Abstract
The main goal of the international study I-MOVE (Influenza Monitoring of Vaccine Effectiveness) implemented in Poland is to identify and evaluate the activity types of influenza virus and to determine the effectiveness of vaccination against influenza in the 2014–2015 influenza season. The study is based on selecting patients with flu symptoms and collecting biological samples for laboratory examination. Detection, typing, and subtyping of influenza viruses were carried out by the National Center for Influenza Virus Research at National Institute of Public Health – National Institute of Hygiene, serving as a reference center, and also in selected laboratories of the Regional Sanitary Epidemiological Stations. Molecular biology methods, such as reverse transcription polymerase chain reaction (RT-PCR), were applied in this study. A total of 218 samples were collected. A hundred and twenty six samples, representing 57.8 % of the total, were confirmed with influenza virus infection. Influenza type A virus was detected in 54 samples, which included 16 samples of A/H1N1/pdm09 subtype and 11 samples of A/H3N2/ subtype. The remaining 27 samples positive for influenza type A were not subtyped. Influenza type B virus was detected in 57 samples, which appeared to be the dominant strain in this study. Furthermore, several cases of concurrent infection with influenza type B virus and the A/H1N/pdm09 or A/H3N2/ subtype were observed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Since the time of Hong Kong pandemic in 1968–1969, circulation of two subtypes of influenza A virus and influenza B virus with a different intensity is recorded every year. In Poland, during several recent seasons influenza cases were registered from a few hundred to a few million suspected cases of influenza and influenza-like infections (ILI) (Bednarska et al. 2015; Czarkowski et al. 2014). Each season, influenza is identified by circulation of different subtypes of influenza type A virus. In the 2014/2015 season, the A/H3N2/ was dominant, while the major etiological agent of ILI was respiratory syncytial virus. In addition, since the 2011/2012 influenza season, various types of co-infection were recorded, both within the influenza viruses and viruses that cause ILI, regardless of patients’ age. In the 2014–2015 influenza season, there was a three-component vaccine available; an inactivated split and subunit antigen of the following composition: A/California/7/2009 (H1N1) pdm09 – like virus, A/Texas/50/2012 (H3N2) – like virus, and B/Massachusetts/2012 – like virus (MMWR 2014). In Poland, the percentage of vaccinated population remains very low, as it amounted to a dismal 3.6 % in the season in question.
The international study I-MOVE (Influenza Monitoring of Vaccine Effectiveness) coordinated by the Epiconcept and European Center for Disease Prevention and Control (ECDC) has been conducted in European countries since 2007. Poland has entered the project as of the 2010/2011 influenza season (Kissling et al. 2012). For this case-controlled project, patients are qualified of every age who meet criteria of the ILI definition and from whom swabs are collected for laboratory examination conducted with reverse transcription polymerase chain reaction (RT-PCR) (Valenciano et al. 2015).
The aim of this article was to evaluate the activity of influenza virus and to determine the effectiveness of vaccination against influenza in the 2014–2015 season conducted within the framework of I-MOVE study in Poland. The control group included patients with ILI symptoms who had negative influenza test results.
2 Methods
The study was approved by an institutional Ethics Committee and was conducted in accord with the principles of the Declaration of Helsinki for Human Research. The study group consisted of 126 patients; 74 women and 52 men. The control group consisted of 91 patients, 46 women and 45 men, who visited the doctor’s office for reasons other than influenza, who did not meet the ILI definition and had negative influenza RT-PCR results. The participants of both groups were stratified into four age-groups: 0–4, 5–14, 15–64, and 65+ years of age. The study material consisted of throat and nasal swabs obtained from patients who met the ILI criteria as set by the European Union. A total of 218 persons were enrolled into the study, recruited by 22 general practitioners (GP), collaborating in the study. Specimens were sent to the laboratory with attached information regarding the date of a visit to GP and swabbing. Specimens were stored at −80 °C until analysis.
2.1 RNA Isolation
Influenza virus RNA was isolated using a Maxwell 16 Viral Total Nucleic Acid Purification Kit (Promega Corporation, Madison, WI) from 200 μl of clinical samples suspended in phosphate-buffered saline, according to the manufacturer’s instructions for low elution volume (LEV) cartridges. The RNA was eluted with 50 μl of RNase-free water.
2.2 Reverse Transcription PCR
Influenza A virus subtypes were determined using RT-PCR with a Light Thermocycler 2.0 System (Roche Diagnostics; Rotkreuz, Switzerland). RT-PCR reactions were conducted in capillary tubes of 20 μl volume using 0.5 μl (20 nM) of primers and 0.5 μl (5 nM) of probes for each reaction. Primers and probes were obtained through the Influenza Reagent Resource (IRR) program from the US Center for Disease Control (CDC) – Influenza Reagent Resource (IRR). The reaction mixture, containing reaction buffer, MgSO4, bovine serum albumin (BSA), RNase-free H2O, and SuperScript® III/Platinum® Taq Mix (Invitrogen by Life Technologies – Thermo Fisher Scientific, Carlsband, CA), was incubated with 5 μl of RNA sample in each capillary tube. RNA from the 2014/2015 vaccine viruses: A/California/7/2009(H1N1)pdm09 and A/Texas/50/2012(H3N2), and B/Massachusetts/2/2012 were introduced as positive controls. The negative control consisted of RNase-free water. Before DNA amplification, the RNA templates were reverse transcribed to obtain the corresponding cDNA at 50 °C for 30 min. The cDNA was then subjected to initial denaturation (1 cycle of 95 °C for 2 min), followed by further amplification steps of denaturation at 95 °C for 15 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 20 s repeated in 45 cycles.
3 Results and Discussion
Since the beginning of the influenza season in the middle of September 2014 (week 38 according to the ISO week date standard (ISO-8601)), Poland experienced only sporadic ILI cases. From week 43 to 47 of 2014, a widespread virus transmission began in the population and, consequently, we experienced a mild-to-moderate epidemic of influenza between week 48 of 2014 and week 15 of 2015, with the peak in weeks 10–13 of 2015. The influenza activity, which was mainly driven by influenza virus type A (51.4 %), subsided thereafter. There were no fatal cases of influenza recorded in the epidemic season of 2014–2015.
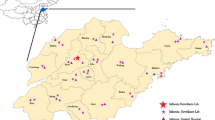
The majority of influenza cases taken into account in the present study concerned the after-peak period; still, however, with a substantially high, sustained level of transmission. Of the 218 cases that were medically attended, ILI was identified in 126 cases. In this group, there were only 36 (30.8 %) patients aged over 60 and eight of them tested positive for pandemic influenza A(H1N1). There were significant regional differences in the number of samples taken from patients who displayed the symptoms of ILI (Fig. 1). These differences distributed by the voivodeships of Poland are illustrated in Fig. 1. The differences lay down in the following way: Mazovian – 21, Lodz – 16, Silesian – 19, Subcarpathian – 62, Podlaskie – 21, Pomeranian – 20, Warmian-Masurian – 12, Lower Silesian – 31, and Greater Poland – 16 samples. Clinical material was tested to detect influenza virus type A and type B. Laboratory examination of the swabs collected from the Mazovian and Lodz provinces was performed in the Department of Influenza Research, National Influenza Center of the National Institute of Public Health-National Institute of Hygiene in Warsaw. Samples from other voivodeships were examined in the Provincial Sanitary-Epidemiological Stations. One hundred and twenty six samples, representing 57.8 % of the total, were confirmed with influenza virus infection, in the remaining 92 (42.2 %) samples influenza virus was not confirmed.
Influenza type A virus was detected in 54 samples, which included 16 samples of the subtype A/H1N1/pdm09 and 11 samples of the subtype A/H3N2/. The remaining 27 samples positive for influenza type A were not subtyped (Fig. 2).
In 57 samples influenza type B virus was detected, which appeared to be the dominant strain in this study. Only did five voivodeships confirm infections caused by influenza type B. The Subcarpathian voivodeship recorded 39 positive tests for influenza virus type B, which represented 68.4 % of all confirmed influenza virus type B samples; type B virus infection constituted a fraction of that percentage in other voivodeships.
It is worthy of note that co-infections of influenza type B and either subtype A/H1N1/podm09 or subtype A/H3N2/ of influenza type A were also confirmed. Co-infections occurred in the Mazovian and Lodz voivodeships of Poland; 7 and 8 cases, respectively (Fig. 1 and Fig. 2). There was no association between the patient’s age and the type of co-infection. In both voivodeships where the co-infections occurred, the age of the infected varied widely from 31 to 88 years.
Concerning the influenza type A virus, subtype A/H1N1/pdm09 predominated as it was detected in the following five voivodeships: Lodz, Mazovian, Lower Silesian, Silesian, and Greater Poland. That is in line with the data from the Global Influenza Surveillance and Response System and from the Department of Influenza Research, National Influenza Center of the National Institute of Public Health-National Institute of Hygiene in Warsaw, the organizations involved in the supervision of the 2014/2015 epidemic season, which confirmed the overall predominance of influenza virus type A, subtype A/H3N2/ in that season (Broberg et al. 2015).
4 Conclusions
-
The main circulating strain of influenza virus in the 2014/2015 epidemic season in Poland was type A, subtype A/H3N2/, although subtype B was a strain frequently detected in some geographic regions.
-
Substantial influenza morbidity in the 2014/2015 epidemic season implies the possibility of some mismatch between the genetic and antigenic features of the circulating virus and those included in the vaccine. That mismatch should not disenchant either from obtaining vaccination in the future seasons or from continuing efforts by savvy medical professional to increase vaccination coverage rate.
References
Bednarska K, Hallmann-Szelińska E, Kondratiuk K, Brydak LB (2015) Evaluation of the activity of influenza and influenza like viruses in epidemic season, 2013–2014. Adv Exp Med Biol 857:1–7
Broberg E, Snacken R, Adlhoch C, Beauté J, Galinska M, Pereyaslov D, Brown C, Penttigen P, Buda S, Schweiger B (2015) Start of the 2014/2015 influenza season in Europe: drifted influenza A(H3N2) viruses circulate as dominant subtype. Euro Surveill 20(4):1–5
Czarkowski MP, Hallmann-Szelińska E, Staszewka E, Bednarska K, Kondratiuk K, Brydak LB (2014) Influenza in Poland in 2011–2012 and in 2011/2012 and 2012/2013 epidemic season. Przegl Epidemiol 68(3):455–463
Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, Karron RA, Walter EB (2014) Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) –United States, 2014–15 Influenza season. MMWR 63(32):691–697
Kissling E, Valenciano M, I-MOVE case–control studies team (2012) Early estimates of seasonal influenza vaccine effectiveness in Europe among target groups for vaccination: results from the I-MOVE multicentre case-control study, 2011/12. Euro Surveill 17(15):pii=20146
Valenciano M, Kissling E, Reuss A, Jiménez-Jorge S, Horváth JK, Donnell JMO, Pitigoi D, Machado A, Pozo F, I-MOVE Multicentre Case Control Study Team (2015) The European I-MOVE Multicentre 2013–2014 Case-control Study. Homogeneous moderate influenza vaccine effectiveness against A(H1N1)pdm09 and heterogeneous results by country against A(H3N2). Vaccine 33(24):2813–2822
Acknowledgements
This work was funded in parts by Specific Contract No 1-ECD.5235, implementing activities to the Framework Contract No/ECDC/2014/026, and NIPH-NIH thematic grant 5/EM.1
Conficts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hallmann-Szelińska, E., Bednarska, K., Korczyńska, M., Paradowska-Stankiewicz, I., Brydak, L.B. (2016). Virological Characteristics of the 2014/2015 Influenza Season Based on Molecular Analysis of Biological Material Derived from I-MOVE Study. In: Pokorski, M. (eds) Allergy and Respiration. Advances in Experimental Medicine and Biology(), vol 921. Springer, Cham. https://doi.org/10.1007/5584_2016_236
Download citation
DOI: https://doi.org/10.1007/5584_2016_236
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42003-5
Online ISBN: 978-3-319-42004-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)