Abstract
The farnesoid X receptor (FXR, NR1H4) is a bile acid (BA)-activated transcription factor, which is essential for BA homeostasis. FXR and its hepatic and intestinal target genes, small heterodimer partner (SHP, NR0B2) and fibroblast growth factor 15/19 (Fgf15 in mice, FGF19 in humans), transcriptionally regulate BA synthesis, detoxification, secretion, and absorption in the enterohepatic circulation. Furthermore, FXR modulates a large variety of physiological processes, such as lipid and glucose homeostasis as well as the inflammatory response. Targeted deletion of FXR renders mice highly susceptible to cholic acid feeding resulting in cholestatic liver injury, weight loss, and increased mortality. Combined deletion of FXR and SHP spontaneously triggers early-onset intrahepatic cholestasis in mice resembling human progressive familial intrahepatic cholestasis (PFIC). Reduced expression levels and activity of FXR have been reported in human cholestatic conditions, such as PFIC type 1 and intrahepatic cholestasis of pregnancy. Recently, two pairs of siblings with homozygous FXR truncation or deletion variants were identified. All four children suffered from severe, early-onset PFIC and liver failure leading to death or need for liver transplantation before the age of 2. These findings underscore the central role of FXR as regulator of systemic and hepatic BA levels. Therefore, targeting FXR has been exploited in different animal models of both intrahepatic and obstructive cholestasis, and the first FXR agonist obeticholic acid (OCA) has been approved for the treatment of primary biliary cholangitis (PBC). Further FXR agonists as well as a FGF19 analogue are currently tested in clinical trials for different cholestatic liver diseases. This chapter will summarize the current knowledge on the role of FXR in cholestasis both in rodent models and in human diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bile acid homeostasis
- Cholestasis
- Farnesoid X receptor (FXR)
- FGF19
- Fibroblast growth factor
- Obeticholic acid
- Primary biliary cholangitis (PBC)
- Primary sclerosing cholangitis (PSC)
1 Introduction
Cholestasis describes the impaired formation and/or secretion of bile into the small intestine. Clinically, cholestasis can be classified into intrahepatic or extrahepatic as well as into obstructive or nonobstructive. Obstructive cholestasis can affect the intrahepatic and the extrahepatic biliary tree or both. Common causes of biliary obstruction are malignancies, gallstones, cysts, or strictures, the latter including biliary atresia (European Association for the Study of the Liver 2009). Intrahepatic cholestasis may result from defective BA synthesis, from impaired secretory functions of hepatocytes and/or cholangiocytes, or from obstruction of the intrahepatic biliary tree distal of the canal of Hering (European Association for the Study of the Liver 2009). Hepatocytic bile formation comprises the synthesis of BAs from cholesterol as well as the secretion of conjugated BAs across the canalicular membrane and is controlled by FXR signaling.
FXR belongs to the superfamily of nuclear receptors (NRs), which act as ligand-activated transcription factors (Forman et al. 1995; Makishima et al. 1999; Parks et al. 1999; Wang et al. 1999). Human primary and secondary BAs such as chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), lithocholic acid (LCA), and their taurine and glycine conjugates serve as ligands for FXR (Makishima et al. 1999; Parks et al. 1999; Wang et al. 1999). The role of FXR as a master regulator of BA homeostasis is underscored by the high expression of the receptor in the liver, ileum, as well as kidney and by dysregulation of BA homeostasis in FXR knockout mice (Forman et al. 1995; Sinal et al. 2000).
In hepatocytes, BAs suppress the transcription of the rate-controlling enzyme in BA synthesis, CYP7A1 as well as CYP8B1, which is the enzyme controlling cholic acid (CA) production (Russell 2003). One of the FXR downstream targets is the small heterodimer partner (SHP, NR0B2), which is an atypical NR since it lacks a DNA-binding domain and acts as a corepressor inhibiting coactivator binding and directly repressing transcriptional activity of other NRs, such as liver receptor homolog-1 (LRH-1, NR5A2), hepatocyte nuclear factor 4 (HNF-4α), and retinoid X receptor (RXR) (Goodwin et al. 2000; Anakk et al. 2011; Kerr et al. 2002; Wang et al. 2002; Lee et al. 2000; Seol et al. 1996; Lee and Moore 2002; Lu et al. 2000). SHP is recruited to the promotor of target genes with the help of other NRs (Kliewer and Mangelsdorf 2015). HNF-4α and LRH-1 bind to the BA-response elements (BAREs) of the CYP7A1 and CYP8B1 promoters and recruit SHP to this site (Goodwin et al. 2000; Lu et al. 2000; Kliewer and Mangelsdorf 2015; Stroup et al. 1997; Yang et al. 2002; Zhang and Chiang 2001; Keitel et al. 2008). Targeted deletion of SHP impaired but did not completely abolish the BA-dependent suppression of CYP7A1, indicating that several redundant (and also SHP-independent) mechanisms facilitate BA-mediated repression of CYP7A1 transcription (Kerr et al. 2002; Wang et al. 2002). BA reuptake into enterocytes in the terminal ileum (see below) triggers activation of FXR and expression of its target gene fibroblast growth factor 15 (Fgf15, which corresponds to FGF19 in humans), which circulates to the liver via portal blood, where it binds to the FGF receptor 4 (FGFR4) on hepatocytes (Fig. 1) (Kliewer and Mangelsdorf 2015; Inagaki et al. 2005). Binding of Fgf15/FGF19 leads to dimerization of FGFR4 and autophosphorylation and activation of the c-Jun N-terminal kinase (JNK) pathway resulting in repression of CYP7A1 transcription (Holt et al. 2003). However, SHP is also required for efficient repression of CYP7A1 by Fgf15/FGF19, since overexpression of Fgf15 triggered only a minor, not significant reduction in Cyp7a1 mRNA levels in SHP KO mice (Kliewer and Mangelsdorf 2015; Inagaki et al. 2005). While mice with liver-specific targeted deletion of FXR retained the ability to suppress Cyp7a1 transcription when treated with the synthetic FXR agonist GW4064, mice with targeted deletion of FXR exclusively in the intestine were resistant to Cyp7a1 repression in response to GW4064 (Kim et al. 2007). In contrast suppression of Cyp8b1 was dependent on FXR in the liver, and recombinant Fgf15 application had no effect on Cyp8b1 mRNA levels while significantly lowering Cyp7a1 expression (Kliewer and Mangelsdorf 2015; Kim et al. 2007). Thus, activation of the FXR-Fgf15/FGF19 pathway in the intestine predominates over the FXR-SHP pathway in the liver in repression of CYP7A1 expression (Kliewer and Mangelsdorf 2015; Kim et al. 2007; Modica et al. 2012).
FGF19 signaling in liver and intestine. Bile acids (BAs) are synthesized in the liver from cholesterol under control of CYP7A1. High hepatic BA concentrations activate FXR signaling thereby repressing CYP7A1 expression. In the intestine, FXR is activated by BAs resulting in increased FGF19 expression, which circulates to the liver and binds to FGF receptor 4 (FGFR4) and its coreceptor β-klotho on hepatocytes, resulting in suppression of CYP7A1 and reduced BA synthesis. Furthermore, FGF19 triggers gallbladder filling. For detailed description and references, refer to text
In hepatocytes bile is formed by ATP-dependent transport of bile constituents such as BAs, phospholipids, cholesterol, and bilirubin from the hepatocyte into the bile canaliculus. The bile salt export pump (BSEP, ABCB11) secretes BAs across the canalicular membrane, which is the major driving force for BA-dependent bile flow (Gerloff et al. 1998; Häussinger et al. 2004; Kullak-Ublick et al. 2004). Intracellular accumulation of BAs induces BSEP transcription via FXR enhancing their own secretion in a feed-forward manner. Organic anions and bilirubin conjugates are secreted into bile by MRP2 (ABCC2), while phospholipids are flopped from the inner to the outer leaflet of the canalicular membrane by MDR3 (ABCB4, Mdr2 in mice) (Smit et al. 1993; van Helvoort et al. 1996; Kamisako et al. 1999; Keppler et al. 2000). Both human multidrug resistance-associated protein 2 (MRP2, ABCC2) and the multidrug resistance protein 3 (MDR3, ABCB4) are at least in part positively regulated by FXR (Fig. 2) (Huang et al. 2003; Kast et al. 2002). Hence, FXR signaling triggers bile flow and secretion of cholephilic compounds (Keitel et al. 2008).
Bile formation and regulation by FXR. Bile acids (BAs) are synthesized from cholesterol within hepatocytes and secreted by the bile salt export pump (BSEP) across the apical/canalicular membrane into the bile canaliculus. BAs form mixed micelles together with phosphatidylcholine transported by the phospholipid floppase MDR3 and cholesterol exported by ABCG5/G8. The ATPase FIC1 is a phosphatidylserine flippase maintaining membrane asymmetry. CFTR exports chloride from cholangiocytes which is subsequently exchanged against bicarbonate via the anion exchanger 2 (AE2), leading to formation of the bicarbonate umbrella, which protects cholangiocytes from toxic BA concentrations. ASBT acts as symporter for BAs and sodium, facilitating uptake of BAs into cholangiocytes. The organic solute transporter OSTα/β secretes BAs across the basolateral membrane of both cholangiocytes and hepatocytes. Different transport proteins are located in the basolateral/sinusoidal membrane of hepatocytes. The main importer for BAs is the sodium taurocholate cotransporting peptide (NTCP). However, both OATP1B1 and OATP1B3 can facilitate uptake of BAs and organic anions into hepatocytes. MRP4 and OSTα/β act as alternate BA exporters under cholestatic conditions. Bilirubin is mainly imported via OATP1B3 and transported across the canalicular membrane into bile by MRP2. Under cholestatic conditions bilirubin can be exported into sinusoidal blood via MRP3. FXR represses (red) de novo BA synthesis, hepatic BA uptake, and uptake of BAs into cholangiocytes via ASBT. In contrast, export mechanisms are positively regulated (green) by FXR. For a detailed description and references, refer to text
In cholangiocytes as well as in enterocytes, bile acid uptake is mediated by the apical sodium-dependent bile salt transporter (ASBT, SLC10A2) (Dawson et al. 2003; Lazaridis et al. 1997). ASBT transcription is differentially modulated between species with human ASBT expression being regulated by FXR (Fig. 2) (Kalaany and Mangelsdorf 2006; Neimark et al. 2004; Sinha et al. 2008). Basolateral export of BAs is facilitated in cholangiocytes as well as in enterocytes by the organic solute transporters α/β (OSTα/β, SLC51A/B) (Landrier et al. 2006; Wagner et al. 2003; Kazgan et al. 2014).
Hepatic BA uptake across the sinusoidal membrane is mediated via several transport proteins with more than 80% of conjugated BAs being imported by the sodium-dependent taurocholate cotransporting peptide (NTCP, SLC10A1) (Hagenbuch and Meier 1994; Kullak-Ublick 2003; Kullak-Ublick et al. 2000). Comparable to CYP7A1 and ASBT, BAs repress NTCP expression in a FXR-SHP-dependent mechanism (Denson et al. 2001; Jung et al. 2004). Nevertheless, further SHP-independent mechanisms of NTCP transcriptional regulation must exist, since NTCP mRNA levels in SHP KO mice were unaltered (Wang et al. 2002). Further BA uptake transporters in the basolateral hepatocyte membrane belong to the family of organic anion transporters (OATPs). While OATP1B1 expression is downregulated by FXR, OATP1B3 expression is increased (Fig. 2) (Jung and Kullak-Ublick 2003; Jung et al. 2002). The repression of BA uptake (via NTCP, OATP1B1 (Fig. 2)) in combination with suppression of BA de novo synthesis may protect hepatocytes from intracellular accumulation of toxic BAs under cholestatic conditions. Alternate BA secretion across the basolateral membrane into sinusoidal blood is facilitated by multidrug resistance-associated protein 4 (MRP4, ABCC4) and the organic solute transporter α/β (OSTα/β). Both transport systems are upregulated under cholestatic conditions in rodents and humans (Denk et al. 2004; Keitel et al. 2005; Schuetz et al. 2001; Boyer et al. 2006). While BAs induce the expression of OSTα/β via FXR (Fig. 2), the upregulation of MRP4 by BAs is FXR-independent and is observed both on the translational and posttranslational levels (Wagner et al. 2003; Schuetz et al. 2001; Boyer et al. 2006).
In hepatocytes, BAs activate FXR which subsequently inhibits de novo BA synthesis, enhances conjugation and detoxification, and increases BA efflux both across the canalicular and basolateral membranes. In the terminal ileum, FXR triggers expression of Fgf15/FGF19 which via FGFR4 on hepatocytes suppresses BA synthesis (Fig. 1). Therefore, FXR sensing and signaling in both, the liver and intestine, prevents BA overload and thus liver damage (Kliewer and Mangelsdorf 2015; Keitel et al. 2008).
2 FXR Knockout Mice Are More Susceptible to Cholestasis
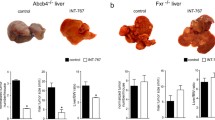
Mice with targeted deletion of Fxr displayed no obvious phenotype when held under standard housing conditions and fed a regular chow diet (Sinal et al. 2000). However, lack of Fxr resulted in increased (about eightfold) levels of serum BAs. Supplementation of the diet with 1% cholic acid (CA) led to wasting, hypothermia, and an increased mortality of about 30% by day 7 in the Fxr knockout mice (KO), none of which was observed in the wild-type animals (Sinal et al. 2000). Serum BA levels were 23-fold higher in Fxr KO mice than in wild-type animals, which may be attributed to the impaired secretion of BAs into bile resulting in accumulation of BAs within hepatocytes, increased elimination of BAs across the basolateral membrane, and thus reduced excretion into feces (Sinal et al. 2000). Hepatic BA overload is aggravated in Fxr KO mice by impaired suppression of Cyp7a1 and Cyp8b1 mRNA levels and failure to upregulate Bsep expression in response to CA feeding (Sinal et al. 2000; Zollner et al. 2003). However, the overall BA pool was reduced in the absence of FXR both in chow-fed and in CA-fed cohorts. Upregulation of the detoxification enzyme Cyp3a11 increased BA hydroxylation and enhanced secretion into urine via Mrp4, which represents a protective adaptive mechanism in Fxr KO mice (Cho et al. 2010; Marschall et al. 2006). In contrast to CA feeding, common bile duct ligation (CBDL), which serves as model of extrahepatic obstructive cholestasis, was associated with similar mortality rates in Fxr KO and wild-type animals (Wagner et al. 2003). Overall, Fxr KO mice were relatively resistant toward CBDL-induced liver damage (Wagner et al. 2003; Stedman et al. 2006). Potential mechanisms of protection in this experimental setting are the lower BA concentrations in Fxr KO mice as well as the reduced expression of Bsep, which result in diminished bile flow, reduced biliary pressure, less bile infarcts, and thus reduced liver injury (Wagner et al. 2003; Stedman et al. 2006). Histologically livers from Fxr KO mice showed disseminated necrosis as compared to wild-type livers, which displayed marked bile infarcts after CBDL (Wagner et al. 2003; Stedman et al. 2006). Interestingly, upregulation of Mrp2, Mrp3, and Mrp4 as a consequence of CA feeding or CBDL was independent of Fxr in mice livers, while induction of Bsep expression was strictly dependent on Fxr (Wagner et al. 2003; Zollner et al. 2003).
Alpha-naphthylisothiocyanate (ANIT) administration recapitulates a cholangiocellular intrahepatic cholestasis. Hepatocytes facilitate GSH conjugation of ANIT, which can then be secreted into bile via Mrp2 (Abcc2), where GSH dissociates from ANIT leading to biliary injury (Cui et al. 2009; Dietrich et al. 2001). Similar to BA overload by feeding and CBDL, ANIT-treated Fxr KO mice failed to induce Bsep and Ostα expression and displayed impaired suppression of Ntcp mRNA (Cui et al. 2009). Conversely, intraperitoneal injection of the FXR agonist GW4064, prior to oral ANIT administration, ameliorated liver injury in wild-type mice (Cui et al. 2009). These findings underscore the essential role of Fxr signaling in protection from cholestatic liver injury.
Fxr and Shp double-knockout mice (DKO) were generated and were expected to phenocopy Fxr KO mice under the assumption that SHP is an exclusive downstream target of FXR (Anakk et al. 2011). In contrast to Fxr KO mice, Fxr-Shp DKO mice displayed hepatomegaly, increased hepatocyte proliferation, hepatocyte necrosis, and ductular proliferation resulting in elevated serum levels of AST, ALT, bilirubin, and BAs. These changes became evident as early as 3 weeks of age (Anakk et al. 2011). A strong induction of both Cyp7a1 and Cyp8b1 was observed, while Cyp27a1 was significantly reduced, consistent with increased BA synthesis resulting in elevation of hepatic and serum BA levels (Anakk et al. 2011). Furthermore, Bsep was downregulated in livers of DKO mice contributing to retention of BAs within the hepatocyte and thus hepatocyte injury (Anakk et al. 2011). Expression of basolateral BA efflux transporters Mrp3 and Mrp4 was induced, while mRNA levels of basolateral BA uptake transporters Ntcp and Oatp1 were reduced in DKO mice as an adaptive mechanism to counteract BA overload within the hepatocyte. In contrast to single-knockout mice for Fxr, Shp, or Bsep, these DKO phenocopy multiple features of children with progressive familial intrahepatic cholestasis (PFIC) and thus serve as a model for early-onset intrahepatic cholestasis syndromes (Sinal et al. 2000; Anakk et al. 2011; Wang et al. 2001, 2002, 2003).
3 Genetic Variants in Human FXR Are Associated with a Spectrum of Cholestatic Disorders
Genetic variants in the FXR gene were first identified in women with intrahepatic cholestasis of pregnancy (ICP) (van Mil et al. 2007). ICP is the most common liver disease in pregnant women and affects 0.1–2.0% of pregnancies in Europe (Bacq 2011; Joshi et al. 2010; Lammert et al. 2000; Wikstrom Shemer et al. 2013; Keitel et al. 2016; Williamson and Geenes 2014). ICP usually presents with pruritus, elevated serum BAs, and transaminase levels late in pregnancy (up to 80% become clinically evident after 28 weeks of gestation) (Lammert et al. 2000; Keitel et al. 2016; Williamson and Geenes 2014; Bacq et al. 2012). Perinatal complications such as spontaneous or iatrogenic premature delivery, meconium staining of the amniotic fluid, respiratory distress, low Apgar scores, and even stillbirth are more frequent in women with ICP, especially if maternal BA levels exceed 40 μmol/l (Keitel et al. 2016; Geenes et al. 2014; Glantz et al. 2004; Rioseco et al. 1994; Williamson et al. 2004). Analysis of long-term outcome of women, who had previous ICP, demonstrated an increased incidence of various liver, biliary, pancreatic, metabolic, and immune-mediated diseases, including nonalcoholic liver cirrhosis, cholelithiasis, cholecystitis and cholangitis, gallstone-associated pancreatitis, hepatocellular and biliary cancer, as well as diabetes and cardiovascular and thyroid disease (Keitel et al. 2016; Marschall et al. 2013; Ropponen et al. 2006; Wikstrom Shemer et al. 2015). ICP is precipitated in genetically susceptible women by gestational hormones as well as environmental factors, which are not fully understood (Lammert et al. 2000). While most of the genetic risk for ICP has been linked to variants in the MDR3 (ABCB4) gene, also variants associated with ICP have been identified in the genes encoding BSEP (ABCB11), FIC1 (ATP8B1), MRP2 (ABCC2), tight junction protein 2 (TJP2), as well as FXR (NR1H4) (van Mil et al. 2007; de Vree et al. 1998; Dixon et al. 2009, 2014, 2017; Gudbjartsson et al. 2015; Jacquemin et al. 1999; Müllenbach et al. 2003, 2005; Pauli-Magnus et al. 2004; Wasmuth et al. 2007; Keitel et al. 2006). Overall a study of FXR genetic contribution to ICP identified four variants: c.-1G>T (rs56163822, MAF 4.45%), c.1A>G (p.M1V, rs138943609, MAF 0.023%), c.238T>C (p.W80R), and c.518T>C (p.M173T, rs6155050, MAF 0.39%), of which c-1G>T and c.518C>T were also present in the respective control cohort (van Mil et al. 2007). (Reference sequence for FXR is NM_001206979.1; minor allele frequencies (MAF) were taken from http://gnomad.broadinstitute.org/.) The variants c.-1G>T, c.1A>G (p.M1V), and c.518C>T (p.M173T) resulted in reduced target gene expression in vitro (van Mil et al. 2007). In contrast to BSEP (ABCB11) and MDR3 (ABCB4), variants in FXR are less frequently detected in ICP.
Recently four individuals from two families were identified, who carried either a homozygous truncation variant (c.526C>T, p.R176*) within the FXR DNA-binding domain or a homozygous in-frame insertion variant c.419_420insAAA (p.Y139_N140insK), resulting in a 31.7 kb deletion affecting the zinc-binding module of the FXR DNA-binding domain (Gomez-Ospina et al. 2016). Immunohistochemistry of the patients’ livers revealed complete absence of FXR and BSEP staining, while MDR3 could be detected in all livers (Gomez-Ospina et al. 2016). This finding again underscores the strict dependency of BSEP expression on the presence of FXR as suggested by rodent studies (Wagner et al. 2003). All four patients developed clinically apparent signs of jaundice, cholestasis, and liver damage within the first 6 weeks of life, while the parents carrying only one affected allele were asymptomatic (Gomez-Ospina et al. 2016). Liver disease was rapidly progressive in the affected children resulting in early death of both children with the in-frame insertion at age of 5 weeks and 8 months, respectively, and the need for liver transplantation at the age of 4.4 months and 22 months of the patients with the truncation variant (Gomez-Ospina et al. 2016). The absence of BSEP in these children partially phenocopies the phenotype of BSEP deficiency (PFIC2) with normal to low serum levels for gamma-glutamyltransferase (GGT) despite elevated AST, ALT, and bilirubin levels. However, FXR deficiency was characterized by vitamin K-independent coagulopathy, high AFP serum levels, and reduced FGF19 levels (Gomez-Ospina et al. 2016). The latter are explained by absence of FXR from the intestine. After successful liver transplantation, the two affected children now express wild-type FXR only in the donor liver and not in other organs such as the intestine, kidney, or adrenal glands (Gomez-Ospina et al. 2016). During the observed time period after transplantation (about 8 years in patient 1 and 11 months in patient 2), no overt pathology became apparent in other organs (Gomez-Ospina et al. 2016). Therefore, it will be interesting to monitor these patients for signs of extrahepatic FXR deficiency in the future.
4 FXR Expression and Function Is Altered in Different Forms of Intrahepatic Cholestasis
Altered FXR expression has been observed not only in patients with severe genetic variants in the FXR gene but also in patients with PFIC1 disease due to variants in the FIC1 (ATP8B1) gene. Absence of FIC1, which is a hallmark of severe PFIC1, was associated with reduced FXR activity and FXR expression resulting in impaired target gene transactivation in an intestinal cell line (Chen et al. 2004). While the experiments of this study were restricted to intestinal changes, reduced BSEP expression in the liver may contribute to the phenotype and explain the similar clinical presentation of FIC1- and BSEP-related PFIC subtypes (PFIC1 and PFIC2, respectively) (Chen et al. 2004). Whether the downregulation of FXR in PFIC1 is a direct or an indirect effect of cholestasis remains elusive (Cai et al. 2009).
As described above ICP is associated with altered BA homeostasis and elevated maternal serum BA levels (Milona et al. 2010). Elevation of 17β-estradiol and its metabolites during pregnancy impairs transactivation of FXR target genes, such as Cyp7a1, Cyp8b1, or Bsep, through estrogen receptor α (ERα) (Milona et al. 2010). ERα in turn directly interacts with FXR on the protein level, thus preventing the binding of FXR to the FXR-response element in the target gene promotor (Milona et al. 2010; Song et al. 2014). In addition, sulfated progesterone metabolites, which are elevated in normal pregnancy and further raised in ICP patients, also impair FXR activation and target gene expression (Keitel et al. 2016; Abu-Hayyeh et al. 2013, 2016; Abu-Hayyeh and Williamson 2015). Attenuated FXR signaling may therefore contribute to hypercholanemia, dyslipidemia, and gallstone formation during pregnancy (Keitel et al. 2016; Abu-Hayyeh et al. 2013; Abu-Hayyeh and Williamson 2015).
5 Targeting FXR in Cholestasis: Lessons from Rodents
Disruption of Fxr or combined deletion of Fxr and Shp triggers development of cholestasis of varying severity, while stimulation of Fxr promotes transcription of BA detoxification enzymes and hepatobiliary transport proteins facilitating BA clearance and represses enzymes relevant for BA synthesis thus lowering BA levels in hepatocytes (Sinal et al. 2000; Anakk et al. 2011; Marschall et al. 2006; Guo et al. 2003; Liu et al. 2003; Ananthanarayanan et al. 2001). Therefore, activation of FXR signaling with BA and non-BA ligands has emerged as attractive therapeutic target for different cholestatic liver diseases.
Intraperitoneal injection of 17α-ethinylestradiol (E217α) for 5 days mimics intrahepatic cholestasis induced by drugs or pregnancy (ICP). Bile flow was lowered by about 50% after 5 days of E217α injection (Fiorucci et al. 2005). Simultaneous administration of the CDCA-derived FXR agonist 6-ethyl chenodeoxycholic acid (6-ECDCA, also known as INT7-747 or obeticholic acid (OCA)) or the synthetic agonist GW4064 completely restored the E217α-induced reduction in bile flow (Fiorucci et al. 2005). The increase in bile flow in response to OCA or GW4064 was accompanied by an upregulation of Bsep, Mrp2, and Mdr2 mRNA expression in liver tissue of these rats (Fiorucci et al. 2005). In a further model, intraperitoneal administration of GW4064 protected against alpha-naphthylisothiocyanate induced cholangiocellular cholestasis in both mice and rats (Cui et al. 2009; Liu et al. 2003). In rats, intraperitoneal GW4064 injection about 24 h prior to a single oral dose of ANIT significantly reduced serum levels of AST, ALT, LDH, alkaline phosphatase (ALP), and BAs, which was accompanied by a significant induction of Bsep, Mdr2, Mrp2, and SHP expression in liver tissue (Liu et al. 2003). Injection of GW4064 to rats resulted in a significant upregulation of Mdr2 (Abcb4), Bsep (Abcb11), and SHP mRNA expression in liver tissue of these animals (Liu et al. 2003). Upregulation of BSEP, MDR3, and SHP was also observed in human hepatocytes after 12 h of GW4064 stimulation (Liu et al. 2003). Injection of GW4064 24 h after ligation of the common bile duct (CBDL) significantly reduced serum levels for AST, ALT, and LDH but not for ALP, BAs, or bilirubin as compared to vehicle-treated CBDL rats. Liver histology revealed lower numbers of bile infarcts in GW4064-treated CBDL rats as compared to CBDL alone (Liu et al. 2003). However, the beneficial effect of Fxr activation in obstructive cholestasis has been controversial, since Fxr KO mice are relatively protected from liver damage induced by CBDL (Wagner et al. 2003; Stedman et al. 2006). This conflict that both targeted deletion and systemic stimulation of Fxr protect from obstructive cholestasis may relate to the effects of Fxr signaling in the liver and intestine. Liver damage in response to CBDL results from increased pressure in the biliary tree and retention of bile acids within the liver (Wagner et al. 2003). Inhibition of BA synthesis and stimulation of basolateral efflux of bile constituents from the hepatocyte represent protective mechanisms against BA overload (Wagner et al. 2003; Stedman et al. 2006). These mechanisms are already induced in Fxr KO mice but can also be triggered by transactivation of Fxr in the intestine leading to Fgf15-mediated endocrine suppression of hepatic BA synthesis (Modica et al. 2012; Wagner et al. 2003; Keitel et al. 2005; Stedman et al. 2006). Overexpression of Fxr in the intestine resulted in upregulation of Fxr target gene expression such as Fgf15, Shp, Ostα, and Ostβ in the terminal ileum (Modica et al. 2012). In the liver a complete repression of Cyp7a1 and an about 30% reduction in BA pool size were observed in these animals (Modica et al. 2012). CBDL in mice overexpressing Fxr in the intestine ameliorated liver damage as measured by reduced levels for AST, ALT, ALP, BAs, and bilirubin as well as less bile infarcts (Modica et al. 2012). Besides the pronounced protective effect on the liver, Fxr overexpression inhibited bacterial overgrowth and translocation and promoted intestinal barrier integrity (Modica et al. 2012; Inagaki et al. 2006). Overexpression of Fxr in the intestine also protected from ANIT-induced cholestasis (Modica et al. 2012). Mdr2 (Abcb4) KO mice are characterized by defective phospholipid secretion into bile exposing cholangiocytes to toxic levels of BAs, which results in biliary damage and progressive sclerosing cholangitis (Fickert et al. 2002, 2004). Mdr2 KO mice are commonly used as model for primary sclerosing cholangitis (PSC) and ABCB4 (MDR3)-related disease (Fickert et al. 2002, 2004). Overexpression of Fxr in the intestine of Mdr2 KO mice reversed and attenuated liver damage in these animals as shown by reduced serum levels of AST, ALT, ALP, and BAs as well as on histology (Modica et al. 2012). In contrast double-knockout mice for Mdr2 and Fxr suffered from aggravated liver damage with severe elevation of AST, ALT, ALP, and BAs (Modica et al. 2012). This finding is in line with the report that feeding of INT-767 (6α-ethyl-3α,7α,23-trihydroxy-24-nor-5 β-cholan-23-sulfate), which is a dual agonist for FXR as well as for the G protein-coupled bile acid receptor TGR5 (GPBAR1), reduced liver injury in Mdr2 KO mice; however, the beneficial effects were almost exclusively attributed to Fxr activation (Baghdasaryan et al. 2011).
Intraperitoneal injection of recombinant human FGF19 efficiently suppressed Cyp7a expression and protein levels and lowered the BA pool by 30% in wild-type mice (Modica et al. 2012). Administration of recombinant FGF19 to wild-type mice 4 days prior to CBDL ameliorated liver injury, demonstrating that FGF19 protects against cholestasis through the reduction in BA synthesis and BA pool size (Modica et al. 2012). Thus, the FXR target gene Fgf15/FGF19 alone seems to be sufficient for treatment of different cholestatic disorders (Modica et al. 2012).
FGF19 and its receptor fibroblast growth factor receptor 4 (FGFR4) are not only relevant for normal liver regeneration but have been linked to hepatocarcinogenesis in both rodents and humans (Alvarez-Sola et al. 2017; Uriarte et al. 2013, 2015; Padrissa-Altes et al. 2015; Zhou et al. 2014). Transgenic overexpression of human FGF19 in skeletal muscle resulted in HCC development by the age of 10 months in 80% of female mice (Nicholes et al. 2002). Using an adeno-associated virus (AAV)-mediated gene delivery system via tail vein injection, overexpression of human FGF19 could be achieved in the liver and induced HCC development in mice in a strain-dependent manner in up to 100% of animals (Zhou et al. 2014). Induction of hepatocarcinogenesis in Fgf15 wild-type and KO mice using diethylnitrosamine (DEN) and carbon tetrachloride (CCL4) injection led to more pronounced fibrosis and tumor development in Fgf15 wild-type mice as compared to KO mice (Uriarte et al. 2015). In humans, focal amplifications of the FGF19 gene have been observed in about 15% of HCCs, overexpression of FGF19 was found in about 25% of HCCs, while high levels of FGFR4 expression were present in 30–50% of HCCs, further underscoring the relevance of FGF19-FGFR4 signaling in hepatocarcinogenesis (Alvarez-Sola et al. 2017; Ho et al. 2009; Sawey et al. 2011).
Conversely, long-term administration of dual FXR/TGR5 agonist INT-767 to Mdr2 KO mice improved liver injury and prevented spontaneous HCC development (Cariello et al. 2017). This beneficial effect was not observed in Fxr KO mice, which are also prone to spontaneous HCC development underscoring that FXR signaling is essential for the protection of Mdr2 KO mice (Cariello et al. 2017). The mechanisms why activation of FXR pathways can both prevent and promote tumor development remain unclear at the moment.
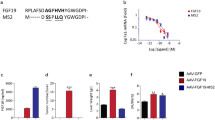
Since FGF19-FGFR4 signaling has been implicated in HCC tumorigeneses, safety concerns have been raised regarding FXR- and FGF19-based therapies especially in precancerous conditions such as advanced liver fibrosis, nonalcoholic steatohepatitis, or primary sclerosing cholangitis (PSC). Deletion of 5 amino acids (P24-S28) and introduction of 3 amino acid substitutions (A30S, G31S, and H33L) resulted in a FGF19 variant (denoted as M70, later named NGM282), which enables CYP7A1 suppression but prevents the proliferative and tumorigenic signaling. M70 cannot trigger phosphorylation and activation of signal transducer and activator of transcription 3 (STAT3) and its target genes cyclin D1 and survivin (Zhou et al. 2014). In contrast, suppression of CYP7A1 and reduction in serum BA levels were comparable to wild-type FGF19 (Zhou et al. 2014). Therefore, this FGF19 variant (M70) was tested in animal models of cholestasis. Twice daily subcutaneous injection of M70 starting 4 days prior to CBDL prevented liver injury and lowered serum AST, ALT, ALP, bilirubin, and BA levels (Luo et al. 2014). On liver histology M70 treatment was associated with fewer and smaller bile infarcts when compared to vehicle-treated CBDL mice (Luo et al. 2014). A similar beneficial effect of M70 was observed in ANIT-induced cholangiocellular cholestasis (Luo et al. 2014). M70 was administered twice daily subcutaneously for 4 days prior to oral ANIT application (Luo et al. 2014). With regard to changes in gene expression, M70 suppressed Cyp7a1 and Bsep mRNA levels to similar extent as wild-type FGF19 (Luo et al. 2014). Overall, the beneficial effect of M70 was indistinguishable from wild-type FGF19 in these models of cholestasis (Luo et al. 2014).
Treatment of 12-week-old Mdr2 KO mice with FGF19 or M70 by AAV injection not only inhibited expression of Cyp7a1, Cyp27a1, and Bsep and thus cholestasis but also significantly reduced hepatic inflammation, biliary fibrosis, and overall liver injury (Zhou et al. 2016). Furthermore, while FGF19 treatment led to the formation of HCCs by week 32, no HCCs were observed in livers of M70-treated Mdr2 KO mice. M70 also reduced ductular proliferation and ameliorated hepatosplenomegaly in Mdr2 KO mice with established cholangiopathy, thus reverting the phenotype of these mice (Zhou et al. 2016). This finding suggested a potential application of M70 in MDR3 disease (PFIC3) as well as for cholangiopathies.
Administration of M70 to healthy volunteers subcutaneously for 7 days resulted in a significant reduction of serum C4 levels as marker for CYP7A1 activity, demonstrating feasibility of targeting FGF19 signaling in humans (Luo et al. 2014).
6 Targeting FXR Signaling in Cholestasis: Current and Future Clinical Applications
Since agonists of FXR as well as its downstream target FGF19 were beneficial in different rodent models of intra- and extrahepatic cholestasis, different FXR modulators as well as a nontumorigenic FGF19 analogue (M70, NGM282) have been developed. The first clinical trials with FXR ligands and the FGF19 analogue (M70, NGM282) were carried out in patients with primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC).
6.1 Treatment of Primary Biliary Cholangitis (PBC) with FXR Agonists and an FGF19 Analogue
PBC affects predominantly middle-aged women, has an incidence of about 5 in 100,000 inhabitants per year, and is characterized histologically by a chronic, nonsuppurative inflammation of the small intrahepatic bile ducts, which can result in the destruction of the bile ducts, in the development of portal fibrosis and ultimately cirrhosis and its complications (Boonstra et al. 2012a; Carey et al. 2015). Transplant-free survival of untreated PBC ranges around 6–10 years (Carey et al. 2015). Ursodeoxycholic acid (UDCA) in a dose of 13–15 mg/kg BW is recommended as first-line treatment for all PBC patients since it has been shown to lower serum liver tests but also to improve liver histology, it delays disease progression, and it improves transplant-free survival (Beuers et al. 2015; Corpechot et al. 2008, 2011; European Association for the Study of the Liver 2017; Hirschfield et al. 2018a; Leuschner et al. 1996; Poupon et al. 1991, 1999). However, depending on which combination of laboratory values and thus which scoring system is used, only 26–86% of PBC patients show a positive response to UDCA at 12 months after starting treatment (Corpechot et al. 2008, 2011; Lammers et al. 2015; Kuiper et al. 2009; Pares et al. 2006). Thus, 30–50% of PBC patients have an inadequate UDCA response and are at risk of disease progression. In addition, there is a small group of patients who cannot tolerate UDCA treatment. These two groups of patients have been in need for further therapies (Bahar et al. 2018).
The FXR agonist obeticholic acid (OCA, formerly 6-ECDCA, INT-747) was evaluated in phase II studies for PBC, both as monotherapy and in combination with UDCA (Kowdley et al. 2018; Hirschfield et al. 2015). Patient selection comprised treatment-naive patients and those with insufficient laboratory response to UDCA. Treatment was carried out over 12 weeks with different OCA doses (10 mg up to 50 mg/day) (Kowdley et al. 2018; Hirschfield et al. 2015). In both trials, OCA triggered a reduction in ALP values from the baseline, which was significant as compared to the placebo group (Kowdley et al. 2018; Hirschfield et al. 2015). In the phase III study (POISE), 216 PBC patients with intolerance toward UDCA and/or inadequate response to UDCA and ALP levels above 1.67 times the upper limit of normal (ULN) and/or bilirubin levels (between 1 and 1.9-times ULN) were randomized to placebo, OCA at 10 mg daily, or OCA at 5 mg daily. The 5 mg OCA group could be titrated to 10 mg after 24 weeks depending on the response (Nevens et al. 2016). Over 90% of the study participants received UDCA maintenance therapy (Nevens et al. 2016). The primary endpoint of the study was a drop in ALP values below 1.67 times the ULN after 12 months of therapy. This goal was achieved by 46% in the 5–10 mg OCA group, by 47% in the 10 mg OCA group, and also by 10% of the placebo-treated patients (Nevens et al. 2016). In 77% of patients in the two OCA treatment groups, ALP values fell by more than 15%, which was the secondary endpoint of the study (Nevens et al. 2016). This endpoint was also reached by 29% of the patients in the placebo group. The most common side effect of OCA was itching, which was dose-related and was the cause of premature discontinuation of the study by eight patients corresponding to about 10% of the OCA-treated patients (Nevens et al. 2016). This OCA-specific adverse effect was already known from phase II studies, which is why a lower OCA starting dose was used in the phase III trial as compared to the phase II trials (Kowdley et al. 2018; Hirschfield et al. 2015; Nevens et al. 2016). Based on this data, FDA and EMEA granted conditional approval of OCA in PBC patients with inadequate response or intolerance to UDCA. The recommended starting dose of OCA is 5 mg daily, which should be titrated up to 10 mg daily after 3 months depending on decrease in ALP values and side effects, especially itch intensity.
Despite the fact that the clinical trials did not include patients with decompensated liver cirrhosis (Child-Pugh stages B and C), a dosing recommendation for these patients was included, starting with 5 mg once weekly and titration up to 10 mg twice weekly (Bahar et al. 2018). After marketing approval, safety announcements have been issued relating to 19 PBC patients with Child-Pugh B/C cirrhosis, who had received daily instead of weekly dosing and developed subsequent liver injury or even death (Bahar et al. 2018). Based on data from the phase III study, it can be assumed that about 50% of patients with inadequate response to UDCA will decrease ALP levels below 1.5 to 1.67 times the ULN and thus experience a significant improvement of prognosis; however, long-term trials and real-world data will have to confirm whether these beneficial effects can be translated into clinical routine (Bahar et al. 2018). However, even with addition of OCA, some PBC patients will not lower ALP values sufficiently and thus require further treatment options. The main unwanted effect of OCA is pruritus, which is dose-dependent and may be partially explained by activation of the membrane-bound G protein-coupled bile acid receptor TGR5. Taurine- and glycine-conjugated OCA have an EC50 of 0.2 and 0.3 μM for TGR5 activation, respectively, which should be reached in patients, since OCA undergoes enterohepatic circulation and is enriched in the BA pool (Tully et al. 2017).
Further non-bile acid FXR modulators, including cilofexor (formerly GS-9674, Px-104) and tropifexor (formerly LJN452), are currently evaluated in phase II trials in PBC patients (Bahar et al. 2018; Massafra et al. 2018).
The FGF19 analogue NGM282 (formerly M70) has been investigated in a randomized, double-blind, placebo-controlled phase II trial in PBC patients with inadequate response to UDCA over 28 days (Mayo et al. 2018). Overall, 45 patients were randomized to receive subcutaneous daily doses of 0.3 mg or 3 mg NGM282 or placebo (Mayo et al. 2018). Almost 50% of patients receiving NGM282 achieved a 15% or greater reduction in serum ALP levels as compared to baseline, while only 7% of patients in the placebo group reached this endpoint by day 28 (Mayo et al. 2018). Moreover, AST and ALT levels were significantly reduced by NGM282 treatment, as were C4 levels for the 3 mg treatment group. Discontinuation of study medication resulted in an increase in ALP, AST, ALT, and GGT values, which returned to baseline values (Mayo et al. 2018). NGM282 was well tolerated with most adverse events being classified as grade I and grade II and similar to placebo-treated patients. However, diarrhea and loose stools were more frequent in the NGM282 treatment groups (Mayo et al. 2018). Changes in pruritus severity or quality of life as assessed by the PBC-40 questionnaire during the study period were comparable between patients receiving either NGM282 or placebo (Mayo et al. 2018).
6.2 Treatment of Primary Sclerosing Cholangitis (PSC) with FXR Agonists and an FGF19 Analogue
PSC affects predominantly men with a median age at diagnosis around 40 years, has an incidence of about 1 in 100,000 persons per year, and is characterized histologically by a chronic inflammation and fibro-obliterative destruction of intra- and/or extrahepatic bile ducts, resulting in progressive biliary fibrosis, cirrhosis, and its complications (Boonstra et al. 2012b; Lazaridis and LaRusso 2016). PSC is strongly associated with inflammatory bowel disease (up to 80% of patients have both conditions) and is a predisposing factor for colon, cholangiocellular, and gallbladder cancer (Lazaridis and LaRusso 2016; Eaton et al. 2013; Hirschfield et al. 2013). The FXR agonist OCA has recently been evaluated in a phase II placebo-controlled double-blind trial in patients with PSC. The data obtained in this trial is not published yet; however, preliminary results have been added to clinicaltrials.gov (NCT02177136). Overall, 77 PSC patients were randomized to receive either placebo, 1.5 mg OCA daily titrated to 3 mg after 12 weeks, or 5 mg OCA titrated to 10 mg after 12 weeks. The overall trial duration was 24 weeks. In both OCA treatment groups, a significant reduction in ALP values was observed as compared to baseline and placebo.
Cilofexor (formerly GS-9675, Px104) is an orally available selective (EC50 43 nM) nonsteroidal agonist for FXR, which was evaluated in a randomized, placebo-controlled phase II trial over 12 weeks in PSC (Trauner et al. 2019). Fifty-two patients with large-duct PSC as demonstrated by magnetic resonance cholangiopancreatography or endoscopic retrograde cholangiopancreatography within the previous 12 months and ALP levels greater than 1.67 times the ULN were randomized to receive placebo, cilofexor 30 mg once daily, or cilofexor 100 mg once daily for 12 weeks. Randomization was stratified to the presence of UDCA maintenance therapy. The study offered a 96-week open-label extension with 100 mg cilofexor daily to all patients completing the 12 weeks of blinded treatment (Trauner et al. 2019). Treatment with cilofexor showed a significant (for the 100 mg dose) and dose-dependent decrease in ALP values as compared to baseline (−21% relative ALP reduction for 100 mg, −6% relative ALP reduction for 30 mg), which was not observed in placebo-treated patients (+3% ALP relative increase versus baseline) (Trauner et al. 2019). Treatment with cilofexor also resulted in a dose-dependent significant reduction of AST, ALT, and GGT values from baseline. Overall, cilofexor was well tolerated, and only three patients (14%) discontinued drug treatment. A significant reduction in serum BA levels as well as of C4 levels was observed in the 100 mg cilofexor group (Trauner et al. 2019). Pruritus was observed in 14–20% of patients treated with cilofexor, while 40% of patients in the placebo group reported pruritus (Trauner et al. 2019). Importantly, bowel disease remained stable during the 12 weeks trial (Trauner et al. 2019). Larger studies with FXR agonists are needed to evaluate whether the reduction in ALP will result in a clinically meaningful outcome improvement for PSC patients and to determine potential long-term side effects, especially with regard to malignancy development.
The FGF19 analogue (NGM282, formerly M70) has also been recently evaluated in a randomized, double-blind, placebo-controlled phase II trial in PSC patients (Hirschfield et al. 2018b). Sixty-two patients were randomized to receive placebo, NGM282 1 mg or 3 mg per subcutaneous injection once daily for 12 weeks. The primary outcome was the change in ALP levels from baseline at 12 weeks. Neither dose of NGM282 led to significant reduction in ALP levels from baseline by week 12 (Hirschfield et al. 2018b). However, NGM282 treatment resulted in a dose-dependent highly significant reduction of BA synthesis (C4 levels) as well as serum BA levels. Furthermore, AST and ALT values were significantly lower at end of treatment in the 3 mg NGM282 group (Hirschfield et al. 2018b). Furthermore, NGM282 significantly and dose-dependently improved serum markers for liver fibrosis (ELF score and Pro-C3) as compared to placebo-treated patients (Hirschfield et al. 2018b). 81–95% of patients treated with NGM282 experienced adverse effects, most of which were grade 1 or grade 2 and were mainly related to injection site or diarrhea. Three patients reported serious adverse events, one of which was potentially related to NGM282 (bowel obstruction in the follow-up period, which resolved) (Hirschfield et al. 2018b). Overall, the drug was well tolerated. Antibodies against FGF19 were not found in any of the patients, who tested positive for antidrug antibodies (Hirschfield et al. 2018b). Further trials in PSC will be needed to determine if ALP reduction is an adequate study endpoint. Also, longer trials will be needed to determine if the anti-fibrotic effects as well as the changes in BA metabolism will prevent disease progression and improve outcome in PSC patients treated with NGM282 (Hirschfield et al. 2018b).
7 Summary and Perspectives
FXR and its hepatic and intestinal target genes transcriptionally regulate BA synthesis, detoxification, secretion, and absorption in the enterohepatic circulation. Activation of FXR protects from BA overload and liver damage by suppression of BA de novo synthesis, reduction in hepatic uptake of BAs, as well as through stimulation of secretion across the canalicular and basolateral hepatocyte membranes. These mechanisms made FXR and its signaling pathways attractive targets for the treatment of cholestatic liver diseases. While the first in class FXR agonist obeticholic acid has already been approved for the treatment of PBC in patients with insufficient response toward UDCA or with UDCA intolerance, further nonsteroidal and more selective FXR modulators are being developed. These agents may avoid prolonged systemic activation of FXR, allow for a tissue-specific targeting, and also dissociate different FXR signaling effects. The first of these nonsteroidal FXR agonists have progressed into clinical phase II trials in PBC (tropifexor). In PSC both OCA and cilofexor have reached clinical phase II trials. Furthermore, the nontumorigenic FGF19 analogue NGM282 has been tested in patients with PBC as well as PSC with favorable results. Thus, over the next years, more agents targeting the FXR-FGF19 pathway will progress into phase III trials to determine efficacy, safety, and tolerability, and the future will show whether the results seen in the phase II trials translate into clinically meaningful outcome improvements for patients with cholestatic liver diseases.
References
Abu-Hayyeh S, Williamson C (2015) Progesterone metabolites as farnesoid X receptor inhibitors. Dig Dis 33(3):300–306
Abu-Hayyeh S, Papacleovoulou G, Lovgren-Sandblom A, Tahir M, Oduwole O, Jamaludin NA, Ravat S, Nikolova V, Chambers J, Selden C, Rees M, Marschall HU, Parker MG, Williamson C (2013) Intrahepatic cholestasis of pregnancy levels of sulfated progesterone metabolites inhibit farnesoid X receptor resulting in a cholestatic phenotype. Hepatology 57(2):716–726
Abu-Hayyeh S, Ovadia C, Lieu T, Jensen DD, Chambers J, Dixon PH, Lovgren-Sandblom A, Bolier R, Tolenaars D, Kremer AE, Syngelaki A, Noori M, Williams D, Marin JJ, Monte MJ, Nicolaides KH, Beuers U, Oude-Elferink R, Seed PT, Chappell L, Marschall HU, Bunnett NW, Williamson C (2016) Prognostic and mechanistic potential of progesterone sulfates in intrahepatic cholestasis of pregnancy and pruritus gravidarum. Hepatology 63(4):1287–1298
Alvarez-Sola G, Uriarte I, Latasa MU, Urtasun R, Barcena-Varela M, Elizalde M, Jimenez M, Rodriguez-Ortigosa CM, Corrales FJ, Fernandez-Barrena MG, Berasain C, Avila MA (2017) Fibroblast growth factor 15/19 in hepatocarcinogenesis. Dig Dis 35(3):158–165
Anakk S, Watanabe M, Ochsner SA, McKenna NJ, Finegold MJ, Moore DD (2011) Combined deletion of Fxr and Shp in mice induces Cyp17a1 and results in juvenile onset cholestasis. J Clin Invest 121(1):86–95
Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ (2001) Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem 276(31):28857–28865
Bacq Y (2011) Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol 35(3):182–193
Bacq Y, Sentilhes L, Reyes HB, Glantz A, Kondrackiene J, Binder T, Nicastri PL, Locatelli A, Floreani A, Hernandez I, Di Martino V (2012) Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta-analysis. Gastroenterology 143(6):1492–1501
Baghdasaryan A, Claudel T, Gumhold J, Silbert D, Adorini L, Roda A, Vecchiotti S, Gonzalez FJ, Schoonjans K, Strazzabosco M, Fickert P, Trauner M (2011) Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2−/− (Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO output. Hepatology 54(4):1303–1312
Bahar R, Wong KA, Liu CH, Bowlus CL (2018) Update on new drugs and those in development for the treatment of primary biliary cholangitis. Gastroenterol Hepatol (N Y) 14(3):154–163
Beuers U, Trauner M, Jansen P, Poupon R (2015) New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol 62(1 Suppl):S25–S37
Boonstra K, Beuers U, Ponsioen CY (2012a) Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol 56(5):1181–1188
Boonstra K, van Erpecum KJ, van Nieuwkerk KM, Drenth JP, Poen AC, Witteman BJ, Tuynman HA, Beuers U, Ponsioen CY (2012b) Primary sclerosing cholangitis is associated with a distinct phenotype of inflammatory bowel disease. Inflamm Bowel Dis 18(12):2270–2276
Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N (2006) Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol 290(6):G1124–G1130
Cai SY, Gautam S, Nguyen T, Soroka CJ, Rahner C, Boyer JL (2009) ATP8B1 deficiency disrupts the bile canalicular membrane bilayer structure in hepatocytes, but FXR expression and activity are maintained. Gastroenterology 136(3):1060–1069
Carey EJ, Ali AH, Lindor KD (2015) Primary biliary cirrhosis. Lancet 386(10003):1565–1575
Cariello M, Peres C, Zerlotin R, Porru E, Sabba C, Roda A, Moschetta A (2017) Long-term administration of nuclear bile acid receptor FXR agonist prevents spontaneous hepatocarcinogenesis in Abcb4(−/−) mice. Sci Rep 7(1):11203
Chen F, Ananthanarayanan M, Emre S, Neimark E, Bull LN, Knisely AS, Strautnieks SS, Thompson RJ, Magid MS, Gordon R, Balasubramanian N, Suchy FJ, Shneider BL (2004) Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid X receptor activity. Gastroenterology 126(3):756–764
Cho JY, Matsubara T, Kang DW, Ahn SH, Krausz KW, Idle JR, Luecke H, Gonzalez FJ (2010) Urinary metabolomics in Fxr-null mice reveals activated adaptive metabolic pathways upon bile acid challenge. J Lipid Res 51(5):1063–1074
Corpechot C, Abenavoli L, Rabahi N, Chretien Y, Andreani T, Johanet C, Chazouilleres O, Poupon R (2008) Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology 48(3):871–877
Corpechot C, Chazouilleres O, Poupon R (2011) Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol 55(6):1361–1367
Cui YJ, Aleksunes LM, Tanaka Y, Goedken MJ, Klaassen CD (2009) Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice. Toxicol Sci 110(1):47–60
Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, Maeda N, Parks JS (2003) Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem 278(36):33920–33927
de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O, Oude Elferink RP, Hadchouel M (1998) Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci U S A 95(1):282–287
Denk GU, Soroka CJ, Takeyama Y, Chen WS, Schuetz JD, Boyer JL (2004) Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. J Hepatol 40(4):585–591
Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, Karpen SJ (2001) The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology 121(1):140–147
Dietrich CG, Ottenhoff R, de Waart DR, Oude Elferink RP (2001) Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology 167(1):73–81
Dixon PH, van Mil SW, Chambers J, Strautnieks S, Thompson RJ, Lammert F, Kubitz R, Keitel V, Glantz A, Mattsson LA, Marschall HU, Molokhia M, Moore GE, Linton KJ, Williamson C (2009) Contribution of variant alleles of ABCB11 to susceptibility to intrahepatic cholestasis of pregnancy. Gut 58(4):537–544
Dixon PH, Wadsworth CA, Chambers J, Donnelly J, Cooley S, Buckley R, Mannino R, Jarvis S, Syngelaki A, Geenes V, Paul P, Sothinathan M, Kubitz R, Lammert F, Tribe RM, Ch’ng CL, Marschall HU, Glantz A, Khan SA, Nicolaides K, Whittaker J, Geary M, Williamson C (2014) A comprehensive analysis of common genetic variation around six candidate loci for intrahepatic cholestasis of pregnancy. Am J Gastroenterol 109(1):76–84
Dixon PH, Sambrotta M, Chambers J, Taylor-Harris P, Syngelaki A, Nicolaides K, Knisely AS, Thompson RJ, Williamson C (2017) An expanded role for heterozygous mutations of ABCB4, ABCB11, ATP8B1, ABCC2 and TJP2 in intrahepatic cholestasis of pregnancy. Sci Rep 7(1):11823
Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD (2013) Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology 145(3):521–536
European Association for the Study of the Liver (2009) EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol 51(2):237–267
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver (2017) EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol 67(1):145–172
Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, Marschall HU, Tsybrovskyy O, Zatloukal K, Denk H, Trauner M (2002) Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology 123(4):1238–1251
Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K, Marschall HU, Denk H, Trauner M (2004) Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 127(1):261–274
Fiorucci S, Clerici C, Antonelli E, Orlandi S, Goodwin B, Sadeghpour BM, Sabatino G, Russo G, Castellani D, Willson TM, Pruzanski M, Pellicciari R, Morelli A (2005) Protective effects of 6-ethyl chenodeoxycholic acid, a farnesoid X receptor ligand, in estrogen-induced cholestasis. J Pharmacol Exp Ther 313(2):604–612
Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW (1995) Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81(5):687–693
Geenes V, Chappell LC, Seed PT, Steer PJ, Knight M, Williamson C (2014) Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology 59(4):1482–1491
Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ (1998) The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem 273(16):10046–10050
Glantz A, Marschall HU, Mattsson LA (2004) Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology 40(2):467–474
Gomez-Ospina N, Potter CJ, Xiao R, Manickam K, Kim MS, Kim KH, Shneider BL, Picarsic JL, Jacobson TA, Zhang J, He W, Liu P, Knisely AS, Finegold MJ, Muzny DM, Boerwinkle E, Lupski JR, Plon SE, Gibbs RA, Eng CM, Yang Y, Washington GC, Porteus MH, Berquist WE, Kambham N, Singh RJ, Xia F, Enns GM, Moore DD (2016) Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nat Commun 7:10713
Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA (2000) A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6(3):517–526
Gudbjartsson DF, Helgason H, Gudjonsson SA, Zink F, Oddson A, Gylfason A, Besenbacher S, Magnusson G, Halldorsson BV, Hjartarson E, Sigurdsson GT, Stacey SN, Frigge ML, Holm H, Saemundsdottir J, Helgadottir HT, Johannsdottir H, Sigfusson G, Thorgeirsson G, Sverrisson JT, Gretarsdottir S, Walters GB, Rafnar T, Thjodleifsson B, Bjornsson ES, Olafsson S, Thorarinsdottir H, Steingrimsdottir T, Gudmundsdottir TS, Theodors A, Jonasson JG, Sigurdsson A, Bjornsdottir G, Jonsson JJ, Thorarensen O, Ludvigsson P, Gudbjartsson H, Eyjolfsson GI, Sigurdardottir O, Olafsson I, Arnar DO, Magnusson OT, Kong A, Masson G, Thorsteinsdottir U, Helgason A, Sulem P, Stefansson K (2015) Large-scale whole-genome sequencing of the Icelandic population. Nat Genet 47(5):435–444
Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB Jr, Kliewer SA, Gonzalez FJ, Sinal CJ (2003) Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem 278(46):45062–45071
Hagenbuch B, Meier PJ (1994) Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest 93(3):1326–1331
Häussinger D, Kubitz R, Reinehr R, Bode JG, Schliess F (2004) Molecular aspects of medicine: from experimental to clinical hepatology. Mol Aspects Med 25(3):221–360
Hirschfield GM, Karlsen TH, Lindor KD, Adams DH (2013) Primary sclerosing cholangitis. Lancet 382(9904):1587–1599
Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC Jr, Pares A, Trauner M, Marschall HU, Adorini L, Sciacca C, Beecher-Jones T, Castelloe E, Bohm O, Shapiro D (2015) Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology 148(4):751–761 e8
Hirschfield GM, Dyson JK, Alexander GJM, Chapman MH, Collier J, Hubscher S, Patanwala I, Pereira SP, Thain C, Thorburn D, Tiniakos D, Walmsley M, Webster G, Jones DEJ (2018a) The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut 67(9):1568–1594
Hirschfield GM, Chazouilleres O, Drenth JP, Thorburn D, Harrison SA, Landis CS, Mayo MJ, Muir AJ, Trotter JF, Leeming DJ, Karsdal MA, Jaros MJ, Ling L, Kim KH, Rossi SJ, Somaratne RM, DePaoli AM, Beuers U (2018b) Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double-blind, placebo-controlled phase II trial. J Hepatol 70(3):483–493
Ho HK, Pok S, Streit S, Ruhe JE, Hart S, Lim KS, Loo HL, Aung MO, Lim SG, Ullrich A (2009) Fibroblast growth factor receptor 4 regulates proliferation, anti-apoptosis and alpha-fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. J Hepatol 50(1):118–127
Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang d Y, Mansfield TA, Kliewer SA, Goodwin B, Jones SA (2003) Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev 17(13):1581–1591
Huang L, Zhao A, Lew JL, Zhang T, Hrywna Y, Thompson JR, de PN, Royo I, Blevins RA, Pelaez F, Wright SD, Cui J (2003) Farnesoid X receptor activates transcription of the phospholipid pump MDR3. J Biol Chem 278(51):51085–51090
Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA (2005) Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2(4):217–225
Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA (2006) Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A 103(10):3920–3925
Jacquemin E, Cresteil D, Manouvrier S, Boute O, Hadchouel M (1999) Heterozygous non-sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet 353(9148):210–211
Joshi D, James A, Quaglia A, Westbrook RH, Heneghan MA (2010) Liver disease in pregnancy. Lancet 375(9714):594–605
Jung D, Kullak-Ublick GA (2003) Hepatocyte nuclear factor 1alpha: a key mediator of the effect of bile acids on gene expression. Hepatology 37(3):622–631
Jung D, Podvinec M, Meyer UA, Mangelsdorf DJ, Fried M, Meier PJ, Kullak-Ublick GA (2002) Human organic anion transporting polypeptide 8 promoter is transactivated by the farnesoid X receptor/bile acid receptor. Gastroenterology 122(7):1954–1966
Jung D, Hagenbuch B, Fried M, Meier PJ, Kullak-Ublick GA (2004) Role of liver-enriched transcription factors and nuclear receptors in regulating the human, mouse, and rat NTCP gene. Am J Physiol Gastrointest Liver Physiol 286(5):G752–G761
Kalaany NY, Mangelsdorf DJ (2006) LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol 68:159–191
Kamisako T, Leier I, Cui Y, Kînig J, Buchholz U, Hummel-Eisenbeiss J, Keppler D (1999) Transport of monoglucuronosyl and bisglucuronosyl bilirubin by recombinant human and rat multidrug resistance protein 2. Hepatology 30(2):485–490
Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA (2002) Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem 277(4):2908–2915
Kazgan N, Metukuri MR, Purushotham A, Lu J, Rao A, Lee S, Pratt-Hyatt M, Lickteig A, Csanaky IL, Zhao Y, Dawson PA, Li X (2014) Intestine-specific deletion of SIRT1 in mice impairs DCoH2-HNF-1alpha-FXR signaling and alters systemic bile acid homeostasis. Gastroenterology 146(4):1006–1016
Keitel V, Burdelski M, Warskulat U, Kuhlkamp T, Keppler D, Häussinger D, Kubitz R (2005) Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology 41(5):1160–1172
Keitel V, Vogt C, Häussinger D, Kubitz R (2006) Combined mutations of canalicular transporter proteins cause severe intrahepatic cholestasis of pregnancy. Gastroenterology 131(2):624–629
Keitel V, Kubitz R, Häussinger D (2008) Endocrine and paracrine role of bile acids. World J Gastroenterol 14(37):5620–5629
Keitel V, Dröge C, Stepanow S, Fehm T, Mayatepek E, Kohrer K, Häussinger D (2016) Intrahepatic cholestasis of pregnancy (ICP): case report and review of the literature. Z Gastroenterol 54(12):1327–1333
Keppler D, Kamisako T, Leier I, Cui Y, Nies AT, Tsujii H, König J (2000) Localization, substrate specificity, and drug resistance conferred by conjugate export pumps of the MRP family. Adv Enzyme Regul 40:339–349
Kerr TA, Saeki S, Schneider M, Schaefer K, Berdy S, Redder T, Shan B, Russell DW, Schwarz M (2002) Loss of nuclear receptor SHP impairs but does not eliminate negative feedback regulation of bile acid synthesis. Dev Cell 2(6):713–720
Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ (2007) Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res 48(12):2664–2672
Kliewer SA, Mangelsdorf DJ (2015) Bile acids as hormones: the FXR-FGF15/19 pathway. Dig Dis 33(3):327–331
Kowdley KV, Luketic V, Chapman R, Hirschfield GM, Poupon R, Schramm C, Vincent C, Rust C, Pares A, Mason A, Marschall HU, Shapiro D, Adorini L, Sciacca C, Beecher-Jones T, Bohm O, Pencek R, Jones D, Obeticholic Acid PBC Monotherapy Study Group (2018) A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology 67(5):1890–1902
Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, Houben MH, Witteman BJ, van Erpecum KJ, van Buuren HR, Dutch PBCSG (2009) Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology 136(4):1281–1287
Kullak-Ublick GA (2003) ABC transporter regulation by bile acids: where PXR meets FXR. J Hepatol 39(4):628–630
Kullak-Ublick GA, Stieger B, Hagenbuch B, Meier PJ (2000) Hepatic transport of bile salts. Semin Liver Dis 20(3):273–292
Kullak-Ublick G, Stieger B, Meier PJ (2004) Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology 126(1):322–342
Lammers WJ, Hirschfield GM, Corpechot C, Nevens F, Lindor KD, Janssen HL, Floreani A, Ponsioen CY, Mayo MJ, Invernizzi P, Battezzati PM, Pares A, Burroughs AK, Mason AL, Kowdley KV, Kumagi T, Harms MH, Trivedi PJ, Poupon R, Cheung A, Lleo A, Caballeria L, Hansen BE, van Buuren HR, Global PBCSG (2015) Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology 149(7):1804–1812 e4
Lammert F, Marschall HU, Glantz A, Matern S (2000) Intrahepatic cholestasis of pregnancy: molecular pathogenesis, diagnosis and management. J Hepatol 33(6):1012–1021
Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA (2006) The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am J Physiol Gastrointest Liver Physiol 290(3):G476–G485
Lazaridis KN, LaRusso NF (2016) Primary sclerosing cholangitis. N Engl J Med 375(12):1161–1170
Lazaridis KN, Pham L, Tietz P, Marinelli RA, de Groen PC, Levine S, Dawson PA, LaRusso NF (1997) Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest 100(11):2714–2721
Lee YK, Moore DD (2002) Dual mechanisms for repression of the monomeric orphan receptor liver receptor homologous protein-1 by the orphan small heterodimer partner. J Biol Chem 277(4):2463–2467
Lee YK, Dell H, Dowhan DH, Hadzopoulou-Cladaras M, Moore DD (2000) The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression. Mol Cell Biol 20(1):187–195
Leuschner M, Guldutuna S, You T, Hubner K, Bhatti S, Leuschner U (1996) Ursodeoxycholic acid and prednisolone versus ursodeoxycholic acid and placebo in the treatment of early stages of primary biliary cirrhosis. J Hepatol 25(1):49–57
Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, Jones SA (2003) Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest 112(11):1678–1687
Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ (2000) Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6(3):507–515
Luo J, Ko B, Elliott M, Zhou M, Lindhout DA, Phung V, To C, Learned RM, Tian H, DePaoli AM, Ling L (2014) A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med 6(247):247ra100
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B (1999) Identification of a nuclear receptor for bile acids. Science 284(5418):1362–1365
Marschall HU, Wagner M, Bodin K, Zollner G, Fickert P, Gumhold J, Silbert D, Fuchsbichler A, Sjovall J, Trauner M (2006) Fxr(−/−) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. J Lipid Res 47(3):582–592
Marschall HU, Wikstrom Shemer E, Ludvigsson JF, Stephansson O (2013) Intrahepatic cholestasis of pregnancy and associated hepatobiliary disease: a population-based cohort study. Hepatology 58(4):1385–1391
Massafra V, Pellicciari R, Gioiello A, van Mil SWC (2018) Progress and challenges of selective Farnesoid X Receptor modulation. Pharmacol Ther 191:162–177
Mayo MJ, Wigg AJ, Leggett BA, Arnold H, Thompson AJ, Weltman M, Carey EJ, Muir AJ, Ling L, Rossi SJ, DePaoli AM (2018) NGM282 for treatment of patients with primary biliary cholangitis: a multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Commun 2(9):1037–1050
Milona A, Owen BM, Cobbold JF, Willemsen EC, Cox IJ, Boudjelal M, Cairns W, Schoonjans K, Taylor-Robinson SD, Klomp LW, Parker MG, White R, van Mil SW, Williamson C (2010) Raised hepatic bile acid concentrations during pregnancy in mice are associated with reduced farnesoid X receptor function. Hepatology 52(4):1341–1349
Modica S, Petruzzelli M, Bellafante E, Murzilli S, Salvatore L, Celli N, Di Tullio G, Palasciano G, Moustafa T, Halilbasic E, Trauner M, Moschetta A (2012) Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology 142(2):355–365 e1–4
Müllenbach R, Linton KJ, Wiltshire S, Weerasekera N, Chambers J, Elias E, Higgins CF, Johnston DG, McCarthy MI, Williamson C (2003) ABCB4 gene sequence variation in women with intrahepatic cholestasis of pregnancy. J Med Genet 40(5):e70
Müllenbach R, Bennett A, Tetlow N, Patel N, Hamilton G, Cheng F, Chambers J, Howard R, Taylor-Robinson SD, Williamson C (2005) ATP8B1 mutations in British cases with intrahepatic cholestasis of pregnancy. Gut 54(6):829–834
Neimark E, Chen F, Li X, Shneider BL (2004) Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology 40(1):149–156
Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U, Trauner M, Jones DE, Floreani A, Hohenester S, Luketic V, Shiffman M, van Erpecum KJ, Vargas V, Vincent C, Hirschfield GM, Shah H, Hansen B, Lindor KD, Marschall HU, Kowdley KV, Hooshmand-Rad R, Marmon T, Sheeron S, Pencek R, MacConell L, Pruzanski M, Shapiro D, Group PS (2016) A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med 375(7):631–643
Nicholes K, Guillet S, Tomlinson E, Hillan K, Wright B, Frantz GD, Pham TA, Dillard-Telm L, Tsai SP, Stephan JP, Stinson J, Stewart T, French DM (2002) A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am J Pathol 160(6):2295–2307
Padrissa-Altes S, Bachofner M, Bogorad RL, Pohlmeier L, Rossolini T, Bohm F, Liebisch G, Hellerbrand C, Koteliansky V, Speicher T, Werner S (2015) Control of hepatocyte proliferation and survival by Fgf receptors is essential for liver regeneration in mice. Gut 64(9):1444–1453
Pares A, Caballeria L, Rodes J (2006) Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology 130(3):715–720
Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284(5418):1365–1368
Pauli-Magnus C, Lang T, Meier Y, Zodan-Marin T, Jung D, Breymann C, Zimmermann R, Kenngott S, Beuers U, Reichel C, Kerb R, Penger A, Meier PJ, Kullak-Ublick GA (2004) Sequence analysis of bile salt export pump (ABCB11) and multidrug resistance p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy. Pharmacogenetics 14(2):91–102
Poupon RE, Balkau B, Eschwege E, Poupon R (1991) A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med 324(22):1548–1554
Poupon RE, Bonnand AM, Chretien Y, Poupon R (1999) Ten-year survival in ursodeoxycholic acid-treated patients with primary biliary cirrhosis. The UDCA-PBC Study Group. Hepatology 29(6):1668–1671
Rioseco AJ, Ivankovic MB, Manzur A, Hamed F, Kato SR, Parer JT, Germain AM (1994) Intrahepatic cholestasis of pregnancy: a retrospective case-control study of perinatal outcome. Am J Obstet Gynecol 170(3):890–895
Ropponen A, Sund R, Riikonen S, Ylikorkala O, Aittomaki K (2006) Intrahepatic cholestasis of pregnancy as an indicator of liver and biliary diseases: a population-based study. Hepatology 43(4):723–728
Russell DW (2003) The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72:137–174
Sawey ET, Chanrion M, Cai C, Wu G, Zhang J, Zender L, Zhao A, Busuttil RW, Yee H, Stein L, French DM, Finn RS, Lowe SW, Powers S (2011) Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell 19(3):347–358
Schuetz EG, Strom S, Yasuda K, Lecureur V, Assem M, Brimer C, Lamba J, Kim RB, Ramachandran V, Komoroski BJ, Venkataramanan R, Cai H, Sinal CJ, Gonzalez FJ, Schuetz JD (2001) Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem 276(42):39411–39418
Seol W, Choi HS, Moore DD (1996) An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science 272(5266):1336–1339
Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ (2000) Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102(6):731–744
Sinha J, Chen F, Miloh T, Burns RC, Yu Z, Shneider BL (2008) Beta-Klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. Am J Physiol Gastrointest Liver Physiol 295(5):G996–G1003
Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA (1993) Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75(3):451–462
Song X, Vasilenko A, Chen Y, Valanejad L, Verma R, Yan B, Deng R (2014) Transcriptional dynamics of bile salt export pump during pregnancy: mechanisms and implications in intrahepatic cholestasis of pregnancy. Hepatology 60(6):1993–2007
Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez JG, Evans RM, Downes M (2006) Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc Natl Acad Sci U S A 103(30):11323–11328
Stroup D, Crestani M, Chiang JY (1997) Identification of a bile acid response element in the cholesterol 7 alpha-hydroxylase gene CYP7A. Am J Physiol 273(2 Pt 1):G508–G517
Trauner M, Gulamhusein A, Hameed B, Caldwell S, Shiffman ML, Landis C, Eksteen B, Agarwal K, Muir A, Rushbrook S, Lu X, Xu J, Chuang JC, Billin AN, Chung C, Li G, Subramanian GM, Myers RP, Bowlus CL, Kowdley KV (2019) The nonsteroidal FXR agonist cilofexor (GS-9674) improves markers of cholestasis and liver injury in patients with PSC. Hepatology. https://doi.org/10.1002/hep.30509
Tully DC, Rucker PV, Chianelli D, Williams J, Vidal A, Alper PB, Mutnick D, Bursulaya B, Schmeits J, Wu X, Bao D, Zoll J, Kim Y, Groessl T, McNamara P, Seidel HM, Molteni V, Liu B, Phimister A, Joseph SB, Laffitte B (2017) Discovery of tropifexor (LJN452), a highly potent non-bile acid FXR agonist for the treatment of cholestatic liver diseases and nonalcoholic steatohepatitis (NASH). J Med Chem 60(24):9960–9973
Uriarte I, Fernandez-Barrena MG, Monte MJ, Latasa MU, Chang HC, Carotti S, Vespasiani-Gentilucci U, Morini S, Vicente E, Concepcion AR, Medina JF, Marin JJ, Berasain C, Prieto J, Avila MA (2013) Identification of fibroblast growth factor 15 as a novel mediator of liver regeneration and its application in the prevention of post-resection liver failure in mice. Gut 62(6):899–910
Uriarte I, Latasa MU, Carotti S, Fernandez-Barrena MG, Garcia-Irigoyen O, Elizalde M, Urtasun R, Vespasiani-Gentilucci U, Morini S, de Mingo A, Mari M, Corrales FJ, Prieto J, Berasain C, Avila MA (2015) Ileal FGF15 contributes to fibrosis-associated hepatocellular carcinoma development. Int J Cancer 136(10):2469–2475
van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, van Meer G (1996) MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell 87(3):507–517
van Mil SW, Milona A, Dixon PH, Mullenbach R, Geenes VL, Chambers J, Shevchuk V, Moore GE, Lammert F, Glantz AG, Mattsson LA, Whittaker J, Parker MG, White R, Williamson C (2007) Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology 133(2):507–516
Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, Zatloukal K, Guo GL, Schuetz JD, Gonzalez FJ, Marschall HU, Denk H, Trauner M (2003) Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology 125(3):825–838
Wang H, Chen J, Hollister K, Sowers LC, Forman BM (1999) Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3(5):543–553
Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, Helgason CD, Ackerley C, Phillips MJ, Ling V (2001) Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci U S A 98(4):2011–2016
Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, Chua SS, Wei P, Heyman RA, Karin M, Moore DD (2002) Redundant pathways for negative feedback regulation of bile acid production. Dev Cell 2(6):721–731
Wang L, Han Y, Kim CS, Lee YK, Moore DD (2003) Resistance of SHP-null mice to bile acid-induced liver damage. J Biol Chem 278(45):44475–44481
Wasmuth HE, Glantz A, Keppeler H, Simon E, Bartz C, Rath W, Mattsson LA, Marschall HU, Lammert F (2007) Intrahepatic cholestasis of pregnancy: the severe form is associated with common variants of the hepatobiliary phospholipid transporter ABCB4 gene. Gut 56(2):265–270
Wikstrom Shemer E, Marschall HU, Ludvigsson JF, Stephansson O (2013) Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG 120(6):717–723
Wikstrom Shemer EA, Stephansson O, Thuresson M, Thorsell M, Ludvigsson JF, Marschall HU (2015) Intrahepatic cholestasis of pregnancy and cancer, immune-mediated and cardiovascular diseases: a population-based cohort study. J Hepatol 63(2):456–461
Williamson C, Geenes V (2014) Intrahepatic cholestasis of pregnancy. Obstet Gynecol 124(1):120–133
Williamson C, Hems LM, Goulis DG, Walker I, Chambers J, Donaldson O, Swiet M, Johnston DG (2004) Clinical outcome in a series of cases of obstetric cholestasis identified via a patient support group. BJOG 111(7):676–681
Yang Y, Zhang M, Eggertsen G, Chiang JY (2002) On the mechanism of bile acid inhibition of rat sterol 12alpha-hydroxylase gene (CYP8B1) transcription: roles of alpha-fetoprotein transcription factor and hepatocyte nuclear factor 4alpha. Biochim Biophys Acta 1583(1):63–73
Zhang M, Chiang JY (2001) Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J Biol Chem 276(45):41690–41699
Zhou M, Wang X, Phung V, Lindhout DA, Mondal K, Hsu JY, Yang H, Humphrey M, Ding X, Arora T, Learned RM, DePaoli AM, Tian H, Ling L (2014) Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res 74(12):3306–3316
Zhou M, Learned RM, Rossi SJ, DePaoli AM, Tian H, Ling L (2016) Engineered fibroblast growth factor 19 reduces liver injury and resolves sclerosing cholangitis in Mdr2-deficient mice. Hepatology 63(3):914–929
Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall HU, Zatloukal K, Denk H, Trauner M (2003) Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol 38(6):717–727
Acknowledgment
Our own studies are supported by DFG through SFB974 “Communication and systems relevance in liver damage and regeneration.”
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Keitel, V., Dröge, C., Häussinger, D. (2019). Targeting FXR in Cholestasis. In: Fiorucci, S., Distrutti, E. (eds) Bile Acids and Their Receptors. Handbook of Experimental Pharmacology, vol 256. Springer, Cham. https://doi.org/10.1007/164_2019_231
Download citation
DOI: https://doi.org/10.1007/164_2019_231
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22004-4
Online ISBN: 978-3-030-22005-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)