Abstract
Sulforaphane is a promising agent under preclinical evaluation in many models of disease prevention. This bioactive phytochemical affects many molecular targets in cellular and animal models; however, amongst the most sensitive is Keap1, a key sensor for the adaptive stress response system regulated through the transcription factor Nrf2. Keap1 is a sulfhydryl-rich protein that represses Nrf2 signaling by facilitating the polyubiquitination of Nrf2, thereby enabling its subsequent proteasomal degradation. Interaction of sulforaphane with Keap1 disrupts this function and allows for nuclear accumulation of Nrf2 and activation of its transcriptional program. Enhanced transcription of Nrf2 target genes provokes a strong cytoprotective response that enhances resistance to carcinogenesis and other diseases mediated by exposures to electrophiles and oxidants. Clinical evaluation of sulforaphane has been largely conducted by utilizing preparations of broccoli or broccoli sprouts rich in either sulforaphane or its precursor form in plants, a stable β-thioglucose conjugate termed glucoraphanin. We have conducted a series of clinical trials in Qidong, China, a region where exposures to food- and air-borne carcinogens has been considerable, to evaluate the suitability of broccoli sprout beverages, rich in either glucoraphanin or sulforaphane or both, for their bioavailability, tolerability, and pharmacodynamic action in population-based interventions. Results from these clinical trials indicate that interventions with well characterized preparations of broccoli sprouts may enhance the detoxication of aflatoxins and air-borne toxins, which may in turn attenuate their associated health risks, including cancer, in exposed individuals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Developing rational chemoprevention strategies requires well-characterized agents, suitable cohorts, and reliable intermediate biomarkers of cancer or cancer risk [1]. Sulforaphane is one promising agent under preclinical and clinical evaluation. Sulforaphane was isolated from broccoli guided by bioassays for the induction of the cytoprotective enzyme NQO1 [2]. The inducible expression of NQO1 is now recognized to be regulated principally through the Keap1–Nrf2–ARE signaling pathway [3]. This pathway in turn is an important modifier of susceptibility to electrophilic and oxidative stresses, factors central to the processes of chemical carcinogenesis and other chronic degenerative diseases [4]. Sulforaphane is a potent inducer of Nrf2 signaling and blocks the formation of dimethylbenz[a]anthracene-evoked mammary tumors in rats as well as other tumor types in various animal models [5, 6]. In some instances these protective effects are lost in Nrf2-disrupted mice [7, 8]. In addition to increasing cellular capacity for detoxifying electrophiles and oxidants, sulforaphane has been shown to induce apoptosis, inhibit cell cycle progression, and inhibit angiogenesis [9–11]. Collectively, these actions serve to impede tumor growth. However, not all of the molecular actions of sulforaphane are triggered at the same concentrations. For example, activation of Nrf2 signaling occurs at substantially lower concentrations than does induction of apoptosis [2, 12]. The overall potent and multimodal actions of sulforaphane make it appealing to use in both preventive and therapeutic settings.
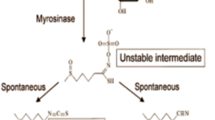
Broccoli and other cruciferous vegetables (e.g., cabbage, kale, and Brussels sprouts), primary sources of sulforaphane, are widely consumed in many parts of the world. Epidemiological evidence from prospective cohort studies and retrospective case-control studies suggest that consumption of a diet rich in crucifers reduces the risk of several types of cancers as well as some chronic degenerative diseases [13, 14]. There is growing evidence that the protective effects of crucifers against disease may be attributable largely to their content of glucosinolates (β-thioglucose N-hydroxysulfates) [15]. Glucosinolates in plant cells are hydrolyzed to bioactive isothiocyanates by the β-thioglucosidase myrosinase [15]. Myrosinase is released from intracellular vesicles following crushing of the plant cells by chewing, food preparation, or damage by insects. This hydrolysis is also mediated in a less predictable manner by β-thioglucosidases in the microflora of the human gut [16]. Young broccoli plants are an especially good source of glucosinolates, with levels 20–50 times those found in mature market-stage broccoli [17]. The principal glucosinolate contained in broccoli is glucoraphanin, which is hydrolyzed by myrosinase to sulforaphane (see Fig. 1).
Glucoraphanin in broccoli is converted to sulforaphane either by plant myrosinases, or if the plant myrosinases have been denatured by cooking, by bacterial myrosinases in the human colon. Sulforaphane is passively absorbed and rapidly conjugated with glutathione by glutathione S-transferases (GSTs), then metabolized sequentially by γ-glutamyl-transpeptidase (GTP), cysteinyl-glycinease (GCase), and N-acetyltransferase (NAT). The conjugates are actively transported into the systemic circulation where the mercapturic acid and its precursors are urinary excretion products. Deconjugation may also occur to yield the parent isothiocyanate, sulforaphane. The mercapturic acid and cysteine conjugate forms are the major urinary metabolites of sulforaphane [18]. For the beverages used in the Qidong interventions enumerated in Table 1, sulforaphane was generated enterically from glucoraphanin through the action of thioglucosidases in the gut microflora (glucoraphanin-rich, GRR), or prereleased by treatment of aqueous broccoli sprout extract with myrosinase from the daikon plant Raphanus sativus (sulforaphane-rich, SFR)
Human populations are continuously exposed to varying amounts of chemicals or manufacturing by-products that are carcinogenic in animal models; over 100 such compounds have been designated as human carcinogens by the International Agency for Research on Cancer [21] and the National Toxicology Program [22]. Exposures to these exogenous agents occur through the environmental vectors of food, water and air. In some cases the pathway to reducing cancer burden from these exposures is obvious – eliminate exposures. However, in some instances, exposures are largely unavoidable, such as exposures to aflatoxins and other mycotoxins in food, or require substantial behavioral changes (e.g., smoking cessation) or economic investments (e.g., clean air in developing megacities) that are exceedingly difficult to implement in individuals or populations. In these settings, effective, tolerable, low cost, and practical approaches to chemoprevention with foods rich in glucosinolates serving as precursors for anticarcinogenic isothiocyanates, such as glucoraphanin and its cognate isothiocyate sulforaphane in broccoli, may be especially desirable.
This chapter highlights recent studies on the mechanisms of action of sulforaphane as an inducer of Nrf2-regulated genes and their roles in attenuating or blocking carcinogenesis. These studies, in turn, have supported the development and conduct of a series of clinical trials in Qidong, China for the optimization of dose and formulation regimens seeking to reduce body burdens of environmental carcinogens in residents of this region. In Qidong, exposures to food-borne and air-borne toxins and carcinogens can be considerable. Heptatocellular carcinoma can account for up to 10% of the adult deaths in some rural townships there. Chronic infection with hepatitis B virus, coupled with exposure to aflatoxins, likely contributes to this high risk of liver cancer [23]. As vaccination programs and economic development take hold, risk factors for liver cancer are diminishing in Qidong; however, development is likely leading to increased exposures to air-borne chemicals with uncertain but potentially adverse health outcomes.
2 Keap1–Nrf2 Signaling
Environmental carcinogens typically undergo metabolic activation in target cells to form reactive electrophiles that damage DNA. Several completed clinical trials have attempted to reduce the burden of DNA damage imparted by environmental exposures to heterocyclic amines [24], tobacco smoke [25], and aflatoxins [19, 26, 27]. The end points for these trials were short-term biomarker modulations of carcinogen metabolism and/or DNA adducts and other forms of DNA damage. In these studies, modulation of these biomarkers is presumptive evidence for a cancer risk reduction, a concept that has been well validated in animal models [28]. Multiple strategies for modifying the bioactivation and/or detoxication of environmental carcinogens have been developed [4]. Disruption of Nrf2 signaling in mice leads to increased sensitivity to carcinogenesis by environmental agents [7, 29], increased burden of carcinogen-DNA adducts in target tissues [30–32], loss of chemopreventive efficacy of anticarcinogens such as sulforaphane, oltipraz, and CDDO-Im [7, 29, 32], and highlights a critical role for this adaptive stress response pathway as a critical determinant of susceptibility, and hence, a target for prevention.
The Keap1–Nrf2 signaling pathway provides a broad based cytoprotective response towards disruption of cellular homeostasis by extrinsic and intrinsic stresses. The current model of Keap1–Nrf2 interactions, as addressed in recent reviews [33, 34], involves the Kelch domains of a Keap1 homodimer functionally interacting with two different sites within the Neh2 domain of Nrf2, the ETGE, or high affinity “hinge” site and the DLG, the lower affinity “latch” site (see Fig. 2). Under normal cellular conditions, Tong et al. [35] propose that Nrf2 first interacts with the Keap1 dimer through the ETGE hinge interaction, tethering Nrf2 to the Keap1 homodimer, and subsequently the Cul3–Rbx1 complex which, following the stable interaction of Nrf2 to Keap1 through the DLG latch motif, leads to the appropriate orientation of proteins to facilitate the ubiquitination and subsequent proteasomal targeting as well as destruction of Nrf2. Upon cellular stress or pharmacologic induction, the ability of Keap1 to maintain both points of contact, the hinge and the latch, is thought to be disrupted by the alteration of the tertiary or quaternary structure of the Keap1 homodimer, accomplished via alterations of the many reactive cysteines within Keap1 through oxidation or covalent modification [36, 37]. The disruption of this efficient turnover of Nrf2 allows for the accumulation of the protein and permits Nrf2 to translocate into the nucleus. Once within the nucleus, Nrf2 forms heterodimers with small Maf proteins, and drives the transcription of genes with a functional antioxidant response element (ARE) within their promoters [3, 38]. These genes include, but are not limited to, conjugation/detoxication proteins, antioxidative enzymes, anti-inflammation proteins, the proteasome, and cellular chaperones, creating a general cytoprotective response following pathway activation [39]. Recently, the response of Nrf2 has been broadened in scope, with studies documenting interactions between Nrf2 and Notch signaling [40], p53/p21 [41], p62 based autophagy [42, 43], aryl hydrocarbon receptor signaling [44], NF-κB [45, 46], and other processes [47]. These interactions provide the means to elicit the broad-based cell survival responses that now typify the pathway.
Scheme of Keap1–Nrf2 interactions. Under homeostatic conditions, Nrf2 is bound by Keap1 through the “hinge” ETGE) and “latch” (DLG) domains of Nrf2. Upon association, Nrf2 is ubiquitinated by the Cul2/Rbx1/E2 ubiquitin ligase complex, marking it for proteasomal degradation. Induction of Nrf2 signaling by sulforaphane through thiocarbamylation at Cys 151may lead to disruption of the Cul3 association with Keap1 and abrogation of Nrf2 ubiquitination. Newly synthesized Nrf2 thereby escapes proteasomal degradation and translocates to the nucleus where it accumulates and activates the transcription of its target genes
3 Keap1 Is Targeted by Sulforaphane
Sulforaphane is – or is amongst – the most potent naturally occurring inducers of Nrf2 signaling, exhibiting efficacy in the high nanomolar range in cell cultures. Its potency may reflect in part a capacity to accumulate in cells as an interchangeable conjugate with glutathione [48]. Keap1 is a cysteine-rich protein that serves as the sensor regulating activation of Nrf2 signaling by various chemical classes of anticarcinogens, all of which are thiol regents [49]. Hong et al. [50] observed that sulforaphane modified multiple Keap1 domains, whereas the model electrophiles but less potent pathway activators dexamethasone mesylate and biotinylated iodoacetic acid modified Keap1 preferentially in the central linker domain [49]. Some of the differences between sulforaphane modification patterns and those of other electrophiles probably reflect differences in electrophile chemistry. Dexamethasone mesylate and biotinylated iodoacetic acid are SN2 type electrophiles that alkylate by nucleophilic displacement of a leaving group. Thiols react with sulforaphane by addition to the isothiocyanate carbon to yield thionoacyl adducts. The acylation reaction occurs much more rapidly than does alkylation, although these adducts are subjected to dissociation and rearrangement. A follow-up analysis by Hu et al. [51] using a modified sample preparation protocol has determined C151 to be one of four cysteine residues preferentially modified by sulforaphane. These chemical mapping results are consistent with in vivo observations reported by multiple investigators in which C151 has also been determined to be the primary target for modification by sulforaphane [52, 53]. In cells in which cysteine 151 of Keap1 has been mutated to serine, nuclear accumulation and subsequent induction of Nrf2 target genes by sulforaphane are severely abrogated.
As depicted in Fig. 2, the Nrf2 signaling pathway is activated in response to the modification of Keap1 C151 by an increased amount of newly synthesized Nrf2 translocating to the nucleus, a result of decreased Keap1-mediated Nrf2 ubiquitination, and subsequent proteasomal degradation. This decrease in Nrf2 ubiquitination appears to arise from a diminished interaction between Keap1 and Cul3 upon the modification of C151, as shown by co-immunoprecipitation experiments in cells expressing mutant Keap1 (C151W) or treated with sulforaphane [36].
4 Gene Expression Changes Evoked by Sulforaphane in Animal and Human Cells
Extensive microarray-based studies have and continue to define the battery of Nrf2-regulated genes in the context of different species, tissues, cell types, and responses to small molecule activators of the pathway (reviewed in [33, 54]). These studies typically employ both genetic and pharmacologic perturbations of pathway activity to define the nature and range of induced or repressed genes. Several early studies focused on the comparative effects of sulforaphane or vehicle treatment in Nrf2-disrupted or wild-type mice in small intestine [55] and liver [56]. Patterns of elevated expression of Nrf2-regulated genes reflected those seen with other inducers such as 1,2-dithiole-3-thione [57] or with genetic upregulation via hepatic-specific disruption of Keap1 [58] in the liver. Families of genes elevated in response to sulforaphane include electrophile detoxication enzymes, enzymes involved in free radical metabolism, glutathione homeostasis, generation of reducing equivalents and lipid metabolism, solute transporters, subunits of the 26S proteasome, nucleotide excision repair proteins, and heat shock proteins. Bioavailability and Nrf2-dependent pharmacodynamic action of sulforaphane have been demonstrated in a number of extrahepatic tissues [59, 60]. More recent studies have evaluated the Nrf2 transcriptional program in human cells [61, 62]. Recently, Agyeman et al. [63] analyzed the transcriptomic and proteomic changes in human breast epithelial MCF10A cells following sulforaphane treatment or Keap1 knockdown with siRNA using microarray and stable isotopic labeling with amino acids in culture, respectively. Strong concordance between the transcriptomic and proteomic profiles was observed. As seen in other studies with human cells, induction of aldo-keto reductase family members was most vigorous. Figure 3 demonstrates that aldo-keto reductases AKR1C1/2, AKR1C3, and AKR1B10, as well as the prototypic Nrf2-regulated enzyme NQO1, are substantively induced by sulforaphane following treatment of primary human mammary organoid cultures prepared from reduction mammaplasty specimens. Thus, an Nrf2 regulated response to sulforaphane in humans that recapitulates at least in part that observed in rodent models is evident.
5 Clinical Trials in Qidong with Broccoli Sprout Preparations
Extensive work by Talalay and colleagues has characterized the pharmacokinetics and safety in humans of ingestion of sulforaphane-rich (SFR) or glucoraphanin-rich (GRR) hot water extracts prepared from broccoli sprouts [16, 64, 65]. In many cases, freeze-dried standardized sprout extracts from specifically selected cultivars and seed sources grown in a prescribed manner were utilized to provide consistency of preparations across multiple studies. First and foremost, these studies have established the safety of these GRR and SFR preparations. Dose limiting factors center on taste, gastric irritation, and flatulence. Second, they have demonstrated a linear uptake and elimination of sulforaphane following administration of a wide range of doses as an SFR beverage. Third, bioavailability of sulforaphane was substantially better when administered as an SFR vs a GRR beverage. This latter result points to a limited capacity for the microbial thioglucosidases of the human gut to catalyze the conversion of glucoraphanin to sulforaphane. Subsequently, dozens of clinical trials are underway or completed utilizing broccoli or broccoli sprout preparations, as indicated by a review of the clinicaltrials.gov website. Summarized below and in Table 1 are the key findings in a series of four clinical trials we have conducted in Qidong, China with broccoli sprout derived beverages. All trials were approved by Institutional Review Boards in the United States and China.
Inasmuch as the initial hospital-based studies with broccoli sprout beverages were conducted in Baltimore amongst Caucasian and African-American participants, our first initiative in Qidong sought to address whether and to what extent the Chinese could convert, absorb, and excrete sulforaphane following administration of a GRR beverage. In 2002, 12 volunteers from the village of He Zuo in Qidong refrained from eating cruciferous and other green vegetables over a 4-day period. Extensive dietary logs were maintained and daily home visits to witness food preparation confirmed the absence of these vegetables from the diet. On the evening of the 3rd day, each volunteer consumed a GRR beverage containing 225 μmol glucoraphanin. Overnight, 12-h urine samples were collected during the run-in and post-intervention phases of the study. Using a cyclocondensation assay to measure sulforaphane and other isothiocyanate metabolites, average total excretion levels of 0.23, 0.32, 0.26, and 12.17 μmol of isothiocyanates were detected in the overnight voids. This greater than 40-fold increase reflects an excretion of sulforaphane metabolites as 5.4% of the administered dose of sulforaphane (in the form of its precursor glucoraphanin).
In 2003 a beverage formed from hot water infusions of 3-day old broccoli sprouts grown on site, containing defined concentrations of glucosinolates as the stable precursor of the sulforaphane, was evaluated for its ability to alter the disposition of aflatoxin. Exposures to aflatoxin, common in this community, likely arose from fungal contamination of their dietary staples. In this clinical study, also conducted in He Zuo, 200 healthy adults drank beverages containing either 400 or <3 μmole glucoraphanin nightly for 2 weeks. Urinary levels of aflatoxin-N 7-guanine, formed from depurination of the primary hepatic DNA adduct, were similar between the two intervention arms. A nonsignificant 9% decrease was seen in participants randomized to receive GRR compared to placebo beverage. However, measurement of urinary levels of sulforaphane metabolites indicated striking interindividual differences in bioavailability. This outcome may reflect individual differences in the rates of hydrolysis of glucoraphanin to sulforaphane by the intestinal microflora of the study participants. Accounting for this variability, a significant inverse association was observed for excretion of total sulforaphane metabolites and aflatoxin-N 7-guanine adducts in the 100 individuals receiving broccoli sprout glucosinolates [19]. This preliminary study illustrated the potential use of an inexpensive, easily implemented, food-based method for secondary prevention in a population at high risk for aflatoxin exposures.
One of several challenges in design of clinical chemoprevention trials is the selection of an adequate dose, type of formulation, and dose schedule of the intervention agent. A cross-over clinical trial was undertaken in He Zuo, Qidong in 2009 to compare the bioavailability and tolerability of sulforaphane from two broccoli sprout-derived beverages: one GRR and the other SFR (see Fig. 1). Sulforaphane was generated from glucoraphanin contained in the GRR beverage by gut microflora or formed by treatment of GRR with myrosinase from daikon sprouts to provide an SFR beverage [18]. Bulk amounts of freeze-dried powders of GRR and SFR were prepared in a commercial facility to provide a consistent composition throughout the study. Fifty healthy, eligible participants were requested to refrain from crucifer vegetable consumption and randomized into two treatment arms. The study design was as follows: 5-day run-in period, 7-day administration of beverages, 5-day washout period, and 7-day administration of the opposite intervention. Isotope dilution mass spectrometry was used to measure levels of glucoraphanin, sulforaphane, and sulforaphane thiol conjugates in urine samples collected daily throughout the study (see Fig. 1). Bioavailability, as measured by urinary excretion of sulforaphane and its metabolites, was substantially greater with the SFR (mean ~70%) than with GRR (mean ~5%) beverages. In addition, inter-individual variability in excretion was considerably lower with SFR than with GRR beverage. Elimination rates were considerably slower with GRR, allowing for achievement of steady-state dosing as opposed to bolus dosing with SFR [18].
An emerging problem in this region of China is outdoor air pollution. Analysis of urine samples for levels of phenanthrene tetraol, a metabolite of the polycyclic aromatic hydrocarbon and pollutant phenanthrene, from samples collected in the 2003 Qidong study indicated levels four to five times higher than measured in urine samples collected from urban residents of Minneapolis – St. Paul, Minnesota at the same time [19]. Urinary levels of phenanthrene tetraol remained high in the 2009 Qidong samples [20]. Therefore, urinary excretion of the mercapturic acids of the air-borne toxins acrolein, crotonaldehyde, ethylene oxide, and benzene were also measured in urine samples from both pre- and post-interventions using liquid chromatography tandem mass spectrometry. Statistically significant increases of 20–50% in the levels of excretion of glutathione-derived conjugates of acrolein, crotonaldehyde, and benzene were seen in individuals receiving SFR, GRR, or both compared with their preintervention baseline values. No significant differences were seen between the effects of SFR vs GRR. Intervention with broccoli sprouts may enhance detoxication of airborne pollutants and attenuate their associated health risks [20].
Optimal dosing formulations in future studies might consider blends of sulforaphane and glucoraphanin as SFR and GRR mixtures to achieve peak concentrations for activation of some targets and prolonged inhibition of others implicated in the protective actions of sulforaphane. With that view in mind, a placebo-controlled intervention in 291 participants with a blend of 40 μmol SFR and 600 μmol GRR has been completed in early 2012 in He He, Qidong. This study will assess the impact of the broccoli sprout beverage on internal dose biomarkers of air pollution, and, in particular, evaluate the sustainability of the intervention over several months in terms of tolerability and efficacy. Although it is apparent that the Keap1–Nrf2 pathway can be activated in humans over the short term, it remains to be determined whether or not the pathway becomes refractory to repeated activation stimuli. Collectively, this series of clinical trials have defined paradigms for using biomarkers of exposures to environmental carcinogens as intermediate endpoints in the evaluation of agents for the prevention of chronic diseases. In particular, prevention trials of whole foods or simple extracts offer prospects for reducing an expanding global burden of cancer effectively with minimal cost, in contrast to promising isolated phytochemicals or pharmaceuticals [66].
References
Kelloff GJ, Lieberman R, Steele VE et al (2001) Agents, biomarkers, and cohorts for chemopreventive agent development in prostate cancer. Urology 57:46–51
Zhang Y, Talalay P, Cho CG, Posner GH (1992) A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA 89:2399–2403
Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD (2003) Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J 374:337–348
Kensler TW (1997) Chemoprevention by inducers of carcinogen detoxication enzymes. Environ Health Perspect 105(Suppl 4):965–970
Dinkova-Kostova AT (2007) Chemoprotection against cancer: an idea whose time has come. Altern Ther Health Med 13:S122–S127
Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P (1994) Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA 91:3147–3150
Fahey JW, Haristoy X, Dolan PM et al (2002) Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci USA 99:7610–7615
Xu C, Huang MT, Shen G et al (2006) Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res 66:8293–8296
Juge N, Mithen RF, Traka M (2007) Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci 64:1105–1127
Higdon JV, Delage B, Williams DE, Dashwood RH (2007) Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res 55:224–236
Zhang Y, Tang L (2007) Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol Sin 28:1343–1354
Gamet-Payrastre L, Li P, Lumeau S et al (2000) Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res 60:1426–1433
Riboli E, Norat T (2003) Epidemiologic evidence of the protective effects of fruit and vegetables on cancer risk. Am J Clin Nutr 78:559–695
World Cancer Research Fund/American Institute for Cancer Research (2007) Food, nutrition, physical activity and the prevention of cancer: a global perspective. American Institute for Cancer Research, Washington, DC
Fahey JW, Zalcmann AT, Talalay P (2001) The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56:5–51
Shapiro TA, Fahey JW, Wade KL et al (1998) Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev 7:1091–1100
Fahey JW, Zhang Y, Talalay P (1997) Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA 94:10367–10372
Egner PA, Chen JG, Wang JB et al (2011) Bioavailability of sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev Res 4:384–395
Kensler TW, Chen JG, Egner PA et al (2005) Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol Biomarkers Prev 14:2605–2613
Kensler TW, Ng D, Carmella SG et al (2012) Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis 33:101–107
International Agency for Research on Cancer (IARC) (2011) Agents classified by the IARC monographs, volumes 1–100. IARC Press, Lyon, France
US Department of Health and Human Services, Public Health Service, National Toxicology Program (2011) Report on Carcinogens, 12th edn
Kensler TW, Roebuck BD, Groopman JD, Wogan GN (2011) Aflatoxin: a 50-year odyssey of mechanistic and translational toxicology. Toxicol Sci 120(S1):S28–S48
Shaughnessy DT, Gangarosa LM, Schliebe B et al (2011) Inhibition of fried meat-induced colorectal DNA damage and altered systemic genotoxicity in humans by crucifera, chlorophyllin, and yogurt. PLoS One 6:e18707
Hecht SS, Carmella SG, Murphy SE (1999) Effects of watercress consumption on urinary metabolites of nicotine in smokers. Cancer Epidemiol Biomarkers Prev 8:907–913
Wang JS, Shen X, He X et al (1999) Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People’s Republic of China. J Natl Cancer Inst 91:347–354
Egner PA, Wang JB, Zhu YR et al (2001) Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc Natl Acad Sci USA 98:14601–14606
Kensler TW, Groopman JD, Wogan GN (1996) Use of carcinogen-DNA and carcinogen-protein adduct biomarkers for cohort selection and as modifiable end points in chemoprevention trials. IARC Sci Publ 139:237–248
Ramos-Gomez M, Kwak MK, Dolan PM et al (2001) Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA 98:3410–3415
Aoki Y, Sato H, Nishimura N et al (2001) Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol 173:154–160
Ramos-Gomez M, Dolan PM, Itoh K, Yamamoto M, Kensler TW (2003) Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in mice. Carcinogenesis 24:461–467
Yates MS, Kwak MK, Egner PA et al (2006) Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-, 12-dioxooleana-1, 9(11)-dien-28-oyl]imidazole. Cancer Res 66:2488–2494
Hayes JD, McMahon M (2009) NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci 34:176–188
Taguchi K, Motohashi H, Yamamoto M (2011) Molecular mechanisms of the keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 16:123–140
Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M (2006) Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol 26:2887–2900
Zhang DD, Hannink M (2003) Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for the stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23:8137–8151
Holland R, Hawkins AD, Eggler AL et al (2008) Prospective type 1 and type 2 disulfides of Keap1 protein. Chem Res Toxicol 21:2015–2060
Malhotra D, Portales-Casamar E, Singh A et al (2010) Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIPSeq profiling and network analysis. Nucleic Acids Res 38:5718–5734
Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116
Wakabayashi N, Shin S, Slocum SL, Agoston ES, Wakabayashi J, Kwak MK, Misra V, Biswal S, Yamamoto M, Kensler TW (2010) Regulation of notch1 signaling by nrf2: implications for tissue regeneration. Sci Signal 3(130):ra52
Chen W, Sun Z, Wang XJ et al (2009) Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell 34:663–673
Komatsu M, Kurokawa H, Waguri S et al (2010) The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12:213–223
Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD (2010) A noncanonical mechanism of activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol 30:3275–3285
Shin S, Wakabayashi N, Misra V et al (2007) NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol Cell Biol 27:7188–7197
Li W, Khor TO, Xu C et al (2008) Activation of Nrf2-antioxidant signaling attenuates NFKappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol 76:1485–1489
Song MY, Kim EK, Moon WS et al (2009) Sulforaphane protects against cytokine- and streptozotocin-induced beta-cell damage by suppressing the NF-kappaB pathway. Toxicol Appl Pharmacol 235:57–67
Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW (2010) When NRF2 talks, who’s listening? Antioxid Redox Signal 13:1649–1663
Zhang Y (2000) Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis 21:1175–1182
Dinkova-Kostova AT, Holtzclaw WD, Cole RN et al (2002) Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA 99:11908–11913
Hong F, Sekhar KR, Freeman ML, Liebler DC (2005) Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem Res Toxicol 18:1917–1926
Hu C, Eggler AL, Mesecar AD, van Breemen RB (2011) Modification of keap1 cysteine residues by sulforaphane. Chem Res Toxicol 24:515–521
Kobayashi M, Li L, Iwamoto N et al (2009) The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol 29:493–502
McMahon M, Lamont DJ, Beattie KA, Hayes JD (2010) Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc and alkenals. Proc Natl Acad Sci USA 107:18838–18843
Kwak MK, Kensler TW (2010) Targeting Nrf2 signaling for cancer chemoprevention. Toxicol Appl Pharmacol 244:66–76
Thimmulappa RK, Mai KH, Srisuma S et al (2002) Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide array. Cancer Res 62:5196–5203
Hu R, Xu C, Shen G et al (2006) Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL6J/Nrf2(−/−) mice. Cancer Lett 243:170–192
Kwak MK, Wakabayashi N, Itoh K et al (2003) Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem 278:8135–8145
Yates MS, Tran QT, Dolan PM et al (2009) Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis 30:1024–1031
Cornblatt BS, Ye L, Dinkova-Kostova AT et al (2007) Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 28:1485–1490
Clarke JD, Hsu A, Williams DE et al (2011) Metabolism and distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm Res 28:3171–3179
Devling TW, Lindsay CD, McLellan LI et al (2005) Utility of siRNA against Keap1 as a strategy to stimulate a cancer chemopreventive phenotype. Proc Natl Acad Sci USA 102:7280–7285
Jeong WS, Keum YS, Chen C et al (2005) Differential expression and stability of endogenous nuclear factor E2-related factor 2 (Nrf2) by natural chemopreventive compounds. J Biochem Mol Biol 38:167–176
Agyeman AS, Chaerkaedy R, Shaw PG et al (2012) Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res Treat 132:175–187
Shapiro TA, Fahey JW, Dinkova-Kostova AT et al (2006) Safety, tolerance and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer 55:53–62
Ye L, Dinkova-Kostova AT, Wade KL et al (2002) Quantitative determination of dithiolcarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta 316:43–53
Fahey JW, Talalay P, Kensler TW (2012) Notes from the field: “green” chemoprevention as frugal medicine. Cancer Prev Res 5:179–188
Acknowledgments
This work has been supported by USPHS grants P01 ES006052, R01 CA93780, R01 CA94076, Breast SPORE P50 CA088843, Center grant ES003819, Department of Defense W81XWH-08-1-0176, and the Prevent Cancer Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kensler, T.W. et al. (2012). Keap1–Nrf2 Signaling: A Target for Cancer Prevention by Sulforaphane. In: Pezzuto, J., Suh, N. (eds) Natural Products in Cancer Prevention and Therapy. Topics in Current Chemistry, vol 329. Springer, Berlin, Heidelberg. https://doi.org/10.1007/128_2012_339
Download citation
DOI: https://doi.org/10.1007/128_2012_339
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-34574-6
Online ISBN: 978-3-642-34575-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)