Abstract
Increasing longevity, coupled with rising frailty and sarcopenia of aging, significantly affects function and quality of life of older adults. This review discusses the definition, assessment, and management of frailty and sarcopenia, and examines the relationship between them. Medline, Scopus and Psychoinfo databases were searched using the keywords frailty, sarcopenia, aging, and functional disability. The findings are that frailty and sarcopenia are often assessed clinically with such methods such as DeXA, CT scan, MRI, bioelectrical impedance, or anthropometry. Frailty and sarcopenia differentially affect older adults. Both conditions are characterized by decreased energy reserves and resistance to external and internal stressors, resulting in susceptibility to fatigue, comorbidity, sedentary life style, functional decline, hospitalization, quality of life, and even death. The estimated prevalence of frailty with sarcopenia is relatively low; however, the condition requires early detection and careful management.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
This article reviews the body of knowledge related to frailty and sarcopenia. According to a recent report by the WHO, the proportion of people aged over 65 increases faster than that of any other age group (WHO 2015). Today, life expectancy of a 65-year old person is 3–4 years more than it was 20 years ago; meaning more people are getting old, and more old people are getting older. Aging is accompanied by gradual, yet progressive changes in all biological systems, and it affects physical, cognitive, psychological, and social abilities. More specifically, the aging process is accompanied by changes in physical activity, decline in lean body mass, and reduced muscle mass and strength, leading to primary sarcopenia (Cruz-Jentoft et al. 2014; Landi et al. 2014).
Primary sarcopenia is an age-related syndrome mainly represented by a reduction in muscle mass and strength and difficulties performing daily living activities. Frailty is a physiological decline in many biological systems resulting in poor health, weight loss, moderate to severe dependency in daily living activities, recurrent hospitalizations, and death (Turner et al. 2014; Rodríguez-Mañas et al. 2013; Heuberger 2011; Sternberg et al. 2011; Xue 2011; Lang et al. 2009; Walston et al. 2006; Fried et al. 2001; Hamerman 1999). The link between frailty and sarcopenia is not well-described in the literature. The conditions are interrelated conditions and lead to difficulties performing daily living activities and increased disability (Peters et al. 2012; Raîche et al. 2008; Ravaglia et al. 2008; Saliba et al. 2001). A review by McCleary et al. (2014) has stressed that older adults with frailty or sarcopenia are prone to complications during cancer treatment. Guidelines published in 2010 by the American College of Surgeons indicate the importance of assessing both frailty and sarcopenia prior to oncologic surgery in the elderly (Chow et al. 2012).
Despite the challenges in clarifying the definition of these conditions, assessing them is even more complex and has less consensus. Some studies evaluated the presence of frailty or sarcopenia by using functional assessments, such as the Katz Index (Figueiredo et al. 2013), the Short Physical Performance Battery (SPPB) (Chang et al. 2014), and the Timed Up and Go (TUG) test (Hassani et al. 2015). Furthermore, it has been shown that function is the most important prognostic measure in predicting co-morbidities and mortality (Inouye et al. 1998). It is also reasonable to assume the presence of a cognitive decline related to frailty with or without primary sarcopenia (Bowen 2012; Vermeulen et al. 2011). Therefore, assessment of the older adult should performed be according to The International Classification of Functioning, Disability and Health (ICF) (Azzopardi et al. 2016; Cao and Morley 2016). This includes several psychosocial, cognitive, functional, recreational, and participation domains. Early screening for frailty may be valuable for preventing or minimizing sarcopenia and hopefully reversing it. The prevalence of these individual and combined medical diagnoses is of interest because of their association with health prevention, prognosis, and intervention. The purpose of this review is to describe frailty and sarcopenia, define their assessment and management, and to examine the relationship between them.
2 Sarcopenia Definition
The term sarcopenia was first defined by Irwin Rosenberg in 1989 (Rosenberg 1989). The currently accepted definition includes three phases of the health condition, as introduced by the European Working Group on Sarcopenia in Older People (EWGSOP). This group has developed a practical clinical definition and diagnostic criteria for age-related sarcopenia (Cruz-Jentoft et al. 2014). The first phase is defined as pre-sarcopenia, where a reduction in muscle mass is observed. This is followed by decreased muscle strength (i.e., sarcopenia), followed by the third phase, severe sarcopenia, that occurs when, in addition to reduction in muscle mass and strength, physical performance declines. Another definition of sarcopenia has been introduced in Rome, Italy in November 2009 by the International Working Group on Sarcopenia. That definition emphasizes the evaluation of walking speed and measuring muscle mass (Fielding et al. 2011). Recently, the term muscle quality (MQ) has been suggested in the clinical setting to describe the ratio between muscle power and muscle mass (Barbat-Artigas et al. 2012). Thus, MQ can be regarded as a clinical marker of muscle efficiency. Whatever the definition, the prevalence of sarcopenia is reported as up to one-third of older adults (Walston et al. 2006; Fried et al. 2001).
3 Anthropometric Assessment
The most frequent methods used in clinical practice are anthropometric. These are noninvasive, quantitative techniques for determining body composition by analyzing specific dimensions, such as height and weight; skin-fold thickness; and waist, hip, and chest circumference. Body mass index (BMI) is one of the most popular and important indices to assess body composition (Kulkarni et al. 2013). References for cut-off points for low or high BMI for males and females were established in diverse populations (Yu et al. 2015; Araujo et al. 2010). Interestingly, the cut-off points for Caucasians are higher than for Asians (Silva et al. 2010).
Anthropometrics are simple, clinical tools that can be easily used for sarcopenia because they are the most portable, commonly applicable, inexpensive, and non-invasive techniques for assessing the size, proportion, and composition of the human body. However, their validity is limited when applied to individuals due to a large prediction errors and because the cut-off points to identify low muscle mass still need to be defined. Therefore, if a patient is identified as being at-risk for sarcopenia based on anthropometrics, additional measurements of muscle mass with dual-energy X-ray absorptiometry (DeXA) is recommended (Villani et al. 2013, 2014).
3.1 Leg and Arm Circumference
Limb circumferences correlate with appendicular muscle mass and reflect both health and nutritional status. They also predict physical performance, health and survival in older people (Landi et al. 2010, 2014; Rolland et al. 2003). The validity of measuring lower limb circumference to detect sarcopenia is still debatable and the inter-tester reliability is weak.
3.2 Skin Fold Thickness
Loss of skin elasticity and subcutaneous fat can provide a general idea of body composition. The association between obesity and sarcopenia is widely reported (Visser et al. 2002). It is accepted that decreased muscle mass and/or muscle strength occurs with adipose cell infiltration into muscle or through lipogenesis of satellite cells (adult stem cells) that differentiate to adipocytes instead of myocytes (Vettor et al. 2009). A caliper is used to measure subcutaneous tissue. The skin fold pinch can be taken at many sites around the body. The most common are the triceps, a site 1 cm below the inferior angle of the scapula, and another site immediately above the iliac crest (top of hip bone) on the most lateral aspect. Because of high errors involved, it is usually not appropriate to convert skin fold measures to percentage body fat (%BF). Therefore, it is best to use the sum of the three sites to monitor and compare body fat measures. Interestingly, a study by Tamura et al. (2012) has suggested evaluating sarcopenia of the lingual muscles by measuring tongue thickness. The idea behind this is that elderly people often suffer from malnutrition caused by dysphagia. This often leads to sarcopenia, which may compromise oral function. Thus, tongue thickness could be related to nutritional status. However, it must be measured using ultrasonography.
4 Muscle Mass Assessment
Decreased muscle mass is defined as a slow, progressive reduction in the cross-sectional area CSA) of muscle fibers (Wei et al. 2016). Initially, the sarcoplasm volume decreases, then the fast twitch (type II) muscle fibers get shorter, followed by a shrinking diameter of slow twitch (type I) muscle fibers (Drey 2011). Corresponding to this reduction in fiber CSA, is a progressive increase in dense interstitial collagen and adipose connective tissue (Scott et al. 2015). At a later stage, termed the ultra-structural level, sarcopenic fibers show evidence of disorganization, including misalignment of Z bands, dissociation of the T system and a displacement of sarcosomes due to distraction of intermediate filaments that hold the sarcosome in place. These changes are similar to those seen in muscular dystrophy (Malatesta et al. 2014). Structural changes also occur in the extracellular matrix, including an increase in collagen concentration and a change in the elastic fiber system. These changes in the elastic fiber result in increased tissue stiffness, which also plays a role in the overall decrease in muscle function.
When measuring muscle mass, the cross-sectional area, the interstitial connective tissue, and the subcutaneous adipose tissue should all be considered. In the absence of a gold standard, a few general and specific outcome measures are used to assess muscle mass. The general, indirect tools to assess muscle mass include biochemical markers, nutrition intake, body mass index (BMI), and bioelectrical impedance analysis (BIA). Tools that are more direct, yet non-invasive include imaging techniques such as magnetic resonance imaging (MRI), DeXA, and computed tomography (CT) scan, as well as anthropometrics to assess leg and arm circumferences and skin fold thickness.
4.1 Biochemical Markers
Certain biological molecules, assessed in venous blood serum, have been mentioned as possible biochemical markers to detect decreased muscle mass. These include elevated expression of activin A (>0.35 ng/ml) (Ding et al. 2016) and myostatin (Wang and Mitch 2014; Hittel et al. 2009), and a decreased (<4.2 ng/mL) level of N-terminal peptide of procollagen type III (P3NP) (Fragala et al. 2014). Although, these molecules are being studied for their ability to indicate reduced muscle mass, current data suggest that it is premature to recommend their use in daily practice (Beaudart et al. 2016).
4.2 Imaging Assessment
Imaging is considered a valid tool, yet it is expensive and not always accessible to assess muscle mass. Techniques include MRI, DeXA, and CT. Imaging studies allow the assessment of body composition to be included in standard clinical or supportive care (Mitsiopoulos et al. 1998).
4.2.1 Dual-Energy X-Ray Absorptiometry (DeXA)
DeXA is a low-radiation technique to measure and estimate appendicular skeletal lean mass. Yet it cannot assess intra-muscular fat (Cruz-Jentoft et al. 2014; Levine et al. 2000). DeXA scan can only produce two-dimensional images and therefore cannot distinguish between subcutaneous and visceral adipose tissue.
Measuring appendicular skeletal lean mass can provide the ratio of individual body composition by calculating the sum of the non-fat and non-bone mass of the four limbs. As proposed by the European Society of Parenteral and Enteral Nutrition Special Interest Group (Cruz-Jentoft et al. 2014), two standard deviations below the mean level of young individuals (matched for age and gender) is considered a cut-off point for low muscle mass due to sarcopenia (e.g., appendicular mass relative to height squared, which is ≤7.23 kg/m2 in men and ≤5.67 kg/m2 in women). DeXA and BMI screening for muscle mass provide valid information. They were found to be very sensitive for estimating appendicular skeletal muscle mass of the lower limbs. Adjustment of anthropometric measurements for age, sex, or BMI could provide a better correlation with DeXA-measured lean mass (Dunsky et al. 2014).
4.2.2 Magnetic Resonance Imaging (MRI)
To determine whole body composition, MRI is used to provide valid, quantified volumetric information about soft and hard tissues such as muscle, subcutaneous adipose, intermuscular adipose and bone (Buford et al. 2012). The main advantages of MRI are that it provides excellent spatial resolution and differentiates body mass composition without radiation exposure. However, as with CT scan, DeXA, and BIA, MRI cannot analyze skeletal muscle quality. Yet it is more expensive compared to the other methods and its use is limited by local availability and technical expertise.
4.2.3 Computed Tomography (CT)
CT scan for assessing body composition has been shown to be superior to DeXA (Levine et al. 2000). CT can provide information on specific lean body mass, total body lean body mass, subcutaneous volume, visceral fat mass, total fat mass, subcutaneous fat-to-muscle ratio, and visceral-to-subcutaneous adipose tissue ratio (Mourtzakis et al. 2008). Major advantages of CT scans are high accuracy and reproducible results. Disadvantages, however, include radiation exposure and a higher cost compared to BIA and DeXA.
4.2.4 Bioelectrical Impedance Analysis (BIA)
BIA is a widely-available, inexpensive, user-friendly method that estimates the volume of fat and lean body mass based on the relation between the volume of a conductor and its electrical resistance. BIA involves placing electrodes on the hand and foot and measuring the impedance of a low-level electric current. The impedance is higher for fat and bone compared with soft tissue. Impedance can be affected by hydration status and fluid intake. Measurement accuracy and reference values have been established for older individuals (Reiss et al. 2016; Kim et al. 2015; Kim and Kim 2013).
5 Muscle Strength Assessment
The physiological meaning of reduced muscle strength is a loss of force-generating capacity. Usually, this loss is not correlated with morphological changes. In sarcopenia, mass reduction occurs to a greater extent and faster than strength reduction does. In clinical settings, hand or leg dynamometers are widely used for measuring muscle strength.
Isokinetic machines are used for research purposes, and in many cases isokinetic knee extensor torque is evaluated at 60°, 90°, and 120° (Bottaro et al. 2005). Isometric leg extension torque is well-correlated with handgrip strength and functional reach test (Jenkins et al. 2014). As with anthropometrics, this is easy to perform, inexpensive and does not require special training.
Isometric leg extension, to measure quadriceps femoris muscle strength, should be done while seated on a standard chair with the leg placed at 45° in knee extension. The manual muscle tester (MMT) is an ergonomic hand-held device for objectively quantifying muscle strength. The test is performed with the clinician applying force to the patient’s limb. The clinical objective of the test is to overcome or ‘break’ the patient’s resistance. The MMT records the peak force and the time required to achieve the ‘break’ point, while providing reliable, accurate, and stable muscle strength readings that conform to most manual muscle testing protocols.
Hand grip strength should be measured while the patient is seated on a standard chair with the elbow flexed at 90°. Two measures should be taken with each arm, while the individual is encouraged to squeeze as hard as possible for 3–4 s for each trial. The higher of the two measurements is recorded. There are several types of hydraulic devices, such as the Jamar dynamometer (Patterson Medical, Warrenville, IL), which is the gold standard for this measurement, and pneumatic dynamometers such as the Martin Vigorimeter (Gebrüder Martin GmbH & Co., Tuttlingen, Germany) which is mostly used for patients with hand deformity (e.g., rheumatoid arthritis).
6 Sarcopenia – Functional Assessment
Functional performance is an integrative outcome of the overall effect of health and it reflects the ability of an individual to perform the physical tasks necessary for activities of daily living (Rosen and Reuben 2011). The Katz Index is widely used in community living and skilled nursing facilities to assess ability to dress, shower/bathe, sit down and rise from a chair, eat, and walk indoors (Katz 1983). The maximum score is 15, with a score of five indicating no functional limitations, a score of 6–10 indicating some functional limitations, and a score of 11 or more indicating several functional limitations.
Gait speed measurement is also frequently used in outpatient clinics. No special equipment is required, as it only needs a stop watch and a flat surface. References are suggested by the EWGSOP (Chiles Shaffer et al. 2016). In the 4-min gait speed test, men and women with a gait speed <0.8 m/s are described as having poor physical performance (Cuesta et al. 2015). The International Working Group on Sarcopenia has indicated that a diagnosis of sarcopenia is consistent with a gait speed of less than 1 m/s, or less than 400 m during a 6-min walking test (Fielding et al. 2011). There are several gait speed tests to use for sarcopenic individuals, but the Timed Up and Go (TUG) test (Bijlsma et al. 2014), the Short Physical Performance Battery (SPPB) (Steffl et al. 2016), and the five times sit-to-stand test (FTSST) (Lord et al. 2002) are the most widely used. These tests best correlate with mobility and disability. In the TUG test, individuals are asked to rise from a standard armchair, walk to a marker 3 m away, turn and walk back, and sit down again. The SPPB is a 10-min test with a maximum score of 12 points. It assesses gait speed (over 3–4 m) and individuals with a score ≤ 8 are characterized as having poor physical performance. The five times sit-to-stand test (FTSST) provides a reliable and valid indication of lower body strength and is commonly used. This timed test requires participants to rise from an armless chair, 43 cm high without using their arms and return to the seated position, five consecutive times. The test begins when the participant stands up from the initial sitting position at the go command and ends when the participant is in the final fully upright position at the end of the fifth stand.
A Japanese study has developed a screening tool to diagnose sarcopenia. The model is based on gender, demographic variables, blood profile especially albumin level, chronic diseases, physical activity information and anthropometrics (Ishii et al. 2014).
6.1 Sarcopenia Management
Patient-centered care has a key role in the management of sarcopenia. Since sarcopenia is frequently found in association with co-morbidities, e.g., osteoporosis, type II diabetes mellitus, chronic heart failure, poor balance, etc., treatment of these conditions is indispensable if the management of sarcopenia is to succeed. The intervention should involve a combination of physical exercises, dietary regimen, and nutritional supplements.
6.1.1 Physical Exercises (PE)
The PE and progressive resistance training have a strong effect on muscle strength, muscle mass, and physical performance in older people (Reid et al. 2015). There are no specific exercise protocols designed for individuals with sarcopenia. Therefore, general recommendations and guidelines for PE for elderly people suggested by WHO (2017) can serve as an initial protocol. General recommendations regarding the PE intervention in older people have also been suggested by the Asian Working Group for Sarcopenia (AWGS) (Chen et al. 2016b). To improve muscle function, the intervention should be for at least 3 months. Supervised resistance exercise or combined exercise programs should be recommended for sarcopenic or sedentary community-dwelling people. Progressive resistance, aerobic training predominantly effects muscle mass and muscle strength. Yet endurance aerobic training also is crucial to improve the function of the capillary bed in and around the muscle fibers and to increase local and systemic circulation. To improve muscle function, aerobic training should be performed least three times a week, for at least 150 min weekly, and for 12 consecutive weeks. Supervised, progressive resistance exercise should be performed 2–3 times a week, for a minimum of 30 min per week, for at least 3 months (Shad et al. 2016).
6.1.2 Diet and Nutrition
The European Union Geriatric Medicine Society (EUGMS), in cooperation with the dietary protein aging study group (PROT-AGE Study Group), has recently published nutritional recommendations for individuals with sarcopenia (Bauer et al. 2013). In general, some observational studies suggest that adequate protein intake (0.8–1.2 g/kg/day) and other dietary supplements (e.g., long-chains (omega−3 and 6) polyunsaturated fatty acids (PUFAs)) (Da Boit et al. 2017), β-hydroxy β-methylbutyrate (HMB), creatine, and vitamin D, combined with resistance exercise, may help preserve muscle mass in healthy older people. Supplementation with creatine, protein, or leucine, combined with exercise, seems to have a positive influence on physical performance (Martone et al. 2015).
A meta-analysis on diet has suggested that vitamin D supplementation could increase lower limb muscle strength (Stockton et al. 2011). A diet rich in protein or protein supplementation, mainly 60–90 min after physical exercise, accelerates muscle absorption of amino acids, especially leucine (Moore and Soeters 2015). Physical activity performed in the evening expands the overnight muscle protein synthetic response to pre-sleep protein absorption and permits more of amino acids to be used for de novo muscle protein synthesis during overnight sleep in older men (Holwerda et al. 2016).
7 Role of Clinicians and Primary Care Physicians
Despite that the definition and assessment of sarcopenia still lacks consensus, primary care physicians should consider a diagnosis of sarcopenia in older individuals (>65 years) with risk factors (e.g., diabetes, cancer, cardiovascular and pulmonary disease, osteoporosis, and poor balance). The Charlson Comorbidity Index (CCI) can be used (Perkins et al. 2004). The CCI uses a weighted scoring system (1–6 points) based on the presence of comorbid diseases. For instance, myocardial infarction or peripheral vascular disease equals 1 point, diabetes mellitus equals 2 points, liver disease 3 points, and cancer or acquired immune deficiency syndrome (AIDS) is 6 points.
When considering preventive health interventions, family physicians, geriatric physicians, and clinical dieticians should address comorbidities, functional status, activity level and risk factors. They should also check and inquire about caloric intake, protein quality, minerals (e.g., iron, magnesium), and serum vitamin B and vitamin D levels. The nutritional risk assessment can be carried out with the Mini Nutritional Assessment Short Form. The values range from 0 to 14 points and scores <11 identify patients at risk of poor nutrition.
8 Frailty Definition, Assessment, and Management
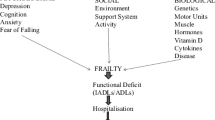
The definition of frailty is even more complex, with less consensus than sarcopenia. When Fried et al. (2001) have described the frailty phenotype and its association with mortality and morbidity, they noted a potential link between frailty and sarcopenia. However, frailty definitions vary, and it is largely conceptualized as increased vulnerability across multiple systems (Sirola et al. 2011; Rockwood and Mitnitski 2007). Based on an expert opinion statement (Rodríguez-Mañas et al. 2013), seven variables have been selected to define frailty: polypharmacy (Gnjidic et al. 2012), chronic heart failure (Phan et al. 2008), diabetes mellitus (Bourdel-Marchasson and Berrut 2005), subjective self-health assessment (Theou et al. 2015), physical activity questionnaire (Santos et al. 2015), mini-mental status evaluation (MMSE) (Bieniek et al. 2016), general health questionnaire (GHQ) (Kahlon et al. 2015), and a questionnaire to measure mood or depression (Bielderman et al. 2013). The variables outlined above are detailed below.
Polypharmacy, excluding nutraceuticals, is defined as the use of three or more prescribed medications. Chronic heart failure is reported as an independent predictor for frailty and is associated with other morbidities. Diabetes as a metabolic condition is associated with frailty. A subjective health assessment is widely used to assess frailty. This questionnaire addresses two main issues: (1) how is your health generally (very good/good/not so good/not good at all/bad) and (2) how is your health today as compared to your health a year ago (better/the same/not as good). Physical activity is one of the most powerful predictors for disability in daily living. The questionnaire on physical activity provides information about physical habits, frequency, duration, and average length of activity sessions. The MMSE is used to evaluate cognitive function ability. The maximum score is 30 and less than 24 points is considered a deficient cognition. The GHQ is mostly used to assess general health and also mood or depression. A score < 4 indicates no mood disturbance, 4–8 indicates mild disturbance, and 8–12 points a significant disturbance.
8.1 Frailty Assessment
Frailty is measurable. It is usually assessed late in life, in particular to evaluate the need for immediate care, help, or rehabilitation. With the worldwide increase in life expectancy, early detection of individuals at-risk may initiate an action to deter this health condition. To-date, comprehensive geriatric assessment (CGA) appears to be the most evidence-based process to detect and assess frailty (Chen et al. 2016a). Thus, CGA can provide a better understanding of the complex nature of frailty and support the development of healthcare practices to improve outcomes.
Frailty is mostly manifested by a low body weight, with a low hematocrit and serum albumin level < 3.4 g/dL (Blodgett et al. 2016). Several indices are commonly used to assess frailty. Woo et al. (2015) have suggested the FRAIL scale as the initial approach in detecting frailty in the community, enabling the targeted intervention to retard decline and future disability. The Fried Index (Fried et al. 2001) incorporates self-reported data of five criteria: unintended weight loss, exhaustion, leisure time activity, and some physical tests like hand grip strength. The Gill Frailty Index (Searle et al. 2008; Rothman et al. 2008) focuses entirely on a lower body physical performance measuring physical ability by sit-to-stand test and 20 m walking speed. This index, contrary to the Fried index, only consists of observed and measured physical performance tests. The main advantages of these instruments are that a single clinician can administer them with minimal safety concerns. The time to complete and score the Fried Index is 15–20 min and that for the Gill Index is less than 2 min. Kim et al. (2014) have reported an interesting observation on these two instruments. Individuals who were frail according to Gill Index may meet the Fried frailty criteria. Thus, a valid and reliable tool for frailty assessment is still an open issue that awaits a more comprehensive approach.
The Groningen Frailty Indicator (GFI) (Steverink et al. 2001) is a 15-item screening instrument that assesses frailty among home-dwelling elderly populations. It includes a combination of a professional and self-assessment questionnaire, addressing items that assess disability and can predict poor outcomes. It incorporates grades of frailty. The GFI is widely used in clinical practice and in clinical studies. A score of 4 or higher out of the 15 items represents moderate-to-severe frailty.
8.2 Frailty Management
Caring for frail elderly people is complicated and when they become ill it is even a more challenging and frustrating task. The elderly’s illnesses are often nonspecific, unrecognized, and poorly documented. Treatment is complex and success is often unclear. Moreover, frailty status often requires end of life and sometimes palliative care. As noted above, frail people are vulnerable to common stressors, tend to have multiple interrelated medical and health problems, underweight, muscle weakness, impaired function, and high risk for falls and fractures. Therefore, the magnitude of their needs and the complexity of their health issues, require special focus and skills, along with a comprehensive, systematic, geriatric assessment to meet the challenges presented.
The basic concept of appropriate frailty management is that the clinician should move away from organs’ impairment and pathology-based approaches toward the International Classification of Functioning, Disability and Health (ICF) approach, and thus include biopsychosocial aspects, as recommended by the French Society of Geriatrics and Gerontology (Rolland et al. 2011). The initial treatment goal is optimal management of any underlying illness or poor health condition that may increase frailty. Complex alterations of pharmacokinetics and pharmacodynamics occur in frail elderly patients (Cesari et al. 2015). Thus, recommended approach is of a kind ‘to start low and go slow’. Another important challenge is monitoring of the progress of interventions, particularly in frail people with unusual disease appearances. Simple tracking of activity level in the frail elderly may however be helpful since when a frail person gets better he becomes more mobile and when the situation worsens less mobile.
8.3 Nutrition for Frailty
Although nutrition is considered a major factor in managing frailty, evidence of the effect of nutrition is often derived from short-term studies in selected samples; large clinical trials are lacking. Currently, there is no robust evidence for nutritional recommendations for individuals with frailty. To gain weight, a daily diet approach should be first to maintain balanced nutrient intake (Solon-Biet et al. 2015). Clinical studies show that a low-carbohydrate diet is beneficial for human health (Rosedale et al. 2009). A high-fat diet is associated with increased mortality and increased incidence of many metabolic diseases, including sarcopenic obesity, type II diabetes and cardiovascular problems (Baulderstone et al. 2012; Schrager et al. 2007). On the other hand, diets rich in unsaturated fatty acids lead to reduced blood levels of harmful low-density lipoproteins and increase the level of protective high-density lipoproteins (Mensink et al. 2003). Moreover, diets rich in natural, unsaturated fatty acids lower blood pressure, improve insulin sensitivity, and reduce the risks of cardiovascular and metabolic diseases (Da Silva et al. 2015). The Nutritional Geometric Framework (NGF) indicates the effects of a nature of various nutrient dimensions on lifespan and mortality (Raubenheimer et al. 2016). However, NGF has mostly been applied to the influence of protein intake relative to carbohydrates. Even if the results of randomized controlled trials are inconsistent regarding the effects of protein supplementation on physical function, several observational studies have suggested that maintaining adequate protein intake may help preserve energy in older people (Beck et al. 2016; Suominen et al. 2015). The frail elderly who have acute or chronic diseases need a higher dietary protein intake in a range of 1.2–1.5 g/kg/day (Chang 2017).
Hormone replacement therapy with testosterone, dehydroepiandrosterone (DHEA), or growth hormone has not proven beneficial (Morley and Malmstrom 2013; Lunenfeld 2006; Morley et al. 2005). Some drugs, which may be beneficial for treatment of fatigue, such as amantadine, methylphenidate, and modafinil, require further evidence-based evaluation and exploration (Mücke et al. 2016).
9 Relationship between Frailty and Sarcopenia
Sarcopenia and frailty often co-exist and both entail a physical function impairment as a core component. As indicated, numerous tools and instruments are available to assess sarcopenia. However, it is important for the clinician to be aware of which health aspects are being measured when assessing a person for frailty. This review stresses the importance of distinguishing and appropriately assessing older people who are frail from those who suffer from sarcopenia. The British Geriatrics Society believes that older people should be assessed for frailty and/or sarcopenia during all interactions with health and social care professionals (Turner et al. 2014). This would allow health care professionals to examine the benefits and risks of interventions and to allow individuals and their care givers to make rational decisions about the factors affecting their health.
Frailty, like sarcopenia, has been used interchangeably with disability (Rockwood et al. 2000). Yet despite the overlap in symptoms, frailty may be a universal condition of whole body wasting, including underweight and weakness, that directly affects functioning and recovery. Sarcopenia, on the other hand, is a more age-related, slowly progressive decline in muscle mass and strength, moderately affecting daily living activities. Frailty can be reversible, but this is not true for sarcopenia, which is a normal physiological change. Frailty can be understood as a continuum with two modifiable phases. Non-frail people can become pre-frail, and later on they become frail. There is a reverse way possible from frailty to pre-frailty status, and even potentially to a non-frail status. Frailty can be described as an ‘acute’ body condition, whereas sarcopenia is a normal, irreversible process resulting in programmed muscle death, i.e., muscle apoptosis. Therefore, even though, e.g., if an 80-year-old person in a pre-frailty condition, deteriorated to frailty, this could be halted or reversed, whereas for a person of the same age who already has some degree of sarcopenia, it is almost impossible to halt the physiological deterioration due to apoptosis.
Although a link between frailty and sarcopenia has been noted in a few studies (Mijnarends et al. 2015; Garatachea and Lucia 2013), this review focuses on the association of frailty and functional impairment and of sarcopenia and functional impairment. Yet both conditions result in disability in later life. Although low muscle mass increases the risk for fatigability, and low muscle strength and endurance increase functional disability, frailty increases the risk for underweight, functional disability, and other outcomes such as osteoporosis (Rosen and Klibanski 2009). It is well-documented that frail older adults are vulnerable, with minimal reserve capacity, and increased risk for malnutrition, institutionalization, and death (Payette et al. 2000). Sarcopenic older adults mostly demonstrate increased risk for gait, balance deficits, and falls. For these reasons, frailty care has mostly focused on nutrition and weight gain, whereas sarcopenia research has concentrated on physical interventions to increase muscle mass and strength. The management is different for frail versus sarcopenic older adults. Frail individuals should consume calories to increase body weight, sarcopenic individuals should increase their physical activity by performing resistance and endurance exercises. To gain weight, frail people’s diet should include carbohydrate, lipids, and proteins. In addition, several studies on dietary patterns have shown positive effects of enhanced nutritional intake also on general health, e.g., lower blood pressure, reduced risk of coronary heart failure, reduced risk of type 2 diabetes, and cancers (Asp and Bryngelsson 2008). Sarcopenic people who need to gain muscle mass should consume protein, particularly 1.5–2 h after performing physical exercises.
Frail people often demonstrate low body weight or low BMI (<25 kg/m2), despite evidence of abdominal obesity (Buch et al. 2016), with a greater likelihood of functional limitations and difficulties performing daily living activities (Byard 2015). The mechanism of interaction of the frail state with obesity has not been clearly defined, but it might involve hormonal dysregulation and inflammatory pathways, as well as oxidative stress. Insulin resistance, associated with abdominal obesity, may promote abnormal ‘colonization’ and emergence of ectopic fat in muscle, which is associated with functional limitations (Auyeung et al. 2013). The Foundation for the National Institutes of Health Sarcopenia Project validated cut points and reported that older adults with sarcopenia have a low-to-moderate BMI, which appears to protect against functional limitations (Batsis et al. 2015).
10 Clinical and Practical Recommendations
-
I propose the assessment of muscle mass primarily with dual-energy X-ray absorptiometry (DeXA). In case the technique is unavailable, anthropometry can be easily used in primary care settings as an initial screening tool for patients with low muscle mass. Patients should then be referred for further evaluation in clinical specialty settings.
-
Physical performance should be primarily assessed by measuring gait speed. The Short Physical Performance Battery (SPPB) test might be limited by administration time, but might also be useful to identify men and women with low physical performance.
-
A comprehensive geriatric assessment (CGA) appears the most evidence-based process for detecting and assessing frailty.
-
The Groningen Frailty Indicator, the Fried Index, and the Gill Frailty Index are valid and reliable tools for assessing and monitoring frailty.
-
Whereas further studies are required to provide a full evidence-based guidance to clinicians, current management should include physical activity advice, particularly progressive resistance training, treatment and prevention of vitamin D deficiency, and adequate and balanced energy intake emphasizing dietary protein.
-
Emphasis on the importance of education and increased awareness of clinicians concerning the potential deleterious effects of frailty and sarcopenia.
-
Careful attention given to individuals older than 65 years with low BMI or abdominal obesity.
11 Summary and Conclusions
This review describes differences, similarities, and commonalities in the definition, assessment, and management of frailty and sarcopenia. Despite the similarities between the two, frailty and sarcopenia are two separate health conditions. Therefore, it is important to diagnose and manage them as separate entities.
-
The definition of sarcopenia is agreed upon by the European Working Group on Sarcopenia in Older People (gait speed, handgrip strength, and muscle mass), and the International Working Group on Sarcopenia (gait speed and muscle mass).
-
The consensus definition of frailty include the Fried phenotype (weight loss, exhaustion, physical inactivity, handgrip strength, and walk time) (Fried et al. 2001) and the Rockwood phenotype (use of walking aid, activities of daily living, incontinence, and cognitive impairment) (Rockwood et al. 2000).
-
Primary sarcopenia is age-related, but it is frequently found in association with comorbidities, such as osteoporosis, malnutrition, and type 2 diabetes mellitus. Thus, it should be considered a consequence of the coexisting pathological conditions, i.e., ‘secondary sarcopenia’.
-
Several tools are currently available for measuring muscle mass, muscle strength, and physical performance, which are of use for the diagnosis and follow-up of sarcopenia. However, these tools remain to be fully adopted for widespread use in clinical daily practice.
-
The development of pharmaceutical therapies for sarcopenia and frailty has been delayed, in part because of the lack of consensus regarding the definitions of the two conditions.
Physicians and other healthcare professionals have an important role to play in the assessment and management of sarcopenia to reduce its impact on individuals’ well-being, the development of disability, and on health resource utilization. This review suggests that frailty and sarcopenia differentially affect functional capability, morbidity, quality of life, and disability in daily living activities. Frailty is characterized by low weight and reflects nutritional status, whereas sarcopenia – the loss of muscle mass – is more accurate and can be a quantitative, global marker of frailty. This review also highlights the importance of frailty and sarcopenia in predicting post-operative outcomes among individuals undergoing surgery for cancer.
References
Araujo AB, Chiu GR, Kupelian V, Hall SA, Williams RE, Clark RV, McKinlay JB (2010) Lean mass, muscle strength, and physical function in a diverse population of men: a population-based cross-sectional study. BMC Public Health 10:508
Asp NG, Bryngelsson S (2008) Health claims in Europe: new legislation and PASSCLAIM for substantiation. J Nutr 138(6):1210S–1215S
Auyeung TW, Lee JS, Leung J, Kwok T, Woo J (2013) Adiposity to muscle ratio predicts incident physical limitation in a cohort of 3,153 older adults – an alternative measurement of sarcopenia and sarcopenic obesity. Age (Dordr) 35(4):1377–1385
Azzopardi RV, Vermeiren S, Gorus E, Habbig AK, Petrovic M, Van Den Noortgate N, De Vriendt P, Bautmans I, Beyer I, Gerontopole Brussels Study Group (2016) Linking frailty instruments to the international classification of functioning, disability, and health: a systematic review. J Am Med Dir Assoc 17(11):1066.e1–1066.e11
Barbat-Artigas S, Rolland Y, Zamboni M, Aubertin-Leheudre M (2012) How to assess functional status: a new muscle quality index. J Nutr Health Aging 16(1):67–77
Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ (2015) Sarcopenia, sarcopenic obesity, and functional impairments in older adults: national health and nutrition examination surveys 1999–2004. Clin Nutr 35(12):1031–1039
Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta D, Visvanathan R, Volpi E, Boirie Y (2013) Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc 14(8):542–559
Baulderstone L, Yaxley A, Luszcz M, Miller M (2012) Diet liberalization in older Australians decreases frailty without increasing the risk of developing chronic disease. J Frailty Aging 4:174–182
Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, Bautmans I, Bertière MC, Brandi ML, Al-Daghri NM, Burlet N, Cavalier E, Cerreta F, Cherubini A, Fielding R, Gielen E, Landi F, Petermans J, Reginster JY, Visser M, Kanis J, Cooper C (2016) Sarcopenia in daily practice: assessment and management. BMC Geriatr 16(1):170
Beck AM, Dent E, Baldwin C (2016) Nutritional intervention as part of functional rehabilitation in older people with reduced functional ability: a systematic review and meta-analysis of randomised controlled studies. J Hum Nutr Diet 29(6):733–745
Bielderman A, van der Schans CP, van Lieshout MR, de Greef MH, Boersma F, Krijnen WP, Steverink N (2013) Multidimensional structure of the Groningen frailty indicator in community-dwelling older people. BMC Geriatr 13:86
Bieniek J, Wilczyński K, Szewieczek J (2016) Fried frailty phenotype assessment components as applied to geriatric inpatients. Clin Interv Aging 11:453–459
Bijlsma AY, Meskers CG, van den Eshof N, Westendorp RG, Sipilä S, Stenroth L, Sillanpää E, McPhee JS, Jones DA, Narici MV, Gapeyeva H, Pääsuke M, Voit T, Barnouin Y, Hogrel JY, Butler Browne G, Maier AB (2014) Diagnostic criteria for sarcopenia and physical performance. Age (Dordr) 36(1):275–285
Blodgett JM, Theou O, Howlett SE, Wu FC, Rockwood K (2016) A frailty index based on laboratory deficits in community-dwelling men predicted their risk of adverse health outcomes. Age Ageing 45(4):463–468
Bottaro M, Russo AF, de Oliveira RJ (2005) The effects of rest interval on quadriceps torque during an isokinetic testing protocol in elderly. J Sports Sci Med 4(3):285–290
Bourdel-Marchasson I, Berrut G (2005) Caring the elderly diabetic patient with respect to concepts of successful aging and frailty. Diabetes Metab 2:5S13–5S19
Bowen ME (2012) The relationship between body weight, frailty, and the disablement process. J Gerontol B Psychol Sci Soc Sci 67(5):618–626
Buch A, Carmeli E, Boker LK, Marcus Y, Shefer G, Kis O, Berner Y, Stern N (2016) Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age – an overview. Exp Gerontol 76:25–32
Buford TW, Lott DJ, Marzetti E, Wohlgemuth SE, Vandenborne K, Pahor M, Leeuwenburgh C, Manini TM (2012) Age-related differences in lower extremity tissue compartments and associations with physical function in older adults. Exp Gerontol 47:38–44
Byard RW (2015) Forensic issues at the extremes of weight: from frailty syndrome through frail obesity to morbid obesity. J Forensic Legal Med 35:38–39
Cao L, Morley JE (2016) Sarcopenia is recognized as an independent condition by an international classification of disease, tenth revision, clinical modification (ICD-10-CM) code. J Am Med Dir Assoc 17(8):675–677
Cesari M, Fielding R, Bénichou O, Bernabei R, Bhasin S, Guralnik JM, Jette A, Landi F, Pahor M, Rodriguez-Manas L, Rolland Y, Roubenoff R, Sinclair AJ, Studenski S, Travison T, Vellas B (2015) Pharmacological interventions in frailty and sarcopenia: report by the international conference on frailty and sarcopenia research task force. J Frailty Aging 4(3):114–120
Chang SF (2017) Frailty is a major related factor for at risk of malnutrition in community-dwelling older adults. J Nurs Scholarsh 49(1):63–72
Chang SF, Yang RS, Lin TC, Chiu SC, Chen ML, Lee HC (2014) The discrimination of using the short physical performance battery to screen frailty for community-dwelling elderly people. J Nurs Scholarsh 46(3):207–215
Chen LK, Hwang AC, Liu LK, Lee WJ, Peng LN (2016a) Frailty is a geriatric syndrome characterized by multiple impairments: a comprehensive approach is needed. J Frailty Aging 5(4):208–213
Chen LK, Lee WJ, Peng LN, Liu LK, Arai H, Akishita M, Asian Working Group for Sarcopenia (2016b) Recent advances in sarcopenia research in Asia: 2016 update from the Asian working Group for Sarcopenia. J Am Med Dir Assoc 17(8):767.e1–767.e7
Chiles Shaffer N, Ferrucci L, Shardell M, Simonsick EM, Studenski S (2016) Agreement and predictive validity using less-conservative foundation for the National Institutes of Health sarcopenia project weakness Cutpoints. J Am Geriatr Soc. doi:10.1111/jgs.14706
Chow WB, Rosenthal RA, Merkow RP, Ko CY, Esnaola NF (2012) Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg 215(4):453–466
Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JP, Sieber C, Stout JR, Studenski SA, Vellas B, Woo J, Zamboni M, Cederholm T (2014) Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the international sarcopenia initiative (EWGSOP and IWGS). Age Ageing 43(6):748–759
Cuesta F, Formiga F, Lopez-Soto A, Masanes F, Ruiz D, Artaza I, Salvà A, Serra-Rexach JA, Rojano I, Luque X, Cruz-Jentoft AJ (2015) Prevalence of sarcopenia in patients attending outpatient geriatric clinics: the ELLI study. Age Ageing 44(5):807–809
Da Boit M, Sibson R, Sivasubramaniam S, Meakin JR, Greig CA, Aspden RM, Thies F, Jeromson S, Hamilton DL, Speakman JR, Hambly C, Mangoni AA, Preston T, Gray SR (2017) Sex differences in the effect of fish oil supplementation on the adaptive response to resistance exercise training in older people: a randomized control trial. Am J Clin Nutr 105(1):151–158
Da Silva MS, Julien P, Pérusse L, Vohl MC, Rudkowska I (2015) Natural rumen-derived trans fatty acids are associated with metabolic markers of cardiac health. Lipids 50(9):873–882
Ding H, Zhang G, Sin KW, Liu Z, Lin RK, Li M, Li YP (2016) Activin a induces skeletal muscle catabolism via p38β mitogen-activated protein kinase. J Cachexia Sarcopenia Muscle. doi:10.1002/jcsm.12145
Drey M (2011) Sarcopenia – pathophysiology and clinical relevance. Wien Med Wochenschr 161(17–18):402–408
Dunsky A, Zach S, Zeev A, Goldbourt U, Shimony T, Goldsmith R, Netz Y (2014) Level of physical activity and anthropometric characteristics in old age – results from a national health survey. Eur Rev Aging Phys Act 11(2):149–157
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12(4):249–256
Figueiredo CS, Assis MG, Silva SL, Dias RC, Mancini MC (2013) Functional and cognitive changes in community-dwelling elderly: longitudinal study. Brazil J Phys Ther 7(3):297–306
Fragala MS, Jajtner AR, Beyer KS, Townsend JR, Emerson NS, Scanlon TC, Oliveira LP, Hoffman JR, Stout JR (2014) Biomarkers of muscle quality: N-terminal propeptide of type III procollagen and C-terminal agrin fragment responses to resistance exercise training in older adults. J Cachex Sarcopenia Muscle 5(2):139–148
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, MA MB, Cardiovascular Health Study Collaborative Research Group (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156
Garatachea N, Lucia A (2013) Genes, physical fitness and ageing. Ageing Res Rev 12(1):90–102
Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, McLachlan AJ, Cumming RG, Handelsman DJ, Le Couteur DG (2012) Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 65(9):989–995
Hamerman D (1999) Toward an understanding of frailty. Ann Intern Med 130(11):945–950
Hassani A, Kubicki A, Brost V, Mourey F, Yang F (2015) Kinematic analysis of motor strategies in frail aged adults during the timed up and go: how to spot the motor frailty? Clin Interv Aging 10:505–513
Heuberger RA (2011) The frailty syndrome: a comprehensive review. J Nutr Gerontol Geriatr 30(4):315–368
Hittel DS, Berggren JR, Shearer J, Boyle K, Houmard JA (2009) Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 58(1):30–38
Holwerda AM, Kouw IW, Trommelen J, Halson SL, Wodzig WK, Verdijk LB, van Loon LJ (2016) Physical activity performed in the evening increases the overnight muscle protein synthetic response to pre-sleep protein ingestion in older men. J Nutr 146(7):1307–1314
Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J (1998) Importance of functional measures in predicting mortality among older hospitalized patients. JAMA 279(15):1187–1193
Ishii S, Tanaka T, Shibasaki K, Ouchi Y, Kikutani T, Higashiguchi T, Obuchi SP, Ishikawa-Takata K, Hirano H, Kawai H, Tsuji T, Iijima K (2014) Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int 14(Suppl 1):93–101
Jenkins ND, Buckner SL, Bergstrom HC, Cochrane KC, Goldsmith JA, Housh TJ, Johnson GO, Schmidt RJ, Cramer JT (2014) Reliability and relationships among handgrip strength, leg extensor strength and power, and balance in older men. Exp Gerontol 58:47–50
Kahlon S, Pederson J, Majumdar SR, Belga S, Lau D, Fradette M, Boyko D, Bakal JA, Johnston C, Padwal RS, McAlister FA (2015) Association between frailty and 30-day outcomes after discharge from hospital. Can Med Assoc J 187(11):799–804
Katz S (1983) Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 31(12):721–727
Kim M, Kim H (2013) Accuracy of segmental multi-frequency bioelectrical impedance analysis for assessing whole-body and appendicular fat mass and lean soft tissue mass in frail women aged 75 years and older. Eur J Clin Nutr 67:395–400
Kim H, Higgins PA, Canaday DH, Burant CJ, Hornick TR (2014) Frailty assessment in the geriatric outpatient clinic. Geriatr Gerontol Int 14(1):78–83
Kim M, Shinkai S, Murayama H, Mori S (2015) Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr Gerontol Int 15:1013–1022
Kulkarni B, Kuper H, Taylor A, Wells JC, Radhakrishna KV, Kinra S, Ben-Shlomo Y, Smith GD, Ebrahim S, Byrne NM, Hills AP (2013) Development and validation of anthropometric prediction equations for estimation of lean body mass and appendicular lean soft tissue in Indian men and women. J Appl Physiol 115:1156–1162
Landi F, Russo A, Liperoti R, Pahor M, Tosato M, Capoluongo E, Bernabei R, Onder G (2010) Midarm muscle circumference, physical performance and mortality: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE study). Clin Nutr 29:441–447
Landi F, Onder G, Russo A, Liperoti R, Tosato M, Martone AM, Capoluongo E, Bernabei R (2014) Calf circumference, frailty and physical performance among older adults living in the community. Clin Nutr 33(3):539–544
Lang PO, Michel JP, Zekry D (2009) Frailty syndrome: a transitional state in a dynamic process. Gerontology 55(5):539–549
Levine JA, Abboud L, Barry M, Reed JE, Sheedy PF, Jensen MD (2000) Measuring leg muscle and fat mass in humans: comparison of CT and dual-energy X-ray absorptiometry. J Appl Physiol 88:452–456
Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A (2002) Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci 57(8):M539–M543
Lunenfeld B (2006) Endocrinology of the aging male. Minerva Ginecol 58(2):153–170
Malatesta M, Cardani R, Pellicciari C, Meola G (2014) RNA transcription and maturation in skeletal muscle cells are similarly impaired in myotonic dystrophy and sarcopenia: the ultrastructural evidence. Front Aging Neurosci 6:196
Martone AM, Lattanzio F, Abbatecola AM, Carpia DL, Tosato M, Marzetti E, Calvani R, Onder G, Landi F (2015) Treating sarcopenia in older and oldest old. Curr Pharm Des 21(13):1715–1722
McCleary NJ, Dotan E, Browner I (2014) Refining the chemotherapy approach for older patients with colon cancer. J Clin Oncol 32:2570–2580
Mensink RP, Zock PL, Kester AD, Katan MB (2003) Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta analysis of 60 controlled trials. Am J Clin Nutr 77(5):1146–1155
Mijnarends DM, Schols JM, Meijers JM, Tan FE, Verlaan S, Luiking YC, Morley JE, Halfens RJ (2015) Instruments to assess sarcopenia and physical frailty in older people living in a community (care) setting: similarities and discrepancies. J Am Med Dir Assoc 16(4):301–308
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 85:115–122
Moore DR, Soeters PB (2015) The biological value of protein. Nestle Nutr Inst Workshop Ser 82:39–51. doi:10.1159/000382000
Morley JE, Malmstrom TK (2013) Frailty, sarcopenia, and hormones. Endocrinol Metab Clin N Am 42(2):391–405
Morley JE, Kim MJ, Haren MT (2005) Frailty and hormones. Rev Endocr Metab Disord 6(2):101–108
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33(5):997–1006
Mücke M, Mochamat CH, Peuckmann-Post V, Minton O, Stone P, Radbruch L (2016) Pharmacological treatments for fatigue associated with palliative care: executive summary of a Cochrane collaboration systematic review. J Cachex Sarcopenia Muscle 7(1):23–27
Payette H, Coulombe C, Boutier V, Gray-Donald K (2000) Nutrition risk factors for institutionalization in a free-living functionally dependent elderly population. J Clin Epidemiol 53(6):579–587
Perkins AJ, Kroenke K, Unützer J, Katon W, Williams JW Jr, Hope C, Callahan CM (2004) Common comorbidity scales were similar in their ability to predict health care costs and mortality. J Clin Epidemiol 57(10):1040–1048
Peters LL, Boter H, Buskens E, Slaets JP (2012) Measurement properties of the Groningen frailty indicator in home-dwelling and institutionalized elderly people. J Am Med Dir Assoc 13(6):546–5451
Phan HM, Alpert JS, Fain M (2008) Frailty, inflammation, and cardiovascular disease: evidence of a connection. Am J Geriatr Cardiol 17(2):101–107
Raîche M, Hébert R, Dubois MF (2008) PRISMA-7: a case-finding tool to identify older adults with moderate to severe disabilities. Arch Gerontol Geriatr 47(1):9–18
Raubenheimer D, Simpson SJ, Le Couteur DG, Solon-Biet SM, Coogan SC (2016) Nutritional ecology and the evolution of aging. Exp Gerontol 86:50–61
Ravaglia G, Forti P, Lucicesare A, Pisacane N, Rietti E, Bianchin M, Dalmonte E (2008) Physical activity and dementia risk in the elderly: findings from a prospective Italian study. Neurology 70(19 Pt 2):1786–1794
Reid KF, Martin KI, Doros G, Clark DJ, Hau C, Patten C, Phillips EM, Frontera WR, Fielding RA (2015) Comparative effects of light or heavy resistance power training for improving lower extremity power and physical performance in mobility-limited older adults. J Gerontol A Biol Sci Med Sci 70(3):374–380
Reiss J, Iglseder B, Kreutzer M, Weilbuchner I, Treschnitzer W, Kässmann H, Pirich C, Reiter R (2016) Case finding for sarcopenia in geriatric inpatients: performance of bioimpedance analysis in comparison to dual X-ray absorptiometry. BMC Geriatr 16:52
Rockwood K, Mitnitski A (2007) Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 62(7):722–727
Rockwood K, Hogan DB, MacKnight C (2000) Conceptualisation and measurement of frailty in elderly people. Drugs Aging 17(4):295–302
Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, Gonzalez-Colaço Harmand M, Bergman H, Carcaillon L, Nicholson C, Scuteri A, Sinclair A, Pelaez M, Van der Cammen T, Beland F, Bickenbach J, Delamarche P, Ferrucci L, Fried LP, Gutiérrez-Robledo LM, Rockwood K, Rodríguez Artalejo F, Serviddio G, Vega E, FOD-CC Group (Appendix 1) (2013) Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci 68(1):62–67
Rolland Y, Lauwers-Cances V, Cournot M, Nourhashémi F, Reynish W, Rivière D, Vellas B, Grandjean H (2003) Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc 51:1120–1124
Rolland Y, Benetos A, Gentric A, Ankri J, Blanchard F, Bonnefoy M, de Decker L, Ferry M, Gonthier R, Hanon O, Jeandel C, Nourhashemi F, Perret-Guillaume C, Retornaz F, Bouvier H, Ruault G, Berrut G (2011) Frailty in older population: a brief position paper from the French society of geriatrics and gerontology. Geriatr Psychol Neuropsychiatr Vieil 9(4):387–390
Rosedale R, Westman EC, Konhilas JP (2009) Clinical experience of a diet designed to reduce aging. J Appl Res 9(4):159–165
Rosen CJ, Klibanski A (2009) Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med 122(5):409–414
Rosen SL, Reuben DB (2011) Geriatric assessment tools. Mt Sinai J Med 78(4):489–497
Rosenberg IR (1989) Summary comments. Am J Clin Nutr 50:1231–1233
Rothman MD, Leo-Summers L, Gill TM (2008) Prognostic significance of potential frailty criteria. J Am Geriatr Soc 56(12):2211–2116
Saliba D, Elliott M, Rubenstein LZ, Solomon DH, Young RT, Kamberg CJ, Roth C, MacLean CH, Shekelle PG, Sloss EM, Wenger NS (2001) The vulnerable elders survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc 49(12):1691–1699
Santos PH, Fernandes MH, Casotti CA, Coqueiro Rda S, Carneiro JA (2015) The profile of fragility and associated factors among the elderly registered in a family health unit. Cien Saude Colet 20(6):1917–1924
Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, Ferrucci L (2007) Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol (1985) 102(3):919–925
Scott D, Trbojevic T, Skinner E, Clark RA, Levinger P, Haines TP, Sanders KM, Ebeling PR (2015) Associations of calf inter- and intra-muscular adipose tissue with cardiometabolic health and physical function in community-dwelling older adults. J Musculoskelet Neuronal Interact 15(4):350–357
Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K (2008) A standard procedure for creating a frailty index. BMC Geriatr 8:24
Shad BJ, Wallis G, van Loon LJ, Thompson JL (2016) Exercise prescription for the older population: the interactions between physical activity, sedentary time, and adequate nutrition in maintaining musculoskeletal health. Maturitas 93:78–82
Silva AM, Shen W, Heo M, Gallagher D, Wang Z, Sardinha LB, Heymsfield SB (2010) Ethnicity-related skeletal muscle differences across the lifespan. Am J Hum Biol 22(1):76–82
Sirola J, Pitkala KH, Tilvis RS, Miettinen TA, Strandberg TE (2011) Definition of frailty in older men according to questionnaire data (RAND-36/SF-36): the Helsinki businessmen study. J Nutr Health Aging 15(9):783–787
Solon-Biet SM, Mitchell SJ, de Cabo R, Raubenheimer D, Le Couteur DG, Simpson SJ (2015) Macronutrients and caloric intake in health and longevity. J Endocrinol 226(1):R17–R28
Steffl M, Musalek M, Kramperova V, Petr M, Kohlikova E, Holmerova I, Volicer L (2016) Assessment of diagnostics tools for sarcopenia severity using the item response theory (IRT). J Nutr Health Aging 20(10):1051–1055
Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A (2011) The identification of frailty: a systematic literature review. J Am Geriatr Soc 59(11):2129–2138
Steverink N, Slaets J, Schuurmans H, Van Lis M (2001) Measuring frailty: developing and testing the GFI (Groningen frailty indicator). Gerontologist 41:236e237
Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL (2011) Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int 22(3):859–871
Suominen MH, Puranen TM, Jyväkorpi SK, Eloniemi-Sulkava U, Kautiainen H, Siljamäki-Ojansuu U, Pitkalä KH (2015) Nutritional guidance improves nutrient intake and quality of life, and may prevent falls in aged persons with Alzheimer disease living with a spouse (NuAD trial). J Nutr Health Aging 19(9):901–907
Tamura F, Kikutani T, Tohara T, Yoshida M, Yaegaki K (2012) Tongue thickness relates to nutritional status in the elderly. Dysphagia 27(4):556–561
Theou O, O’Connell MD, King-Kallimanis BL, O’Halloran AM, Rockwood K, Kenny RA (2015) Measuring frailty using self-report and test-based health measures. Age Ageing 44(3):471–477
Turner G, Clegg A, British Geriatrics Society, Age UK, Royal College of General Practioners (2014) Best practice guidelines for the management of frailty: a British geriatrics society, Age UK and Royal College of general practitioners report. Age Ageing 43(6):744–747
Vermeulen J, Neyens JC, van Rossum E, Spreeuwenberg MD, de Witte LP (2011) Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr 11:33. doi:10.1186/1471-2318-11-33
Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R, Federspil G (2009) The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab 297(5):E987–E998
Villani AM, Miller M, Cameron ID, Kurrle S, Whitehead C, Crotty M (2013) Body composition in older community-dwelling adults with hip fracture: portable field methods validated by dual-energy X-ray absorptiometry. Brit J Nutr 109(7):1219–1229
Villani AM, Crotty M, Cameron ID, Kurrle SE, Skuza PP, Cleland LG, Cobiac L, Miller MD (2014) Appendicular skeletal muscle in hospitalized hip-fracture patients: development and cross-validation of anthropometric prediction equations against dual-energy X-ray absorptiometry. Age Ageing 43:857–862
Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB (2002) Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 50:897–904
Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP (2006) Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging research conference on frailty in older adults. J Am Geriatr Soc 54(6):991–1001
Wang XH, Mitch WE (2014) Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 10(9):504–516
Wei N, Pang MY, Ng SS, Ng GY (2016) Optimal frequency/time combination of whole body vibration training for improving muscle size and strength of people with age-related muscle loss (sarcopenia): a randomized controlled trial. Geriatr Gerontol Int. doi:10.1111/ggi.12878
WHO (2015) Facts about ageing http://www.who.int/ageing/about/facts/en/. Accessed on 23 Jan 2017
WHO (2017) http://www.who.int/dietphysicalactivity/factsheet_olderadults/en/. Accessed on 23 Jan 2017
Woo J, Yu R, Wong M, Yeung F, Wong M, Lum C (2015) Frailty screening in the community using the FRAIL scale. J Am Med Dir Assoc 16(5):412–419
Xue QL (2011) The frailty syndrome: definition and natural history. Clin Geriatr Med 27(1):1–15
Yu S, Appleton S, Chapman I, Adams R, Wittert G, Visvanathan T, Visvanathan R (2015) An anthropometric prediction equation for appendicular skeletal muscle mass in combination with a measure of muscle function to screen for sarcopenia in primary and aged care. J Am Med Dir Assoc 16:25–30
Conflicts of Interest
The author declares no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Carmeli, E. (2017). Frailty and Primary Sarcopenia: A Review. In: Pokorski, M. (eds) Clinical Research and Practice. Advances in Experimental Medicine and Biology(), vol 1020. Springer, Cham. https://doi.org/10.1007/5584_2017_18
Download citation
DOI: https://doi.org/10.1007/5584_2017_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-65444-7
Online ISBN: 978-3-319-65445-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)