Abstract
Hashimoto’s thyroiditis (HT) is a very common autoimmune disease of the thyroid. In addition to genetic background, several viruses, including herpesviruses, have been suggested to play a role as possible environmental triggers of disease, but conclusive data are still lacking. Previous results showed that HT patients have an increased cellular immune response directed against the HHV-6 U94 protein and increased NK activity directed against HHV-6 infected thyrocytes.
In this study, we characterized the antiviral antibody response and the NK cells activity and subtype in HHV-6 infected HT patients. The results showed that HT subjects have increased prevalence and titer of anti-U94 antibodies and a higher amount of CD3-CD56brightCD16−NK cell percentages compared to controls. Furthermore, the cell activation of CD3−CD56bright NK cells in HT patients significantly correlates with TPO and Tg Ab levels.
The results suggest that HHV-6 might contribute to HT development, increasing NK cell secretion of inflammatory cytokines that could sustain the persistence of an inflammatory status in HT patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Hashimoto’s thyroiditis (HT) is a very common autoimmune disease of the thyroid. In addition to genetic background, several viruses, including herpesviruses, have been suggested to play a role as possible environmental triggers of disease, but conclusive data are still lacking.
HHV-6 infection is common and has a worldwide distribution (Caselli and Di Luca 2007). Viral strains cluster in two variants that were recently classified as different viral species, on the basis of characteristic biological differences: HHV-6A, with still unknown disease association, and HHV-6B, the etiologic agent of roseola (exanthem subitum), a childhood benign febrile disease. HHV-6 species in vitro replicate most efficiently in primary T-cells and in selected T-cell lines. However, the in vivo tropism of HHV-6 is considerably broader, including macrophages, endothelial cells, salivary glands, and brain (Caruso et al. 2002; Di Luca et al. 1994; Thomas et al. 2008). After primary infection, HHV-6 establishes a latent infection and resides mainly in peripheral blood mononuclear cells (PBMCs) and in macrophages (Di Luca et al. 1994; Kondo et al. 1991). During latency, HHV-6 expresses specific viral transcripts. In particular, expression of U94, in the absence of other viral lytic transcripts, is considered a molecular marker of viral latency (Caselli et al. 2006; Rotola et al. 1998).
HHV-6 has been tentatively associated to several chronic autoimmune inflammatory processes (Scotet et al. 1999), including Sjogren syndrome (Fox et al. 1989; Ranger-Rogez et al. 1994), multiple sclerosis (Alvarez-Lafuente et al. 2010; Challoner et al. 1995; Rotola et al. 2004), rheumatoid arthritis and systemic lupus erythematosus (Alvarez-Lafuente et al. 2005; Krueger et al. 1991). In addition, recent case reports suggested that HHV-6 infection might be related to the onset of autoimmune disorders, including purpura fulminans, severe autoimmune acquired protein S deficiency (Boccara et al. 2009), autoimmune connective tissue diseases (Broccolo et al. 2009), and severe autoimmune hepatitis (Potenza et al. 2008). Our analysis of fine needle thyroid aspirates (FNAs) and blood from HT patients and controls showed that HHV-6A prevalence and load are highly increased in thyroid tissue of HT patients (Caselli et al. 2012b). Furthermore, HT-derived thyrocytes harbor active HHV-6A, whereas the virus is strictly latent in the few virus-positive controls. We also reported that HHV-6A infects thyroid cells, inducing de novo expression of HLA-II surface antigens. Consequently, HT patients have increased CD4+ and CD8+ T-cell responses to HHV-6 U94 protein and infected thyrocytes become a target for innate Natural Killer (NK) cell killing. NK cells comprise about 10–15 % of all circulating lymphocytes and are able to lyse target cells that have lost the protective signal mediated by human leukocyte antigen (HLA) class I surface molecules (Storkus et al. 1987) as in viral infections. In particular, non-productive HHV-6A and HHV-6B infection is known to lead to the up-regulation of HLA (Human Leukocyte Antigens)-A, -B, -C molecules on dendritic cells (Bertelsen et al. 2010; Gustafsson et al. 2013), via autocrine IFN (Interferon)-α signaling, as well as the up-regulation of HLA-DR and CD86 molecules. This modification may result in the inability of NK cells to recognize target infected cells, as they still present HLA expression. Moreover, HHV-6A infection suppress DC stimulation of allogenic T cell proliferation. The ability to block innate and adaptive immune responses might be a successful strategy by which HHV-6A avoids the induction of appropriate host defense mechanisms, and thus facilitating persistent infection. Human NK cells can be divided into two subsets based on their cell-surface density of CD56 molecule in CD56bright and CD56dim, each with distinct phenotypic properties. There is evidence to suggest that these NK-cell subsets have unique functional attributes and, therefore, distinct roles in the human immune response. The CD56dim NK cell subset is more naturally cytotoxic while CD56bright NK-cell subset has the ability to produce abundant cytokines following activation and has low natural cytotoxicity (Cooper and Caligiuri 2001). NK cell activities have been evaluated during HHV-6 infection. NK cell activation was high in the acute phase of HHV-6 infection and declined gradually during convalescence. These results suggest that NK cells play a major role in resolving acute phase infection while specific lymphocyte activity develops later (Kumagai et al. 2006). In this study we analyzed changes in the activity and subtype of NK cells in peripheral blood cells from HT patients.
Clinical samples derived from 8 HT patients and 8 patients with benign follicular epithelial lesions (controls). The 8 HT patients included 2 males and 6 females, with a mean age of 57 ± 15 years (range 37–78 years), with anti-thyroperoxidase antibodies (TPO Ab) > 35 IU/ml (mean value = 835 IU/ml, range 343–3000 IU/ml), and anti-thyroglobulin antibodies (Tg Ab) > 115 IU/ml (mean value = 205 IU/ml, range 120–366 IU/ml). The 8 control patients included 4 males and 4 females with a mean age of 64 ± 18 years (range 30–91 years) (there was no statistically significant difference between the two groups), and showed TPO Ab < 35 IU/ml (mean value = 10 IU/ml, range 8–13 IU/ml), and Tg Ab < 115 IU/ml (mean value = 16 IU/ml, range 11–21 IU/ml). None of the patients enrolled in the study presented other autoimmune diseases.
Patients were characterized for HHV-6 viral load in their peripheral blood mononuclear cells (PBMCs) and thyroid FNAs, obtained as part of routine clinical work from patients undergoing FNAs for diagnostic purposes, and were used after receiving approval from the Local Ethical Committee of the University of Ferrara and S. Anna Hospital of Ferrara. The patients provided written informed consent for both FNA procedure (which is part of the clinical practice) and for biomolecular analyses, to which purpose the samples were anonymized. PBMCs were isolated by Ficoll-Hypaque gradients. DNA was isolated from FNAs and PBMCs as described (Caselli et al. 2012a). HHV-6 DNA presence and load were analyzed by PCR and real time quantitative PCR (qPCR) specific for the U94 and U42 genes (Caruso et al. 2009); samples were considered positive when 1 ug of cell DNA harbored more than 100 copies of viral DNA (Caselli et al. 2012a). Amplification of the house-keeping human RNase P gene was used as a control. All clinical samples were analyzed in a randomized and blinded fashion. NK cell activity and number was measured by flow cytometer.
NK cells were characterized with a specific anti-CD panel (CD3-PerCp-Cy5.5, CD56-FITC, CD107a-PE, CD16-PE) (e-Bioscience). For the CD107a degranulation assay, that shows NK cell activation status, cells were stained with CD107a-PE (e-Bioscience) after 1 h of incubation at 37 °C and 3 h of treatment with Golgi Stop solution (Becton Dickinson) (Rizzo et al. 2012). Ten thousand events were acquired. Cell viability was assessed by propidium iodide staining. Anti-isotype controls (Exbio) were performed.
HT and control subjects were also characterized for their antibody response against HHV-6 (whole virus) or its U94/Rep protein, by testing plasma samples by specific ELISA assays (Caselli et al. 2002). As a control, the plasma samples derived from 12 healthy donors were also assayed.
The results showed that HHV-6 was more prevalent in HT FNAs (8/8, 100 %) than in FNAs derived from controls (2/8, 25 %) (p < 0.001) (Table 1). Furthermore, viral load was higher in HT specimens (mean 1.2 × 104 copies/μg of cellular DNA, range 8 × 102–4.7 × 104 copies/μg DNA) than in the few controls which resulted positive for HHV-6 (mean 3.9 × 102 copies/μg DNA, range 2.2–5.7 × 102 copies/μg DNA) (p < 0.01). Similar results were obtained in PBMCs. In particular, HHV-6 was detected in 8/8 HT PBMCs (100 %) and only in 3/8 PBMCs derived from controls (37 %) (p < 0.01) (Table 1). Furthermore, viral load was higher in HT specimens (mean 1.8 × 104 copies/μg of cellular DNA, range 1.8 × 102–3.9 × 104 copies/μg DNA) than in the few controls which resulted positive for HHV-6 (mean 3.7 × 102 copies/μg DNA, range 2.8–4.9 × 102 copies/μg DNA) (p < 0.01). Where possible, virus species characterization, performed as previously described (Caselli et al. 2012a), showed the presence of HHV-6A in the thyroid tissue and of HHV-6B in PBMCs (data not shown), confirming the previously observed different tropism of the two viruses (Caselli et al. 2012a).
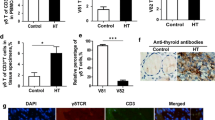
The anti-HHV-6 antibody response, evaluated by ELISA using a whole virus lysate (obtained by treatment of purified virions with 0.25 % Triton followed by brief sonication) as the antigen, showed no significant differences in antibody prevalence or titer between HT and control subjects or healthy donors, as well as the anti-tetanus toxin/toxoid (TT) IgG response, used as a control (measured by an ELISA kit, Alpha Diagnostic) (Fig. 1). On the contrary, the antibody response specifically directed against the HHV-6 U94/Rep protein was more prevalent in HT patients (8/8) than in controls (6/8), and especially the titer was significantly higher in HT vs control subjects (1:1624 vs 1:543) (p < 0.01), who showed prevalence and titer values similar to those of healthy donors (10/12; titer 1:442) (Fig. 1). These results confirmed that HT patients not only have a specific anti-U94/Rep cellular immune response (Caselli et al. 2012a), but also develop specific antibodies against this virus protein.
Anti-HHV6 antibody response in HT and control subjects. Humoral response against (a) whole HHV-6 virus, (b) Tetanus Toxoid (TT), and (c) HHV-6 U94/Rep protein were evaluated by ELISA in Hashimoto’s thyroiditis (HT), control (CTR) and healthy donors (HD) groups. Results are expressed as (a) mean absorbance at OD405nm ± standard deviation, (b) mean Ab titer (U/ml) ± standard deviation, and (c) mean Ab titer (dilution−1) ± standard deviation. *p ≤ 0.01, obtained with two tailed Student t test
The analysis of CD3−CD56bright NK cell percentages reported a higher amount of these cells in the samples from HT patients compared with controls (p = 0.02; two tailed Student t test) (Fig. 2a). The activation status of CD3−CD56bright NK cells was higher in HT patients compared with controls (p = 0.01; two tailed Student t test) (Fig. 2b). On the contrary, CD3−CD56dim NK cells did not present differences in the two groups of subjects (Table 2). Since NK cells are also subdivided into different populations based on the relative expression of CD16, we analyzed the levels of this surface markers. CD16 is a Fc receptor that, upon recognition of antibody-coated cells, delivers a potent signal to NK cells, which eliminate targets through direct killing and cytokine production. When we considered CD16 expression (Fig. 2c, Table 2), we observed that almost all CD3−CD56dim NK cells are CD16+, as previously reported (Poli et al. 2009). On the contrary, there was a slight difference in the percentage of CD3−CD56brightCD16− NK cells between the two groups (Fig. 2c, Table 2).
Cell percentage of (a) CD3−CD56bright NK cells and (b) CD3−CD56brightCD107a+ in HT and control (CTR) subjects. Data are reported as Mean ± standard deviation. *p value obtained with two tailed Student t test. (c) Representative dot plots for CD56 and CD16 staining in HT and control (CTR) subjects. Cell percentages are reported
When we analyzed the possible association between cell activation of CD3−CD56bright NK cells in HT patients and TPO and Tg Ab levels, we observed a slight correlation between these parameters and CD3−CD56bright NK cell CD107a expression (Fig. 3a, b).
These results indicate that NK cells might have an important role for the control of disease activity and viral infection. In fact, we observed an increased NK cell activity in HT patients characterized by HHV-6 infection in FNAs. Previous researches documented the implication of NK cells in the control of both viral infections (Kumagai et al. 2006; Rizzo et al. 2012; Wu et al. 2015) and autoimmune thyroid disease exacerbation (Hidaka et al. 1992). The increase in CD3−CD56bright NK cells, that are characterized by a cytokine-secreting phenotype, during HHV-6 infection could modify the cytokine environment in HT patients with a possible implication in the disease. In particular, we found an increase in CD3−CD56brightCD16− NK cells, that are known to abundantly produce IFN-γ (Vitale et al. 2004). It is known that cytokines are involved in the pathogenesis of thyroid diseases working in both the immune system and directly targeting the thyroid follicular cells. They are involved in the induction and effector phase of the immune response and inflammation, playing a key role in the pathogenesis of autoimmune thyroid disease. Finally, cytokines can directly damage thyroid cells, leading to functional disorders and may also stimulate the production of nitric oxide and prostaglandin, thus increasing the inflammatory response in HT patients (Mikoś et al. 2014). Moreover, our findings on the increase in CD56bright NK cells in HT patients are in agreement with a previous study that documented the increase in CD56bright NK cells and inflammatory cytokines in the cerebrospinal fluid and serum of a 15-month-old girl with acute necrotizing encephalopathy (ANE) associated with HHV-6 (Kubo et al. 2006).
We are aware that it is difficult to prove etiologic links between viral infections and diseases, especially in the case of a ubiquitous agent such as HHV −6. Moreover, the number of subjects enrolled in this study is limited and a larger cohort is necessary to confirm these results. Nevertheless, our findings indicate that HHV-6 might contribute to HT development, increasing NK cell secretion of inflammatory cytokines sustaining the persistence of an inflammatory status in HT patients.
References
Alvarez-Lafuente R, Fernandez-Gutierrez B, de Miguel S, Jover JA, Rollin R, Loza E, Clemente D, Lamas JR (2005) Potential relationship between herpes viruses and rheumatoid arthritis: analysis with quantitative real time polymerase chain reaction. Ann Rheum Dis 64(9):1357–1359. doi:64/9/1357 [pii]10.1136/ard.2004.033514
Alvarez-Lafuente R, Martinez A, Garcia-Montojo M, Mas A, De Las Heras V, Dominguez-Mozo MI, Maria Del Carmen C, Lopez-Cavanillas M, Bartolome M, Gomez de la Concha E, Urcelay E, Arroyo R (2010) MHC2TA rs4774C and HHV-6A active replication in multiple sclerosis patients. Eur J Neurol 17(1):129–135. doi:ENE2758 [pii]10.1111/j.1468-1331.2009.02758.x
Bertelsen LBPC, Kofod-Olsen E, Oster B, Höllsberg P, Agger R, Hokland M (2010) Human herpesvirus 6B induces phenotypic maturation without IL-10 and IL-12p70 production in dendritic cells. Scand J Immunol 71(6):431–439
Boccara O, Lesage F, Regnault V, Lasne D, Dupic L, Bourdon-Lanoy E, Pannier S, Fraitag S, Audat F, Lecompte T, Hubert P, Bodemer C (2009) Nonbacterial purpura fulminans and severe autoimmune acquired protein S deficiency associated with human herpesvirus-6 active replication. Br J Dermatol 161(1):181–183. doi:BJD9264 [pii]10.1111/j.1365-2133.2009.09264.x
Broccolo F, Drago F, Paolino S, Cassina G, Gatto F, Fusetti L, Matteoli B, Zaccaria E, Parodi A, Lusso P, Ceccherini-Nelli L, Malnati MS (2009) Reactivation of human herpesvirus 6 (HHV-6) infection in patients with connective tissue diseases. J Clin Virol 46 (1):43–46. doi:S1386-6532(09)00191-7 [pii]10.1016/j.jcv.2009.05.010
Caruso A, Rotola A, Comar M, Favilli F, Galvan M, Tosetti M, Campello C, Caselli E, Alessandri G, Grassi M, Garrafa E, Cassai E, Di Luca D (2002) HHV-6 infects human aortic and heart microvascular endothelial cells, increasing their ability to secrete proinflammatory chemokines. J Med Virol 67(4):528–533
Caruso A, Caselli E, Fiorentini S, Rotola A, Prandini A, Garrafa E, Saba E, Alessandri G, Cassai E, Di Luca D (2009) U94 of human herpesvirus 6 inhibits in vitro angiogenesis and lymphangiogenesis. Proc Natl Acad Sci U S A 106(48):20446–20451. doi:0905535106 [pii]10.1073/pnas.0905535106
Caselli E, Di Luca D (2007) Molecular biology and clinical associations of Roseoloviruses human herpesvirus 6 and human herpesvirus 7. New Microbiol 30(3):173–187
Caselli E, Boni M, Bracci A, Rotola A, Cermelli C, Castellazzi M, Di Luca D, Cassai E (2002) Detection of antibodies directed against human herpesvirus 6 U94/REP in sera of patients affected by multiple sclerosis. J Clin Microbiol 40(11):4131–4137
Caselli E, Bracci A, Galvan M, Boni M, Rotola A, Bergamini C, Cermelli C, Dal Monte P, Gompels UA, Cassai E, Di Luca D (2006) Human herpesvirus 6 (HHV-6) U94/REP protein inhibits betaherpesvirus replication. Virology 346(2):402–414
Caselli E, Zatelli MC, Rizzo R, Benedetti S, Martorelli D, Trasforini G, Cassai E, Degli Uberti EC, Di Luca D, Dolcetti R (2012a) Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto’s Thyroiditis. PLoS Pathog 8(10):e1002951. doi:10.1371/journal.ppat.1002951PPATHOGENS-D-12-01343 [pii]
Caselli E, Zatelli M, Rizzo R, Benedetti S, Martorelli D, Trasforini G, Cassai E, degli Uberti EC, Di Luca D, Dolcetti R (2012b) Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto’s thyroiditis. PLoS Pathog 8:e1002951
Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, Schultz ER, Bennett JL, Garber RL, Chang M et al (1995) Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A 92(16):7440–7444
Cooper MAFT, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22(11):633–640
Di Luca D, Dolcetti R, Mirandola P, De Re V, Secchiero P, Carbone A, Boiocchi M, Cassai E (1994) Human herpesvirus 6: a survey of presence and variant distribution in normal peripheral lymphocytes and lymphoproliferative disorders. J Infect Dis 170(1):211–215
Fox RI, Saito I, Chan EK, Josephs S, Salahuddin SZ, Ahlashi DV, Staal FW, Gallo R, Pei-Ping H, Le CS (1989) Viral genomes in lymphomas of patients with Sjogren’s syndrome. J Autoimmun 2(4):449–455
Gustafsson RK, Engdahl EE, Hammarfjord O, Adikari SB, Lourda M, Klingström J, Svensson M, Fogdell-Hahn A (2013) Human herpesvirus 6A partially suppresses functional properties of DC without viral replication. PLoS One 8(3):e58122
Hidaka YAN, Iwatani Y, Kaneda T, Nasu M, Mitsuda N, Tanizawa O, Miyai K (1992) Increase in peripheral natural killer cell activity in patients with autoimmune thyroid disease. Autoimmunity 11:239
Kondo K, Kondo T, Okuno T, Takahashi M, Yamanishi K (1991) Latent human herpesvirus 6 infection of human monocytes/macrophages. J Gen Virol 72(Pt 6):1401–1408
Krueger GR, Sander C, Hoffmann A, Barth A, Koch B, Braun M (1991) Isolation of human herpesvirus-6 (HHV-6) from patients with collagen vascular diseases. Vivo 5(3):217–225
Kubo T SK, Kobayashi D, Motegi A, Kobayashi O, Takeshita S, Nonoyama S (2006) A case of HHV-6 associated acute necrotizing encephalopathy with increase of CD56bright NKcells. Scand J Infect Dis 38(11–12):1122–1125
Kumagai TYT, Yoshida M, Okui T, Ihira M, Nagata N, Yano S, Shiraki K, Yamada M, Ichihara K, Asano Y (2006) Time course characteristics of human herpesvirus 6 specific cellular immune response and natural killer cell activity in patients with exanthema subitum. J Med Virol 78(6):792–799
Mikoś HMM, Obara-Moszyńska M, Niedziela M (2014) The role of the immune system and cytokines involved in the pathogenesis of autoimmune thyroid disease(AITD). Endokrynol Pol 65(2):150–155
Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J (2009) CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 126(4):458–465. doi:IMM3027 [pii]10.1111/j.1365-2567.2008.03027.x
Potenza L, Luppi M, Barozzi P, Rossi G, Cocchi S, Codeluppi M, Pecorari M, Masetti M, Di Benedetto F, Gennari W, Portolani M, Gerunda GE, Lazzarotto T, Landini MP, Schulz TF, Torelli G, Guaraldi G (2008) HHV-6A in syncytial giant-cell hepatitis. N Engl J Med 359(6):593–602. doi:359/6/593 [pii]10.1056/NEJMoa074479
Ranger-Rogez S, Vidal E, Liozon F, Denis F (1994) Primary Sjogren’s syndrome and antibodies to human herpesvirus type 6. Clin Infect Dis 19(6):1159–1160
Rizzo RGV, Casetta I, Caselli E, De Gennaro R, Granieri E, Cassai E, Di Luca D, Rotola A (2012) Altered natural killer cells’ response to herpes virus infection in multiple sclerosis involves KIR2DL2 expression. J Neuroimmunol 251:55–64
Rotola A, Ravaioli T, Gonelli A, Dewhurst S, Cassai E, Di Luca D (1998) U94 of human herpesvirus 6 is expressed in latently infected peripheral blood mononuclear cells and blocks viral gene expression in transformed lymphocytes in culture. Proc Natl Acad Sci U S A 95(23):13911–13916
Rotola A, Merlotti I, Caniatti L, Caselli E, Granieri E, Tola MR, Di Luca D, Cassai E (2004) Human herpesvirus 6 infects the central nervous system of multiple sclerosis patients in the early stages of the disease. Mult Scler 10(4):348–354
Scotet E, Peyrat MA, Saulquin X, Retiere C, Couedel C, Davodeau F, Dulphy N, Toubert A, Bignon JD, Lim A, Vie H, Hallet MM, Liblau R, Weber M, Berthelot JM, Houssaint E, Bonneville M (1999) Frequent enrichment for CD8 T cells reactive against common herpes viruses in chronic inflammatory lesions: towards a reassessment of the physiopathological significance of T cell clonal expansions found in autoimmune inflammatory processes. Eur J Immunol 29(3):973–985
Storkus WJHD, Salter RD, Dawson JR, Cresswell P (1987) NK susceptibility varies inversely with target cell class I HLA antigen expression. J Immunol 138(6):1657–1659
Thomas D, Liakos V, Michou V, Kapranos N, Kaltsas G, Tsilivakos V, Tsatsoulis A (2008) Detection of herpes virus DNA in post-operative thyroid tissue specimens of patients with autoimmune thyroid disease. Exp Clin Endocrinol Diabetes 116(1):35–39
Vitale M, Della Chiesa M, Carlomagno S, Romagnani C, Thiel A, Moretta L, Moretta A (2004) The small subset of CD56brightCD16- natural killer cells is selectively responsible for both cell proliferation and interferon-gamma production upon interaction with dendritic cells. Eur J Immunol 34(6):1715–1722. doi:10.1002/eji.200425100
Wu ZSC, Reichel JJ, Just M, Mertens T (2015) Natural killer cells can inhibit the transmission of human cytomegalovirus in cell culture by using mechanisms from innate and adaptive immune responses. J Virol 89(5):2906–2917
Acknowledgements
This work was supported by grants from University of Ferrara (FAR).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Rizzo, R. et al. (2015). Increase in Peripheral CD3−CD56brightCD16− Natural Killer Cells in Hashimoto’s Thyroiditis Associated with HHV-6 Infection. In: Donelli, G. (eds) Advances in Microbiology, Infectious Diseases and Public Health. Advances in Experimental Medicine and Biology(), vol 897. Springer, Cham. https://doi.org/10.1007/5584_2015_5010
Download citation
DOI: https://doi.org/10.1007/5584_2015_5010
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26319-9
Online ISBN: 978-3-319-26320-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)