Abstract
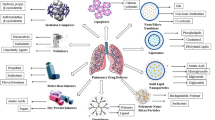
Historically, the inhaled route has been used for the delivery of locally-acting drugs for the treatment of respiratory conditions, such as asthma, COPD, and airway infections. Targeted delivery of substances to the lungs has some key advantages over systemic administration, including a more rapid onset of action, an increased therapeutic effect, and, depending on the agent inhaled, reduced systemic side effects since the required local concentration in the lungs can be obtained with a lower dose. Fortunately, when designed properly, inhaled drug delivery devices can be very effective and safe for getting active agents directly to their site of action.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In what is arguably one of the most impressive works on asthma ever written, “On asthma: Its pathology and treatment” (1860), physician (and asthma sufferer) Henry H. Salter discusses and classifies all treatments then available for this intriguing disease, which he describes as “paroxysmal dyspnoea of a peculiar character, generally periodic, with intervals of healthy respiration between the attacks”. One treatment that particularly stands out today is smoking tobacco. As a depressant, tobacco was claimed to counteract the spasm of the airway musculature (Salter 1868).
Smoking tobacco fell out of grace in the second half of the twentieth century due to its obvious negative effects on the respiratory tract with increasing evidence associating smoking with lung cancer and chronic obstructive pulmonary disease (COPD) (Cornfield et al. 1959, 2009; Auerbach et al. 1966; U.S. Department of Health and Human Services 2010). Nonetheless, smoking has continued to be advocated as an obscure reliever of breathlessness for some patients with asthma. In essence, tobacco may provide some benefit for some patients with asthma, as it has been demonstrated that nicotine can suppress various inflammatory and allergic parameters providing a plausible explanation why some patients with asthma continue to smoke claiming beneficial effects (Mishra et al. 2008). However, the cigarette presents what may be the worst example of a delivery device for administering the nicotine into the body for the “pleasurable” effects sought by smokers as it is associated with the inhalation of many other harmful chemicals producing what are now well-recognised harmful effects on the lungs and elsewhere. Fortunately, when designed properly, inhaled drug delivery methods can be very effective and safe for getting active agents into the body.
2 Inhaled Drug Delivery
The respiratory system offers a unique route for the delivery of drugs to the body. Historically, this route has been used for the delivery of locally acting drugs for the treatment of respiratory conditions, such as asthma, COPD, and airway infections. Targeted delivery of substances to the lungs has some key advantages over systemic administration routes, including a more rapid onset of action, an increased therapeutic effect, and, depending on the agent inhaled, reduced systemic side effects since the required local concentration in the lungs can be obtained with a lower dose (Newhouse and Dolovich 1986; Dolovich et al. 2005).
However, the particular architecture of the lower respiratory tract, with its vast absorptive surface area of approximately 100 m2 (Weibel 1963), also allows inhalation of certain substances into the lung to be an alternative portal to the systemic circulation instead of parenteral or oral administration. The lungs can thus function as a non-invasive systemic delivery route, for example when a rapid effect is desired for pain relievers (Aurora et al. 2011; Farr and Otulana 2006; Fulda et al. 2005; Furyk et al. 2009; Silberstein 2012; Xu et al. 2012), or for substances with low (or no) bioavailability after administration via the gastrointestinal tract (Patton and Byron 2007; Siekmeier and Scheuch 2008). Examples of such substances are therapeutic proteins, which are prone to degradation by metabolic enzymes (e.g. pepsin) in the gastric lumen (Lizio et al. 2000; Zijlstra et al. 2004; Laube et al. 1998; Heinemann et al. 2000; Bosquillon et al. 2004), or substances that are metabolised extensively upon first passage through the gastrointestinal wall and the liver (the first-pass effect) (Zheng et al. 1999).
However, the architecture of the lungs also poses the main challenge for pulmonary drug delivery, as it has evolved to prevent foreign matter from reaching the peripheral parts of the lungs. Therefore, aerosols must meet a strict set of physical and chemical requirements for inhaled drug delivery to be successful. Moreover, a device is needed for aerosol generation and facilitation of its delivery to the lungs. This makes inhaled drug delivery much more complex than oral or parenteral administration.
2.1 Aerosol Deposition in the Respiratory Tract
Aerosolised compounds can only exert their effects when they first pass the oropharynx and subsequently come in contact with the airway surface following inhalation into the respiratory tract. Transport of the particles in the aerosol towards these surfaces, i.e. their deposition, results from a balance between the forces that act on the inhaled particles. Four types of forces are involved in particle deposition in the respiratory tract: inertial, gravitational, and diffusional forces, as well as the drag force of the moving air that counteracts deposition (Hinds 1982; Frijlink and De Boer 2004).
Impaction as a result of high particle inertia is the predominant deposition mechanism in the upper airways, where the air velocity is high and the airflow turbulent. Particles of a sufficient mass (sufficiently high inertia) cannot follow the changes of airflow direction at the bifurcations fast enough and collide with the opposing airway surface. The probability of impaction increases with the square of the particle diameter, particle density, and particle velocity. Generally, particles with an aerodynamic diameter larger than 5–10 μm (at particle velocities above 30–50 L/min) have the highest probability of depositing in the throat by inertial impaction.
Deposition by sedimentation is the settling of particles under the influence of gravity. The gravitational force increases with the mass (cubic particle diameter) and the stationary settling velocity (counteracted by the drag force) with the square of the particle diameter. Furthermore, sedimentation is a time-dependent process, which implies that the longer a particle resides in an airway duct, the higher the probability that it gets deposited by sedimentation. Therefore, this mechanism prevails when both the air velocity is low and of the same order of magnitude as the settling velocity, and the residence time is high, which is the case in the peripheral airways.
Diffusion, or Brownian motion, is the random movement of particles within a gas resulting from collisions with gas molecules. Diffusion increases with decreasing particle diameter and only very fine particles (smaller than 0.5 μm) are deposited by diffusion. Like sedimentation, diffusion is a time-dependent process. Hence, deposition by diffusion occurs mainly in the peripheral airways, although the relatively limited residence time of particles in the respiratory tract in combination with their random movements results in a very low deposition probability and very fine particles are likely to be exhaled instead of depositing.
2.2 The Aerodynamic Particle Diameter and Particle Size Distribution
In the descriptions above, four parameters were identified that determine whether a particle deposits in the respiratory tract and by which mechanism: the particle diameter, the particle density, the particle velocity, and the residence time in the airways.
Small, spherical particles with high density can exhibit the same aerodynamic behaviour as much larger spheres with a lower density. Hence, expressing the size of such particles in their geometric diameter is not useful for predicting their fate after inhalation. Therefore, the concept of an aerodynamic diameter has been introduced, which standardises for particle density and shape (Hinds 1982). By definition, the aerodynamic diameter of a particle is the diameter of a sphere of unit density that settles with the same velocity in still air (thus under the influence of gravity) as the particle in question. Particles with the same aerodynamic diameter exhibit the same inertial behaviour.
In general, particles with an aerodynamic diameter of 1–5 μm are regarded as suitable for inhalation. To express particle size, the mass median aerodynamic diameter (MMAD: the aerodynamic diameter below which 50% of the emitted mass is contained) is commonly used, in combination with the geometric standard deviation (GSD) as measure for the size distribution. Yet this parameter provides no information on how much of the dose is converted into an aerosol. More meaningful parameters are the fine particle fraction (FPF) and fine particle dose (FPD), which express the portion of the dose (in percentage and mass, respectively) with an aerodynamic diameter below a specified size, usually below 5 μm.
2.3 Patient-Related Factors That Influence Aerosol Deposition
Aerosol deposition patterns are not solely determined by the aerodynamic size distribution of the particles. Particle velocity and residence time, which have already been mentioned as parameters that affect deposition, depend on the inhalation manoeuvre of the patient. Another important determinant is the geometry of the respiratory tract. With decreasing airway diameter, the deposition probability of all three mechanisms increases. This implies that deposition patterns are different in populations with reduced airspaces, such as children (smaller airways), asthma and COPD patients (narrowed and obstructed airways), or patients with bronchiectasis (dilated airways filled with sputum).
Inhalation is the result of expansion of the chest, which creates a pressure difference between the lungs and the atmosphere, in response to which air flows into the lungs. The harder the patient inhales, the faster the particles travel into the lungs, initially with the same velocity as the air that is inhaled. At higher velocities, particle deposition shifts more towards inertial impaction, as more (finer) particles cannot follow the changes in airflow direction at bifurcations.
The inhaled air functions as the medium for aerosol transport into the lungs. To reach the alveolar region, the inhaled volume has to be sufficiently large. This can be accomplished by either exhaling maximally prior to deeply inhaling once, or by tidally breathing in the aerosol over a prolonged period of time. In the latter case, mixing of the freshly inhaled air containing the aerosol with the air that is already present in the lungs is required to enable deposition of the aerosol particles in the peripheral parts of the respiratory tract (Bennett and Smaldone 1987; Nikander et al. 2010).
Patients can be instructed to inhale in the most appropriate way. However, not all patients have the capacity – either cognitive or physical, or both – to follow these instructions. When an inhalation device is not used properly, device performance is negatively affected, which inevitably results in altered aerosol deposition, and thus in less drug reaching the target area, and possibly increased chance of developing unwanted side effects. It is thus of utmost importance that inhalation devices are user-friendly and optimised for specific patient populations.
3 Delivery Devices for Inhalation
Inhalation products are complex drug delivery systems consisting of a formulation of the drug and a delivery device that converts the formulation into an inhalable aerosol.
Devices for inhaled drug delivery have two basic functions, namely aerosol formation and facilitation of aerosol transport into the lungs. A distinction is made between passive and active devices. A passive device derives the energy required for aerosol formation from the inhaled air stream, i.e. from the patient, while active devices create the aerosol independently of the patient’s inhalation. Inhalation devices can be further categorised in various ways, such as single-dose versus multi-dose, or disposable versus reusable. Multi-dose devices may (but do not necessarily) provide benefits for chronic therapy, such as cost reduction, portability, and portability, ease of use and convenience. For irregular administrations and one-time applications, disposable devices may be more suitable. Furthermore, aspects such as the risk of device contamination acting as a reservoir for microbial growth and allowing the development of antibiotic resistance may affect the choice for a multi- or single-dose device.
Traditionally, three types of inhalation devices can be distinguished: pressurised metered dose inhalers (pMDIs), nebulisers, and dry powder inhalers (DPIs). More recently, new types of devices have emerged, such as the Soft Mist Inhaler (Boehringer Ingelheim), which combine functional characteristics of the traditional classifications.
3.1 Pressurised Metered Dose Inhalers
Pressurised metered dose inhalers are the most often-prescribed inhalation devices for symptom management of patients with asthma or COPD (Laube et al. 2011). pMDIs were developed in the 1950s as the first portable multi-dose inhaled drug delivery system. Their basic design consists of a canister that is closed off by a metering valve, an actuation mechanism, and a mouthpiece. The canister holds a propellant under pressure, in which the drug and any excipients are dissolved or suspended (Smyth 2005).
The principal excipient present in all pMDI formulations is the propellant, which is required for aerosolisation of the drug, but which also acts as solvent or suspension medium. In addition, co-solvents, solubilisers, and stabilisers may be present. The first generation pMDIs contained chlorofluorocarbons (CFCs) as propellants, which have been phased out from use due to their ozone depleting properties since the 1987 Montreal Protocol on Substances that Deplete the Ozone Layer. The Montreal protocol has led to replacement of CFCs by hydrofluoroalkanes (HFAs) in pMDIs (Bell and Newman 2007). Some important differences exist between CFC-pMDIs and HFA-pMDIs. On average, the plumes from HFA-pMDIs have a lower velocity and a higher temperature than CFC-pMDI plumes (Gabrio et al. 1999), which may affect patient experience (Laube et al. 2011). In addition, the particles generated with HFA-pMDIs can be smaller, which has led to the development of so-called extra-fine particle products (e.g. Foster (budesonide/formoterol), Chiesi). By virtue of the lower plume velocity and smaller aerosol particle size, HFA-pMDIs show an enhanced lung deposition (Leach et al. 2002; Leach 1998; Goldin et al. 1999; Barnes et al. 2011).
The pMDI is actuated by pressing the canister down, resulting in the release of a fixed amount of the contents that disperses into small particles by rapid expansion of the propellant in the nozzle region. This actuation mechanism is one of the main disadvantages of conventional pMDIs, as actuation takes place independently of the patient’s inhalation. Dose release and inhalation should be synchronic, or the entire dose deposits in the back of the patient’s throat. Therefore, good actuation-inhalation (“hand-lung”) coordination is required, which cannot be taught to all patients. To allow for more generalised use of pMDIs, two alternatives have been introduced: the breath-actuated pMDI (e.g. Teva’s Redihaler) and the use of a valved holding chamber (VHC). Breath-actuated pMDIs still require the patient to comprehend how to perform the desired inhalation manoeuvre. When no comprehension is to be expected at all, for example in very young children, a VHC can be used.
VHCs (e.g. Trudell’s AeroChamber, GSK’s Babyhaler) are extension devices with a one-way valve incorporated into the mouthpiece, allowing for the patient to inhale a static aerosol instead of a plume. The patient can keep the device in his mouth while breathing in and out, as the exhalation into the VHC is directed away from the aerosol-holding chamber via the one-way valve. This way, the aerosol can be inhaled in multiple breaths. Even though less mouth deposition can be expected when using a VHC, the final lung deposition is still low due to losses in the VHC by various mechanisms, including impaction and sedimentation of the aerosol (Bisgaard et al. 2002). For the sake of an easier name, VHCs are sometimes incorrectly grouped with spacers. Spacers (e.g. GSK’s Volumatic) are simpler types of extension devices that have no valve and function solely by increasing the distance between the pMDI and the throat of the patient. This lack of a valve strongly diminishes the spacer’s applicability in patients who cannot follow inhalation instructions, as it bears the risk of the patient exhaling into the device and thereby spoiling the aerosol.
3.2 Nebulisers
Nebulisers generate aerosols from aqueous solutions or suspensions of the drug. Their use is mainly confined to situations that do not allow for the use of a pMDI or DPI, for example when the patient is unconscious, or for therapeutic agents for which no pMDI or DPI formulation is available (yet) (Le Brun et al. 2000). Also for drugs requiring high doses, such as antibiotics, nebulisation was the only available delivery option until recently.
Basically, two different nebuliser types exist: jet and ultrasonic. Jet nebulisers produce aerosols with a two-fluid nozzle. The relatively wide size distribution of the droplets from such nozzles is adjusted to the desired range by removal of the largest droplets, which occurs through impaction against a flow body in the aerosol stream (baffle). Many variables can influence the droplet size distribution, including the physicochemical properties of the solution (McCallion et al. 1995; MacNeish et al. 1997; Coates et al. 1997; Le Brun et al. 1999; Lexmond et al. 2013), the jet pressure adjusted for the nozzle (Lexmond et al. 2013; de Boer et al. 2003; Newman et al. 1986; Niven and Brain 1994), and the breathing manoeuvre of the patient, or when tested in the lab, the suction flow rate (Le Brun et al. 1999; de Boer et al. 2003). The lung deposition efficiency of jet nebulisers is low, resulting in long administration times (Le Brun et al. 2000). Other major drawbacks of jet nebulisers are the long preparation and cleaning times, as well as the large residual volumes in the nebuliser cups, which may result in considerable waste of the formulation.
Ultrasonic nebulisers produce droplets by applying high-frequency pulses from an oscillating piezo-element to the solution, thereby creating standing waves on the liquid surface from which droplets are released. The droplet size distribution depends largely on the oscillation frequency, which is mostly in the order of magnitude between 1.3 and 2.4 kHz. Unlike jet nebulisers, ultrasonic nebulisers do not require rather bulky compressors or other pressurised air systems. In the more recently developed vibrating mesh nebulisers, the piezo technology is combined with a perforated membrane (mesh), which is in contact with the drug formulation. Two different principles are available; those in which the oscillation is applied to the membrane itself and those in which the oscillation comes from a horn transducer that vibrates in the liquid reservoir. Vibrating mesh nebulisers deliver more condense aerosols than jet nebulisers, which increases the output rate and reduces the administration time. They are often equipped with chip technology to adjust the nebulisation procedure to the solution to be administered and to the breathing manoeuvre of the patient (adaptive aerosol delivery), or to monitor patient adherence and compliance (Nikander et al. 2010; Geller and Kesser 2010; Bennett 2005; Fischer et al. 2009; McCormack et al. 2012). Examples of vibrating mesh nebulisers are the I-Neb (Philips Respironics), Aeroneb (Nektar Therapeutics), Micro Air (Omron Healthcare), and eFlow Rapid (Pari).
Nebulisers generally are reusable devices. Consequently, they have to be cleaned and disinfected on a regular basis. Improper cleaning can lead to deterioration of nebuliser performance (Rottier et al. 2009). Moreover, good hygiene is paramount because nebuliser formulations consist of water mostly, and are thus highly sensitive to microbiological contamination.
3.3 The Soft Mist Inhaler
Like the vibrating mesh nebulisers, the Soft Mist Inhaler (SMI) is a more recent development in inhalation devices. The SMI is a nebuliser, as it disperses a solution of the active agent into fine droplets. It differs from the traditional nebulisers in that it is a hand-held, portable device that does not require an external power source, but is actuated by a mechanical spring. The instantaneous formation of the aerosol is comparable to a pMDI; thus, proper actuation-inhalation coordination is necessary (Lavorini 2013). However, it takes longer before the entire aerosol is generated (1.5 s versus 0.21–0.36 s for an HFA-pMDI) and the aerosol is emitted as a slow-moving mist, allowing for a relatively high lung deposition (Dalby et al. 2004).
3.4 Dry Powder Inhalers
Dry powder inhalers are the only inhalation devices that contain the drug in the dry state. DPIs typically consist of a powder formulation, a dose metering mechanism that either contains or measures a single dose of the therapeutic, a powder de-agglomeration principle, and a mouthpiece (Frijlink and De Boer 2004). Most DPIs are passive (breath-actuated) devices, so actuation and inhalation do not have to be coordinated as with pMDIs. Being operated by the breath of the patient means that a certain minimal inspiratory effort from the patient is required for proper dose release from the DPI.
Various DPIs containing different therapeutics are commercially available. These devices can be classified into three types. The first are multi-dose DPIs, which contain the powder formulation in bulk in a reservoir, from which a dose is metered upon use by the patient. The second category includes devices that contain multiple pre-metered (sealed) doses within the device, which are called multiple unit-dose devices. Lastly, single-dose DPIs exist that are loaded with a single dose of the powder formulation, which is prepared either by the manufacturer (disposable devices) or by the patient immediately before use (capsule-based devices).
Delivery of the powder formulation to the lungs occurs through consecutive processes within the DPI, which are (typically) initiated by the inhalation manoeuvre of the patient. After entrainment of the powder formulation from the dose metering system, de-agglomeration or dispersion takes place, eventually resulting in an aerosol of small, inhalable particles of the active agent (and any excipients). The effectiveness of these processes, and hence the effectiveness of aerosol formation, is dependent on the powder formulation, the DPI (especially the powder dispersion/de-agglomeration mechanism), and the patient’s inspiratory effort (inspiratory flow rate and inhaled volume).
Particularly for DPIs, the inhalation manoeuvre is highly important and the inhalation profile that is needed depends on the working principle of the inhaler and the desired deposition site in the respiratory tract (Frijlink and De Boer 2004). Whether a patient is able to perform the inhalation manoeuvre required for a particular type of DPI depends on patient characteristics like age and clinical condition (i.e. type and severity of disease), which may present them with physical limitations or insufficient understanding of how to handle the device (Price et al. 2013; Brocklebank et al. 2001; Lavorini et al. 2008; Pedersen et al. 2010; Lexmond et al. 2014a).
DPIs are versatile and applicable to a wide array of drugs because of the large dose range that can be covered, the dry state of the formulation, and the various formulation approaches that are available. DPIs are also very complex delivery systems, consisting of an inextricable combination of device and formulation. For that reason, the development of DPI products is often a next-level approach and they are generally only available for well-established therapies.
4 Choosing the Appropriate Device
The interaction between patient and device is the most important interaction to acknowledge when prescribing an inhaled drug product, since the device has to be prepared and used correctly to achieve sufficient lung deposition required for the desired therapeutic effect. In other words, an inhalation product is only as good as the patient’s ability to use it, or their motivation to use and maintain it correctly. The options are of course limited by the therapies that are available. Asthma and COPD medications are the best-established inhaled therapies, for which numerous options are available – not only in the number of active substances available, but also in the large number of devices. This is exemplified by the short-acting β2-agonist salbutamol, where in the UK alone at least four nebuliser solutions are available (two with, and two without a preservative), three HFA-pMDIs, one breath-actuated pMDI, and five DPIs, most of these in various dosages.
When there is ample choice, the prescriber should opt for the therapy that has the highest chance of success. This success is not only dependent on the patient’s ability to use a specific device, but also on their preferences, cooperativeness, willingness, and possibly familiarity with the device. If a patient has used a specific device correctly for years, their therapy may not be improved by switching to a device that by itself is better than the one they have been using, because they have to learn and adopt new handling instructions, and possibly also a new inhalation manoeuvre. However, a patient who is competent and willing may very well benefit from putting effort into learning a new technique and switching to the new device.
For all types of devices, a challenge facing inhaled drug development is that a large proportion of patients have been reported to use their devices incorrectly, resulting in suboptimal therapy (Lavorini et al. 2008; Giraud and Roche 2002; Melani et al. 2012). These proportions increase when patients use multiple devices, especially if this includes different types of devices (van der Palen et al. 1999; Price et al. 2012). Therefore, it is advisable to limit the number of (different) devices per patient, if possible. Fixed dose combination products may have therapeutic benefits in this respect, provided that the combination is rational – i.e. the combined drugs have complementary pharmacological effects and comparable dosing frequency, and preferably the same target area in the respiratory tract. Various combination products are already available (e.g. the ICS/LABA combinations; budesonide/formoterol, fluticasone proprionate/salmeterol, beclomethasone dipropionate/formoterol, fluticasone furoate/vilanterol for the maintenance treatment of asthma and COPD, and the LAMA/LABA combinations umeclidinium/vilanterol, aclidinium/formoterol, glycopyrronium/indacaterol, currently only for the treatment of COPD) and there are many others currently in late stage development, including triple therapy for the treatment of patients with COPD treatment (LAMA/LABA/ICS) (Cazzola et al. 2012).
Further to increasing the chance of successful therapy, choice of device also allows the most cost effective products to be used, as pharmacoeconomic considerations are increasingly an important aspect of delivering healthcare. This generally implies that the moment a cheaper alternative product becomes available, patients are often switched to the less expensive product. However, because of the precarious balance between patient use and device performance, switching may not always be in the best interest of the patient. For example, if the effectiveness of the therapy is reduced due to the switch to a cheaper device causing suboptimal control of symptoms, perhaps resulting in hospitalisation, then overall costs of healthcare may even increase over the long term. Optimal healthcare is therefore better looked at from the overall cost-effectiveness of treatment, rather than concentrating on just minimising the direct costs of the medication.
When the choice is limited or there is none at all, it is the physician’s – and pharmacist’s – duty to facilitate and ensure the patient is receiving the maximal therapeutic benefit from whatever inhaled medicine has been prescribed. Training and regular checking of the technique used by the patient or their carer are essential in this respect (Broeders et al. 2009; Papi et al. 2011). Enabling maximal therapeutic benefit includes prescribing appropriate accessories for specific age groups or devices, such as facemasks for infants and toddlers or VHCs for use with pMDIs.
4.1 Effectiveness; Effect of Training and Compliance
Key to effective inhalation therapy is correct inhaler and inhalation technique, which comprise both correct handling of the device and the performance of a correct inhalation manoeuvre. What makes this so hard is that practically each individual device has its own mode of operation. Overall, the same procedures apply to the different types of inhalation devices, but the steps can vary significantly between devices, especially for DPIs. Therefore, general guidelines on the use of a type of inhaler lack practicality and usefulness and should be avoided.
Correct inhaler and inhalation technique can only be expected when the patient is properly trained in using their particular device. Training is a joint effort involving both the healthcare provider and the patient, which starts with teaching and learning the manoeuvre, followed by repeated demonstration by the patient and, if necessary, adjusting the technique by the healthcare provider (Lavorini et al. 2008; Papi et al. 2011). Needless to say, the healthcare provider must completely understand and master the technique for every specific inhalation device that they deal with. Unfortunately, this is often not the case (Self et al. 2007), which can be attributed at least partly to poor appreciation of the complexities of inhaler technology. This can be resolved by additional training for healthcare providers, as well as appointing physician assistants and nurse practitioners specialised in pulmonary diseases and inhalation therapies.
Besides correct technique, patient compliance is just as important for effective inhalation therapy (Cochrane et al. 2000). Compliance is expressed as the level at which the patient’s behaviour complies with the prescribed therapy. Indeed, flawless inhaler technique is useless when a patient does not take their medication. Noncompliance is not necessarily deliberate (it includes forgetting to take medication) and it is not necessarily harmful either. However, if a patient has good reasons not to adhere to their prescribed therapy, for example because they have side effects they cannot tolerate, discussing alternatives with their physician is better than altering the dose or dose regimen themselves. Taking any concerns of the patient seriously and engaging in dialogue can often be the key to improving compliance and thereby the effectiveness of the therapy.
4.2 Off-Label Use
Off-label use is defined as the intentional use of a medicinal product for a medical purpose that is not in accordance with the authorised product information (European Medicines 2012). This definition also applies to medical devices and thus to inhaled delivery devices. Off-label use of inhaled delivery devices, mostly nebulisers, can especially be found in clinical research.
Preparing a formulation for nebulisation can be very straightforward, which is the main reason why nebulisers are often used in the early stages of clinical development, as for example seen in studies on pulmonary vaccination and lung cancer therapy (Wauthoz et al. 2010; Otterson et al. 2010). Also for individualised medicine, pulmonary administration of the therapeutic can be accomplished by nebulisation of a simple solution of the compound (Máiz et al. 2009). It should be stressed though that in such cases, compatibility of the formulation and the nebuliser cannot simply be assumed. Stability, for example, can be compromised when the stresses induced by the nebulisation process may damage the formulation, which can be the case for large molecules or complex particulate systems (Niven and Brain 1994; Khatri et al. 2001; Münster et al. 2000; Albasarah et al. 2010; Elhissi et al. 2006, 2007; Amini et al. 2014; Kleemann et al. 2007; Hertel et al. 2014).
However, it is not just complex molecules that may present challenges when nebulised. Sometimes even the most straightforward small-molecule formulation can be incompatible with the chosen delivery device as shown for adenosine 5′-monophosphate (AMP), which is used as a bronchial challenge agent in asthma research and diagnostics. The high AMP concentrations required in some patients to evoke bronchoconstriction were shown to greatly affect nebuliser performance (Lexmond et al. 2013). This study presents a good example of a simple and fast, but inadequate solution to a problem that is more complex than anticipated and has led to the development of an alternative dry powder formulation of adenosine where much higher doses of this challenge agent can be delivered to the airways (Lexmond et al. 2014b, c).
5 Summary
There have been major advances in the development of effective drugs for the treatment of asthma and COPD over the past two decades. Furthermore, since the introduction of the first pMDI for delivering drugs topically to the airways, there have also been considerable improvements to inhalation devices and to our understanding of the complexity of formulating inhaled medicines. However, with the development of new drug classes, some of which are not traditional low molecular weight compounds, but larger molecules such as peptides (Larche 2014), proteins (O’Byrne 2013), and even oligonucleotides (Fonseca and Kline 2009), major challenges remain to ensure that these novel pharmacological entities can be delivered safely and effectively for the benefit of patients.
References
Albasarah YY, Somavarapu S, Taylor KMG (2010) Stabilizing protein formulations during air-jet nebulization. Int J Pharm 402(1–2):140–145
Amini MA, Faramarzi MA, Gilani K, Moazeni E, Esmaeilzadeh-Gharehdaghi E, Amani A (2014) Production, characterisation, and in vitro nebulisation performance of budesonide-loaded PLA nanoparticles. J Microencapsul 64:1–7
Auerbach O, Stout AP, Hammond EC, Garfinkel L (1966) Emphysema and other pulmonary changes in smokers. Postgrad Med 40(1):95–100
Aurora SK, Silberstein SD, Kori SH, Tepper SJ, Borland SW, Wang M et al (2011) MAP0004, orally inhaled DHE: a randomized, controlled study in the acute treatment of migraine. Headache 51(4):507–517
Barnes N, Price D, Colice G, Chisholm A, Dorinsky P, Hillyer EV et al (2011) Asthma control with extrafine-particle hydrofluoroalkane-beclometasone vs. large-particle chlorofluorocarbon-beclometasone: a real-world observational study. Clin Exp Allergy 41(11):1521–1532
Bell J, Newman S (2007) The rejuvenated pressurised metered dose inhaler. Expert Opin Drug Deliv 4(3):215–234
Bennett WD (2005) Controlled inhalation of aerosolised therapeutics. Expert Opin Drug Deliv 2(4):763–767
Bennett WD, Smaldone GC (1987) Human variation in the peripheral air-space deposition of inhaled particles. J Appl Physiol 62(4):1603–1610
Bisgaard H, Anhoj J, Wildhaber JH (2002) Spacer devices. In: Bisgaard H, O’Callaghan C, Smaldone GC (eds) Drug delivery to the lung. p 511
Bosquillon C, Préat V, Vanbever R (2004) Pulmonary delivery of growth hormone using dry powders and visualization of its local fate in rats. J Control Release 96(2):233–244
Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L et al (2001) Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess 5(26)
Broeders MEAC, Sanchis J, Levy ML, Crompton GK, Dekhuijzen PNR (2009) The ADMIT series--issues in inhalation therapy. 2. Improving technique and clinical effectiveness. Prim Care Respir J 18(2):76–82
Cazzola M, Page CP, Calzetta L, Matera MG (2012) Pharmacology and therapeutics of bronchodilators. Pharmacol Rev 64(3):450–504
Coates AL, MacNeish CF, Meisner D, Kelemen S, Thibert R, MacDonald J et al (1997) The choice of jet nebulizer, nebulizing flow, and addition of albuterol affects the output of tobramycin aerosols. Chest 111(5):1206–1212
Cochrane MG, Bala MV, Downs KE, Mauskopf J, Ben-Joseph RH (2000) Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest 117(2):542–550
Cornfield J, Haenszel W, Hammond E, Lilienfield A, Shimkin M, Wynder E (1959) Smoking and lung cancer - recent evidence and a discussion of some questions. J Natl Cancer Inst 22:173–203
Cornfield J, Haenszel W, Hammond EC, Lilienfeld AM, Shimkin MB, Wynder EL (2009) Smoking and lung cancer: recent evidence and a discussion of some questions. Int J Epidemiol 38(5):1175–1191
Dalby R, Spallek M, Voshaar T (2004) A review of the development of Respimat Soft Mist Inhaler. Int J Pharm 283(1–2):1–9
de Boer AH, Hagedoorn P, Frijlink HW (2003) The choice of a compressor for the aerosolisation of tobramycin (TOBI®) with the PARI LC PLUS® reusable nebuliser. Int J Pharm 268(1–2):59–69
Dolovich MB, Ahrens RC, Hess DR, Anderson P, Dhand R, Rau JL et al (2005) Device selection and outcomes of aerosol therapy: evidence-based guidelines. Chest 335–371
Elhissi AMA, Karnam KK, Danesh-Azari M-R, Gill HS, Taylor KMG (2006) Formulations generated from ethanol-based proliposomes for delivery via medical nebulizers. J Pharm Pharmacol 58(7):887–894
Elhissi AMA, Faizi M, Naji WF, Gill HS, Taylor KMG (2007) Physical stability and aerosol properties of liposomes delivered using an air-jet nebulizer and a novel micropump device with large mesh apertures. Int J Pharm 334(1–2):62–70
European Medicines Agency (2012) Guideline on good pharmacovigilance practices (GVP) Module VI – Management and reporting of adverse reactions to medicinal. p 90
Farr SJ, Otulana BA (2006) Pulmonary delivery of opioids as pain therapeutics. Adv Drug Deliv Rev 58(9–10):1076–1088
Fischer A, Stegemann J, Scheuch G, Siekmeier R (2009) Novel devices for individualized controlled inhalation can optimize aerosol therapy in efficacy, patient care and power of clinical trials. Eur J Med Res 14(Suppl 4):71–77
Fonseca DE, Kline JN (2009) Use of CpG oligonucleotides in treatment of asthma and allergic disease. Adv Drug Deliv Rev 61(3):256–262
Frijlink H, De Boer A (2004) Dry powder inhalers for pulmonary drug delivery. Expert Opin Drug Deliv 1(1):67–86
Fulda G, Giberson F, Fagraeus L (2005) A prospective randomized trial of nebulized morphine compared with patient-controlled analgesia morphine in the management of acute thoracic pain. J Trauma 59(2):383–388
Furyk JS, Grabowski WJ, Black LH (2009) Nebulized fentanyl versus intravenous morphine in children with suspected limb fractures in the emergency department: a randomized controlled trial. Emerg Med Australas 21(3):203–209
Gabrio BJ, Stein SW, Velasquez DJ (1999) A new method to evaluate plume characteristics of hydrofluoroalkane and chlorofluorocarbon metered dose inhalers. Int J Pharm 186(1):3–12
Geller DE, Kesser KC (2010) The I-neb Adaptive Aerosol Delivery System enhances delivery of alpha1-antitrypsin with controlled inhalation. J Aerosol Med Pulm Drug Deliv 23(Suppl 1):S55–S59
Giraud V, Roche N (2002) Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J 19(2):246–251
Goldin JG, Tashkin DP, Kleerup EC, Greaser LE, Haywood UM, Sayre JW et al (1999) Comparative effects of hydrofluoroalkane and chlorofluorocarbon beclomethasone dipropionate inhalation on small airways: assessment with functional helical thin-section computed tomography. J Allergy Clin Immunol 104(6):S258–S267
Heinemann L, Klappoth W, Rave K, Hompesch B, Linkeschowa R, Heise T (2000) Intra-individual variability of the metabolic effect of inhaled insulin together with an absorption enhancer. Diabetes Care 23(9):1343–1347
Hertel S, Pohl T, Friess W, Winter G (2014) Prediction of protein degradation during vibrating mesh nebulization via a high throughput screening method. Eur J Pharm Biopharm 87(2):386–394
Hinds W (1982) Aerosol technology: properties, behavior, and measurement of airborne particles. Wiley-Interscience, New York, 442 pp
Khatri L, Taylor KM, Craig DQ, Palin K (2001) An assessment of jet and ultrasonic nebulisers for the delivery of lactate dehydrogenase solutions. Int J Pharm 227(1–2):121–131
Kleemann E, Schmehl T, Gessler T, Bakowsky U, Kissel T, Seeger W (2007) Iloprost-containing liposomes for aerosol application in pulmonary arterial hypertension: formulation aspects and stability. Pharm Res 24(2):277–287
Larche M (2014) Mechanisms of peptide immunotherapy in allergic airways disease. Ann Am Thorac Soc 11:S292–S296
Laube B, Georgopoulos A, Adams G (1998) Preliminary study of the efficacy of insulin aerosol delivered by oral inhalation in diabetic patients. J Am Med Assoc 269:2106–2109
Laube BL, Janssens HM, de Jongh FHC, Devadason SG, Dhand R, Diot P et al (2011) What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J 37(6):1308–1331
Lavorini F (2013) The challenge of delivering therapeutic aerosols to asthma patients. ISRN Allergy 102418
Lavorini F, Magnan A, Dubus JC, Voshaar T, Corbetta L, Broeders M et al (2008) Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med 102(4):593–604
Le Brun PP, de Boer AH, Gjaltema D, Hagedoorn P, Heijerman HG, Frijlink HW (1999) Inhalation of tobramycin in cystic fibrosis. Part 2: optimization of the tobramycin solution for a jet and an ultrasonic nebulizer. Int J Pharm 189(2):215–225
Le Brun PP, de Boer AH, Heijerman HG, Frijlink HW (2000) A review of the technical aspects of drug nebulization. Pharm World Sci 22(3):75–81
Leach C (1998) Improved delivery of inhaled steroids to the large and small airways. Respir Med 92(Suppl):3–8
Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ (2002) Lung deposition of hydrofluoroalkane-134a beclomethasone is greater than that of chlorofluorocarbon fluticasone and chlorofluorocarbon beclomethasone: a cross-over study in healthy volunteers. Chest 122(2):510–516
Lexmond AJ, Hagedoorn P, Frijlink HW, de Boer AH (2013) Challenging the two-minute tidal breathing challenge test. J Aerosol Med Pulm Drug Deliv 26:1–7
Lexmond AJ, Kruizinga TJ, Hagedoorn P, Rottier BL, Frijlink HW, De Boer AH (2014a) Effect of inhaler design variables on paediatric use of dry powder inhalers. PLoS One 9(6), e99304
Lexmond AJ, Hagedoorn P, van der Wiel E, ten Hacken NHT, Frijlink HW, de Boer AH (2014b) Adenosine dry powder inhalation for bronchial challenge testing, part 1: inhaler and formulation development and in vitro performance testing. Eur J Pharm Biopharm 86(1):105–114
Lexmond AJ, van der Wiel E, Hagedoorn P, Bult W, Frijlink HW, ten Hacken NHT et al (2014c) Adenosine dry powder inhalation for bronchial challenge testing, part 2: proof of concept in asthmatic subjects. Eur J Pharm Biopharm 88:148–152
Lizio R, Klenner T, Borchard G, Romeis P, Sarlikiotis AW, Reissmann T et al (2000) Systemic delivery of the GnRH antagonist cetrorelix by intratracheal instillation in anesthetized rats. Eur J Pharm Sci 9(3):253–258
MacNeish CF, Coates AL, Meisner D, Thibert R, Kelemen S, Vadas EB (1997) A comparison of pulmonary availability between Ventolin (albuterol) nebules and Ventolin (albuterol) respirator solution. Chest 111(1):204–208
Máiz L, Lamas A, Fernández-Olmos A, Suárez L, Cantón R (2009) Unorthodox long-term aerosolized ampicillin use for methicillin-susceptible Staphylococcus aureus lung infection in a cystic fibrosis patient. Pediatr Pulmonol 44(5):512–515
McCallion ONM, Taylor KMG, Thomas M, Taylor AJ (1995) Nebulization of fluids of different physicochemical properties with air-jet and ultrasonic nebulizers. Pharm Res 12(11):1682–1688
McCormack P, Southern KW, McNamara PS (2012) New nebulizer technology to monitor adherence and nebulizer performance in cystic fibrosis. J Aerosol Med Pulm Drug Deliv 25(6):307–309
Melani AS, Canessa P, Coloretti I, DeAngelis G, DeTullio R, Del Donno M et al (2012) Inhaler mishandling is very common in patients with chronic airflow obstruction and long-term home nebuliser use. Respir Med 106(5):668–676
Mishra NC, Rir-sima-ah J, Langley RJ, Singh SP, Pena-Philippides JC, Koga T et al (2008) Nicotine primarily suppresses lung Th2 but not goblet cell and muscle cell responses to allergens. J Immunol 180(11):7655–7663
Münster AB, Bendstrup E, Jensen JI, Gram J (2000) Jet and ultrasonic nebulization of single chain urokinase plasminogen activator (scu-PA). J Aerosol Med 13(4):325–333
Newhouse M, Dolovich M (1986) Control of asthma by aerosols. N Engl J Med 315:870–874
Newman SP, Pellow PGD, Clarke SW (1986) Droplet size distributions of nebulised aerosols for inhalation therapy. Clin Phys Physiol Meas 7(2):139–146
Nikander K, Prince I, Coughlin S, Warren S, Taylor G (2010) Mode of breathing – tidal or slow and deep – through the I-neb Adaptive Aerosol Delivery (AAD) System. J Aerosol Med Pulm Drug Deliv 23(Suppl 1):S37–S43
Niven RW, Brain JD (1994) Some functional aspects of air-jet nebulizers. Int J Pharm 104(1):73–85
O’Byrne PM (2013) Role of monoclonal antibodies in the treatment of asthma. Can Respir J 20(1):23–25
Otterson GA, Villalona-Calero MA, Hicks W, Pan X, Ellerton JA, Gettinger SN et al (2010) Phase I/II study of inhaled doxorubicin combined with platinum-based therapy for advanced non-small cell lung cancer. Clin Cancer Res 16(8):2466–2473
Papi A, Haughney J, Virchow JC, Roche N, Palkonen S, Price D (2011) Inhaler devices for asthma: a call for action in a neglected field. Eur Respir J 37(5):982–985
Patton JS, Byron PR (2007) Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov 6(1):67–74
Pedersen S, Dubus JC, Crompton GK (2010) The ADMIT series--issues in inhalation therapy. 5) Inhaler selection in children with asthma. Prim Care Respir J 19(3):209–216
Price D, Chrystyn H, Kaplan A, Haughney J, Román-Rodríguez M, Burden A et al (2012) Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res 4(4):184–191
Price D, Bosnic-Anticevich S, Briggs A, Chrystyn H, Rand C, Scheuch G et al (2013) Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir Med 107(1):37–46
Rottier BL, van Erp CJP, Sluyter TS, Heijerman HGM, Frijlink HW, De Boer AH (2009) Changes in performance of the Pari eFlow rapid and Pari LC Plus during 6 months use by CF patients. J Aerosol Med Pulm Drug Deliv 22(3):263–269
Salter H (1868) On asthma; its pathology and treatment. Philadelphia
Self TH, Arnold LB, Czosnowski LM, Swanson JM, Swanson H (2007) Inadequate skill of healthcare professionals in using asthma inhalation devices. J Asthma 44(8):593–598
Siekmeier R, Scheuch G (2008) Systemic treatment by inhalation of macromolecules–principles, problems, and examples. J Physiol Pharmacol 53–79
Silberstein S (2012) MAP0004: dihydroergotamine mesylate inhalation aerosol for acute treatment of migraine. Expert Opin Pharmacother 13(13):1961–1968
Smyth H (2005) Propellant-driven metered-dose inhalers for pulmonary drug delivery. Expert Opin Drug Deliv 2(1):53–74
U.S. Department of Health and Human Services (2010) How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease. A report of the Surgeon General. Centers for Disease Control and Prevention (US), Atlanta (GA). 7, Pulmonary Diseases. 704 pp
van der Palen J, Klein JJ, van Herwaarden CL, Zielhuis GA, Seydel ER (1999) Multiple inhalers confuse asthma patients. Eur Respir J 14(5):1034–1037
Wauthoz N, Deleuze P, Hecq J, Roland I, Saussez S, Adanja I et al (2010) In vivo assessment of temozolomide local delivery for lung cancer inhalation therapy. Eur J Pharm Sci 39(5):402–411
Weibel ER (1963) Morphometry of the human lung. Springer-Verlag, 151 pp
Xu X, Wang X, Ge W, Pan L, Zheng M (2012) The pharmacokinetics of inhaled morphine delivered by an ultrasonic nebulizer in ventilated dogs. J Aerosol Med Pulm Drug Deliv 25(1):41–46
Zheng Y, Marsh KC, Bertz RJ, El-Shourbagy T, Adjei AL (1999) Pulmonary delivery of a dopamine D-1 agonist, ABT-431, in dogs and humans. Int J Pharm 191(2):131–140
Zijlstra GS, Hinrichs WLJ, de Boer AH, Frijlink HW (2004) The role of particle engineering in relation to formulation and de-agglomeration principle in the development of a dry powder formulation for inhalation of cetrorelix. Eur J Pharm Sci 23(2):139–149
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lexmond, A., Forbes, B. (2016). Drug Delivery Devices for Inhaled Medicines. In: Page, C., Barnes, P. (eds) Pharmacology and Therapeutics of Asthma and COPD. Handbook of Experimental Pharmacology, vol 237. Springer, Cham. https://doi.org/10.1007/164_2016_67
Download citation
DOI: https://doi.org/10.1007/164_2016_67
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52173-2
Online ISBN: 978-3-319-52175-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)