as growth rate constant (k, h−1) and lag phase (λ, h) by deriving according to growth models, as previously reported (Yang et al. 2007; Howell et al. 2012; Braissant et al. 2013).
3 Results
3.1 Antimicrobial Assay Against Planktonic Bacteria
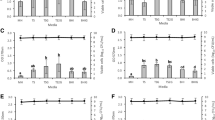
Antimicrobial activity of levofloxacin was tested in real time against planktonic S. pyogenes (strain ATCC 19615). Figure 1 shows the recorded heat flow (μW) (Fig. 1a) produced by S. pyogenes at each time point due to exothermic metabolic processes and the total heat (Fig. 1b), which is the cumulative amount of heat (J) produced over the experimental time. This parameter expresses the area under the heat flow curve, and it is indeed obtained by the mathematical integration of the instantaneous heat curve. The shape of the total heat curve is similar to the bacterial growth curve. Indeed, the total heat represents a proxy for bacterial replication and reaches a maximum value as the bacterial metabolic activity starts to diminish. The incubation with levofloxacin determined a dose-dependent reduction of heat produced by bacteria, as compared to the metabolic activity of the untreated control (GC). The MHIC of levofloxacin against planktonic S. pyogenes was 1 μg/ml.
Microcalorimetric analysis of planktonic S. pyogenes (ATCC 19615) co-incubated with different concentrations of levofloxacin. (a) Heat flow and (b) heat plot. Numbers represent concentrations of levofloxacin (μg/ml). Circled value represents the MHIC. GC growth control, NC negative control. Representative data of replicated experiments are reported
Moreover, the thermokinetic parameters of S. pyogenes growth during incubation with various concentrations of levofloxacin are listed in Table 1. The growth rate constants (k) gradually decreased with increasing concentrations of antibiotic from 1.35 ± 0.01 h−1 to 0 h−1, when bacteria were incubated with levofloxacin ranging from 0.125 to 1 μg/ml, respectively. S. pyogenes growth was completely inhibited during the monitoring time when the concentration of antibiotic reached 1 μg/ml, showing an inhibitory ratio (I) of 100%. A growth inhibition exceeding the 50% was already observed after treatment with 0.25 μg/ml levofloxacin.
3.2 Antimicrobial Assay Against Biofilm Bacteria
The activity of levofloxacin was also tested in real time against 24-h-old biofilms of S. pyogenes (strain ATCC 19615). As shown in Fig. 2, all the tested concentrations of levofloxacin (ranging from 128 to 1024 μg/ml) inhibited the replication of bacteria from the biofilm, resulting in a suppression of the heat production during 24-h incubation in the microcalorimeter (Fig. 2a and b). Therefore, the MHICb was ≤128 μg/ml.
Microcalorimetric analysis of S. pyogenes (ATCC 19615) biofilm co-incubated with different concentrations of levofloxacin. (a) The curve corresponds to the instantaneous heat produced by viable bacteria present in the biofilm of the growth control (GC) and (b) to the total heat produced during the whole experiment. The absence of heat production corresponds to biofilm co-incubated with antibiotic. Numbers represent concentrations of levofloxacin (μg/ml). GC growth control, NC negative control. Representative data of replicated experiments are reported. (c) Evaluation of biofilm survival after anti-biofilm treatment by CFUs counting of the sonicated beads. Arrow indicates the MBEC. T0, CFUs/ml on glass beads before anti-biofilm treatment; GC biofilm growth control, NC negative control; (mean ± SD, n = 3)
Then, the analysis of viable bacteria attached on the beads was performed by colony counting after bead sonication and plating of the sonication fluids. As shown in Fig. 2c, an increase of ≈ 2 log10 CFUs/ml was observed in the GC samples, as compared to the CFUs/ml calculated after sonication of the bead before the treatment (T0). Moreover, a dose-dependent reduction of S. pyogenes CFUs/ml was observed for all samples treated with levofloxacin, as compared to the GC (Fig. 2c). A concentration of 1024 μg/ml levofloxacin was able to kill all sessile cells, as no colonies were observed after sonication and plating (plating detection limit = 20 CFUs/ml).
In order to confirm the data observed by colony counting, a set of beads was washed after 24-h treatment with antibiotic and inoculated in fresh medium (without any antibiotic) for a second round of calorimetric analysis. As shown in Fig. 3, a heat signal was observed for all the samples pre-treated with levofloxacin ranging from 128 to 512 μg/ml, suggesting that residual bacteria were present on the beads and therefore replicated in fresh medium. By contrast, biofilm pre-treated with 1024 μg/ml levofloxacin showed no heat production.
Microcalorimetric analysis of S. pyogenes (ATCC 19615) biofilm treated with different concentrations of antibiotic. Each curve shows (a) the heat produced by viable bacteria present in the biofilm after 24 h of antibiotic treatment or no treatment (GC) and (b) to the total heat produced during the whole experiment. Numbers represent concentrations of levofloxacin (μg/ml). Circled value represents the MBBC. GC growth control, NC negative control. Representative data of replicated experiments are reported
As reported in Table 2, a longer lag phase (λ) of ~20 h, ~25 h, and ~35 h was observed when biofilm was treated with increasing antibiotic concentrations (from 128 to 512 μg/ml, respectively), as compared to the growth lag phase displayed by the untreated biofilm (~5 h), suggesting a gradually decreased number of viable bacteria left on the beads. This increase in the lag phase obtained from IMC data analysis was also consistent with the bactericidal effect of the drug observed after plating the sonication fluid and counting bacterial colonies. Additionally, Table 2 reports also on the Pmax (μW), Tmax (h), and Htot (J) related to the metabolic activity of viable bacteria in the biofilm after the antibiotic treatment. Similarly, values of maximum heat flow peaks progressively increased, while their corresponding Tmax decreased, when biofilms were treated with more diluted antibiotic doses. The total heat (after 48 h) produced from the samples treated with 128 and 256 μg/ml of levofloxacin did not vary deeply from the growth control (4.13 ± 0.72, 4.04 ± 0.16, and 4.05 ± 0.13 J, respectively). However, higher concentrations of levofloxacin (512 and 1024 μg/ml) resulted in a decrease in the total heat produced (2.83 ± 2.01 and 0.28 ± 0.03 J, respectively). Lastly, the time needed to reach the detection threshold of 10 μW (TTD) was longer when biofilms were treated with increasing amounts of drug (from ~20 h to ~30 h, respectively, after treatment with 128 and 512 μg/ml of drug). The treatment with 1024 μg/ml of levofloxacin resulted in a deep reduction of bacterial cell viability. Indeed, the heat flow value never exceeded 10 μW, thus remaining undetectable during the 48 h monitoring.
3.3 Biofilm Prevention Assay
The evaluation of the biofilm preventing activity of levofloxacin is represented in Fig. 4, whereas Table 3 reports on the corresponding parameters of Pmax (μW), Tmax (h), Htot (J), and TTD. The heat flow observed during 48 h IMC monitoring is related to the metabolic activity of viable bacteria attached on the beads during the co-incubation with the antibiotic. By contrast, the absence of heat flow after 48 h would correlate with the lack of viable cells attached to the porous bead and, consequently, with no biofilm formation on the abiotic surface. An alternative explanation could be that specifically concentrated antimicrobials, giving an undetectable heat flow signal, might have timely suppressed free-swimming microbes before surface colonization, thus avoiding biofilm development. As observed for S. pyogenes (strain ATCC 19615), a concentration of levofloxacin ≥256 μg/ml did not determine the total reduction of heat flow, even though a noteworthy decrease in heat production and a significant temporal shift thereof could be clearly appreciated. Hence, our results suggest that, despite the high antibiotic dose tested, levofloxacin did not successfully prevent the formation of biofilm on the beads.
Microcalorimetric analysis of levofloxacin preventing S. pyogenes (ATCC 19615) biofilm formation. Each curve shows (a) the heat produced by adherent cells on glass beads formed during the 24 h co-incubation with levofloxacin and (b) the total heat produced during the whole experiment. Numbers represent concentrations (μg/ml) of levofloxacin. GC growth control; NC negative control. Representative data of replicated experiments are reported
As reported in Table 3, Pmax of newly formed biofilms after non-exposure and exposure to levofloxacin concentrated up to 32 μg/ml did not vary profoundly (Pmax ranged between 254.82 ± 6.52 and 219.81 ± 20.96 μW). Differently, when bacteria were co-incubated with drug concentrations ranging from 64 to 256 μg/ml, Pmax of the different curves decreased to values lower than 200 μW (Pmax between 169.96 ± 27.22 and 64.38 ± 37.73 μW). The time at which Pmax were observed differed without following regular shift, suggesting a certain extent of variability among newly developed biofilms. By contrast, the Htot after 48-h monitoring showed similar values among drug-exposed and unexposed biofilms (around ~4.65 J), except for biofilm grown during incubation with the highest tested concentration (256 μg/ml), which produced a Htot of 2.27 ± 1.65 J. The analyzed heat flow curves exceeded the threshold of 10 μW in the first half of the monitoring. Exception was observed for the heat values given by biofilm newly formed during incubation with 128 and 256 μg/ml levofloxacin, which indeed reached the detection limit after 26.90 ± 2.67 and 32.91 ± 5.62 μW, respectively.
3.4 Formulation of Levofloxacin-Loaded Physical Hydrogel, Drug Release by Agar Diffusion Assay, and Antibiotic Activity by IMC
The simultaneous drug loading and hydrogel formulation was successfully achieved upon addition of levofloxacin solution to HA and mixing at room temperature. The biomaterial fully swelled within a time span of 24 h (Fig. 5a), and a burst release of ~1 mg/ml levofloxacin in the release medium was observed (Fig. 5b) within the first 8 h of incubation at 37 °C. As depicted in the microcalorimetric curves, when levofloxacin-loaded gels (HA/Levo gel) were incubated with planktonic S. pyogenes (strain ATCC 19615) for 24 h, the bacterial metabolism was completely inhibited, resulting in undetectable heat flow values (Fig. 5c and d). By contrast, free-swimming bacteria incubated with control HA-gels formulated with PBS (HA/PBS gel without antibiotic) showed an unaltered metabolic activity similar to what observed for microbial cells incubated with neither material nor pure levofloxacin (GC).
Formulation of levofloxacin-loaded physical hydrogel and drug release studies. (a) Gross picture of drug-loaded HA hydrogel after gelification (HA gel), upon addition of release buffer on top of the hydrogel (T0) and after a 24-h release at 37 °C. (b) Released active levofloxacin (μg/ml) during 24 h incubation in release buffer at 37 °C. Data are expressed as mean±SD, n = 3. (c) Heat flow and (d) heat curves resulted from 24-h real-time IMC analysis of antimicrobial activity of levofloxacin released from hydrogels against planktonic S. pyogenes (ATCC 19615). GC growth control, HA/PBS gel PBS/hyaluronic acid hydrogel (control gel without levofloxacin), HA/Levo gel levofloxacin-loaded hyaluronic acid hydrogel, NC negative control. Representative data of replicated experiment are reported
4 Discussion
The analysis of the antimicrobial susceptibility in planktonic and sessile cells is a crucial step in either the development of a new drug or the establishment of an effective therapy (Balouiri et al. 2016). Therefore, a high-throughput system for either screening the antimicrobial efficacy of different molecules or evaluating the minimal concentration able to kill or reduce pathogenic microorganisms is desirable.
Here, we have reported on IMC, a nonconventional approach to investigate the susceptibility of S. pyogenes (strain ATCC 19615) to levofloxacin by employing a 48-channel microcalorimeter. IMC is a fast and simple method to evaluate in real time the viability of both free-floating and sessile bacteria during and after the treatment with an antibiotic. The IMC relies on the continuous measurement of the heat instantaneously produced by metabolically active cells. Hence, the effect of antimicrobials may be evaluated in terms of metabolism/growth inhibition, as long as the active compound is co-incubated with the tested strain (Figs. 1, 2a and b), or bactericidal activity, when treated samples are examined for the presence of viable/replicating bacteria after removal of the antimicrobial agent (Figs. 3 and 4). Due to its high sensitivity, IMC can detect low numbers of bacterial cells (detection limit 104–105 CFUs/ml) that would be otherwise undetectable even using standard optical density (600 nm) measurements (detection limit 107–108 CFUs/ml) (Braissant et al. 2015a). Although it is not considered a standardized method yet, IMC techniques have been demonstrated to generate data in agreement with those obtained after performing standard conventional tests (Gonzalez Moreno et al. 2017; Butini et al. 2018; Di Luca et al. 2017; Mihailescu et al. 2014; Oliva et al. 2014). This holds true also with our results (Fig. 2c and 3), where colonies count after the anti-biofilm treatment showed a dose-dependent activity of levofloxacin consistent with the effect observed on the heat production of samples treated under the same conditions.
Moreover, IMC is a noninvasive method that allows to collect the samples after the measurement and to then proceed with further analysis, such as plating for colony counting.
As opposed to standard methods to assess planktonic antimicrobial susceptibility like macro-broth dilution, agar disc diffusion (CLSI 2018), and E-test, which are performed at end point, IMC immediately generates data about dynamic processes, as microbial cells replicate during the co-incubation with the active agents. In addition, the aforementioned tests are not specifically designed to evaluate the effective concentration of antimicrobials able to eradicate a sessile community of microorganisms involved in most infections.
By using IMC alone and in combination with sonication, plating, and colony-counting procedures, we defined three parameters related to the anti-biofilm activity of an antimicrobial compound: namely, the MHICb, MBBC, and MBEC. All these values can be used to evaluate and compare the anti-biofilm activities of different compounds. Antimicrobial agents displaying lower values are therefore more efficient in the anti-biofilm treatment. Moreover, further descriptive parameters can be inferred by calorimetric analysis when sub-inhibiting concentrations of antibiotics are tested, such as Pmax (μW), Tmax (h), Htot (J), and TTD (h), which are related to the metabolic activity of viable bacteria in the biofilm. Given that one bacterial cell can generate a heat of ~2 pW (Higuera-Guisset et al. 2005), a real-time estimation of the amount of metabolically active cells in the biofilm might be made. As an example, the number of CFUs calculated at the maximum peak of heat flow (Pmax), which approximately corresponds to the end of the exponential growth phase, may provide important data on the amount of active biofilm cells remained on the porous glass bead, in our case, after the anti-biofilm treatment. Concurrently, information regarding the time at which the logarithmic phase ceases (Tmax) could also be easily recruited. Nevertheless, the connection between heat flow and cell count must be considered with care, as it exists only at early growth stages (Fan et al. 2008). Indeed, decreases in heat production following Pmax can be mostly related not to a decrease in cell number but rather to a reduced metabolic rate, a possible switch from aerobic to anaerobic processes or the gradual depletion and sequential use of carbon sources (Braissant et al. 2013). Lastly, data on the cumulative heat (Htot) produced by the tested microorganism could be used both as end point datum and as a real-time parameter. In fact, Htot can be employed to calculate the overall percentage reduction of the total heat produced by treated strains (compared to an untreated control), as well as to monitor the switch from biomass building (initial slope) to biofilm maintenance phases (plateau) during the analysis (Astasov-Frauenhoffer et al. 2012). The time to detection (TTD), namely, the temporal interval lying between the experiment start and the exponentially increasing heat flow production exceeding the threshold of 10 μW (Trampuz et al. 2007), also provides real-time information on the metabolic change from lag to log phase of microbial replication. Deeper analysis for acquiring microbiologically and pharmacologically relevant data has also been described and applied (Braissant et al. 2013). Indeed, as we also reported for planktonic and biofilm S. pyogenes treated with a fluoroquinolone, the calculated growth rate (k) and lag phase (λ) provide valuable basis for comparison between metabolic differences and bacterial cell counts of treated and untreated samples. The tendency of an antimicrobial compound to act in a more microbiostatic or microbicidal manner could be therefore efficiently investigated (Astasov-Frauenhoffer et al. 2014), thus promptly supplying trustful therapeutic guidelines for the management of clinical cases.
The relevance of studying the effect of antimicrobial sub-inhibitory concentrations on biofilms remarkably emerged in the recent years. Indeed, similarly to the induction of resistance mechanism in planktonic cells, sub-MHICb may foster biofilm formation (Rachid et al. 2000), rather than inhibit it. These data can be also obtained by analyzing and comparing the activity of sub-inhibitory antimicrobial concentrations by IMC (von Ah et al. 2009) and evaluate the amount of heat produced.
In general, IMC allows for a fast and reliable investigation of biofilm-forming strains, without the need for expensive disposable materials or toxic reagents. Moreover, compared to standardized methods for microbial biofilms studies such as crystal violet assay, resazurin fluorescence dye (Dalecki et al. 2016), and quantification assays based on surface scraping, the microcalorimetric method involves neither biofilm staining nor physical harsh manipulation.
As reported by different authors, a critical step in testing antibiotic is to avoid contamination (Mah 2014). Indeed, in the majority of susceptibility tests, it is difficult to detect the presence of a contaminant microorganism, which might ultimately alter the assay results. Conversely, the outcomes obtained from microcalorimetric measurements enable differentiating among different microorganisms, since each microbial strain displays a “fingerprint” in the form of a characteristic shape of the heat flow curve monitored in real time. A contaminated sample could be therefore more easily identified.
The main advantage of microcalorimetry is to accommodate any type of sample that can fit in ampoules specifically designed depending on the instrument (in our case, 4 ml ampoules). To test implants and other biomedical materials, this feature allows for the insertion of a label-free solid sample into a microcalorimetric ampoule, as we demonstrated by conveniently testing a biphasic gentamicin-loaded calcium sulfate/hydroxyapatite bone graft substitute in our recent work (Butini et al. 2018). Similarly, further studies also highlighted the advantageous application of IMC for the investigation of bacterial adhesion and biofilm formation on titanium and zirconia implant surfaces (Roehling et al. 2017) and for the monitoring of antimicrobial properties of coatings or porous materials (Braissant et al. 2015b). Moreover, following a procedure similar to what recently described (Casadidio et al. 2018), here we confirmed the suitability of this technique for an easy formulation of antimicrobial-loaded hydrogels and, subsequently, a real-time monitoring of microbial response to the released agent. Ordinarily assessed through specifically adjusted agar diffusion methods (Marchesan et al. 2013; De Giglio et al. 2011) requiring several hours of incubation, the real-time analysis of the antimicrobial activity of loaded jellified materials still faces many difficulties. Moreover, the poor mechanical properties of physical hydrogels (i.e., hyaluronic acid hydrogels) might represent a challenging step in antimicrobial susceptibility tests on inoculated agar plates, as the lack of structural stability given by the absence of chemical cross-links could hinder the analysis. Hence, the use of IMC proved to provide a fair improvement in these experimental procedures, as it allows for undemanding polymer dissolution in glass ampoules, simultaneous drug loading and following minimal workload on the gel network.
However, IMC also carries some limitations, mostly related to critical steps within the operating procedure. As highlighted in the experimental protocol, special attention must be paid to ampoules sealing and cap shaping prior to insertion in the minicalorimeters to avoid evaporation and subsequent errors in the measurement. Understandably, this essential step prevents gas and medium exchanges inside the closed ampoules, therefore impairing oxygen and nutrients availability to the inoculated cells. Hence, it is necessary to carefully evaluate the data obtained after more than 5 days of long incubation in the minicalorimeters. Another critical step in sample preparation is the washing of biofilm formed on glass beads that is performed to remove any trace of planktonic bacteria and active molecules. During liquid aspiration in washing steps, the contact between vacuum aspirator and the bead could damage the biofilm structure, thus leading to an erroneous interpretation of the outcomes (e.g., heat flow detection of planktonic bacteria diffusing out from the altered biofilm matrix or false biofilm eradication due to aspiration of residual attached cells). Thus, careful positioning of the aspirating pipette on the side of the test tube slightly inclined is appropriate. Lastly, in addition to drawbacks related to the experimental procedure, the cost of the instrument might be a heavy limiting factor in the choice of application of this technique.
In general, as isothermal microcalorimeters (as other types of calorimeters) record the net heat flow of all processes producing or consuming heat in an IMC ampoule (also non-specific signals), it is important to include a negative control recording heat flow from ampoules containing only the medium and/or material without any specimen.
In our previous works, we have used this method to evaluate the antimicrobial activity of different compounds (including antibiotics (Gonzalez Moreno et al. 2017), bacteriophages (Tkhilaishvili et al. 2018) and antimicrobial peptides (Bormann et al. 2017)) against different planktonic and biofilm-embedded microorganisms, such as bacteria (Gonzalez Moreno et al. 2017), fungi (Furustrand Tafin et al. 2013), and parasites (Wenzler et al. 2012). Moreover, IMC demonstrated to be suitable for investigating the ability of different materials to prevent biofilm formation of different bacterial strains (Butini et al. 2018).
In conclusion, IMC is a nondestructive technique that permits the real-time analysis of microbial viability in the presence or absence of compounds with an antimicrobial activity. The susceptibility of planktonic and, more importantly, biofilm cells to antimicrobials can be conveniently assessed without using time-consuming procedures and potentially harmful reagents. Hence, the wide applicability of this ultra-sensitive method provides further advances in the field of clinical microbiology and biomedical sciences, fostering the scientific research toward the development of new drugs and antimicrobial biomaterials, besides supporting physicians in choosing effective antimicrobial therapies in the daily clinical practice.
Abbreviations
- CAMHB+2.5% LHB:
-
Cation Adjusted Müller Hinton Broth supplemented with 2.5% lysate horse blood
- CFUs:
-
Colony-forming units
- GC:
-
Growth control
- HA:
-
Hyaluronic acid
- HA/Levo:
-
Levofloxacin-loaded hyaluronic acid hydrogel
- HA/PBS:
-
Phosphate buffered saline/hyaluronic acid hydrogel
- Htot :
-
Total heat produced
- IMC:
-
Isothermal microcalorimetry
- k:
-
Growth rate constant
- λ:
-
Lag phase
- MBBC:
-
Minimum biofilm bactericidal concentration
- MBEC:
-
Minimum biofilm eradicating concentration
- MBPC:
-
Minimum biofilm preventing concentration
- MHIC:
-
Minimum heat inhibiting concentration
- MHICb :
-
Minimum heat inhibiting concentration for biofilm
- MIC:
-
Minimum inhibiting concentration
- Pmax :
-
Maximum heat flow peak
- PBS:
-
Phosphate buffered saline
- S. pyogenes :
-
Streptococcus pyogenes
- Tmax :
-
Time of the maximum heat flow peak
- TTD:
-
Time to detection
- TSA:
-
Trypticase soy agar
- TSA+2.5% LHB:
-
Trypticase soy agar supplemented with 2.5% lysate horse blood
References
Astasov-Frauenhoffer M, Braissant O, Hauser-Gerspach I, Daniels AU, Weiger R, Waltimo T (2012) Isothermal microcalorimetry provides new insights into biofilm variability and dynamics. FEMS Microbiol Lett 337(1):31–37. https://doi.org/10.1111/1574-6968.12007
Astasov-Frauenhoffer M, Braissant O, Hauser-Gerspach I, Weiger R, Walter C, Zitzmann NU, Waltimo T (2014) Microcalorimetric determination of the effects of amoxicillin, metronidazole, and their combination on in vitro biofilm. J Periodontol 85(2):349–357. https://doi.org/10.1902/jop.2013.120733
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6(2):71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Bjarnsholt T (2013) The role of bacterial biofilms in chronic infections. APMIS Suppl (136):1–51. https://doi.org/10.1111/apm.12099
Bjarnsholt T, Ciofu O, Molin S, Givskov M, Hoiby N (2013) Applying insights from biofilm biology to drug development – can a new approach be developed? Nat Rev Drug Discov 12(10):791–808. https://doi.org/10.1038/nrd4000
Bormann N, Koliszak A, Kasper S, Schoen L, Hilpert K, Volkmer R, Kikhney J, Wildemann B (2017) A short artificial antimicrobial peptide shows potential to prevent or treat bone infections. Sci Rep 7(1):1506. https://doi.org/10.1038/s41598-017-01698-0
Braissant O, Bachmann A, Bonkat G (2015a) Microcalorimetric assays for measuring cell growth and metabolic activity: methodology and applications. Methods 76:27–34. https://doi.org/10.1016/j.ymeth.2014.10.009
Braissant O, Bonkat G, Wirz D, Bachmann A (2013) Microbial growth and isothermal microcalorimetry: growth models and their application to microcalorimetric data. Thermochim Acta 555:64–71. https://doi.org/10.1016/j.tca.2012.12.005
Braissant O, Chavanne P, de Wild M, Pieles U, Stevanovic S, Schumacher R, Straumann L, Wirz D, Gruner P, Bachmann A (2015b) Novel microcalorimetric assay for antibacterial activity of implant coatings: the cases of silver-doped hydroxyapatite and calcium hydroxide. J Biomed Mater Res B Appl Biomater 103(6):1161–1167
Braissant O, Wirz D, Gopfert B, Daniels AU (2010) Use of isothermal microcalorimetry to monitor microbial activities. FEMS Microbiol Lett 303(1):1–8. https://doi.org/10.1111/j.1574-6968.2009.01819.x
Buchholz F, Harms H, Maskow T (2010a) Biofilm research using calorimetry–a marriage made in heaven? Biotechnol J 5(12):1339–1350
Buchholz F, Wolf A, Lerchner J, Mertens F, Harms H, Maskow T (2010b) Chip calorimetry for fast and reliable evaluation of bactericidal and bacteriostatic treatments of biofilms. Antimicrob Agents Chemother 54(1):312–319
Butini ME, Cabric S, Trampuz A, Di Luca M (2018) In vitro anti-biofilm activity of a biphasic gentamicin-loaded calcium sulfate/hydroxyapatite bone graft substitute. Colloids Surf B Biointerfaces 161:252–260. https://doi.org/10.1016/j.colsurfb.2017.10.050
Casadidio C, Butini ME, Trampuz A, Di Luca M, Censi R, Di Martino P (2018) Daptomycin-loaded biodegradable thermosensitive hydrogels enhance drug stability and foster bactericidal activity against Staphylococcus aureus. Eur J Pharm Biopharm 130:260–271. https://doi.org/10.1016/j.ejpb.2018.07.001
Ciofu O, Rojo-Molinero E, Macià MD, Oliver A (2017) Antibiotic treatment of biofilm infections. APMIS 125(4):304–319. https://doi.org/10.1111/apm.12673
CLSI (2018) Performance standards for antimicrobial susceptibility testing (28th ed.). CLSI supplement M100. Wayne PA Clinical and Laboratory Standards Institute
Coenye T, Nelis HJ (2010) In vitro and in vivo model systems to study microbial biofilm formation. J Microbiol Methods 83(2):89–105. https://doi.org/10.1016/j.mimet.2010.08.018
Dalecki AG, Crawford CL, Wolschendorf F (2016) Targeting biofilm associated Staphylococcus aureus using resazurin based drug-susceptibility assay. J Vis Exp (111). https://doi.org/10.3791/53925
De Giglio E, Cometa S, Ricci MA, Cafagna D, Savino AM, Sabbatini L, Orciani M, Ceci E, Novello L, Tantillo GM, Mattioli-Belmonte M (2011) Ciprofloxacin-modified electrosynthesized hydrogel coatings to prevent titanium-implant-associated infections. Acta Biomater 7(2):882–891. https://doi.org/10.1016/j.actbio.2010.07.030
Di Luca M, Navari E, Esin S, Menichini M, Barnini S, Trampuz A, Casani A, Batoni G (2017) Detection of biofilms in biopsies from chronic rhinosinusitis patients: In Vitro biofilm forming ability and antimicrobial susceptibility testing in biofilm mode of growth of isolated bacteria. In: Advances in experimental medicine and biology. Springer, Boston, pp 1–28. https://doi.org/10.1007/5584_2017_34
Fan D-D, Wang L-H, Shang L-A, Shi H-J, Ma X-X, Mi Y, Xu K-Z (2008) A microcalorimetric method for studying the biological effects of Mg2+ ion on recombinant Escherichia coli. Chem Biochem Eng Q 22(3):363–368
Furustrand Tafin U, Orasch C, Trampuz A (2013) Activity of antifungal combinations against Aspergillus species evaluated by isothermal microcalorimetry. Diagn Microbiol Infect Dis 77(1):31–36. https://doi.org/10.1016/j.diagmicrobio.2013.06.004
Gonzalez Moreno M, Trampuz A, Di Luca M (2017) Synergistic antibiotic activity against planktonic and biofilm-embedded Streptococcus agalactiae, Streptococcus pyogenes and Streptococcus oralis. J Antimicrob Chemother 72(11):3085–3092. https://doi.org/10.1093/jac/dkx265
Higuera-Guisset J, Rodríguez-Viejo J, Chacón M, Muñoz FJ, Vigués N, Mas J (2005) Calorimetry of microbial growth using a thermopile based microreactor. Thermochim Acta 427(1):187–191. https://doi.org/10.1016/j.tca.2004.09.010
Howell M, Wirz D, Daniels A, Braissant O (2012) Application of a microcalorimetric method for determining drug susceptibility in mycobacterium species. J Clin Microbiol 50(1):16–20. https://doi.org/10.1128/JCM.05556-11
Mah TF (2014) Establishing the minimal bactericidal concentration of an antimicrobial agent for planktonic cells (MBC-P) and biofilm cells (MBC-B). J Vis Exp (83):e50854. https://doi.org/10.3791/50854
Marchesan S, Qu Y, Waddington LJ, Easton CD, Glattauer V, Lithgow TJ, McLean KM, Forsythe JS, Hartley PG (2013) Self-assembly of ciprofloxacin and a tripeptide into an antimicrobial nanostructured hydrogel. Biomaterials 34(14):3678–3687. https://doi.org/10.1016/j.biomaterials.2013.01.096
Mihailescu R, Furustrand Tafin U, Corvec S, Oliva A, Betrisey B, Borens O, Trampuz A (2014) High activity of Fosfomycin and Rifampin against methicillin-resistant Staphylococcus aureus biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother 58(5):2547–2553. https://doi.org/10.1128/aac.02420-12
Oliva A, Furustrand Tafin U, Maiolo EM, Jeddari S, Betrisey B, Trampuz A (2014) Activities of fosfomycin and rifampin on planktonic and adherent Enterococcus faecalis strains in an experimental foreign-body infection model. Antimicrob Agents Chemother 58(3):1284–1293. https://doi.org/10.1128/aac.02583-12
Percival SL, Suleman L, Vuotto C, Donelli G (2015) Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol 64(Pt 4):323–334. https://doi.org/10.1099/jmm.0.000032
Rachid S, Ohlsen K, Witte W, Hacker J, Ziebuhr W (2000) Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob Agents Chemother 44(12):3357–3363
Roehling S, Astasov-Frauenhoffer M, Hauser-Gerspach I, Braissant O, Woelfler H, Waltimo T, Kniha H, Gahlert M (2017) In vitro biofilm formation on titanium and zirconia implant surfaces. J Periodontol 88(3):298–307
Solokhina A, Brückner D, Bonkat G, Braissant O (2017) Metabolic activity of mature biofilms of Mycobacterium tuberculosis and other non-tuberculous mycobacteria. Sci Rep 7(1):9225
Tkhilaishvili T, Di Luca M, Abbandonato G, Maiolo EM, Klatt AB, Reuter M et al (2018) Real-time assessment of bacteriophage T3-derived antimicrobial activity against planktonic and biofilm-embedded Escherichia coli by isothermal microcalorimetry. Res Microbiol. https://doi.org/10.1016/j.resmic.2018.05.010
Trampuz A, Steinhuber A, Wittwer M, Leib SL (2007) Rapid diagnosis of experimental meningitis by bacterial heat production in cerebrospinal fluid. BMC Infect Dis 7(1):116. https://doi.org/10.1186/1471-2334-7-116
von Ah U, Wirz D, Daniels AU (2009) Isothermal micro calorimetry – a new method for MIC determinations: results for 12 antibiotics and reference strains of E. coli and S. aureus. BMC Microbiol 9:106. https://doi.org/10.1186/1471-2180-9-106
Wenzler T, Steinhuber A, Wittlin S, Scheurer C, Brun R, Trampuz A (2012) Isothermal microcalorimetry, a new tool to monitor drug action against Trypanosoma brucei and Plasmodium falciparum. PLoS Negl Trop Dis 6(6):e1668. https://doi.org/10.1371/journal.pntd.0001668
Yang LN, Qiu SJ, Xu F, Sun LX, Zhao ZB, Liang JG, Song CG (2007) Microcalorimetric investigation of the growth of the Escherichia coli DH5α in different antibiotics. J Therm Anal Calorim 89(3):875–879. https://doi.org/10.1007/s10973-006-7902-x
Zimmerli W, Trampuz A, Ochsner PE (2004) Prosthetic-joint infections. N Engl J Med 351(16):1645–1654. https://doi.org/10.1056/NEJMra040181
Acknowledgments
An educational grant was provided by the PRO-IMPLANT Foundation (Berlin, Germany). Authors acknowledge Dr. Gerardo Abbandonato for his valuable contribution to microcalorimetric data analysis.
Authors’ Contribution
MEB, MGM, and MDL conceived and designed the work. MEB and MGM performed the experiments. MGM analyzed the data with the contribution of MEB, AT, and MDL. MDL and MEB drafted the manuscript, with the contribution of MGM, MC, AK, and TT. All authors reviewed and revised the first and final drafts of this manuscript.
Declaration of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Butini, M.E. et al. (2018). Real-Time Antimicrobial Susceptibility Assay of Planktonic and Biofilm Bacteria by Isothermal Microcalorimetry. In: Donelli, G. (eds) Advances in Microbiology, Infectious Diseases and Public Health. Advances in Experimental Medicine and Biology(), vol 1214. Springer, Cham. https://doi.org/10.1007/5584_2018_291
Download citation
DOI: https://doi.org/10.1007/5584_2018_291
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35468-8
Online ISBN: 978-3-030-35469-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)