Abstract
Obesity as a worldwide growing challenge is determined by abnormal fat deposition, which may damage general health. Weight loss and control of related risk factors like type2 diabetes, dyslipidemia, hypertension, cardiovascular diseases, and metabolic syndrome is an important concern in obesity management. Different therapeutic approaches such as lifestyle change, medications, and surgery are introduced for obesity treatment. Despite of gaining partially desirable results, the problem is remained unsolved. Therefore, finding a new approach that can overcome previous limitations is very attractive for both researchers and clinicians. Cell-based therapy using adipose-derived stromal cells seems to be a promising strategy to control obesity and related syndromes. To attain this aim, understanding of different type of adipose tissues, main signaling pathways, and different factors involved in development of adipocyte is essential. Recently, several cell-based methods like stem cell administration, brown adipose tissue transplantation, cell lysates and exosomes have been examined on obese mouse models to manage obesity and related disorders. Successful outcome of such preclinical studies can encourage the cell-based clinical trials in the near future.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Obesity is now a global problem and it is called “Globesity” which means many people around the world suffer from this disease (Pietrabissa et al. 2012). Obesity is defined as abnormal or excessive fat accumulation that presents a risk to health [WHO]. The rate of obesity has risen in recent decades and it is predicted to rise even more because of changing lifestyle and demography. In 2016, more than 650 million adults were obese [WHO]. A review study has estimated the range of overweight and obesity among Iranian adults as 27%–38.5% and 12.6%–25.9% respectively (Jafari-Adli et al. 2014).
Obesity is an important risk factor for type2 diabetes, dyslipidemia, hypertension, cardiovascular disease, and some types of cancers (Matsushita and Dzau 2017; Narayanaswami and Dwoskin 2017; Payab et al. 2014; Payab et al. 2017b). Another associated disorder is Metabolic Syndrome which is defined by IDF as central obesity plus any 2 of these 4 parameters: raised triglycerides, reduced HDL cholesterol, raised blood pressure, raised FPG. Thus, obesity can increase the risk of metabolic syndrome (Pi-Sunyer 2009). The life-threatening increase in obesity evoked some main strategies to control it: lifestyle modification, taking medication, and undergoing surgery(Petroni et al. 2017). These conventional methods have some considerable advantages for treatment and management of obesity for instance an average weight loss of 7 to 10Kg in 6 months by lifestyle modification, providing additional weight loss and positive effects on several metabolic parameters such as systolic blood pressure and total cholesterol by taking medication and a dramatic weight loss along with the rapid remission of type 2 diabetes mellitus by surgery (Yanovski and Yanovski 2014). Nonetheless, there are a number of limitations in the long-term efficacy and safety of these types of treatments such as return of lost weight,adverse effects and invasiveness respectively (Pories 2008).
Despite all the significant achievements of the mentioned methods, the obesity is still a major health problem which is needed to develop a novel treatment to enhance the effectiveness of obesity treatment. Nowadays, cell-based products propose promising advances in treatment of several disorders. Accordingly, clinical application of different types of stem cells can help scientists and clinicians to treat diseases include diabetes, disc degeneration, neurodegenerative disorders and obesity (Aghayan et al. 2017). Mesenchymal stem cells (MSCs) are multipotent cells which are considered to be common applicable type of stem cells. These cells can be derived from various sources like bone marrow, blood, umblical cord tissue and adipose tissue and can differentiate into several cell types like chondrocytes, osteoblasts, adipocytes and myoblasts (Augello and De Bari 2010; Tarte et al. 2010). Adipose derived mesenchymal stem cells (ASCs) are considered to be more beneficial, compared to bone marrow derived mesenchymal stem cells: human subcutaneous adipose tissue can be accessed easily and repetitively, the isolation procedure is rather simple, minimally invasive and it provides a large number of isolated cell (Aghayan et al. 2015; Thirumala et al. 2009).
MSCs play an essential role in adipogenesis which is a fundamental part in obesity and this makes them a potential target for therapeutic use(Baptista et al. 2015). In addition, these cells can be used to find a new way of controlling obesity in vitro and in vivo (Lee et al. 2017; Matsushita 2016). There are complex signaling pathways of adipogenesis from MSCs and many studies have determined the pathways governing MSC adipogenesis and realize therapeutic strategies for obesity (James 2013). Much researches have been carried out into the heterogeneity and properties of different white adipose tissue depots and ASCs to find suitable potentials for treatment of obesity (Cleal et al. 2017). Another research area is MSC differentiation into brown adipocyte which is reported to improve obesity hence this sounds promising to identify therapeutic strategies (Vargas-Castillo et al. 2017). However, our knowledge in these fields is not enough as there are contrasts in many results of related studies. MSCs can hopefully be a therapeautic alternative for obesity and further studies are expected to shed some light on all the complexities and open possibilities for a novel treatment for obesity. The authors in this review are trying to show that what are the ultimate results of the related studies and provide a future direction for more researches leading to clinical application of a safe and efficient type of stem cells for obesity treatment.
2 Adipose Tissue as a Secretory Organ
Adipose tissue can be found in mammals and especially in humans. There are two types of adipose tissue that differ in function, structure, color and position:1) white adipose tissue (WAT) and itself includes visceral white adipose tissue (VAT) and subcutaneus white adipose tissue (SAT), 2) brown adipose tissue (BAT). Moreover, investigators have disovered another type of adipose tissue, the color of which is between BAT and WAT and it is called beige adipose tissue (brite adipose tissue) (Illouz et al. 2011). All of them are differentiated from an identical origin, MSC. First of all, MSC which can differentiate into adipocyte, osteoblasts, myoblasts, and connective tissue is isolated from various tissues like bone marrow and adipose tissue. Secondly, MSC results in adipoblast that differentiates into brown and white preadipocyte in the presence of specific stimuli. After that, preadipocyte differentiates into brown or white adipocyte (Fig. 1 ;Esteve Rafols 2014).
The development of brown and beige adipocyte (drawn by Rasta Arjmand). Initially, pluripotent stem cell modifies into MSC which can get cluster of differentiation 24 (CD24) and peroxisome proliferator- activated receptor-γ (PPAR-γ) + white preadipocyte and myogenic factor 5 (Myf5) + brown preadypocyte. Secondly, in the face of white preadipocyte and some factors including PPAR-γ, bone morphogenic protein 4 (BMP-4), BMP-2 and fibroblast growth factor 10 (FGF10). Moreover, WAT can come into beige adipocyte throughout exposure to PR domain containing 16 (PRDM16), FGF21, PPAR-γ, Peroxisome proliferator-activated receptor-γ coactivator α (PGCα), irisin, apelin, Cyclooxygenase 2 (Cox2), microRNA 196 (MIR196a) and mir28. Brown preadipocyte is induced to be matured into BAT by way of ministration with some factors, for instance: PRDM16, FGF19, FGF16 and BMP-7 (Unser et al. 2015)
Some of the main duties of the adipose tissue are energy storage, shock absorption, thermal insulation. Additionally, adipose tissue acts as a secretory organ. In fact, due to the link between obesity and metabolic syndrome, attention is also drawn to the adipose tissue system. Adipose tissue secretes proteins which are generally called adipokine include leptin, adiponektine, interlukine 6 (IL6) (Anderson et al. 2003), tumor necrosis factor α (TNFα) and resistine (Gao et al. 2018). They can be made by adipocytes and skeletal muscle cells. Adipokines can perform different physiological activities. In fact, they can be transmitted through the paracrine system to various organs such as the lungs, heart, the skeletal muscle, muscle of vessels and influence activities of these organs.
2.1 Different Types of Adipose Tissue
2.1.1 White Adipose Tissue
In WAT fatty particles are stored in the form of triglycerides molecules and triglycerides consist of two parts: glycerol and fatty acids WAT mass increases with obesity. Moreover, obese people should lose weight and WAT mass by changing lifestyle, excercise training, taking medicine such as dietary supplement, including polyphenols with physician prescription but, because of the pharmacological approachs side effects researchers focus on excercise training and changing lifestyle (Sakurai et al. 2012).
Treating white adipocytes with irisin, a hormone secreted by skeletal muscle, and FGF21 induces browning. It was reported that “the beneficial effects of exercise, reduction of diet induced obesity and decrease of insulin resistance in mice” is related to irisin. Stimulating the conversion of white fat to brown fat by irisin in humans was also proposed. FGF21 increases the expression of uncoupling protein 1 (UCP1) and other brown–fat-related genes in perirenal and inguinal WAT. Adipocytic FGF21 triggers the browning of WAT and activate BAT in response to cold (Lee et al. 2014; Poher et al. 2015).
2.1.2 Brown Adipose Tissue
The role of BAT is fat burning and producing heat in the body. Although the activity of BAT is reduced with age, it does not lose its activity completely and cold exposure can stimulate reactivation of BAT. BAT color is due to differences in the number of mitochondria and nerve fibers in the brown to yellow color range (Vargas-Castillo et al. 2017). The main location of this tissue are sternocleidomastoid muscles, the supraclavicular region, the armpits, the groin muscles, the adrenal glands, between the subscapularis and pectoralis muscles, the para aortic adipose tissue, and around the viscera in the omentum tissue (Aldiss et al. 2017; Harms and Seale 2013; Lidell et al. 2013). The formation of BAT via brown adipogenesis is an important process due to its ability to expend energy as heat with implication in the treatment of metabolic disorders and obesity (Unser et al. 2015).
Adaptive thermogenesis by BAT activation have been described in two ways: 1) cold induced thermogenesis (CIT), 2) diet-induced thermogenesis (DIT) (Silva and Bianco 2008).
2.1.3 Beige Adipose Tissue
UCP1-positive cells have been demonstrated in WAT as the counterpart of BAT cells (Boucher et al. 2016; Brand et al. 2005; Wu et al. 2012). Nowadays, inducible adipose tissue has been intriguing as an alternative therapy for obesity. Beige adipose tissue can be found in diverse zones as supraclavicular, shoulder-blades, axillary, mediastinal, paravertebral, perirenal and peri-aortic regions (Rogers 2015; Wu et al. 2012). There are different methods for development of beige adipocyte, three of which are more significant: “De novo beige adipogenesis, white-to-beige adipocyte trans differentiation, activation of dormant beige adipocytes” (Rui 2017). In these three types of method, inducing inactive beige adipocytes is motivated by cold exposure, β adregenic’s etc. (Barbatelli et al. 2010; Berkowitz et al. 1998; Rosenwald et al. 2013; Wang et al. 2013; Wu et al. 2012). Beige adipose tissue has offered a new approach in treatment of metabolic diseases. It is similar to both WAT and BAT in rather different ways. Researches have been performed on animal and human subjects investigating to demonstrate the features of this type of adipose tissue.
BAT and brite adipose tissue are distinguished from WAT by their high levels of metabolic rates and thermogenic capacity (Bartelt et al. 2011; Stanford et al. 2013). Mitochondrial energetics, lipid droplet dynamics and metabolic fuel mobilization all influence BAT and beige adipocyte thermogenesis. Also UCP1-mediated thermogenesis is a hallmark of BAT and beige adiocyte. Recent findings illustrate that both of these tissues also do thermogenesis by additional UCP1-independent mechanisms (Rui 2017).
BAT has a common origin with muscles. Both of them are derived from dermomyotom of mesodermal layer. Early B cell factor 2 (EBF2) and BMP7 induce dermomyotom to form brown preadipocyte and PRDM16, CCAAT/enhancer-binding protein β (C/EBPβ), EBF2,PPAR-γ, zinc finger protein 516 (Zfp516) play significant role in differentiation brown preadipocyte to brown adiposyte tissue. There are multitude UCP1 in the mitochondria of BAT, that contributes to thermogenesis (Zhang et al. 2016). “UCP1 of BAT dissipates poroton gradient generated and catalizes proton leak throughout the inner mitochondrial membrane” (Vargas-Castillo et al. 2017). This molecular marker is useful to recognize BAT and Beige adipocyte (Rui 2017). UCP1 locates in the inner mitochondrial membrane. Although, there is no UCP1 in brown and beige progenitor cells, but during adipogenesis its expression enhances under the control of genetic program (Rabelo et al. 1996).
Besides the similarity of these three types of adipose tissue they also have some differences which are depicted in Table 1 (Ikeda et al. 2018; Rui 2017).
2.2 Factors Secreted from Adipose Tissue
Adipokines influence inflammation within adipose tissue and visceral endothelial adipose tissue (VEAT)(Lehr et al. 2012). As an adipokine, Leptin, was discovered almost simultaneously with the most important adipokine called adiponectin. Leptin is produced by adipocytes and plays an important role in regulating body weight. Accordingly, damaging hypothalamic methabolic circuits is because of the lack of leptin receptor in myeloid cell which cause increasing body weight, enlarging proinflammatory genes to modify 3 T3-L1 adipocyte as another consequence of leptin in adipose tissue (Gao et al. 2018). The studies of leptin till now have been performed on animals especially on mouse but researches on the physiological effects of leptin such as leptin resistance in the brain have not been discovered. Adiponectin has several effects including insulin-sensitizing,anti-inflammatory, anti-atherogenic and anti-carcinogenic activity (especially in breast cancer) (Feve 2013; Payab et al. 2017a). Adipose tissue in obese individuals acts as endocrine and secretory organ and the result is increasing the rate of secretion of pro inflammatory adepokines including TNFα and IL6 (Anderson et al. 2003). IL6 is produced and secreted by adipocytes and muscle cells which is increased in the plasma following obesity (Carey and Febbraio 2004). TNFα was the first adepokine, the activity of which was being studied and its performance in the human body is still unclear (Halse et al. 2001) It is secreted by the innate immune cells (macrophage) and expressed in adipocyte (Lehr et al. 2012). In the context of the relationship between inflammation and obesity it should be said that obesity is a type of low-grade inflammation (Cao et al. 2008).
2.3 Gene Expression and Adipogenesis
The role of genetics and mechanisms which affect gene function in the process of adipocyte differentiation is absolutely essential and it is considered as an interesting research area to find a novel treatment for obesity. There has been a great effort to identify genetic variants affecting obesity traits. The first gene associated to non syndromic obesity is fat mass and obesity associated (FTO) (Herrera et al. 2011). The association of this gene region with obesity explains 1% of BMI heritability. Also, FTO is reported to be involved in decreased lipolytic effect in adipocyte. Further, more gene loci related to obesity and BMI associated variants have been identified (Speliotes et al. 2010). The pattern of fat distribution, the factors affecting it and the potential risks caused by central and peripheral obesity, need to be fully understood. Accordingly, it was demonstrated that the development or maintenance of specific regional fat depots can be affected by DNA variants. Among 17 novel common obesity loci, 14 loci are related to body fat distribution and some of them are connected with sex in women. In other words, the fat distribution in men and women is influenced by sex-specific genes (Herrera et al. 2011).
There are several genes that regulate the adipogenesis (Fig. 2). BMP family control multiple steps of differentiation processes like adipogenesis. BMP-2 and BMP-4 are adipogenic factor for white fat and direct white adipocyte progenitor cells to white preadipocyte. In contrast, BMP-7 triggers commitment of MSC to brown adipocyte lineage (Chen and Tong 2013; Tseng et al. 2008). In addition to this family, one of the most powerful transcriptional regulators which control the fate of brown fat cells is PRDM16. Over expression of PRDM16 converts skeletal muscle progenitor cells to brown adipocytes (Chen and Tong 2013). Further, it restricts skeletal muscle gene expression in brown fat precursors by its interaction with PGC-1α and PGC-1β, as other transcriptional co-regulators. Among many nuclear factors regulating adipocyte differentiation, PPARγ2 is a key regulator that triggers differentiation to adipocyte. PRDM16 binds to PPAR-γ and coactivates its function (Seale et al. 2008). In addition to this factor, C/EBPs, a transcription factor family, has some members that induce adipogenesis: C/EBP α, β, δ. C/EBPβ and C/EBP δ “are expressed early and transiently” in the adipogenesis process and are induced by cAMP and glucocorticoids respectively(Ming Shi et al. 2000). It was found that ectopic expression of PPAR-γ and C/EBPα alters the program of differentiation of myoblasts and convert them to adipocytes upon hormonal stimulation. This suggests that the mentioned factors have the “dominant role in adipocyte determination and differentiation processes” (Hu et al. 1995).
An Overview of potential mechanisms/pathways underlying MSC white, beige and brown adipogenesis. Brown adipocyte is known to be arised from myogenic lineage which is a different origin to white and beige adipocyte lineage (Chen and Tong 2013). In the developmental pathway leading to the differentiations of white and beige adipocyte, BMP-2 and BMP-4 direct adipoblasts or white adipocyte progenitor cells to pre-adipocyte. Then, in the presence of some factors including PPAR-γ, C/EBPs and PGC-2, pre adipocyte forms committed white pre-adipocyte and then white adipocyte. Whereas, PRDM16 activates brown adipogenesis and differentiation of pre-adipocyte to committed brown pre-adipocyte. It can also promote inducible brown adipocyte or beige adipocyte. On the other hand, BMP-7 drives the brown-fat cell fate and it induces PRDM16, which represses skeletal muscle differentiation. Hence, Myf5+ progenitors are driven to committed brown adipocytes. PRDM16 acts as a key regulator and co-activates PPAR-γ, which results in subsequent induction of PGC-1α, PGC-1β and UCP1 that lead to brown adipocyte (Fruhbeck et al. 2009; Yao et al. 2011). Brown adipocyte may change its phenotype into white by up regulation of Wnt which suppresses the characters of brown adipocyte . In the same way, white adipocyte may transform into brown adipocyte by changing the expression of RIP140, Rb, and p107 (Yao et al. 2011). Receptor interacting ptotein140 (RIP140)is suggested to repress UCP1 enhancer in brown adipocytes (Rosell et al. 2011). Pocket proteins like retinoblastoma protein (pRb) and Retinoblastoma-like 1(p107) have been shown to alter the adipocyte differentiation and evoke white fat phenotype. Therefore, down regulation of these genes may result in trans-differentiation of white adipocytes to brown adipocytes (Yao et al. 2011)
In addition to the mentioned genes, there are some other genes, which intervene in this process. A gene of vertebrate which has regulatory effect on adipogenesis is called transcriptional and immune response regulator (TC1). It down-regulates PPAR-γ and C/EBPα while it up-regulates the wingless-type MMTV integration family member 1(WNT1), inducible signaling path-way protein 2 (Wisp2) and delta like non-canonical notch ligand 1(Dlk1)(Jang et al. 2016). Wisp2 inhibits adipogenesis in both mesenchymal stem cells and preadipocytes (Hammarstedt et al. 2013).
Another gene which can promote expression of PPARγ2, c/ebpb and c/ebpd is chemokine(C-X-C motif) ligand3 (CXCL3), a chemokine produced by different types of cells including adipocytes (Kusuyama et al. 2016).
One of the most compelling topics in this field is about BAT, as the thermogenesis was found intriguing in this type of adipose tissue. The precursor cells of BAT can also differentiate into muscle cell in the presence of some special transcription factors like PPAR-γ, PRDMI6 (which is of great importance in differentiation of adipocytes and is highly expressed in Brown adipocytes) and Euchromatic histone lysine methyltransferase 1 (EHMT1). As it was mentioned before, UCP1 is the hallmark protein for promoting thermogenesis which its transcriptional process is initiated by a number of transcription factors like: thyrioid response element (TRE), PPAR response element(PPRE), retinoic acid response element(RARE) and cAMP response element(CRE). All of these factors influence the expression of UCP1 as it was mentioned before that can be summarized: TRE which is activated by triiodothyronine (T3) is a positive regulator of UCP1, the binding of PPRE to PPAR controls UCP1 gene expression related to brown adipose differentiation, RARE triggers UCP1 expression in BAT and CRE seems vital as a mutation in its sites diminishes the UCP1 expression. RecentlyZfp516 has been added in the list of transcription factors that binds to UCP1 promoter leading to UCP1 expression (Vargas-Castillo et al. 2017).
2.4 Hypertrophy and Hyperplasia
Extensive adipose tissue growth, which can potentially lead to obesity, generally has two mechanisms: hyperplasia or increasing in fat cell number and hypertrophy or enhancement of fat cell size. In the development of obesity, hyperplasia occurs only in the first stages and it is triggered by hypertrophy. Hypertrophy is used for storing additional fat in the progression and is prior to hyperplasia. A study on C57BL/6 mice fed a high-fat diet explained that hypertrophy is the major cause of increased VAT while hyperplasia due to the presence of higher number of adipose progenitor cells in SAT mostly occurs in this type of adipose tissue (Joe et al. 2009). In an animal study on mice, it was suggested that hyperplasia does not contribute to fat mass increase because it occurs in small cells that have small volume of stored fat, whereas hypertrophy is the main contributor to the increase of fat mass. Studies on of genetic and diet effects on these mechanisms in mice has revealed that increase in number of fat cells is affected by genes while enlargement of fat cells is a diet variable (Jo et al. 2009). In regard to the role of obesity in the development of non-insulin-dependent diabetes and relatively, glucose intolerance and insulin resistance, the size of adipocytes might be important as it is positively correlated with glucose intolerance and hyperinsulinemia (Ferrannini and Camastra 1998). Moreover, as the adipocytes get larger, they become more susceptible to inflammation and cell death (Pellegrinelli et al. 2016).
3 Adipose Tissue-Derived Stem Cells and Adipogenesis
3.1 Mesenchymal Stem Cells (MSCs)
MSCs are spindle shape, multipotent cells with self-renewal capacity that can expand thousand folds and differentiate into different cell lineages including adipocyte, osteocyte and chondrocyte (Bianco 2014; Short et al. 2003). MSCs can be isolated from different tissues like bone marrow (Penfornis and Pochampally 2011), adipose tissue (Gimble and Guilak 2003), umbilical cord(Han et al. 2013), Wharton,s jelly (Chatzistamatiou et al. 2014), placenta (Vellasamy et al. 2012), dental pulp (Alkhalil et al. 2015) etc. Regarding to the multipotent capacity, MSCs seem to be substuted injured cells, although more findings are need to confirm this claim (Baksh et al. 2004). Moreover, MSCs secrete various type of growth factors and cytokines that execute significant role in repair, regeneration and immunogenic balance (Caplan and Dennis 2006). Low immunogenicity, as the main characteristic of MSCs, allow the allogenic use of cell products that is very important in cell-based therapies and regenerative medicine (Aggarwal and Pittenger 2005). ASCs are more considerable source in regenerative medicine research and clinical trials.
One of the most significant concern in cell therapy is providing cells from less or non-invasive sources. ASCs can be isolated in large numbers from abandon and waste adipose tissue that obtained by liposuction as less invasive method (Yarak and Okamoto 2010). On the other hand, the superior potential of ASCs is determined in basic and clinical researches (Aghayan et al. 2015).
3.2 Potential Pathways
Adipogenesis is a multi-step process involving expression of some transcription factors resulted in differentiation of fibroblast-like preadipocytes into mature adipocytes(Ali et al. 2013). According to literature, adipogenesis includes two main phases: 1) the determination phase underlying commitment of MSCs into preadipocyte and 2) the terminal differentiation phase that leads to maturate adipocyte (Matsushita and Dzau 2017).
The role of MSCs in adipogenesis is complicated and requires cross talking between major signaling pathways. Several different pathways are studied that involved in different phases of adipogenesis. Here, we described some important signaling pathways that present positive or negative regulatory effect on adipogenic differentiation (Fig. 3).
An overview of positive and negative regulators of MSCs adipogenesis. Adipogenesis pathway can be divided into two main phases: determination phase that characterized by differentiation of MSCs into preadipocytes and terminal differentiation phase that resulted in developing adipocyte phenotype. Several factors are involved in these two different phases of adipogenic differentiation. Among these, Wnt signaling pathway has been showed negative role in both phases of MSC adipogenic development. On the other hand, PPAR-γ and C/EBPα are two main adipogenic transcription factors that stimulate second phase of adipocyte differentiation. More researches are required to reveal all aspects of MSCs differentiation to adipocytes and knowledge of signaling pathway network improve our comprehension to find new strategies for treatment of obesity
3.2.1 Classic Pathways and Adipogenesis
BMPs are the members of Transforming growth factor beta 1 (TGF-β1) family performing different roles in the adipogenic differentiation of MSCs (Chen et al. 2016). BMP-2 and BMP-4 can persuade commitment of C3H10T1/2 stem cells as mouse MSC model into adipocyte. BMPs affect adipogenesis mediated by canonical Smad and p38 MAPK pathways, which lead to overexpression of lysyl oxidase (LOX) as a target gene of adipocyte lineage commitment (Huang et al. 2009). BMP-7 stimulates adipogenesis in human MSCs while, BMP-2 shows inhibitory effects on differentiation of human MSCs to adipocytes (Gori et al. 1999).
Wnt signaling pathway is very important in cell proliferation and differentiation. Up to date, 19 molecules of Wnt are recognized that trigger one of the canonical and noncanonical Wnt/calcium pathways by binding to Frizeld family receptors. The outcome of Wnt pathway on commitment of MSC to adipogenic lineage is well studied and the inhibitory effect of both pathways is determined. The canonical pathway suppresses expression of PPAR-γ mRNA, whereas the noncanonical pathway stimulates histone methyltransferases that prevent PPAR-γ activation via histone H3 lysine 9 (H3K9) methylation (Yuan et al. 2016).
Wnt 1 and Wnt 10b inhibit the expression of PPAR-γ in 3 T3-L1 preadipocytes, which resulted in retaining undifferentiated state (Liu and Farmer 2004). The existence of Wnt 3a in 3 T3-L1 cell culture medium can suppress the expression of adipogenic genes via prevention of PPAR-γ induction (Kawai et al. 2007). Similarly, neutralization of Wnt 5a promote determination of human MSC to preadipocytes by inactivation of PPAR-γ (Bilkovski et al. 2010) and reduction in middle stage adipogenesis was observed after treatment of rat ASCs by 50 ng/mL Wnt5a in the anti-β-catenin and MAPK pathway independent manner (Tang et al. 2018).
Consequently, Wnt signaling pathways act as an adipogenesis blocker, hence Wnt antagonist like secreted frizzled-related protein 1 (SFRP1) can induce invitro adipogenesis and invivo accumulation of adipose tissue (Lagathu et al. 2010). Hedgehog (Hh) signaling pathway controls several biological process during embryogenesis, development, organ patterning, cellular proliferation and differentiation. Treatment of C3H10T1/2 cells whit Hh reduces the amount of adipogenic transcription factors, (Spinella-Jaegle et al. 2001) this effect was emerged via induction of aniadipogenic factors like Gata 2 upstream of Hh (Suh et al. 2006). In the case of human ASCs, Hh pathway does not alter the entire number of adipocytes, while adipogenesis has been impaired, with declined lipid accumulation, a reduction in adipocyte specific markers, and appearance of an insulin-resistant phenotype. It seems that Hh signaling affects the late events of human MSC adipogenesis nor the commitment stage. In spite of evidences related to inhibitory effects of Hh on adipogenic differentiation, elimination of this pathway is essential but not adequate to promote adipogenesis in both human and mouse MSCs (Fontaine et al. 2008).
Notch signaling is a highly conserved pathway that regulates cell proliferation, differentiation, cell death and cell fate determination in several cell types. After binding to ligand, two proteolytic cleavages has been occurred and the emerged notch intracellular domain (NICD) moves into nucleus to stimulate transcription of target genes (Kopan and Ilagan 2009). The expression of notch gene decreased during adipogenic differentiation of human MSCs and inhibition of notch signaling by γ-Secretase inhibitors enhanced MSCs adipogenesis mediated by autophagy activation involving PTEN-PI3K/Akt/mTOR pathway (Song et al. 2015). However, the role of Notch signaling in adipose progenitor cells proliferation is conversional since the inhibition of Notch leads to decrease in human MSC expansion but increase in mouse 3 T3-L1 preadipocyte cell counting (Shan et al. 2017).
3.2.2 Adipogenesis and Master Transcription Factors
PPAR-γ and C/EBPα are two key transcription factors that regulate adipogenic differentiation. PPAR-γ is a nuclear hormone receptors with two distinct isoforms, PPARγ1 and PPARγ2, are detected. Overexpression of PPAR-γ in fibroblast cell line by retroviral vectors caused to appearance of preadipocyte features. The mentioned ability indicates the role of PPARγ2 in early phase of adipogesis and adipocyte determination (Tontonoz et al. 1994). Accordingly, embryonic stem cells are successfully generated by homologues recombination and showed that the null clones cannot undergo adipogenic differentiation. Moreover, creation chimeric mice of PPAR-γ null ES cells demonstrate no distribution of null cells in adipose tissue that prove the in vivio role of PPAR-γ in adipogenesis and fat formation (Rosen et al. 1999).
C/EBPs are basic region-leucine zipper proteins comprised of six isoforms: C/EBPα, C/EBPβ, C/EBPγ, C/EBPδ, C/EBPε and C/EBPζ. C/EBPα and C/EBPβ are highly expressed transcription factors in liver, lung and adipose tissue (Nerlov 2007). C/EBPβ plays an essential role in adipocyte differentiation of 3 T3-L1 cells via inducing the expression of C/EBPα and PPAR-γ genes (Guo et al. 2015).
C/EBPα is vital for adipogenic differentiation, since the expression of C/EBPα antisense RNA in 3 T3-L1 cells interrupts regular differentiation and gene knock out of C/EBPα in mice cause to failure in normal development of adipose tissue (Lin and Lane 1994).
More evidences shows that PPARγ can induce adipogenesis in the C/EBPα null cells but the ability of C/EBPα to trigger similar condition in the absence of PPARγ was not proven (Rosen et al. 2002).
3.2.3 Hyperplasia Involved Signaling Pathways
Adipocyte number increasing (hyperplasia) is related to signaling pathways that involved in cell proliferation. Adipogenic differentiation is arrested and cell growth triggered while hyperplasia occurring in adipose tissue (White and Tchoukalova 2014). In vivo studies via Tc1−/− mice showed that hyperplasia is regulated in stem cell levels and ASCs revealed more proliferative capacity and adipogenesis activity (Jang et al. 2016).
Abdesselem et al. suggested that reduction of sirtuin 1 expression in preadipocytes followed by hyperacetylation of c-Myc leads to uncontrolled fat hyperplasia so, Sirt1/c-Myc signaling pathway might be more studied as a potential therapeutic pathway for obesity (Abdesselem et al. 2016). Moreover, Wnt-β catenin may be the master pathway regulating adipose hyperplasia, and the induction of this pathway beside cross-talking with glucocorticoid-related signalings alter the activity of preadipocytes. Such alteration is appeared in disruption of adipocyte differentiation and increasing cell proliferation (Wong et al. 2016). Furthermore, activation of the protein kinase C which stimulated by endothelin-1 can improve invitro preadipocyte expansion and invivo hyperplasia. On the other hand, Extracellular receptor kinase(ERK)-dependent pathway inhibits the hypertrophy in 3 T3-L1 adipocytes after endothelin-1 treatment (Lien et al. 2016).
The study of transcription factors and signaling pathways involved in adipogenesis can introduce negative or positive regulators in adipogenic differentiation. Such knowledge might paved the path to plan a new approach in controlling obesity.
4 Therapeutic Use of Adipose Tissue-Derived Stem Cells (Adscs) in Obesity
4.1 Experimental Background
Obesity, which is the excess accumulation of adipose tissue, can be concluded from adipogenic differentiation of MSCs. Therefore, this pandemic disease can be managed by inhibiting this process. In this regard, different factors were investigated to promote an alternative therapy of obesity such as IGF-1, glucorticoids and prostaglandins which have stimulatory effects on adipogenesis and androgens like testosteron and dihydrotestosterone, growth hormone and inflammatory cytokines as adipogenesis inhibitors (Ali et al. 2013; Matsushita and Dzau 2017; Zerradi et al. 2014).
4.1.1 Invitro Experiments
Due to the importance of BAT and thermogenesis, several studies have focused on the BAT and induction of preadipocytes to brown adipocytes. Generally, two main approaches could be used to increase BAT mass and activity: 1) invivo infusion of small molecules and growth factors to spark BAT growth, 2) exvivo cell based approach in which progenitor cells are differentiated into brown adipocytes more and then the brown adipocytes will be implanted in patients (Cypess and Kahn 2010). According to several studies, some genes especially BMP7, persuade preadipocytes differentiation to form BAT. BMP-7 treatment in the brown adipocyte cell line arouses the expression of genes including PGC-1α and PGC-1β which are involved in mitochondrial biogenesis and function. Also, treating multipotent C3H10T1/2 cells with BMP-7 shows an increased expression of UCP-1, C/EBPα, β and δ, PPAR-γ and adipocyte protein 2(aP2) (Tseng et al. 2008). In a similar way, when PRDM16 is expressed in white fat cell progenitors, it provokes PGC-1α, UCP-1, and type2 deiodinase expression, then, the brown fat phenotype is activated (Seale et al. 2007).
Interestingly, umbilical cord blood-MSC (CB-MSC) has indicated a low potential for adipogenic differentiation, while the adipose tissue and bone marrow-MSCs are able to develop adipogenic phenotype. Gene expression analysis verified a higher average expression of preadipocyte factor 1 (PREF1), which has been demonstrated to inhibit adipocyte formation, in CB-MSC compared to the other MSC sources. On the other hand, PPARG, perilipin (PLIN), adiponectin (ADIPOQ) and C/EBPA were upregulated in adipose tissue-and BM-MSCs, resulted in adipogenic differentiation. However, the mRNA levels of these genes were remained unchanged in CB-MSC. Although, treating ASCs with umbilical cord blood plasma (CB-plasma) caused adipogenesis inhibition due to high concentration of Pref-1, but siRNA knock down of PREF1 did not induce adipogenesis. Indeed, endogeneous PREF1 expression has not an essential function in impaired adipogenesis in CB-MSCs whereas, Pref-1 in plasma seems to mediate inhibition of adipogenesis (Karagianni et al. 2013).
Additionally, the adipogenic differentiation of MSCs cultured in the presence of TGF-β and cAMP-enhancing agents revealed that this cytokine reduces expression of adipogenic genes like PPAR γ, a disintegrin and metalloproteinase with thrombospondin motif 5 (ADAMTS5) and aldo-keto reductase family 1 member B10 (AKR1B10). In more detail, MSCs were cultured in adipogenic differentiation medium and it was depicted that TGF-β blocked adipocyte transformation of MSCs in a dose-dependent manner. Despite of the significant effect of TGF-β in adipogenesis, systematic treatment with this cytokine is not a realistic option as it has strong inhibitory effect on the immune system. Also, It may cause skin fibrosis and toxicity which were indicated in animals. Since FDA has approved some drugs for the mentioned genes, they are potential targets for treatment of obesity though further studies are required (van Zoelen et al. 2016).
Recently, MicroRNAs (miRNAs) have also shown the regulatory potential and they are involved in cell fate decision. For example, miR-17-5p and miR-106a target BMP-2 and increase adipogenic CEPAα and PPAR-γ to promote adipogenesis of ASCs (Li et al. 2013). In addition, miRNAs can regulate brown adipogenesis as miR-193b-365 and miR-196a regulate brown adipogenesis positively. The miR-193b-365 is called a key regulator of brown fat development since blocking miR-193b and/or miR-365 impaires brown adipogenesis in primary brown preadipocytes. Also, miR-193b is able to induce differentiation of C2C12 myoblasts to brown adipocytes in adipogenic condition (Sun et al. 2011). Likewise, HomeoboxC8 (HOXC8), a white- fat gene that represses the brown adipogenesis, is down regulated by miR-196a in brown adipogenesis of WAT-Progenitor cells. It has been demonstrated that the transgenic miR-196a mice have a lower blood glucose level in the glucose tolerance test and a lower insulin level than wild type mice. Also, transgenic mice exhibite a resistance to obesity despite the increased food intake compared to wild types (Mori et al. 2012). On the other hand, miR-27 is down regulated during brown differentiation of WAT. MiR-27a and b are decreased in the beige differentiation of SAT preadipocytes. What is more, inhibition of miR-27 increases the expression levels of Ucp1, Prdm16,Pparγ, Pparα, cell death-inducing DFFA-like effector a (Cidea), Pgc1α and aP2 in SAT precursors and the brown adipogenic markers in VAT precursors (Sun and Trajkovski 2014). Similarly, miR-133 negatively regulates PRDM16, hence inhibition of miR-133 or its transcriptional regulator Myocyte enhancer factor 2 (Mef2) leads to differentiation of precursors of BAT and SAT to mature adipocytes (Trajkovski et al. 2012; Unser et al. 2015).
Adipokines and adipokine receptors which are expressed in several type of cells have the capacity to affect the adipogenic differentiation. Analyzing the expression of chemokine and chemokine receptor genes in 3 T3-L1 and ST2 cell lines revealed that some mRNAs are highly increased during the differentiation. Among these mRNAs, Cxcl3,which has the greatest effect in adipogenesis, is increased in both 3 T3-L1 preadipocyte differentiation and ST2 MSC in the inducing condition of adipogenesis (Kusuyama et al. 2016).
4.1.2 In-vivo Experiments
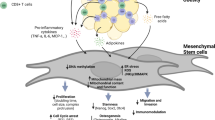
There are several preclinical studies in the field of obesity treatment which used obese mouse models (Fig. 4). Here, we are reviewing some attempts to develop therapeutic way for obesity with more focus on cell-based therapies.
Several useful methods for obesity treatment in preclinical phase. Different strategies have been investigated to treat feature of obesity in mice model. Administration of cell or derived exosomes, injection of inappropriate factors, transplantation of BAT and disruption of WAT are examples of these strategies. Cell-based approaches are more considered to develop as the new promising therapeutic strategies in human obesity. Infusion of ASCs or secreted exosomes led to significant improvement of obesity and related syndromes in mouse models
Rieusset et al. reported that decrease in the activity of PPAR-γ by using its antagonists, protects mice from high-fat diet-induced adipocyte hypertrophy and insulin resistance. In vitro inhibition of PPAR-γ prevents adipocyte differentiation and in vivo inhibition suppresses full development of WAT and BAT (Rieusset et al. 2002). Additionally, PPAR-γ and C/EBPα are up-regulated in ASCs from TC1 deleted mice, whereas the inhibitors of adipogenesis, Wisp2 and Dlk1 can be down-regulated. This data and the point that Tc1−/− mice has more capacity for adipogenesis than wild types may introduce TC1 as a new regulator of ASCs (Jang et al. 2016). Directing the WAT or preadipocytes to form BAT is also a valuable area of research. For instance, implanting BMP-7 treated C3H10T1/2cells into athymic nude mice developed a large number of UCP1 positive brown adipocytes and a small portion of white adipocytes (Tseng et al. 2008).
The expansion, metabolism, viability, and regenerative capacities of ASCs is damaged in obese mice and the cell recovery does not happen even after the weight loss (Perez et al. 2016) since ASCs graft seems to be the appropriate therapeutic option for obesity-related disorders.
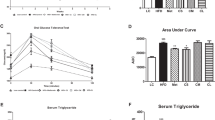
Cao et al. investigated the anti-obesity influence of allogeneic ASCs in high-fat diet-induced obese (DIO) mouse model. Single dose treatment of ASCs led to the reduction in body weights, decrease of the liver inflammation and level of blood glucose and also triglycerides while, the levels of HDL and expression of PPAR-γ was increased (Cao et al. 2015).
In another study, the anti- obesity effects of ASCs but not umbilical cord-derived MSCs have been proved in both dyslipidemia and obese mouse model. This study showed that administration of ASCs could activate AMPK HSL/ACC1 signaling cascades in adipose tissue. The final outcome of such pathways is determined by lipolysis and WAT browning functions (Liu et al. 2016).
Interestingly, the anti-obesity effects of human ASCs treatment as a xenogeneic source has been studied in obese mouse model. Furthermore, ASCs, ASC-lysate and brown adipocyte differentiated from MSCs (M-BA) are compared to analyze therapeutic their effects. Significantly, M-BA due to 60% expression of Ucp1, exhibited the strongest effect on reduction of body weight, triglycerides, cholesterol and increasing the HDL/LDL ratio after injection into high-fat diet (HFD) mice(Lee et al. 2017).
Apart from the agents which were mentioned above, there are other factors affecting adipogenesis including: stem cell microenvironment, surface biochemistry, cell adhesion, geometric and mechanical characteristics and also co-culture. Cell shape influences adipogenesis, as spheroidal MSCs are more capable for adipogenesis than protruded ones. Dynamic loading like cyclic stretching inhibits adipogenesis while static stretching accelerates adipogenic differentiation. Furthermore, adipogenesis is influenced by neighboring cells and the paracrine interactions between them. As a result, mature adipocytes promote adipogenic differentiation as an example (Unser et al. 2015).
Besides the mentioned approaches, BAT transplantation is another strategy in the cell- based therapy. Two different research groups determined that transplantation of BAT into DIO and HFD mice model enhances glucose tolerance, energy balance, insulin sensitivity and reduces fat mass (Liu et al. 2015; Stanford et al. 2013). Moreover, transplantation of BAT into dorsal subcutaneous region of leptin-deficient Ob/Ob mice as a genetically obese model showed similar effects. Improvement of obesity symptoms like reduction of body weight, upregulation of BAT activity, increasing in insulin sensitivity and thermogenesis was observed. Gaining promising results from preclinical studies could introduce BAT transplantation as a novel option for treatment of obesity and diabetes (Liu et al. 2015).
Beside BAT transpalntation strategy, destruction of WAT tissue can be a useful approach in obesity treatment. In this specific case, Anti-angiogenic strategies can be used as a supporting agent since adipogenesis and angiogenesis are very closed and occurred in cell clusters near adipose tissue neovascularisation region (Nishimura et al. 2007). Kolonin et al. designed attractive gene construct by fusion of WAT vasculature receptor and cytotoxic genes. By delivery of such construct to obese mice, WAT tissue is targeted and disrupted and the obesity development reversed via oblation the WAT growth (Kolonin et al. 2004).
Other than all the strategies mentioned above, recently, exosomes have received lots of attention both in basic science and clinic-wise for finding treatment of many diseases. Exosomes are nano vesicles that are secreted from the cells and can act as a key transporter of paracrine factors in angiogenesis, immune regulation and tissue regeneration (Zhao et al. 2018). Extracellular vesicle produced by adipocytes has been studied and it was found that under hypoxic condition, which can be a result of adipocyte hypertrophy, exosomes were enriched in enzymes and were able to stimulate lipid accumulation in target cells (Sano et al. 2014). Brown and Beige adipocyte exosome production, specifically, is enhanced by cAMP treatment in the mouse (Gao et al. 2017).
The immunomodulatory function of MSC-derived exosome was assessed in a study on C57BL/6 male mice. Exosomes secreted from ASCs of WAT (WAT-derived ASCs) polarized M2 macrophages with highly expressing of Arg-1 (due to transferred Signal transducers and activators of transcription 3 (STAT3) from exosomes) and IL-10 thus reduced the inflammatory ability of macrophages. The macrophages then promoted beiging of WAT.and this is why in obese (HFD-fed) mice treated with ADSC-derived exosomes, WAT inflammation and obesity progression were reduced and metabolic hemostasis was improved (Shang et al. 2015; Zhao et al. 2017).
The angiogenic potential of extracellular vesicles (EVs) was also studied. The data suggested that EVs from ASCs of obese individual have lesser pro angiogenic capacity,indicating that circulating fatty acids in obesity alter the function of ASCs. However, platelet-derived growth factor(PDGF) evokes ADSC EV secretion and enhances angiogenic potential (Lopatina et al. 2014).
5 Future Perspective
The pathway of Adipogenesis is divided into two main phases: the determination phase that is characterized by the differentiation of MSCs into the preadipocytes and the phase of terminal differentiation, which leads to the developing adipocyte phenotype. Several factors in these two different phases are involved in the adipogenic differentiation, such as transcription factors, molecular signals, epigenetics, and etc. Among these, a number of factors play an inhibitory role and other categories have stimulatory role. The challenge for future studies is the insight into obtaining the key to identifying these factors and their mechanisms in adipogenesis with the potential of providing new therapeutic goals for treatment of obesity and its comorbidities. In-vitro and in-vivo studies currently support stem cells therapies in the treatment of obesity. The results of studies conducted in this review revealed that the use of stem cells (infusion or injection) can significantly suppress obesity and related diseases such as cardiovascular and diabetes and improves dyslipidemia and insulin resistance.
Since autologous stem cells have been used in previous studies, this treatment does not have the risk of rejection and can be ideal for the treatment of obesity.
Also, future research on molecular control of brown or beige adipogenesis may result in new and novel achievements. Intervention studies such as controlling the adipogenicity from MSC to brown adipocyte or from white to brown adipogenesis can be used as a therapeutic strategy for obesity.
The potential effective and safety of stem cell therapy on obesity and its comorbidities must be further studied.
6 Conclusion
In conclusion, stem cell therapy is a therapeutic option for obesity in the future. However, long term studies are required to evaluate the efficacy and safety of stem cells therapy to find new and novel strategies for the treatment of obesity; and further studies in humans are necessary to investigate the results on animal models. This review showed that current studies are promising tools that can answer many questions in this regard.
In the future, one can hope that stem cell therapy can be used in control adipogenesis along with other obesity treatments and promises a new therapeutic approach in clinical applications.
Abbreviations
- ADAMTS5:
-
A disintegrin and metalloproteinase with thrombospondin motif 5
- ADSC:
-
Adipose derived mesenchymal stem cells
- AKR1B10:
-
Aldo-keto reductase family 1 member B10
- aP2:
-
Adipocyte protein 2
- ASCs:
-
Adipose derived-stem cells
- BAT:
-
Brown adipose tissue
- BMI:
-
Body mass index
- BM-MSCs:
-
Bone marrow mesenchymal stem cells
- BMP4:
-
Bone morphogenic protein 4
- C/EBP α (A)/β/δ:
-
CCAAT/enhancer-binding protein α/β/δ
- CB-MSC:
-
Umbilical cord blood-mesenchymal stem cell
- CB-plasma:
-
Cord blood plasma
- CD24:
-
Cluster of differentiation 24
- Cidea:
-
Cell death-inducing DFFA-like effector a
- CIT:
-
Cold induced thermogenesis
- Cox2:
-
Cyclooxygenase 2
- CRE:
-
cAMP response element
- CXCL3:
-
Chemokine(C-X-C motif) ligand3
- DIO:
-
Diet-induced obese
- DIT:
-
Diet-induced thermogenesis
- Dlk1:
-
Delta like non-canonical notch ligand 1
- EBF2:
-
Early B cell factor 2
- EHMT1:
-
Euchromatic histone lysine methyltransferase 1
- ENG:
-
Endoglin (protein)
- ERK:
-
Extra cellular receptor kinase
- ES:
-
Embryonic stem cell
- EVs:
-
Extracellular vesicles
- FDA:
-
Food and drug administration
- FGF10:
-
Fibroblast growth factor 10
- FPG:
-
Fasting plasma glucose
- FTO:
-
Fat mass and obesity associated(gene)
- H3K9:
-
Histone H3 lysine 9
- HDL:
-
High-density lipoprotein
- HFD:
-
High-fat diet
- Hh:
-
Hedgehog
- HOXC8:
-
HomeoboxC8
- IDF:
-
International diabetes federation
- IGF1:
-
Insulin-like growth factor1
- IL6:
-
Interlukine 6
- LOX:
-
lysyl oxidase
- M-BA:
-
MSC-derived BAT
- Mef2:
-
Myocyte enhancer factor 2
- miR-196a:
-
MicroRNA 196a
- miRNAs:
-
MicroRNAs
- MSC:
-
Mesenchymal stem cells
- Myf5:
-
Myogenic factor 5
- NICD:
-
Notch intracellular domain
- NRs:
-
Number of nuclear receptors
- Pax7:
-
Paired box 7
- PDGF:
-
Platelet-derived growth factor
- PDGPR α /b:
-
Platelet derived growth factor receptor α/β
- PGCα:
-
Peroxisome proliferator-activated receptor-gamma coactivator α
- PLIN:
-
Perilipin
- PPAR-γ/G:
-
Peroxisome proliferator-activated receptor-γ
- PPRE:
-
PPAR response element
- PRb/Rb:
-
Retinoblastoma protein/ retinoblastoma
- PRDM16:
-
PR domain containing 16
- PREF1:
-
Preadipocyte factor 1
- RARE:
-
Retinoic acid response element
- RIP:
-
Receptor interacting protein
- SAT:
-
Subcutaneus white adipose tissue
- SMA:
-
Spinal muscular atrophy
- STAT3:
-
Signal transducers and activators of transcription 3
- TC1:
-
Immune response regulator
- TNFα:
-
Tumor necrosis factor α
- TRE:
-
Thyroid response element
- TGFβ:
-
Transforming growth factor beta
- UCP1:
-
Uncoupling protein 1
- VAT:
-
Visceral white adipose tissue
- VEAT:
-
Visceral endothelial adipose tissue
- WAT:
-
White adipose tissue
- WHO:
-
World health organization
- Wisp2:
-
Inducible signaling path-way protein 2
- WNT1:
-
Wingless-type MMTV integration family member 1
- Zfp516:
-
Zinc finger protein 516
- P107:
-
Retinoblastoma-like 1
References
Abdesselem H, Madani A, Hani A, Al-Noubi M, Goswami N, Hamidane HB, Billing AM, Pasquier J, Bonkowski MS, Halabi N (2016) SIRT1 limits adipocyte hyperplasia through c-Myc inhibition. J Biol Chem 291:2119–2135
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822
Aghayan HR, Goodarzi P, Arjmand B (2015) GMP-compliant human adipose tissue-derived mesenchymal stem cells for cellular therapy. Methods Mol Biol 1283:93–107
Aghayan HR, Arjmand B, Ahmadbeigi N, Gheisari Y, Vasei M (2017) Draft of Iranian National Guideline for cell therapy manufacturing. Arch Iran Med 20:547–550
Aldiss P, Davies G, Woods R, Budge H, Sacks HS, Symonds ME (2017) ‘Browning’ the cardiac and peri-vascular adipose tissues to modulate cardiovascular risk. Int J Cardiol 228:265–274
Ali AT, Hochfeld WE, Myburgh R, Pepper MS (2013) Adipocyte and adipogenesis. Eur J Cell Biol 92:229–236
Alkhalil M, Smajilagic A, Redzic A (2015) Human dental pulp mesenchymal stem cells isolation and osteoblast differentiation. Med Glas (Zenica) 12:27–32
Anderson JW, Kendall CW, Jenkins DJ (2003) Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 22:331–339
Augello A, De Bari C (2010) The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther 21:1226–1238
Baksh D, Song L, Tuan R (2004) Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med 8:301–316
Baptista LS, Silva KR, Borojevic R (2015) Obesity and weight loss could alter the properties of adipose stem cells? World J Stem Cells 7:165–173
Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S (2010) The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298:E1244–E1253
Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmuller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J (2011) Brown adipose tissue activity controls triglyceride clearance. Nat Med 17:200–205
Berkowitz DE, Brown D, Lee KM, Emala C, Palmer D, An Y, Breslow M (1998) Endotoxin-induced alteration in the expression of leptin and beta3-adrenergic receptor in adipose tissue. Am J Phys 274:E992–E997
Bianco P (2014) “Mesenchymal” stem cells. Annu Rev Cell Dev Biol 30:677–704
Bilkovski R, Schulte DM, Oberhauser F, Gomolka M, Udelhoven M, Hettich MM, Roth B, Heidenreich A, Gutschow C, Krone W (2010) Role of WNT-5a in the determination of human mesenchymal stem cells into preadipocytes. J Biol Chem 285:6170–6178
Boucher J, Softic S, EL Ouaamari A, Krumpoch MT, Kleinridders A, Kulkarni RN, O'neill BT, Kahn CR (2016) Differential roles of insulin and IGF-1 receptors in adipose tissue development and function. Diabetes 65:2201–2213
Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, Cornwall EJ (2005) The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J 392:353–362
Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS (2008) Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134:933–944
Cao M, Pan Q, Dong H, Yuan X, Li Y, Sun Z, Dong X, Wang H (2015) Adipose-derived mesenchymal stem cells improve glucose homeostasis in high-fat diet-induced obese mice. Stem Cell Res Ther 6:208
Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem 98:1076–1084
Carey AL, Febbraio MA (2004) Interleukin-6 and insulin sensitivity: friend or foe? Diabetologia 47:1135–1142
Chatzistamatiou TK, Papassavas AC, Michalopoulos E, Gamaloutsos C, Mallis P, Gontika I, Panagouli E, Koussoulakos SL, Stavropoulos-Giokas C (2014) Optimizing isolation culture and freezing methods to preserve Wharton's jelly's mesenchymal stem cell (MSC) properties: an MSC banking protocol validation for the Hellenic Cord Blood Bank. Transfusion 54:3108–3120
Chen M-H, Tong Q (2013) An update on the regulation of adipogenesis. Drug Discov Today: Dis Mech 10:e15–e19
Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X (2016) Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ 23:1128
Cleal L, Aldea T, Chau YY (2017) Fifty shades of white: understanding heterogeneity in white adipose stem cells. Adipocytes 6:205–216
Cypess AM, Kahn CR (2010) Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes 17:143–149
Esteve Rafols M (2014) Adipose tissue: cell heterogeneity and functional diversity. Endocrinol Nutr 61:100–112
Ferrannini E, Camastra S (1998) Relationship between impaired glucose tolerance, non-insulin-dependent diabetes mellitus and obesity. Eur J Clin Investig 28(Suppl 2):3–6 discussion 6-7
Feve B (2013) Adiponectin: an anti-carcinogenic adipokine? Ann Endocrinol (Paris) 74:102–105
Fontaine C, Cousin W, Plaisant M, Dani C, Peraldi P (2008) Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells. Stem Cells 26:1037–1046
Fruhbeck G, Becerril S, Sainz N, Garrastachu P, Garcia-Velloso MJ (2009) BAT: a new target for human obesity? Trends Pharmacol Sci 30:387–396
Gao X, Salomon C, Freeman DJ (2017) Extracellular vesicles from adipose tissue-a potential role in obesity and type 2 diabetes? Front Endocrinol (Lausanne) 8:202
Gao Y, Vidal-Itriago A, Milanova I, Korpel NL, Kalsbeek MJ, Tom RZ, Kalsbeek A, Hofmann SM, Yi CX (2018) Deficiency of leptin receptor in myeloid cells disrupts hypothalamic metabolic circuits and causes body weight increase. Mol Metab 7:155–160
Gimble J, Guilak F (2003) Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 5:362–369
Gori F, Thomas T, Hicok KC, Spelsberg TC, Riggs BL (1999) Differentiation of human marrow stromal precursor cells: bone morphogenetic Protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J Bone Miner Res 14:1522–1535
Guo L, Li X, Tang Q-Q (2015) Transcriptional regulation of adipocyte differentiation: a central role for CCAAT/enhancer-binding protein (C/EBP) β. J Biol Chem 290:755–761
Halse R, Pearson SL, Mccormack JG, Yeaman SJ, Taylor R (2001) Effects of tumor necrosis factor-alpha on insulin action in cultured human muscle cells. Diabetes 50:1102–1109
Hammarstedt A, Hedjazifar S, Jenndahl L, Gogg S, Grunberg J, Gustafson B, Klimcakova E, Stich V, Langin D, Laakso M, Smith U (2013) WISP2 regulates preadipocyte commitment and PPARgamma activation by BMP4. Proc Natl Acad Sci U S A 110:2563–2568
Han Y-F, Tao R, Sun T-J, Chai J-K, Xu G, Liu J (2013) Optimization of human umbilical cord mesenchymal stem cell isolation and culture methods. Cytotechnology 65:819–827
Harms M, Seale P (2013) Brown and beige fat: development, function and therapeutic potential. Nat Med 19:1252
Herrera BM, Keildson S, Lindgren CM (2011) Genetics and epigenetics of obesity. Maturitas 69:41–49
Hu E, Tontonoz P, Spiegelman BM (1995) Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci U S A 92:9856–9860
Huang H, Song T-J, Li X, Hu L, He Q, Liu M, Lane MD, Tang Q-Q (2009) BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci 106:12670–12675
Ikeda K, Maretich P, Kajimura S (2018) The common and distinct features of Brown and Beige adipocytes. Trends Endocrinol Metab 29:191–200
Illouz Y-G, Sterodimas A, Green A C (2011) Role of adipose stem cells therapy in obesity. 133–139
Jafari-Adli S, Jouyandeh Z, Qorbani M, Soroush A, Larijani B, Hasani-Ranjbar S (2014) Prevalence of obesity and overweight in adults and children in Iran; a systematic review. J Diabetes Metab Disord 13:121
James AW (2013) Review of signaling pathways governing MSC osteogenic and Adipogenic differentiation. Scientifica (Cairo) 2013:684736
Jang H, Kim M, Lee S, Kim J, Woo D-C, Kim KW, Song K, Lee I (2016) Adipose tissue hyperplasia with enhanced adipocyte-derived stem cell activity in Tc1(C8orf4)-deleted mice. Sci Rep 6:35884
Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V (2009) Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol 5:e1000324
Joe AW, Yi L, Even Y, Vogl AW, Rossi FM (2009) Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 27:2563–2570
Karagianni M, Brinkmann I, Kinzebach S, Grassl M, Weiss C, Bugert P, Bieback K (2013) A comparative analysis of the adipogenic potential in human mesenchymal stromal cells from cord blood and other sources. Cytotherapy 15:76–88
Kawai M, Mushiake S, Bessho K, Murakami M, Namba N, Kokubu C, Michigami T, Ozono K (2007) Wnt/Lrp/β-catenin signaling suppresses adipogenesis by inhibiting mutual activation of PPARγ and C/EBPα. Biochem Biophys Res Commun 363:276–282
Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W (2004) Reversal of obesity by targeted ablation of adipose tissue. Nat Med 10:625–632
Kopan R, Ilagan MXG (2009) The canonical notch signaling pathway: unfolding the activation mechanism. Cell 137:216–233
Kusuyama J, Komorizono A, Bandow K, Ohnishi T, Matsuguchi T (2016) CXCL3 positively regulates adipogenic differentiation. J Lipid Res 57:1806–1820
Lagathu C, Christodoulides C, Tan CY, Virtue S, Laudes M, Campbell M, Ishikawa K, Ortega F, Tinahones FJ, Fernández-Real J-M (2010) Secreted frizzled-related protein 1 regulates adipose tissue expansion and is dysregulated in severe obesity. Int J Obes 34:1695
Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, Kebebew E, Pacak K, Chen KY, Celi FS (2014) Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 19:302–309
Lee CW, Hsiao WT, Lee OK (2017) Mesenchymal stromal cell-based therapies reduce obesity and metabolic syndromes induced by a high-fat diet. Transl Res 182:61–74.e8
Lehr S, Hartwig S, Sell H (2012) Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl 6:91–101
Li H, Li T, Wang S, Wei J, Fan J, Li J, Han Q, Liao L, Shao C, Zhao RC (2013) miR-17-5p and miR-106a are involved in the balance between osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Stem Cell Res 10:313–324
Lidell ME, Betz MJ, Leinhard OD, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, Virtanen KA, Beuschlein F, Persson A, Borga M, Enerbäck S (2013) Evidence for two types of brown adipose tissue in humans. Nat Med 19:631
Lien CC, Jiang JL, Jian DY, Kwok CF, Ho LT, Juan CC (2016) Chronic endothelin-1 infusion causes adipocyte hyperplasia in rats. Obesity 24:643–653
Lin F-T, Lane MD (1994) CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci 91:8757–8761
Liu J, Farmer SR (2004) Regulating the balance between peroxisome proliferator-activated receptor γ and β-catenin signaling during Adipogenesis A glycogen synthase kinase 3β phosphorylation-defective mutant of β-catenin inhibits EXPRESSION of a subset of adipogenic genes. J Biol Chem 279:45020–45027
Liu X, Wang S, You Y, Meng M, Zheng Z, Dong M, Lin J, Zhao Q, Zhang C, Yuan X, Hu T, Liu L, Huang Y, Zhang L, Wang D, Zhan J, Jong Lee H, Speakman JR, Jin W (2015) Brown adipose tissue transplantation reverses obesity in Ob/Ob mice. Endocrinology 156:2461–2469
Liu GY, Liu J, Wang YL, Liu Y, Shao Y, Han Y, Qin YR, Xiao FJ, Li PF, Zhao LJ, Gu EY, Chen SY, Gao LH, Wu CT, Hu XW, Duan HF (2016) Adipose-derived mesenchymal stem cells ameliorate lipid metabolic disturbance in mice. Stem Cells Transl Med 5:1162–1170
Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G (2014) Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Communication and Signaling : CCS 12:26–26
Matsushita K (2016) Mesenchymal stem cells and metabolic syndrome: current understanding and potential clinical implications. Stem Cells Int 2016:10
Matsushita K, Dzau VJ (2017) Mesenchymal stem cells in obesity: insights for translational applications. Lab Investig 97:1158
Ming Shi XC, Blair H, Yang X, Mcdonald J, Cao X (2000) Tandem repeat of C/EBP binding sites mediates PPARγ2 gene transcription in glucocorticoid-induced adipocyte differentiation
Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, Kaneda Y (2012) Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol 10:e1001314
Narayanaswami V, Dwoskin LP (2017) Obesity: current and potential pharmacotherapeutics and targets. Pharmacol Ther 170:116–147
Nerlov C (2007) The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol 17:318–324
Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S (2007) Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 56:1517–1526
Payab M, Hasani-Ranjbar S, Larijani B (2014) Whether all obese subjects both in metabolic groups and non-metabolic groups should be treated or not. J Diabetes Metab Disord 13:21–21
Payab M, Amoli MM, Qorbani M, Hasani-Ranjbar S (2017a) Adiponectin gene variants and abdominal obesity in an Iranian population. Eat Weight Disord – Studies on Anorexia, Bulimia and Obesity 22:85–90
Payab M, Hasani-Ranjbar S, Merati Y, Esteghamati A, Qorbani M, Hematabadi M, Rashidian H, Shirzad N (2017b) The prevalence of metabolic syndrome and different obesity phenotype in Iranian male military personnel. Am J Mens Health 11:404–413
Pellegrinelli V, Carobbio S, Vidal-Puig A (2016) Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia 59:1075–1088
Penfornis P, Pochampally R (2011) Isolation and expansion of mesenchymal stem cells/multipotential stromal cells from human bone marrow. In: Mesenchymal stem cell assays and applications. Springer, Cham
Perez LM, Suarez J, Bernal A, DE Lucas B, San Martin N, Galvez BG (2016) Obesity-driven alterations in adipose-derived stem cells are partially restored by weight loss. Obesity (Silver Spring) 24:661–669
Petroni ML, Caletti MT, Calugi S, Dalle Grave R, Marchesini G (2017) Long-term treatment of severe obesity: are lifestyle interventions still an option? Expert Rev Endocrinol Metabol 12:391–400
Pietrabissa G, Manzoni GM, Corti S, Vegliante N, Molinari E, Castelnuovo G (2012) Addressing motivation in Globesity treatment: a new challenge for clinical psychology. Front Psychol 3:317
Pi-Sunyer X (2009) The medical risks of obesity. Postgrad Med 121:21–33
Poher AL, Altirriba J, Veyrat-Durebex C, Rohner-Jeanrenaud F (2015) Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Front Physiol 6:4
Pories WJ (2008) Bariatric surgery: risks and rewards. J Clin Endocrinol Metab 93:S89–S96
Rabelo R, Reyes C, Schifman A, Silva JE (1996) Interactions among receptors, thyroid hormone response elements, and ligands in the regulation of the rat uncoupling protein gene expression by thyroid hormone. Endocrinology 137:3478–3487
Rieusset J, Touri F, Michalik L, Escher P, Desvergne B, Niesor E, Wahli W (2002) A new selective peroxisome proliferator-activated receptor gamma antagonist with antiobesity and antidiabetic activity. Mol Endocrinol 16:2628–2644
Rogers NH (2015) Brown adipose tissue during puberty and with aging. Ann Med 47:142–149
Rosell M, Jones MC, Parker MG (2011) Role of nuclear receptor corepressor RIP140 in metabolic syndrome. Biochim Biophys Acta 1812:919–928
Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM (1999) PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4:611–617
Rosen ED, Hsu C-H, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM (2002) C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev 16:22–26
Rosenwald M, Perdikari A, Rülicke T, Wolfrum C (2013) Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 15:659
Rui L (2017) Brown and Beige adipose tissues in health and disease. Compr Physiol 7:1281–1306
Sakurai T, Ogasawara J, Kizaki T, Ishibashi Y, Sumitani Y, Takahashi K, Ishida H, Miyazaki H, Saitoh D, Haga S, Izawa T, Ohno H (2012) Preventive and improvement effects of exercise training and supplement intake in white adipose tissues on obesity and lifestyle-related diseases. Environ Health Prev Med 17:348–356
Sano S, Izumi Y, Yamaguchi T, Yamazaki T, Tanaka M, Shiota M, Osada-Oka M, Nakamura Y, Wei M, Wanibuchi H, Iwao H, Yoshiyama M (2014) Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun 445:327–333
Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM (2007) Transcriptional control of brown fat determination by PRDM16. Cell Metab 6:38–54
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454:961–967
Shan T, Liu J, Wu W, Xu Z, Wang Y (2017) Roles of notch signaling in adipocyte progenitor cells and mature adipocytes. J Cell Physiol 232:1258–1261
Shang Q, Bai Y, Wang G, Song Q, Guo C, Zhang L, Wang Q (2015) Delivery of adipose-derived stem cells attenuates adipose tissue inflammation and insulin resistance in obese mice through remodeling macrophage phenotypes. Stem Cells Dev 24:2052–2064
Short B, Brouard N, Occhiodoro-Scott T, Ramakrishnan A, Simmons PJ (2003) Mesenchymal stem cells. Arch Med Res 34:565–571
Silva JE, Bianco SD (2008) Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid 18:157–165
Song B-Q, Chi Y, Li X, Du W-J, Han Z-B, Tian J-J, Li J-J, Chen F, Wu H-H, Han L-X (2015) Inhibition of notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway. Cell Physiol Biochem 36:1991–2002
Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Lango Allen H, Lindgren CM, Luan J, Magi R, Randall JC, Vedantam S, Winkler TW, Qi L, Workalemahu T, Heid IM, Steinthorsdottir V, Stringham HM, Weedon MN, Wheeler E, Wood AR, Ferreira T, Weyant RJ, Segre AV, Estrada K, Liang L, Nemesh J, Park JH, Gustafsson S, Kilpelainen TO, Yang J, Bouatia-Naji N, Esko T, Feitosa MF, Kutalik Z, Mangino M, Raychaudhuri S, Scherag A, Smith AV, Welch R, Zhao JH, Aben KK, Absher DM, Amin N, Dixon AL, Fisher E, Glazer NL, Goddard ME, Heard-Costa NL, Hoesel V, Hottenga JJ, Johansson A, Johnson T, Ketkar S, Lamina C, Li S, Moffatt MF, Myers RH, Narisu N, Perry JR, Peters MJ, Preuss M, Ripatti S, Rivadeneira F, Sandholt C, Scott LJ, Timpson NJ, Tyrer JP, Van Wingerden S, Watanabe RM, White CC, Wiklund F, Barlassina C, Chasman DI, Cooper MN, Jansson JO, Lawrence RW, Pellikka N, Prokopenko I, Shi J, Thiering E, Alavere H, Alibrandi MT, Almgren P, Arnold AM, Aspelund T, Atwood LD, Balkau B, Balmforth AJ, Bennett AJ, Ben-Shlomo Y, Bergman RN, Bergmann S, Biebermann H, Blakemore AI, Boes T, Bonnycastle LL, Bornstein SR, Brown MJ, Buchanan TA, Busonero F, Campbell H, Cappuccio FP, Cavalcanti-Proenca C, Chen YD, Chen CM, Chines PS, Clarke R, Coin L, Connell J, Day IN, Den Heijer M, Duan J, Ebrahim S, Elliott P, Elosua R, Eiriksdottir G, Erdos MR, Eriksson JG, Facheris MF, Felix SB, Fischer-Posovszky P, Folsom AR, Friedrich N, Freimer NB, Fu M, Gaget S, Gejman PV, Geus EJ, Gieger C, Gjesing AP, Goel A, Goyette P, Grallert H, Grassler J, Greenawalt DM, Groves CJ, Gudnason V, Guiducci C, Hartikainen AL, Hassanali N, Hall AS, Havulinna AS, Hayward C, Heath AC, Hengstenberg C, Hicks AA, Hinney A, Hofman A, Homuth G, Hui J, Igl W, Iribarren C, Isomaa B, Jacobs KB, Jarick I, Jewell E, John U, Jorgensen T, Jousilahti P, Jula A, Kaakinen M, Kajantie E, Kaplan LM, Kathiresan S, Kettunen J, Kinnunen L, Knowles JW, Kolcic I, Konig IR, Koskinen S, Kovacs P, Kuusisto J, Kraft P, Kvaloy K, Laitinen J, Lantieri O, Lanzani C, Launer LJ, Lecoeur C, Lehtimaki T, Lettre G, Liu J, Lokki ML, Lorentzon M, Luben RN, Ludwig B, Manunta P, Marek D, Marre M, Martin NG, Mcardle WL, Mccarthy A, Mcknight B, Meitinger T, Melander O, Meyre D, Midthjell K, Montgomery GW, Morken MA, Morris AP, Mulic R, Ngwa JS, Nelis M, Neville MJ, Nyholt DR, O'donnell CJ, O'rahilly S, Ong KK, Oostra B, Pare G, Parker AN, Perola M, Pichler I, Pietilainen KH, Platou CG, Polasek O, Pouta A, Rafelt S, Raitakari O, Rayner NW, Ridderstrale M, Rief W, Ruokonen A, Robertson NR, Rzehak P, Salomaa V, Sanders AR, Sandhu MS, Sanna S, Saramies J, Savolainen MJ, Scherag S, Schipf S, Schreiber S, Schunkert H, Silander K, Sinisalo J, Siscovick DS, Smit JH, Soranzo N, Sovio U, Stephens J, Surakka I, Swift AJ, Tammesoo ML, Tardif JC, Teder-Laving M, Teslovich TM, Thompson JR, Thomson B, Tonjes A, Tuomi T, Van Meurs JB, Van Ommen GJ, Vatin V, Viikari J, Visvikis-Siest S, Vitart V, Vogel CI, Voight BF, Waite LL, Wallaschofski H, Walters GB, Widen E, Wiegand S, Wild SH, Willemsen G, Witte DR, Witteman JC, Xu J, Zhang Q, Zgaga L, Ziegler A, Zitting P, Beilby JP, Farooqi IS, Hebebrand J, Huikuri HV, James AL, Kahonen M, Levinson DF, Macciardi F, Nieminen MS, Ohlsson C, Palmer LJ, Ridker PM, Stumvoll M, Beckmann JS, Boeing H, Boerwinkle E, Boomsma DI, Caulfield MJ, Chanock SJ, Collins FS, Cupples LA, Smith GD, Erdmann J, Froguel P, Gronberg H, Gyllensten U, Hall P, Hansen T, Harris TB, Hattersley AT, Hayes RB, Heinrich J, Hu FB, Hveem K, Illig T, Jarvelin MR, Kaprio J, Karpe F, Khaw KT, Kiemeney LA, Krude H, Laakso M, Lawlor DA, Metspalu A, Munroe PB, Ouwehand WH, Pedersen O, Penninx BW, Peters A, Pramstaller PP, Quertermous T, Reinehr T, Rissanen A, Rudan I, Samani NJ, Schwarz PE, Shuldiner AR, Spector TD, Tuomilehto J, Uda M, Uitterlinden A, Valle TT, Wabitsch M, Waeber G, Wareham NJ, Watkins H, Wilson JF, Wright AF, Zillikens MC, Chatterjee N, Mccarroll SA, Purcell S, Schadt EE, Visscher PM, Assimes TL, Borecki IB, Deloukas P, Fox CS, Groop LC, Haritunians T, Hunter DJ, Kaplan RC, Mohlke KL, O'connell JR, Peltonen L, Schlessinger D, Strachan DP, Van Duijn CM, Wichmann HE, Frayling TM, Thorsteinsdottir U, Abecasis GR, Barroso I, Boehnke M, Stefansson K, North KE, Mccarthy MI, Hirschhorn JN, Ingelsson E, Loos RJ (2010) Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42:937–948
Spinella-Jaegle S, Rawadi G, Kawai S, Gallea S, Faucheu C, Mollat P, Courtois B, Bergaud B, Ramez V, Blanchet AM (2001) Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci 114:2085–2094
Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ (2013) Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123:215–223
Suh JM, Gao X, Mckay J, Mckay R, Salo Z, Graff JM (2006) Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab 3:25–34
Sun L, Trajkovski M (2014) MiR-27 orchestrates the transcriptional regulation of brown adipogenesis. Metabolism 63:272–282
Sun L, Xie H, Mori MA, Alexander R, Yuan B, Hattangadi SM, Liu Q, Kahn CR, Lodish HF (2011) Mir193b-365 is essential for brown fat differentiation. Nat Cell Biol 13:958–965
Tang Q, Chen C, Zhang Y, Dai M, Jiang Y, Wang H, Yu M, Jing W, Tian W (2018) Wnt5a regulates the cell proliferation and adipogenesis via MAPK-independent pathway in early stage of obesity. Cell Biol Int 42:63–74
Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, Tchirkov A, Rouard H, Henry C, Splingard M, Dulong J, Monnier D, Gourmelon P, Gorin NC, Sensebe L (2010) Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 115:1549–1553
Thirumala S, Goebel WS, Woods EJ (2009) Clinical grade adult stem cell banking. Organogenesis 5:143–154
Tontonoz P, Hu E, Spiegelman BM (1994) Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79:1147–1156
Trajkovski M, Ahmed K, Esau CC, Stoffel M (2012) MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol 14:1330–1335
Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR (2008) New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454:1000–1004
Unser AM, Tian Y, Xie Y (2015) Opportunities and challenges in three-dimensional brown adipogenesis of stem cells. Biotechnol Adv 33:962–979
Van Zoelen EJ, Duarte I, Hendriks JM, Van Der Woning SP (2016) TGFβ-induced switch from adipogenic to osteogenic differentiation of human mesenchymal stem cells: identification of drug targets for prevention of fat cell differentiation. Stem Cell Res Ther 7:123
Vargas-Castillo A, Fuentes-Romero R, Rodriguez-Lopez LA, Torres N, Tovar AR (2017) Understanding the biology of thermogenic fat: is browning a new approach to the treatment of obesity? Arch Med Res 48:401–413
Vellasamy S, Sandrasaigaran P, Vidyadaran S, George E, Ramasamy R (2012) Isolation and characterisation of mesenchymal stem cells derived from human placenta tissue. World J Stem Cells 4:53
Wang QA, Tao C, Gupta RK, Scherer PE (2013) Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19:1338
White UA, Tchoukalova YD (2014) Adipose stem cells and Adipogenesis, pp 15–32
Wong JC, Krueger KC, Costa MJ, Aggarwal A, Du H, Mclaughlin TL, Feldman BJ (2016) A glucocorticoid-and diet-responsive pathway toggles adipocyte precursor cell activity in vivo. Sci Signal 9:ra103-ra103
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, Van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150:366–376
Yanovski SZ, Yanovski JA (2014) Long-term drug treatment for obesity: a systematic and clinical review. JAMA 311:74–86
Yao X, Shan S, Zhang Y, Ying H (2011) Recent progress in the study of brown adipose tissue. Cell Biosci 1:35–35
Yarak S, Okamoto OK (2010) Human adipose-derived stem cells: current challenges and clinical perspectives. An Bras Dermatol 85:647–656
Yuan Z, Li Q, Luo S, Liu Z, Luo D, Zhang B, Zhang D, Rao P, Xiao J (2016) PPARγ and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther 11:216–225
Zerradi M, Dereumetz J, Boulet MM, Tchernof A (2014) Androgens, body fat distribution and Adipogenesis. Curr Obes Rep 3:396–403
Zhang C, Weng Y, Shi F, Jin W (2016) The Engrailed-1 gene stimulates Brown Adipogenesis. Stem Cells Int 2016:7369491
Zhao, H., Shang, Q., Pan, Z., Bai, Y., Li, Z., Zhang, H., Zhang, Q., Guo, C., Zhang, L., Wang, Q. (2017) Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and Beiging in white adipose tissues. Diabetes
Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, Zhang Q, Guo C, Zhang L, Wang Q (2018) Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and Beiging in white adipose tissue. Diabetes 67:235–247
Acknowledgement
The authors would like to acknowledge Rasta Arjmand for her assistance in figure design. We also thank Dr. Mohsen khorshidi, and Maryam Afshari for their kind support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Payab, M. et al. (2018). Stem Cell and Obesity: Current State and Future Perspective. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 2. Advances in Experimental Medicine and Biology(), vol 1089. Springer, Cham. https://doi.org/10.1007/5584_2018_227
Download citation
DOI: https://doi.org/10.1007/5584_2018_227
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-04169-4
Online ISBN: 978-3-030-04170-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)