Abstract
Since their first description in the 1980s, exosomes, small endosomal-derived extracellular vesicles, have been involved in innate and adaptive immunity through modulating immune responses and mediating antigen presentation. Increasing evidence has reported the role of exosomes in host-pathogen interactions and particularly in the activation of antimicrobial immune responses. The growing interest concerning exosomes in infectious diseases, their accessibility in various body fluids, and their capacity to convey a rich content (e.g., proteins, lipids, and nucleic acids) to distant recipient cells led the scientific community to consider the use of exosomes as potential new diagnostic and therapeutic tools. In this review, we summarize current understandings of exosome biogenesis and their composition and highlight the function of exosomes as immunomodulators in pathological states such as in infectious disorders. The potential of using exosomes as diagnostic and therapeutic tools is also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cell-to-cell communication is crucial for maintaining homeostasis within a multicellular organism. In particular, this communication is fundamental in innate and acquired immunities to trigger well-orchestrated immune responses. Among identified mediators, extracellular vesicles (EVs) have achieved a growing interest and are the subject of an increasing number of studies. Several types of EVs have been described to date that have been given different names throughout literature such as microvesicles (also called microparticles or ectosomes) to designate EVs directly released from the plasma membrane, membrane particles, microvesicles, nanoparticles, “exosome-like” microvesicles, tolerosomes, prostasomes, or exosomes to refer to EVs released upon fusion of multivesicular endosomes (MVEs) with the plasma membrane. EVs are traditionally classified according to their intracellular origin, their physical properties, or their protein content. Specific isolation tools and techniques to distinguish EVs from different origins in order to establish a reliable classification are lacking. Therefore, Kowal and coauthors have recently compared the protein content of heterogeneous populations of EVs in order to establish a reliable classification (Kowal et al. 2016). According to the authors, EVs can be firstly classified according to their sedimentation speed, and then EV subpopulations can be distinguished according to their floatation density on iodixanol gradient and their protein content (Kowal et al. 2016).
Exosomes are defined as small EVs (30–100 nm in diameter) pelleting at high speed (ultracentrifugation at 100,000 g) and released upon fusion of MVEs with the plasma membrane (Colombo et al. 2014). In the 1980s, P. Sthal’s and R. Johnstone’s groups originally identified exosomes by their role in elimination of the transferrin receptor via secretion during reticulocyte maturation (Harding et al. 1983; Pan et al. 1985). Since their first description, exosomes have been well-characterized and were shown to be nanovesicles of endocytic origin. Exosomes have been successfully purified from most of body fluids (i.e., serum, saliva, urine, breast milk, etc.) and from cell culture medium (Théry et al. 2006). Analysis of molecular composition of exosomes allowed identification of a rich content with numerous proteins as well as lipids and nucleic acids (Théry et al. 2009). In addition to the molecular composition, numerous groups have been interested in studying the functions of exosomes either in physiological or in pathological states. To date, the most widely documented function of exosomes is their role in immunoregulation. Indeed, exosomes act as crucial regulators in innate immunity since exosomes released from immune cells were shown to be able to stimulate activation, proliferation, and inflammatory responses in various immune recipient cells (Théry et al. 2009). In addition, increasing evidence supports the involvement of exosomes in acquired immunity and particularly in antigen presentation (Théry et al. 2009). The wide range of functions of exosomes in immunoregulation attracts the attention of scientists in fields of research of pathologies such as infectious disorders. As such, exosomes have been shown to be involved in immunoregulation during fungal, parasitic, viral, and bacterial infections, and they can be beneficial either for host defense or for virulence and spread of pathogens. Due to their accessibility in various body fluids and their capacity to convey a complex molecular content even to distant cells, exosomes have been proposed as potential diagnostic, vaccine, and therapeutic tools. However, only a few experiments have been performed to date, in which exosomes were used to diagnose disease, vaccinate, and convey therapeutic molecules.
In this review, we introduce current understandings of biogenesis, secretion, and composition of exosomes. We will then highlight the function of exosomes as immunomodulators in pathological states such as in infectious disorders. The potential of using exosomes as diagnostic, vaccine, and therapeutic tools will also be discussed. It is worthy to note that in several publications cited in this review, other terms rather than “exosomes” were used, which correspond to a mixture of vesicles from different origins.
2 Exosome Biogenesis and Secretion
Exosomes have been isolated from various body fluids such as urine, saliva, bile, breast milk, or blood (Yáñez-Mó et al. 2015). Exosomes are actively secreted by most cell types, in particular, immune cells such as B cells (Clayton et al. 2005), T cells (Nolte-’t Hoen et al. 2009), dendritic cells (DCs) (Théry et al. 1999; Zitvogel et al. 1998), macrophages (Bhatnagar et al. 2007), platelets (Heijnen et al. 1999), and mast cells (Raposo et al. 1997) and from other cell types such as neurons (Fauré et al. 2006), epithelial (Marzesco et al. 2005), endothelial (Song et al. 2014), and mesenchymal stem cells (Lai et al. 2015).
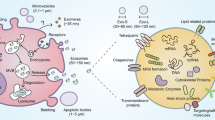
The unique property of exosomes is attributed to their endocytic origin. During exosome biogenesis, extracellular components and membrane receptors are endocytosed in an early endosome (Fig. 1). Then, early endosomes mature into late endosomes (Stoorvogel et al. 1991) and, during this process, small intraluminal vesicles (ILVs) accumulate into MVEs upon budding of the inner membrane of late endosomes, leading to sequestration of proteins, lipids, and cytosolic components. Although MVEs can subsequently fuse with the lysosome to induce cargo degradation (Woodman and Futter 2008), some MVEs can fuse with the plasma membrane, resulting in the release of ILVs as exosomes (Denzer et al. 2000).
Exosome biogenesis. Extracellular proteins and transmembrane receptors are endocyted in an early endosome. Early endosomes mature into late endosomes, and small intraluminal vesicles (ILVs) accumulated into their lumen upon budding of the inner membrane of late endosomes, leading to sequestration of cytosolic components and genetic material. Multivesicular endosomes (MVEs) will then fuse with either lysosomes, leading to the degradation of their content, or the plasma membrane, a process involving SNARE proteins and RAB GTPases, leading to the release of ILVs called “exosomes” into extracellular environment
Although exosome biogenesis is still being defined, a well-described mechanism for ILV formation is driven by the endosomal sorting complexes required for transport (ESCRT), which is composed of four ESCRT complexes (ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III) with associated proteins (e.g., ALIX, VPS34) (Hanson and Cashikar 2012). Firstly identified in endosomal sorting and degradation of ubiquitinated proteins (Davies et al. 2009; Metcalf and Isaacs 2010), ESCRT proteins have been shown to mediate membrane invagination process and ILV formation (Davies et al. 2009; Hurley 2010; Metcalf and Isaacs 2010). Thus, ESCRT-0 binds ubiquitinated proteins, allowing their delivery to MVEs (Raiborg and Stenmark 2002). Then, ESCRT-0 recruits ESCRT-I, which consequently recruits ESCRT-II and ESCRT-III (Babst et al. 2002; Katzmann et al. 2001). By triggering membrane invagination and scission, ESCRT-III enables ILV formation (Wollert et al. 2009). Several studies support the involvement of ESCRT proteins in exosome biogenesis since knockdown of ESCRT proteins has been shown to abolish ILV formation and exosome secretion (Stuffers et al. 2009; Tamai et al. 2010).
Another ESCRT-independent mechanism in exosome biogenesis has been raised, in which siRNA-mediated silencing of ESCRT genes did not abrogate totally exosome release (Stuffers et al. 2009). First, analysis of exosome secretion from oligodendrocytes, central nervous system cells, showed that exosome secretion requires the sphingolipid ceramide (Trajkovic et al. 2008). Another study reported that tetraspanins might be also involved in exosome biogenesis since depletion of the CD63-coding gene in vitro in melanocytes or in vivo in cd63 −/− mice led to a reduction of ILV formation (van Niel et al. 2011).
Once MVEs are formed, they can either fuse with the lysosome to mediate cargo degradation (Woodman and Futter 2008) or with plasma membrane, a process mediated by the cytoskeleton, small GTPases, and fusion machinery (Colombo et al. 2014). Among the GTPases involved in ILV exocytosis, several RAB GTPases, which are members of the Ras GTPase superfamily, have been identified in exosomes such as RAB5, RAB11, RAB27, and RAB35. Indeed, RAB11 inhibition by overexpressing a dominant negative mutant in K562 erythroleukemia cells decreased exosome release (Savina et al. 2002). Moreover, inhibition of RAB35 function resulted in an impaired exosome secretion and accumulation of ILVs (Hsu et al. 2010). Furthermore, shRNA-mediated silencing of RAB27A and RAB27B in HeLa cells decreased exosome secretion (Ostrowski et al. 2010). Recently, it was reported that inhibition of RAL-1 GTPase resulted in a hampered fusion of MVEs with the plasma membrane and consequently in a decreased exosome secretion (Hyenne et al. 2015). The fusion machinery, involving soluble N-ethylmaleimide-sensitive fusion attachment protein (SNAP) receptors (SNARE), has been shown to mediate exosome secretion. SNARE proteins form complexes between vesicular v-SNARE (vesicular-associated membrane proteins or VAMP) proteins and cell membrane t-SNARE proteins (Zylbersztejn and Galli 2011). It was reported that overexpression of the SNARE protein VAMP7 in K562 cells led to an impaired exosome secretion (Fader et al. 2009).
3 Exosome Molecular Composition
Exosomes have been shown to contain proteins, lipids, and nucleic acids (Fig. 2). Protein content of exosomes has been extensively analyzed by several techniques including Western blotting, immune-electron microscopy (immuno-EM), fluorescence-activated cell sorting (FACS), and mass spectrometry (Colombo et al. 2014). Exosomal protein content varies depending on the cell type of origin: for example, B-cell-derived exosomes contain the B-cell receptor (BCR), and DC-derived exosomes contain MCH-II, CD86, and ICAM-1 proteins (Théry et al. 2009). Furthermore, exosomes contain some common proteins such as adhesion molecules [milk fat globule-EGF factor 8 (MFGE8), integrins, and tetraspanins (CD63, CD81, and CD9)], chaperones [heat-shock cognate protein 70 (HSC70), heat-shock protein 90 (HSP90)], proteins involved in membrane trafficking (e.g., RAB GTPases, annexins) and in MVE biogenesis (e.g., clathrin, ALIX, TSG101), etc. (Fig. 2) (Colombo et al. 2014). Recently, proteomic analysis of heterogenous populations of small EVs, separated by a combinatorial approach using differential ultracentrifugation, floatation in a density gradient, and immuno-isolation, confirmed that exosomes can be distinguished from other subpopulations as they are co-enriched in CD63, CD9, and CD81 tetraspanins and endosomal markers (Kowal et al. 2016). Interestingly, proteomic analyses revealed that exosomes contain proteins from different cell compartments such as the plasma membrane, cytosol, or endosomes, while proteins from the nucleus, the mitochondria, the endoplasmic reticulum or the Golgi apparatus are almost missing in exosomes (Lundholm et al. 2014; Théry et al. 2009). These data confirm that exosomes arise from specific subcellular compartments and not from cell fragmentation. The identified exosomal proteins are listed in the online databases ExoCarta (http://www.exocarta.org) (Mathivanan et al. 2012) and Vesiclepedia (http://microvesicles.org/).
Molecular composition of a typical exosome. Common composition including genetic material (in blue box), proteins (in green boxes), and lipids (in yellow box) found in a typical exosome is depicted. Proteins shown in red have been considered as exosomal markers. EF1α1 elongation factor 1-alpha 1, HSC heat-shock cognate, HSP heat-shock protein, ICAM-1 intercellular adhesion molecule 1, MFGE8 milk fat globule EGF factor 8 protein, MHC major histocompatibility complex molecules, MVE multivesicular endosome, TSG101 tumor susceptibility gene 101
Exosomal lipid composition has been characterized, mainly using mass spectrometry or high-performance liquid chromatography (Laulagnier et al. 2004a, b; Llorente et al. 2013; Trajkovic et al. 2008; Wubbolts et al. 2003). As such, exosomes have been shown to be enriched in sphingomyelin, phosphatidylserine, cholesterol, and fatty acids, as compared to plasma membrane (Record et al. 2014). Moreover, exosomes are enriched in GM3 ganglioside (Llorente et al. 2013; Wubbolts et al. 2003), ceramide, and derivatives (Laulagnier et al. 2005; Llorente et al. 2013; Trajkovic et al. 2008). However, lysobisphosphatidic acid (LBPA), a lipid enriched in endosomal compartments and thought to be found in ILVs (Matsuo et al. 2004), is not enriched in exosomes (Laulagnier et al. 2004b; Wubbolts et al. 2003). According to these studies, exosomes seem to display a specific lipid composition (enriched in cholesterol, sphingomyelin, and GM3 ganglioside) similar to that of lipid raft microdomains on plasma membranes. Thus, Tan and colleagues suggested and confirmed that lipid rafts are endocytosed into MVEs and released on exosomes (Tan et al. 2013). Interestingly, it has been shown that exosome biogenesis mechanisms evolve during cell maturation since the lipid content of exosomes derived from reticulocytes is similar to that of donor reticulocytes (enriched in ceramide) but is modified in erythrocytes (Carayon et al. 2011). Exosomal lipids have been included in ExoCarta and Vesiclepedia databases as well.
Numerous groups have analyzed the genetic material in exosomes after the first description of nucleic acids in exosomes by Valadi and colleagues (Valadi et al. 2007). In this pioneer study, exosomes derived from human HMC-1 mast cells and murine MC/9 mast cells were shown to contain multiple and heterogenous RNA species including mRNAs and microRNAs (miRNAs), which were efficiently transferred to recipient cells and biologically active (Valadi et al. 2007). Then, exosomes derived from immune cells have been shown to hold a specific set of miRNAs that can be transferred to recipient cells (Mittelbrunn et al. 2011; Montecalvo et al. 2012). For example, exosomes derived from human THP-1 macrophages convey the miRNA 150, which is handled by recipient endothelial HMEC-1 cells and inhibits the expression of its target gene c-Myb (Zhang et al. 2010). Moreover, high-throughput next-generation sequencing techniques have allowed the identification of other small RNAs in exosomes such as small noncoding RNAs [vault RNA (vtRNA), Y-RNA, transfer RNA (tRNA)] but limited amounts of DNA and ribosomal RNA (Nolte-’t Hoen et al. 2012; van den Boorn et al. 2013).
4 Exosome as Immunomodulators
4.1 Exosomes and Innate Immunity
Exosomes have been involved in modulating innate immune responses (Fig. 3). Raposo and colleagues have reported the release of exosomes from B lymphocytes, suggesting the involvement of exosomes in immune responses (Raposo et al. 1996). Natural killer (NK) cells can be activated through the binding to its surface receptor of HLA-B-associated transcript 3 (BAT3), which is expressed on DC-derived exosomes (Simhadri et al. 2008). Exovesicles derived from mature DCs induce a pro-inflammatory response in intestinal epithelial cells which in turn secrete pro-inflammatory cytokines and chemokines [tumor necrosis factor alpha (TNF-α), regulated on activation, normal T cell expressed and secreted (RANTES), interleukin 8 (IL-8), monocyte chemoattractant protein 1 (MCP-1)] in a TNF-α-dependent pathway (Obregon et al. 2009). Although the ultracentrifugation-based purification method used in this study is consistent with exosome purification, an involvement of other types of EVs cannot be excluded. Moreover, exosomes released from mouse DCs express on their surface TNF superfamily members [TNF, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), and Fas ligand (FasL)], which directly bind to their receptors on NK cells to enhance their cytotoxic activity (Munich et al. 2012). Intradermal injection of wild-type mice with mouse DC-derived exosomes increased the amount of NK cells in the draining lymph node, and this required activation of the natural killer group 2 member D (NKG2D) receptor on NK cells (Viaud et al. 2009). A recent study reported that exosomes released from mouse DCs carry on their surface IL-15Rα, a NKG2D receptor ligand (Viaud et al. 2009). Upon activation, NKG2D induces activation and proliferation of NK cells (Zhang et al. 2015).
Exosomes in innate immunity. Here are summarized main functions of exosomes in innate immunity. BAT3 HLA-B-associated transcript-3, DC dendritic cell, FasL Fas ligand, IEC intestinal epithelial cell, TNF tumor necrosis factor, TRAIL tumor necrosis factor-related apoptosis-inducing ligand, NK natural killer, NKG2D natural killer group 2 member D RANTES regulated on activation, normal T cell expressed and secreted, IL interleukin MCP-1, monocyte chemoattractant protein-1, ? exosomal content undetermined
Exosomes released from macrophages infected with intracellular pathogens, when exposed to uninfected macrophages, induce secretion of pro-inflammatory mediators such as TNF-α and RANTES (Bhatnagar et al. 2007; Bhatnagar and Schorey 2007). Moreover, intranasal injection of mice with exosomes released from mycobacteria-infected macrophages increases secretion of pro-inflammatory mediators (TNF-α and IL-12) and neutrophil and macrophage recruitment in the lung (Bhatnagar et al. 2007). In vitro stimulation of macrophages with exosomes purified from bronchoalveolar lavage fluid of mycobacteria-infected mice provokes an increase of TNF-α secretion (Bhatnagar et al. 2007). It has also been shown that Mycoplasma-infected DCs release exosomes that trigger B-cell proliferation, and this is independent of any antigen presentation (Quah and O’Neill 2007).
4.2 Exosomes and Acquired Immunity
Increasing evidence supports the involvement of exosomes in adaptive immune responses and particularly in antigen presentation (Fig. 4). During antigen presentation, antigen-presenting cells (APCs) such as DCs or B lymphocytes present antigen-MHC complexes to T lymphocytes that are consequently activated. Identification of MHC classes I and II and T-cell co-stimulatory molecules on exosomes released from immune cells (Colombo et al. 2014) led scientists to consider exosomes as new mediators of antigen presentation. Moreover, exosomes have been shown to contain antigens. Exosomes derived from tumor cell lines (Napoletano et al. 2009; Wolfers et al. 2001) or ascites from cancer patients (Andre et al. 2002) carry tumor antigens. Furthermore, macrophages infected with Mycobacterium tuberculosis or Mycobacterium bovis can release exosomes containing bacterial antigens (Giri et al. 2010; Giri and Schorey 2008).
Exosomes in adaptive immunity. Here are summarized main functions of exosomes in adaptive immunity (i.e., in direct antigen presentation, indirect antigen presentation and in cross-dressing). Ag antigen, APC antigen-presenting cell, CMV cytomegalovirus, DC dendritic cell, EBV Epstein-Barr virus, LPS lipopolysaccharide, OVA ovalbumin, ? exosomal content undetermined.
Several works highlighted the capacity of exosomes to perform indirect antigen presentation or cross-presentation (Fig. 4). Antigens conveyed by exosomes are handled by APCs, which complex antigens with their own MHC molecules to present these antigen peptides to T lymphocytes. Stimulation of T lymphocytes with antigen-containing exosomes in the presence of naïve recipient DCs resulted in activation of T cells (Andre et al. 2002; Napoletano et al. 2009; Wolfers et al. 2001). Another study reported that injection of antigen- or peptide-bearing exosomes induced antigen-specific naïve CD4+ T-cell activation in vivo, but in vitro, these exosomes failed to induce antigen-dependent T-cell stimulation unless intermediate DCs were present (Théry et al. 2002). Similarly, exosomes released from mouse mast cells carry antigens and can activate naïve DCs, which in turn activate T cells in vitro (Skokos et al. 2001). Skokos and colleagues injected ovalbumin (OVA)-bearing exosomes released from mast cells, recovered DCs, and showed their ability to activate OVA-specific T-cell hybridomas (Skokos et al. 2003). Moreover, exosomes released from macrophages infected with Mycobacterium tuberculosis or Mycobacterium bovis carry bacterial antigens that, in the presence of intermediate APCs, can activate CD4+ and CD8+ lymphocytes isolated from mycobacteria-immunosensitized mice (Giri and Schorey 2008).
Increasing evidence has shown that exosomes can be involved in direct antigen presentation (Fig. 4). APC-derived exosome-like microvesicles can directly activate naïve CD8+ T cells in vitro (Hwang et al. 2003). Monocyte-derived DCs secrete exosomes containing viral antigens which can activate T lymphocytes in vitro without the presence of DCs (Admyre et al. 2006). Similarly, OVA-containing exosomes derived from mouse OVA-pulsed DCs can directly activate OVA-specific CD8+ T-cell hybridomas (Utsugi-Kobukai et al. 2003). Another study reported that exosomes derived from lipopolysaccharide (LPS)-treated DCs induced a strong antigen-specific T-cell activation both in vitro and in vivo (Segura et al. 2005).
Some evidence showed that pre-formed antigen-MHC complexes carried in exosomes can be directly handled by APCs and presented to T lymphocytes, in a process named “cross-dressing” (Fig. 4) (Yewdell and Dolan 2011). Montecalvo et al. showed that DCs can secrete exosomes containing antigen-MHC complexes (Montecalvo et al. 2008). These exosomes can be internalized, and the antigen-MHC complexes can be directly presented by DCs to activate CD8+ T lymphocytes (André et al. 2004). However, these results are debatable since some studies have shown the disability of exosomes bearing antigen-MHC complexes to perform “cross-dressing” (Coppieters et al. 2009; Wakim and Bevan 2011).
5 Exosomes in Host-Pathogen Interactions
Exosomes secreted during host responses to infection with several pathogen classes including fungi, parasites, viruses, and bacteria have been isolated and characterized. The content and activity of these exosomes, which can be derived from infected host cells or from pathogens, have been analyzed.
5.1 Exosomes in Fungal Infection
Only few studies concerning the involvement of exosomes in fungal infection are available, and these are limited to the analysis of exosomes derived directly from the fungi but not from fungus-infected cells (Fig. 5). EVs released from the yeast Cryptococcus neoformans strain induce cytokine secretion by recipient macrophages as shown by increased TNF-α and tumor growth factor beta (TGF-β) secretion, leading to a restricted fungal infection (Oliveira et al. 2010). It should be noted that the ultracentrifugation-based purification method was used in this study, thus, an involvement of other EVs rather than exosomes cannot be excluded. On another hand, exosomes have been proposed to promote fungal virulence. Indeed, blocking export of exosomes from C. neoformans by knocking down SEC6 (involved in fusion of exocytic vesicles with the plasma membrane) diminished virulence of the yeast in vivo (Panepinto et al. 2009). This decreased virulence was shown to be due to the inability of the yeast to export crucial virulence factors such as laccase (Panepinto et al. 2009).
Exosomes in fungal infection. This figure summarizes the known functions of exosomes in fungal infection. Exosome source and their functional impacts on recipient cells/organisms with underlying mechanism are presented. TNF tumor necrosis factor, TGF-β tumor growth factor beta, ? exosomal content undetermined
5.2 Exosomes in Parasitic Infection
The involvement of exosomes in parasitic infection, including those released from infected host cells and from the parasite, has been analyzed (Fig. 6). The first study of exosomes in parasitic infection is performed by Bhatnagar and colleagues, which showed that exosomes released from macrophages infected with the intracellular protozoan Toxoplasma gondii triggered a pro-inflammatory response in naïve macrophages with an increased secretion of TNF-α (Bhatnagar et al. 2007).
Exosomes/extracellular vesicles in parasitic infection. Here are summarized known functions of exosomes in parasitic infection. Exosome source and their functional impacts on recipient cells/organisms with underlying mechanism are presented. DC dendritic cell, HLA human leukocyte antigen, IFN interferon, Ig immunoglobulin, i.v. intravenous, PBMC peripheral blood mononuclear cells, PfPTP2 Plasmodium falciparum tyrosine phosphatase 2, ? exosomal content undetermined
Exosomes have also been studied in the context of Plasmodium infection. Red cells infected with the malaria-causative parasite Plasmodium falciparum release exosome-like vesicles and microvesicles that contain parasite components (Mantel et al. 2013) and particularly the parasitic protein Plasmodium falciparum tyrosine phosphatase 2 (PfPTP2), which promotes sexual differentiation of the parasite (Regev-Rudzki et al. 2013). An in vivo study reported that microvesicles isolated from the plasma of malaria-infected mice induce a pro-inflammatory response in macrophages in vitro with increased TNF-α secretion (Couper et al. 2010).
Moreover, infection of blood cells with Trypanosoma cruzi provokes the release of microvesicles which, by forming a complex with the complement C3 convertase on the parasite surface, protect the parasite against complement-mediated lysis, resulting in increased parasite survival (Cestari et al. 2012). It was also reported that T. cruzi release EVs carrying parasitic small RNAs which confer susceptibility to infection upon uptake by mammalian epithelial cells (Garcia-Silva et al. 2014). In this study, EVs were isolated using ultracentrifugation method, thus, an involvement of exosomes cannot be excluded. Using the same techniques, it has also been shown that small EVs (e.g., exosomes) released from T. cruzi display a phosphatase activity resulting in increased adhesion and invasion abilities of the parasite in host cells (Neves et al. 2014).
The involvement of exosomes in the context of Leishmania infection has also been studied. Exosomes were proposed to mediate the delivery of Leishmania into macrophages. Indeed, it has been shown that Leishmania spp. release exosomes to deliver proteins to recipient macrophages, inducing a pro-inflammatory response (Silverman et al. 2010b). Similarly, Leishmania-derived exosomes induce secretion of pro-inflammatory cytokines by recipient monocytes (Silverman et al. 2010a). The HSP100 protein has a crucial role in the packaging of Leishmania’s proteins into exosomes since its absence resulted in a modification of exosome content and an impaired pro-inflammatory activity in recipient cells (Silverman et al. 2010b). L. major- and L. donovani-derived exosomes have also been shown to suppress the immune response in vivo since mice pretreated with these exosomes prior to infection showed higher parasite burden compared with untreated mice (Silverman et al. 2010b). Proteomic analyses revealed that exosomes released from Leishmania mexicana-infected macrophages contain GP63 protein, an essential virulence factor (Hassani and Olivier 2013).
5.3 Exosomes in Viral Infection
Exosomes in the context of viral infection have been extensively studied (Fig. 7). The hypothesis of an involvement of exosomes in viral infection resulted from several observations. Numerous viruses such as hepatitis B, hepatitis C, and herpesviruses use the ESCRT machinery (Hurley 2010), to leave the infected host cell (Chen and Lamb 2008; Mori et al. 2008). Moreover, Fang and colleagues reported that human immunodeficiency virus (HIV) budding seems to result from a similar pathway to the exosome biogenesis pathway (Fang et al. 2007). This was later supported by another study showing the involvement of TSG101 and ALIX proteins in virus budding, two major proteins acting in exosome biogenesis (Usami et al. 2009).
Exosomes in viral infection. This figure summarizes the known functions of exosomes in viral infection. Exosome source and their functional impacts on recipient cells/organisms with underlying mechanism are presented. Ag antigen, CMV cytomegalovirus, DC dendritic cell, EBV Epstein-Barr virus, ERK extracellular signal-regulated kinase, ESCRT endosomal sorting complexes required for transport, HCV hepatitis C virus, HIV human immunodeficiency virus, HTLV-1 human T-lymphotropic virus type 1, IFN interferon, LMP-1 latent membrane protein 1, NEF negative regulatory factor, NF-κB nuclear factor-kappa B, PBMC peripheral blood mononuclear cells, TAR trans-activation response element, ? exosomal content undetermined
A role for exosomes released from infected host cells in viral spread and in immunoregulation, which result in an increased infectivity of viruses, has been raised. For instance, exosomes in the context of HIV infection and diffusion has been extensively studied. Several groups reported that CD63 and CD81 tetraspanins, enriched in exosomes, participate in viral budding, viral spread, and in HIV infectivity (Grigorov et al. 2009; Izquierdo-Useros et al. 2009; Jolly and Sattentau 2007; Sato et al. 2008). Particularly, Grigorov and colleagues showed that HIV-1 structural Gag and Env proteins interact with the CD81 tetraspanin in tetraspanin-enriched microdomains on T-cell surface (Grigorov et al. 2009). Furthermore, CD81 expression is crucial for viral replication since shRNA-mediated inhibition of CD81 resulted in an impaired HIV-1 release (Grigorov et al. 2009). It has also been shown that the CD63 tetraspanin is eliminated from the plasma membrane of HIV-1-infected and virion-producing T cells and is embedded on the membrane of released virions (Sato et al. 2008). Interestingly, virion-incorporated CD63 was shown to inhibit HIV-1 infection (Sato et al. 2008). Exosomes have also been shown to convey HIV-1 proteins involved in viral replication cycle such as GAG (Fang et al. 2007) and negative regulatory factor (NEF; (de Carvalho et al. 2014; Lenassi et al. 2010). NEF-containing exosomes induce T-cell apoptosis in vitro, a key feature of HIV infection (Lenassi et al. 2010). Narayanan and colleagues showed that the HIV-1 RNA named transactivating response (TAR) is released into exosomes from infected host cells in vitro and from sera of HIV-infected patients (Narayanan et al. 2013). Moreover, pretreatment of host cells with exosomes derived from HIV-infected cells increased susceptibility of treated cells to HIV infection (Narayanan et al. 2013).
Exosomes have also been studied in the context of infection with other viruses. The latent membrane protein 1 (LMP1), an immunosuppressive protein important in Epstein-Barr virus (EBV) infection (Dukers et al. 2000), was found in exosomes released from EBV-infected B cells, suggesting that exosomes can mediate the immunosuppressive effect of LMP1 during EBV infection (Verweij et al. 2011). Exosomes released from EBV-infected cells also contain the dUTPase enzyme which triggers pro-inflammatory and antiviral responses in recipient DCs and peripheral blood mononuclear cells (PBMCs) (Ariza et al. 2013). Exosomes have also been shown to mediate a functional delivery of viral miRNAs. Indeed, exosomes released from EBV-infected B cells secrete exosomes containing EBV miRNAs which can induce inhibition of known EBV target genes in recipient cells such as C-X-C motif chemokine 11 (CXCL11), a cytokine involved in antiviral responses (Pegtel et al. 2010). Moreover, Jaworski et al. reported the incorporation of the human T-cell leukemia virus type 1 (HTLV-1) TAX protein which is crucial for viral replication (Jaworski et al. 2014). Hepatitis C virus (HCV)-infected cells secrete exosomes containing viral RNAs which can be transferred to recipient DCs, inducing DC activation and secretion of interferon-α (IFN-α) (Dreux et al. 2012). It was also reported that the HCV envelop glycoprotein interacts with the CD81 cell membrane protein and that this complex is released within exosomes (Masciopinto et al. 2004). HCV structural proteins have also been identified in exosomes purified from HCV-infected patients’ plasma (Masciopinto et al. 2004).
5.4 Exosomes in Bacterial Infection
The involvement of exosomes during bacterial infection has been largely studied (Fig. 8). Particularly, the role of exosomes has been extensively analyzed in the context of mycobacterial infection. Exosomes derived from Mycobacterium avium-infected macrophages trigger a pro-inflammatory response in naïve recipient macrophages (Bhatnagar and Schorey 2007). Using antibody-based techniques, these exosomes were shown to contain glycopeptidolipids, a major mycobacterial cell wall constituent (Bhatnagar and Schorey 2007). Wang and colleagues reported that exosomes released from Mycobacterium avium subspecies tuberculosis-infected macrophages induce an increased release of the pro-inflammatory cytokines IFN-γ and TNF-α in naïve recipient macrophages (Wang et al. 2014). Similarly, Mycobacterium tuberculosis- and Mycobacterium bovis-infected macrophages release exosomes inducing a pro-inflammatory response in naïve recipient macrophages (Bhatnagar et al. 2007). The authors highlighted the presence of a mycobacterial lipoprotein mediating this pro-inflammatory message through a Toll-like receptor (TLR)/myeloid differentiation primary response protein 88 (MyD88)-dependent pathway (Bhatnagar and Schorey 2007). These results were confirmed in vivo since exosomes purified from bronchoalveolar lavage fluid of Mycobacterium bovis-infected mice contain the mycobacterial components lipoarabinomannan and the 19-kDa lipoprotein and can trigger a pro-inflammatory response in vitro (Bhatnagar et al. 2007). Similarly, exosomes released from Mycobacterium bovis- or Mycobacterium tuberculosis-infected macrophages in vitro can, when intranasally injected into mice, induce an increased TNF-α and IL-12 secretion as well as neutrophil and macrophage recruitment in the lung (Bhatnagar et al. 2007). Moreover, exosomes released from Mycobacterium tuberculosis-infected macrophages can partially inhibit the ability of naïve macrophages to be activated by IFN-γ (Singh et al. 2011), which is crucial in host response to mycobacterial infection since activated macrophages control intracellular bacterial replication (Flynn et al. 1993).
Exosomes in bacterial infection. This figure summarizes the known functions of exosomes in bacterial infection. Exosome source and their functional impacts on recipient cells/organisms with underlying mechanism are presented. DC dendritic cell, IEC intestinal epithelial cell, LAM lipoarabinomannan, LPS lipopolysaccharide, MAPK mitogen-activated protein kinase, MyD88 myeloid differentiation primary response protein 88, NF-κB nuclear factor-kappa B, PAMP pathogen-associated molecular pattern, TLR Toll-like receptor, ? exosomal content undetermined
The involvement of exosomes in infection with other bacteria has been also analyzed. It was shown that exosomes released from Salmonella Typhimurium-infected macrophages induced a pro-inflammatory response in naïve recipient macrophages (Bhatnagar et al. 2007). The authors showed that the released exosomes contain bacterial LPS (Bhatnagar et al. 2007), which was responsible for this pro-inflammatory response since no inflammatory response was observed in tlr4 −/− macrophages depleted for the LPS receptor TLR4 (Bhatnagar et al. 2007). Furthermore, Mycoplasma-infected cells release exosomes that induced increased IFN-γ secretion in recipient B cells (Yang et al. 2012). Exosomes have also been shown to convey bacterial toxins. In fact, Abrami et al. reported that upon treatment of epithelial cells with the two components of the lethal anthrax toxin, protective antigen (PA) and lethal factor (LF), PA induced the formation of a channel allowing the translocation of LF in the cytosol and in ILVs (Abrami et al. 2013). LF persists in ILVs and is then released in exosomes that can be internalized and consequently delivered in recipient epithelial cells (Abrami et al. 2013).
Our group recently deciphered a previously unknown function of exosomes in the interaction between host cells and Crohn’s disease (CD)-associated adherent-invasive Escherichia coli (AIEC). Increased abundance of invasive E. coli strains in intestinal mucosa of CD patients comparatively to control subjects have been reported (Baumgart et al. 2007; Conte et al. 2006; Darfeuille-Michaud et al. 1998; Martin et al. 2004; Martinez-Medina et al. 2009; Neut et al. 2002; Sasaki et al. 2007; Swidsinski et al. 2002), and these strains were later named AIEC (Darfeuille-Michaud et al. 2004). We showed that AIEC infection induced the release of exosomes by human intestinal epithelial cells and macrophages (Carrière et al. 2016). Characterization of the exosomes released from AIEC-infected cells showed that they are able to trigger a pro-inflammatory response in naïve intestinal epithelial and macrophagic cells with activation of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways and increased pro-inflammatory cytokine secretion (Carrière et al. 2016). In addition, stimulation of human intestinal epithelial cells and macrophages with exosomes released from AIEC-infected cells increased bacterial intracellular replication compared with stimulation with exosomes secreted by uninfected cells (Carrière et al. 2016). Our findings suggest that exosomes are involved in the activation of host innate immune responses upon AIEC infection and in bacterial intracellular replication, two key features of host-AIEC interaction.
6 Exosomes in Disease States: Applications in Diagnostic, Vaccine, and Therapeutic Approaches
6.1 Exosomes: Promising Diagnostic Tools
Exosomes have been successfully purified from numerous body fluids such as blood, urine, bronchoalveolar lavage fluid, and saliva (Admyre et al. 2003; Caby et al. 2005; Pisitkun et al. 2004). Due to their easy recovery and their rich content, exosomes have been proposed as a new diagnostic tool in numerous diseases. Thus, the exosomal Fetuin-A, a protein which is synthesized in the liver and secreted into the blood, has been reported as a novel urinary biomarker for detecting acute kidney injury (Zhou et al. 2006). Especially, the content of tumor-derived exosomes has been extensively analyzed and proposed to diagnose cancers. Szajnik et al. reported that the plasma collected from ovarian cancer patients contains higher levels of exosomal proteins compared to control individuals, and that ovarian cancer patients can be distinguished from healthy individuals by the presence of TGF-β1 and MAGE3/6 in plasma-derived exosomes (Szajnik et al. 2013). Some new proteins previously undescribed have been identified in exosomes isolated from malignant pleural effusions of patients suffering from mesothelium, ovarian, breast and non-small cell lung cancers (Bard et al. 2004). Recently, Melo et al. showed using mass spectrometry analysis that exosomes purified from serum of pancreas cancer patients are enriched in glypican-1 (GPC1), a cell surface proteoglycan (Melo et al. 2015). Using flow cytometry, the authors observed that GPC1+ exosomes enabled distinction between cancer patients from healthy individuals, even in early stages of the disease (Melo et al. 2015). Furthermore, another group developed a powerful multiplex detection chip for blood-based diagnosis of ovarian cancer by multiplexed measurement of three exosomal tumor markers CA-125, EpCAM, and CD24 (Zhao et al. 2015).
Exosomal miRNAs have also been proposed as diagnostic biomarkers since altered miRNA expressions have been reported in numerous diseases (Hu et al. 2012). Indeed, modified miRNA and long noncoding RNA profiles have been identified in exosomes isolated from peritoneum lavage fluid and plasma of patients suffering from gastric cancer (Li et al. 2015; Tokuhisa et al. 2015; Zhou et al. 2015; Zöller 2016). Modified miRNA profiles have also been identified in circulating exosomes derived from patients suffering from glioblastoma (Rabinowits et al. 2009), lung cancer (Rabinowits et al. 2009), and ovarian cancer (Taylor and Gercel-Taylor 2008). The RNA content of exosomes isolated from the blood of patients with dental and neurologic disorders has been analyzed (De Smaele et al. 2010; Miranda et al. 2010; Palanisamy et al. 2010; Rabinowits et al. 2009), and the potential use of exosomal miRNAs as powerful diagnostic biomarkers for Alzheimer’s disease has been raised (Van Giau and An 2016).
Finally, exosomes could be used as diagnostic tools in infectious diseases. The amount of exosomes in the serum of Mycobacterium bovis-infected mice increased proportionally to the bacterial burden (Singh et al. 2012). Moreover, Mycobacterium tuberculosis-infected cells secrete exosomes carrying mycobacterial proteins, suggesting the use of exosomes to diagnose tuberculosis (Kruh-Garcia et al. 2014).
6.2 Exosome-Based Vaccination: An Encouraging Approach
With their immunoregulatory property, exosomes have been proposed and tested as vaccines in cancer and in infectious diseases in order to mobilize the immune system against tumor cells or pathogens.
Dai and colleagues genetically modified human colon adenocarcinoma cells with a recombinant adenovirus encoding human IL-18 and showed that exosomes derived from these cells exhibited more potent capability to induce antitumor immunity compared with exosomes derived from nongenetically modified cells, suggesting that modification of exosomes could be an approach to develop exosome-based tumor vaccines (Dai et al. 2006). A phase I clinical trial reported that ascite-derived exosomes in combination with the granulocyte-macrophage colony-stimulating factor (GM-CSF) used as an adjuvant are safe, well tolerated, and induce a specific antitumor immunity in patients with colorectal cancer (Dai et al. 2008). Several studies reported that murine DC-derived exosomes are able to induce antigen-specific CD4+ and CD8+ T-cell responses both in vitro and in vivo and to enhance antitumor immunity in vivo (Damo et al. 2015; Luketic et al. 2007; Näslund et al. 2013a, b; Segura et al. 2005; Théry et al. 2002; Zitvogel et al. 1998). However, several phase I clinical trials using exosomes released from antigen-loaded DCs from cancer patients for treatment of non-small cell lung cancer and melanoma showed that the use of exosomes in vaccination is safe but does not exhibit a significant impact on tumor growth or in cancer regression (Escudier et al. 2005; Morse et al. 2005; Viaud et al. 2010). Nevertheless, a recent clinical trial revealed a modification of the protein and mRNA composition of exosomes released in glioma patients’ plasma after receiving antitumor vaccines (Muller et al. 2015). This modification has been shown to be correlated with immunological and clinical responses as well as survival, providing a promising approach to evaluate glioma patients’ response to antitumor vaccination (Muller et al. 2015).
Regarding the use of exosomes as vaccines in infectious conditions, it was shown that intravenous injection of exosomes released from DCs infected with the parasite Leishmania major conferred vaccinated mice an effective protection against infection, as shown by a decrease in the number of infected cells in draining nymph lodes (Schnitzer et al. 2010). Moreover, exosomes released from macrophages infected with Mycobacterium bovis or Mycobacterium tuberculosis can activate antigen-specific CD4+ and CD8+ T cells isolated from mycobacteria-immunosensitized mice and promote activation and maturation of DCs (Giri and Schorey 2008). Macrophages treated with Mycobacterium tuberculosis proteins released exosomes that, upon intranasal injection into mice, activated DCs and CD4+ and CD8+ T cells isolated from Mycobacterium tuberculosis-infected mice (Giri et al. 2010). Furthermore, DCs treated with the highly immunogenic diphtheria toxoid (DT) protein secrete exosomes that, once injected intraperitoneally in mice, stimulate a specific DT IgG response (Colino and Snapper 2006). Exosomes have also been suggested to be used as vaccines in parasitosis. Indeed, upon intravenous injection of exosomes released from DCs pulsed with Toxoplasma gondii antigens, anti-Toxoplasma gondii IgM antibodies were detected in the serum of mice (Aline et al. 2004). Moreover, mice were subcutaneously vaccinated before pregnancy with exosomes released from DCs pulsed with Toxoplasma gondii-derived antigens and infected with the parasite during pregnancy (Beauvillain et al. 2009). The results showed that vaccination resulted in effective protection of pups against congenital infection (Beauvillain et al. 2009). Another study showed that infection of epithelial cells with the protozoan parasite Cryptosporidium parvum results in an increased luminal secretion of exosomes (Hu et al. 2013). These exosomes were shown to contain antimicrobial peptides such as cathelicidin-37 and beta-defensin-2 that affect survival of the parasite (Hu et al. 2013). Recently, using proteomic analysis, the parasite Schistosoma mansoni has been shown to secrete exosomes carrying potential virulence factors as well as known vaccine candidates (Sotillo et al. 2015). The use of exosomes as vaccine in Cryptococcus infection has been proposed since extracellular vesicles of the Cryptococcus neoformans yeast strain induce activation of recipient macrophages, improving their abilities to perform phagocytosis and to secrete microbicidal components (Oliveira et al. 2010). Finally, exosomes can constitute a defense mechanism in viral infection. Indeed, Khatua and colleagues identified the secretion in exosomes of the viral cytidine deaminase apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3G (APOBEC3G) (Khatua et al. 2009), a protein known to control the replication of several enteroviruses (Chiu and Greene 2008). The authors reported that APOBEC3G-containing exosomes confer recipient epithelial cells protection against HIV-1 infection (Khatua et al. 2009).
6.3 Exosomes: Promising New Conveyors of Therapeutic Molecules
Cells use exosomes as cargos to deliver proteins and genetic material to neighboring or distant recipient cells. In addition to the use of antigen-pulsed DC-derived exosomes to induce antitumor immune responses (Damo et al. 2015; Escudier et al. 2005; Luketic et al. 2007; Morse et al. 2005; Näslund et al. 2013a, b; Segura et al. 2005; Théry et al. 2002; Viaud et al. 2010; Zitvogel et al. 1998), different strategies for delivering therapeutic molecules using exosomes have been proposed and developed. When being directly incubated with exosomes, curcumin, an anti-inflammatory agent, and antitumor agents such as doxorubicin and paclitaxel have been successfully incorporated into exosomes and have been shown to be effective in vitro and in vivo (Sun et al. 2010; Tian et al. 2014; Yang et al. 2015; Zhuang et al. 2011). Indeed, in a mouse model of sepsis, intraperitoneal injection of curcumin-containing exosomes resulted in the protection of mice against a LPS-induced septic shock (Sun et al. 2010). Similarly, in mouse models of brain inflammation, intranasal administration of curcumin-carrying exosomes led to the uptake of exosomes by microglia cells and consequently to an effective curcumin-mediated anti-inflammatory effect (Zhuang et al. 2011). Moreover, administration of doxorubicin or paclitaxel-containing exosomes to tumor-bearing mice or zebra fishes induced antitumor effects (Tian et al. 2014; Yang et al. 2015). As exosomes can convey genetic material, they have been proposed for the delivery of exogenous RNA in disease states. Using electroporation, a siRNA against gluceraldehyde-3 phosphate dehydrogenase was incorporated into DC-derived exosomes and effectively delivered in vivo, leading to the loss of expression of its target gene (Alvarez-Erviti et al. 2011). Some years later, Ohno and colleagues used this technique to deliver miRNAs in breast cancer (Ohno et al. 2013). Breast cancer is associated with an increased expression of the epidermal growth factor receptor (EGFR) in cancer cells (Woodburn 1999). The authors first transfected an EGF-encoding plasmid into human embryonic kidney HEK293 cells and purified secreted exosomes expressing EGF on their surface. By transfecting the antitumor miRNA let-7a in the EGF-expressing exosomes, they were then successful to deliver let-7a miRNA specifically to EGFR-expressing xenograft breast cancer tissue in immunodeficient rag2 −/− mice (Ohno et al. 2013).
Another strategy based on treating donor cells with drugs has been developed in order to incorporate drugs inside exosomes. This approach enabled the incorporation of antitumor agents such as paclitaxel, etoposide, or carboplatin in HepG2 hepatoma cell line-derived exosomes (Lv et al. 2012). Furthermore, in vitro treatment of NK cells with these exosomes led to an increase of their cytotoxic activity toward cancer cells (Lv et al. 2012).
Finally, two groups have transfected macrophages with plasmids encoding therapeutic proteins such as catalase or glial cell line-derived neurotropic factor (Haney et al. 2013; Zhao et al. 2014). By injecting these macrophages to a mouse model of Parkinson’s disease, the authors observed the release of exosomes carrying modified genetic material and an improvement of motor functions with disease-associated neurodegeneration and neuroinflammation (Haney et al. 2013; Zhao et al. 2014).
7 Conclusion
Although exosomes have become the focus of exponentially growing interest since their first description about 30 years ago, our knowledge of these nanovesicles has only just begun. Working with exosomes remains challenging because of their small size and the fact that other extracellular vesicles (i.e., microvesicles, microparticles, etc.) or biofluid components can be co-extracted with exosomes. Although numerous studies have reported an important role of exosomes in immunoregulation, most of the time, the exosomal component responsible for the functional impact on recipient cells has not been identified. This might be due to the difficulty to identify a relevant candidate among the numerous exosomal proteins, nucleic acids, and lipids. Consequently, the mechanisms underlying the exosome-mediated immune responses observed in recipient cells (i.e., activated or inhibited signaling pathways, etc.) are not always elucidated. Moreover, the role of exosomes as an immunoregulator has been shown only in pathological states, and their functions in homeostasis remain to be elucidated. Finally, although only few experiments and clinical trials have been performed to date, the accessibility of exosomes in various body fluids, the proved safety and feasibility of the use of exosomes in clinical experiments, and the first promising results suggest that exosomes might become a future powerful diagnostic, vaccine, and drug delivery tool for numerous diseases.
Abbreviations
- Ag:
-
Antigen
- AIEC:
-
Adherent-invasive Escherichia coli
- APC:
-
Antigen-presenting cells
- APOBEC3G:
-
Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3G
- BAT3:
-
HLA-B-associated transcript 3
- BCR:
-
B-cell receptor
- CD:
-
Crohn’s disease
- CMV:
-
Cytomegalovirus
- CXCL11:
-
C-X-C motif chemokine 11
- DC:
-
Dendritic cell
- DT:
-
Diphtheria toxoid
- EBV:
-
Epstein-Barr virus
- EF1α1:
-
Elongation factor 1-alpha 1
- EM:
-
Electron microscopy
- ESCRT:
-
Endosomal sorting complexes required for transport
- EV:
-
Extracellular vesicle
- FACS:
-
Fluorescence activated cell sorting
- FasL:
-
Fas ligand
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- GPC1:
-
Glypican-1
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- HLA:
-
Human leukocyte antigen
- HSC:
-
Heat-shock cognate
- HSP:
-
Heat-shock protein
- HTLV-1:
-
Human T-cell leukemia virus type 1
- i.v.:
-
Intravenous
- ICAM-1:
-
Intercellular adhesion molecule 1
- IEC:
-
Intestinal epithelial cell
- IFN:
-
Interferon
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- ILV:
-
Intraluminal vesicles
- LAM:
-
Lipoarabinomannan
- LF:
-
Lethal factor
- LMP1:
-
Latent membrane protein 1
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- MCP-1:
-
Monocyte chemoattractant protein 1
- MFGE8:
-
Milk fat globule-EGF factor 8 protein
- MHC:
-
Major histocompatibility complex molecules
- miRNA:
-
MicroRNA
- MVE:
-
Multivesicular endosome
- MyD88:
-
Myeloid differentiation primary response protein 88
- NEF:
-
Negative regulatory factor
- NF-κB:
-
Nuclear factor-kappa B
- NK:
-
Natural killer
- NKG2D:
-
Natural killer group 2 member D receptor
- OVA:
-
Ovalbumin
- PA:
-
Protective antigen
- PAMP:
-
Pathogen-associated molecular pattern
- PBMC:
-
Peripheral blood mononuclear cells
- PfPTP2:
-
Plasmodium falciparum tyrosine phosphatase 2
- RANTES:
-
Regulated on activation, normal T cell expressed and secreted
- SNARE:
-
Soluble N-ethylmaleimide-sensitive fusion attachment protein (SNAP) receptors
- TAR:
-
Transactivating response
- TGF-β:
-
Tumor growth factor beta
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- tRNA:
-
Transfer RNA
- TSG101:
-
Tumor susceptibility gene 101
- vtRNA:
-
Vault RNA
References
Abrami L, Brandi L, Moayeri M, Brown MJ, Krantz BA, Leppla SH, van der Goot FG (2013) Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep 5:986–996. doi:10.1016/j.celrep.2013.10.019
Admyre C, Grunewald J, Thyberg J, Gripenbäck S, Tornling G, Eklund A, Scheynius A, Gabrielsson S (2003) Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J 22:578–583
Admyre C, Johansson SM, Paulie S, Gabrielsson S (2006) Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur J Immunol 36:1772–1781. doi:10.1002/eji.200535615
Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I (2004) Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun 72:4127–4137. doi:10.1128/IAI.72.7.4127-4137.2004
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345. doi:10.1038/nbt.1807
Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L (2002) Malignant effusions and immunogenic tumour-derived exosomes. Lancet 360:295–305. doi:10.1016/S0140-6736(02)09552-1
André F, Chaput N, Schartz NEC, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu D-H, Tursz T, Amigorena S, Angevin E, Zitvogel L (2004) Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol 172:2126–2136. doi:10.4049/jimmunol.172.4.2126
Ariza ME, Rivailler P, Glaser R, Chen M, Williams MV (2013) Epstein-Barr virus encoded dUTPase containing exosomes modulate innate and adaptive immune responses in human dendritic cells and peripheral blood mononuclear cells. PLoS One 8, e69827. doi:10.1371/journal.pone.0069827
Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD (2002) Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell 3:283–289
Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen L-AA, van Meerbeeck JP, Burgers SA, Hoogsteden HC, Lambrecht BN (2004) Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol 31:114–121. doi:10.1165/rcmb.2003-0238OC
Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, Orsi RH, Wiedmann M, McDonough P, Kim SG, Berg D, Schukken Y, Scherl E, Simpson KW (2007) Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. ISME J 1:403–418. doi:10.1038/ismej.2007.52
Beauvillain C, Juste MO, Dion S, Pierre J, Dimier-Poisson I (2009) Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine 27:1750–1757. doi:10.1016/j.vaccine.2009.01.022
Bhatnagar S, Schorey JS (2007) Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem 282:25779–25789. doi:10.1074/jbc.M702277200
Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS (2007) Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 110:3234–3244. doi:10.1182/blood-2007-03-079152
Caby M-P, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C (2005) Exosomal-like vesicles are present in human blood plasma. Int Immunol 17:879–887. doi:10.1093/intimm/dxh267
Carayon K, Chaoui K, Ronzier E, Lazar I, Bertrand-Michel J, Roques V, Balor S, Terce F, Lopez A, Salomé L, Joly E (2011) Proteolipidic composition of exosomes changes during reticulocyte maturation. J Biol Chem 286:34426–34439. doi:10.1074/jbc.M111.257444
Carrière J, Bretin A, Darfeuille-Michaud A, Barnich N, Nguyen HTT (2016) Exosomes released from cells infected with crohn’s disease-associated adherent-invasive Escherichia coli activate host innate immune responses and enhance bacterial intracellular replication. Inflamm Bowel Dis 22:516–528. doi:10.1097/MIB.0000000000000635
Cestari I, Ansa-Addo E, Deolindo P, Inal JM, Ramirez MI (2012) Trypanosoma cruzi immune evasion mediated by host cell-derived microvesicles. J Immunol 188:1942–1952. doi:10.4049/jimmunol.1102053
Chen BJ, Lamb RA (2008) Mechanisms for enveloped virus budding: can some viruses do without an ESCRT? Virology 372:221–232. doi:10.1016/j.virol.2007.11.008
Cheng Y, Schorey JS (2013) Exosomes carrying mycobacterial antigens can protect mice against an M. tuberculosis Infection. Eur J Immunol 43:3279–3290. doi:10.1002/eji.201343727
Chiu Y-L, Greene WC (2008) The APOBEC3 cytidine deaminases: an innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annu Rev Immunol 26:317–353. doi:10.1146/annurev.immunol.26.021607.090350
Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z (2005) Induction of heat shock proteins in B-cell exosomes. J Cell Sci 118:3631–3638. doi:10.1242/jcs.02494
Colino J, Snapper CM (2006) Exosomes from bone marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen-specific IgG responses in naive recipients in the absence of free antigen. J Immunol Baltim Md 1950(177):3757–3762
Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289. doi:10.1146/annurev-cellbio-101512-122326
Conte MP, Schippa S, Zamboni I, Penta M, Chiarini F, Seganti L, Osborn J, Falconieri P, Borrelli O, Cucchiara S (2006) Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 55:1760–1767. doi:10.1136/gut.2005.078824
Coppieters K, Barral AM, Juedes A, Wolfe T, Rodrigo E, Théry C, Amigorena S, von Herrath MG (2009) No significant CTL cross-priming by dendritic cell-derived exosomes during murine lymphocytic choriomeningitis virus infection. J Immunol 182:2213–2220. doi:10.4049/jimmunol.0802578
Couper KN, Barnes T, Hafalla JCR, Combes V, Ryffel B, Secher T, Grau GE, Riley EM, de Souza JB (2010) Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog 6, e1000744. doi:10.1371/journal.ppat.1000744
Dai S, Zhou X, Wang B, Wang Q, Fu Y, Chen T, Wan T, Yu Y, Cao X (2006) Enhanced induction of dendritic cell maturation and HLA-A*0201-restricted CEA-specific CD8(+) CTL response by exosomes derived from IL-18 gene-modified CEA-positive tumor cells. J Mol Med Berl Ger 84:1067–1076. doi:10.1007/s00109-006-0102-0
Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G (2008) Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther J Am Soc Gene Ther 16:782–790. doi:10.1038/mt.2008.1
Damo M, Wilson DS, Simeoni E, Hubbell JA (2015) TLR-3 stimulation improves anti-tumor immunity elicited by dendritic cell exosome-based vaccines in a murine model of melanoma. Sci Rep 5:17622. doi:10.1038/srep17622
Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel JF (1998) Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn’s disease. Gastroenterology 115:1405–1413. doi:10.1016/S0016-5085(98)70019-8
Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser A-L, Barnich N, Bringer M-A, Swidsinski A, Beaugerie L, Colombel J-F (2004) High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 127:412–421. doi:10.1053/j.gastro.2004.04.061
Davies BA, Lee JRE, Oestreich AJ, Katzmann DJ (2009) Membrane protein targeting to the MVB/lysosome. Chem Rev 109:1575–1586. doi:10.1021/cr800473s
de Carvalho JV, de Castro RO, da Silva EZM, Silveira PP, da Silva-Januário ME, Arruda E, Jamur MC, Oliver C, Aguiar RS, daSilva LLP (2014) Nef neutralizes the ability of exosomes from CD4+ T cells to act as decoys during HIV-1 infection. PLoS One 9, e113691. doi:10.1371/journal.pone.0113691
De Smaele E, Ferretti E, Gulino A (2010) MicroRNAs as biomarkers for CNS cancer and other disorders. Brain Res 1338:100–111. doi:10.1016/j.brainres.2010.03.103
Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ (2000) Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 113:3365–3374
Dreux M, Garaigorta U, Boyd B, Décembre E, Chung J, Whitten-Bauer C, Wieland S, Chisari FV (2012) Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe 12:558–570. doi:10.1016/j.chom.2012.08.010
Dukers DF, Meij P, Vervoort MB, Vos W, Scheper RJ, Meijer CJ, Bloemena E, Middeldorp JM (2000) Direct immunosuppressive effects of EBV-encoded latent membrane protein 1. J Immunol Baltim Md 1950(165):663–670
Escudier B, Dorval T, Chaput N, André F, Caby M-P, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq J-B, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L (2005) Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med 3:10. doi:10.1186/1479-5876-3-10
Fader CM, Sánchez DG, Mestre MB, Colombo MI (2009) TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta 1793:1901–1916. doi:10.1016/j.bbamcr.2009.09.011
Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ (2007) Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol 5, e158. doi:10.1371/journal.pbio.0050158
Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R (2006) Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 31:642–648. doi:10.1016/j.mcn.2005.12.003
Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR (1993) An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 178:2249–2254
Garcia-Silva MR, Cabrera-Cabrera F, Cura das Neves RF, Souto-Padrón T, de Souza W, Cayota A (2014) Gene expression changes induced by Trypanosoma cruzi shed microvesicles in mammalian host cells: relevance of tRNA-derived halves. BioMed Res Int. doi:10.1155/2014/305239
Giri PK, Schorey JS (2008) Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS One 3:e2461. doi:10.1371/journal.pone.0002461
Giri PK, Kruh NA, Dobos KM, Schorey JS (2010) Proteomic analysis identifies highly antigenic proteins in exosomes from M. tuberculosis-infected and culture filtrate protein-treated macrophages. Proteomics 10:3190–3202. doi:10.1002/pmic.200900840
Grigorov B, Attuil-Audenis V, Perugi F, Nedelec M, Watson S, Pique C, Darlix J-L, Conjeaud H, Muriaux D (2009) A role for CD81 on the late steps of HIV-1 replication in a chronically infected T cell line. Retrovirology 6:28. doi:10.1186/1742-4690-6-28
Haney MJ, Zhao Y, Harrison EB, Mahajan V, Ahmed S, He Z, Suresh P, Hingtgen SD, Klyachko NL, Mosley RL, Gendelman HE, Kabanov AV, Batrakova EV (2013) Specific transfection of inflamed brain by macrophages: a new therapeutic strategy for neurodegenerative diseases. PLoS One 8, e61852. doi:10.1371/journal.pone.0061852
Hanson PI, Cashikar A (2012) Multivesicular body morphogenesis. Annu Rev Cell Dev Biol 28:337–362. doi:10.1146/annurev-cellbio-092910-154152
Harding C, Heuser J, Stahl P (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97:329–339
Hassani K, Olivier M (2013) Immunomodulatory impact of leishmania-induced macrophage exosomes: a comparative proteomic and functional analysis. PLoS Negl Trop Dis 7, e2185. doi:10.1371/journal.pntd.0002185
Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ (1999) Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94:3791–3799
Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M, Möbius W, Rhee J, Barr FA, Simons M (2010) Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 189:223–232. doi:10.1083/jcb.200911018
Hu G, Drescher KM, Chen X-M (2012) Exosomal miRNAs: biological properties and therapeutic potential. Front Genet 3:56. doi:10.3389/fgene.2012.00056
Hu G, Gong A-Y, Roth AL, Huang BQ, Ward HD, Zhu G, Larusso NF, Hanson ND, Chen X-M (2013) Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog 9, e1003261. doi:10.1371/journal.ppat.1003261
Hurley JH (2010) The ESCRT complexes. Crit Rev Biochem Mol Biol 45:463–487. doi:10.3109/10409238.2010.502516
Hwang I, Shen X, Sprent J (2003) Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc Natl Acad Sci U S A 100:6670–6675. doi:10.1073/pnas.1131852100
Hyenne V, Apaydin A, Rodriguez D, Spiegelhalter C, Hoff-Yoessle S, Diem M, Tak S, Lefebvre O, Schwab Y, Goetz JG, Labouesse M (2015) RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol 211:27–37. doi:10.1083/jcb.201504136
Izquierdo-Useros N, Naranjo-Gómez M, Archer J, Hatch SC, Erkizia I, Blanco J, Borràs FE, Puertas MC, Connor JH, Fernández-Figueras MT, Moore L, Clotet B, Gummuluru S, Martinez-Picado J (2009) Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood 113:2732–2741. doi:10.1182/blood-2008-05-158642
Jaworski E, Narayanan A, Van Duyne R, Shabbeer-Meyering S, Iordanskiy S, Saifuddin M, Das R, Afonso PV, Sampey GC, Chung M, Popratiloff A, Shrestha B, Sehgal M, Jain P, Vertes A, Mahieux R, Kashanchi F (2014) Human T-lymphotropic virus type 1-infected cells secrete exosomes that contain Tax protein. J Biol Chem 289:22284–22305. doi:10.1074/jbc.M114.549659
Jolly C, Sattentau QJ (2007) Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in T cells take place in tetraspanin-enriched plasma membrane domains. J Virol 81:7873–7884. doi:10.1128/JVI.01845-06
Katzmann DJ, Babst M, Emr SD (2001) Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106:145–155
Khatua AK, Taylor HE, Hildreth JEK, Popik W (2009) Exosomes packaging APOBEC3G confer human immunodeficiency virus resistance to recipient cells. J Virol 83:512–521. doi:10.1128/JVI.01658-08
Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E, Le Moulec S, Moulec SLE, Guigay J, Hirashima M, Guemira F, Adhikary D, Mautner J, Busson P (2009) Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood 113:1957–1966. doi:10.1182/blood-2008-02-142596
Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 113:E968–977. doi:10.1073/pnas.1521230113
Kruh-Garcia NA, Wolfe LM, Chaisson LH, Worodria WO, Nahid P, Schorey JS, Davis JL, Dobos KM (2014) Detection of Mycobacterium tuberculosis peptides in the exosomes of patients with active and latent M tuberculosis infection using MRM-MS. PLoS One 9, e103811. doi:10.1371/journal.pone.0103811
Lai RC, Yeo RWY, Lim SK (2015) Mesenchymal stem cell exosomes. Semin Cell Dev Biol 40:82–88. doi:10.1016/j.semcdb.2015.03.001
Laulagnier K, Grand D, Dujardin A, Hamdi S, Vincent-Schneider H, Lankar D, Salles J-P, Bonnerot C, Perret B, Record M (2004a) PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett 572:11–14. doi:10.1016/j.febslet.2004.06.082
Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux J-F, Kobayashi T, Salles J-P, Perret B, Bonnerot C, Record M (2004b) Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J 380:161–171. doi:10.1042/BJ20031594
Laulagnier K, Vincent-Schneider H, Hamdi S, Subra C, Lankar D, Record M (2005) Characterization of exosome subpopulations from RBL-2H3 cells using fluorescent lipids. Blood Cells Mol Dis 35:116–121. doi:10.1016/j.bcmd.2005.05.010
Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y, Krogan NJ, Plemenitas A, Peterlin BM (2010) HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic Cph Den 11:110–122. doi:10.1111/j.1600-0854.2009.01006
Li Q, Shao Y, Zhang X, Zheng T, Miao M, Qin L, Wang B, Ye G, Xiao B, Guo J (2015) Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol J Int Soc Oncodev Biol Med 36:2007–2012. doi:10.1007/s13277-014-2807-y
Lin Z, Swan K, Zhang X, Cao S, Brett Z, Drury S, Strong MJ, Fewell C, Puetter A, Wang X, Ferris M, Sullivan DE, Li L, Flemington EK (2016) Secreted oral epithelial cell membrane vesicles induce Epstein-Barr virus (EBV) reactivation in latently infected B-cells. J Virol. doi:10.1128/JVI.02830-15
Llorente A, Skotland T, Sylvänne T, Kauhanen D, Róg T, Orłowski A, Vattulainen I, Ekroos K, Sandvig K (2013) Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta 1831:1302–1309
Longatti A, Boyd B, Chisari FV (2014) Virion-independent transfer of replication-competent hepatitis C virus RNA between permissive cells. J Virol 89:2956–2961. doi:10.1128/JVI.02721-14
Luketic L, Delanghe J, Sobol PT, Yang P, Frotten E, Mossman KL, Gauldie J, Bramson J, Wan Y (2007) Antigen presentation by exosomes released from peptide-pulsed dendritic cells is not suppressed by the presence of active CTL. J Immunol Baltim Md 1950(179):5024–5032
Lundholm M, Schröder M, Nagaeva O, Baranov V, Widmark A, Mincheva-Nilsson L, Wikström P (2014) Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS One 9. doi:10.1371/journal.pone.0108925
Lv L-H, Wan Y-L, Lin Y, Zhang W, Yang M, Li G-L, Lin H-M, Shang C-Z, Chen Y-J, Min J (2012) Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 287:15874–15885. doi:10.1074/jbc.M112.340588
Mantel P-Y, Hoang AN, Goldowitz I, Potashnikova D, Hamza B, Vorobjev I, Ghiran I, Toner M, Irimia D, Ivanov AR, Barteneva N, Marti M (2013) Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 13:521–534. doi:10.1016/j.chom.2013.04.009
Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, Englyst H, Williams HF, Rhodes JM (2004) Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology 127:80–93. doi:10.1053/j.gastro.2004.03.054
Martinez-Medina M, Aldeguer X, Lopez-Siles M, González-Huix F, López-Oliu C, Dahbi G, Blanco JE, Blanco J, Garcia-Gil LJ, Darfeuille-Michaud A (2009) Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn’s disease. Inflamm. Bowel Dis 15:872–882. doi:10.1002/ibd.20860
Marzesco A-M, Janich P, Wilsch-Bräuninger M, Dubreuil V, Langenfeld K, Corbeil D, Huttner WB (2005) Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci 118:2849–2858. doi:10.1242/jcs.02439
Masciopinto F, Giovani C, Campagnoli S, Galli-Stampino L, Colombatto P, Brunetto M, Yen TSB, Houghton M, Pileri P, Abrignani S (2004) Association of hepatitis C virus envelope proteins with exosomes. Eur J Immunol 34:2834–2842. doi:10.1002/eji.200424887
Mathivanan S, Fahner CJ, Reid GE, Simpson RJ (2012) ExoCarta 2012: database of exosomal proteins. RNA Lipids Nucleic Acids Res 40:D1241–1244. doi:10.1093/nar/gkr828
Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Fauré J, Blanc NS, Matile S, Dubochet J, Sadoul R, Parton RG, Vilbois F, Gruenberg J (2004) Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303:531–534. doi:10.1126/science.1092425
Meckes DG, Shair KHY, Marquitz AR, Kung C-P, Edwards RH, Raab-Traub N (2010) Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci U S A 107:20370–20375. doi:10.1073/pnas.1014194107
Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R (2015) Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523:177–182. doi:10.1038/nature14581
Metcalf D, Isaacs AM (2010) The role of ESCRT proteins in fusion events involving lysosomes, endosomes and autophagosomes. Biochem Soc Trans 38:1469–1473. doi:10.1042/BST0381469
Miranda KC, Bond DT, McKee M, Skog J, Păunescu TG, Da Silva N, Brown D, Russo LM (2010) Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int 78:191–199. doi:10.1038/ki.2010.106
Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MÁ, Bernad A, Sánchez-Madrid F (2011) Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2:282. doi:10.1038/ncomms1285
Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, Divito SJ, Papworth GD, Watkins SC, Robbins PD, Larregina AT, Morelli AE (2008) Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol Baltim Md 1950(180):3081–3090
Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan MLG, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE (2012) Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119:756–766. doi:10.1182/blood-2011-02-338004
Mori T, Mineta Y, Aoyama Y, Sera T (2008) Efficient secretion of the herpes simplex virus tegument protein VP22 from living mammalian cells. Arch Virol 153:1191–1195. doi:10.1007/s00705-008-0094-x
Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu D-H, Le Pecq J-B, Lyerly HK (2005) A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 3:9. doi:10.1186/1479-5876-3-9
Muller L, Muller-Haegele S, Mitsuhashi M, Gooding W, Okada H, Whiteside TL (2015) Exosomes isolated from plasma of glioma patients enrolled in a vaccination trial reflect antitumor immune activity and might predict survival. Oncoimmunology 4, e1008347. doi:10.1080/2162402X.2015.1008347
Munich S, Sobo-Vujanovic A, Buchser WJ, Beer-Stolz D, Vujanovic NL (2012) Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology 1:1074–1083. doi:10.4161/onci.20897
Napoletano C, Rughetti A, Landi R, Pinto D, Bellati F, Rahimi H, Spinelli GP, Pauselli S, Sale P, Dolo V, De Lorenzo F, Tomao F, Benedetti-Panici P, Frati L, Nuti M (2009) Immunogenicity of allo-vesicle carrying ERBB2 tumor antigen for dendritic cell-based anti-tumor immunotherapy. Int J Immunopathol Pharmacol 22:647–658
Narayanan A, Iordanskiy S, Das R, Van Duyne R, Santos S, Jaworski E, Guendel I, Sampey G, Dalby E, Iglesias-Ussel M, Popratiloff A, Hakami R, Kehn-Hall K, Young M, Subra C, Gilbert C, Bailey C, Romerio F, Kashanchi F (2013) Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J Biol Chem 288:20014–20033. doi:10.1074/jbc.M112.438895
Näslund TI, Gehrmann U, Gabrielsson S (2013a) Cancer immunotherapy with exosomes requires B-cell activation. Oncoimmunology 2, e24533. doi:10.4161/onci.24533
Näslund TI, Gehrmann U, Qazi KR, Karlsson MCI, Gabrielsson S (2013b) Dendritic cell-derived exosomes need to activate both T and B cells to induce antitumor immunity. J Immunol Baltim Md 1950(190):2712–2719. doi:10.4049/jimmunol.1203082
Neut C, Bulois P, Desreumaux P, Membré J-M, Lederman E, Gambiez L, Cortot A, Quandalle P, van Kruiningen H, Colombel J-F (2002) Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn’s disease. Am J Gastroenterol 97:939–946. doi:10.1111/j.1572-0241.2002.05613.x
Neves RFC, Fernandes ACS, Meyer-Fernandes JR, Souto-Padrón T (2014) Trypanosoma cruzi-secreted vesicles have acid and alkaline phosphatase activities capable of increasing parasite adhesion and infection. Parasitol Res 113:2961–2972. doi:10.1007/s00436-014-3958-x
Nolte-’t Hoen ENM, Buschow SI, Anderton SM, Stoorvogel W, Wauben MHM (2009) Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood 113:1977–1981. doi:10.1182/blood-2008-08-174094
Nolte-’t Hoen ENM, Buermans HPJ, Waasdorp M, Stoorvogel W, Wauben MHM, ’t Hoen PAC (2012) Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 40:9272–9285. doi:10.1093/nar/gks658
Obregon C, Rothen-Rutishauser B, Gerber P, Gehr P, Nicod LP (2009) Active uptake of dendritic cell-derived exovesicles by epithelial cells induces the release of inflammatory mediators through a TNF-alpha-mediated pathway. Am J Pathol 175:696–705. doi:10.2353/ajpath.2009.080716
Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M (2013) Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther J Am Soc Gene Ther 21:185–191. doi:10.1038/mt.2012.180
Oliveira DL, Freire-de-Lima CG, Nosanchuk JD, Casadevall A, Rodrigues ML, Nimrichter L (2010) Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect Immun 78:1601–1609. doi:10.1128/IAI.01171-09
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C (2010) Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12:19–30. doi:10.1038/ncb2000
Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J, Wong DT (2010) Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS One 5, e8577. doi:10.1371/journal.pone.0008577
Pan BT, Teng K, Wu C, Adam M, Johnstone RM (1985) Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101:942–948
Panepinto J, Komperda K, Frases S, Park Y-D, Djordjevic JT, Casadevall A, Williamson PR (2009) Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol Microbiol 71:1165–1176. doi:10.1111/j.1365-2958.2008.06588.x
Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MAJ, Hopmans ES, Lindenberg JL, de Gruijl TD, Würdinger T, Middeldorp JM (2010) Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A 107:6328–6333. doi:10.1073/pnas.0914843107
Pisitkun T, Shen R-F, Knepper MA (2004) Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 101:13368–13373. doi:10.1073/pnas.0403453101
Quah BJC, O’Neill HC (2007) Mycoplasma contaminants present in exosome preparations induce polyclonal B cell responses. J Leukoc Biol 82:1070–1082. doi:10.1189/jlb.0507277
Rabinowits G, Gerçel-Taylor C, Day JM, Taylor DD, Kloecker GH (2009) Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer 10:42–46. doi:10.3816/CLC.2009.n.006
Raiborg C, Stenmark H (2002) Hrs and endocytic sorting of ubiquitinated membrane proteins. Cell Struct Funct 27:403–408
Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, Willemsen R, Demmers JAA, Stalin Raj V, Jenster G, Kwekkeboom J, Tilanus HW, Haagmans BL, Baumert TF, van der Laan LJW (2013) Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A 110:13109–13113. doi:10.1073/pnas.1221899110
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183:1161–1172
Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C (1997) Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell 8:2631–2645. doi:10.1091/mbc.8.12.2631
Record M, Carayon K, Poirot M, Silvente-Poirot S (2014) Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta 1841:108–120. doi:10.1016/j.bbalip.2013.10.004
Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, Baum J, Cowman AF (2013) Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 153:1120–1133. doi:10.1016/j.cell.2013.04.029
Sasaki M, Sitaraman SV, Babbin BA, Gerner-Smidt P, Ribot EM, Garrett N, Alpern JA, Akyildiz A, Theiss AL, Nusrat A, Klapproth J-MA (2007) Invasive Escherichia coli are a feature of Crohn’s disease. Lab Investig J Tech Methods Pathol 87:1042–1054. doi:10.1038/labinvest.3700661
Sato K, Aoki J, Misawa N, Daikoku E, Sano K, Tanaka Y, Koyanagi Y (2008) Modulation of human immunodeficiency virus type 1 infectivity through incorporation of tetraspanin proteins. J Virol 82:1021–1033. doi:10.1128/JVI.01044-07
Savina A, Vidal M, Colombo MI (2002) The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci 115:2505–2515
Schnitzer JK, Berzel S, Fajardo-Moser M, Remer KA, Moll H (2010) Fragments of antigen-loaded dendritic cells (DC) and DC-derived exosomes induce protective immunity against Leishmania major. Vaccine 28:5785–5793. doi:10.1016/j.vaccine.2010.06.077
Segura E, Nicco C, Lombard B, Véron P, Raposo G, Batteux F, Amigorena S, Théry C (2005) ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 106:216–223. doi:10.1182/blood-2005-01-0220
Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, Lynn MA, McMaster WR, Foster LJ, Levings MK, Reiner NE (2010a) Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol 185:5011–5022. doi:10.4049/jimmunol.1000541
Silverman JM, Clos J, de’Oliveira CC, Shirvani O, Fang Y, Wang C, Foster LJ, Reiner NE (2010a). An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J. Cell Sci 123:842–852. doi:10.1242/jcs.056465
Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, Kufer TA, Engert A, Pogge von Strandmann E (2008) Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One 3, e3377. doi:10.1371/journal.pone.0003377
Singh PP, LeMaire C, Tan JC, Zeng E, Schorey JS (2011) Exosomes released from M. tuberculosis infected cells can suppress IFN-γ mediated activation of naïve macrophages. PLoS One 6, e18564. doi:10.1371/journal.pone.0018564
Singh PP, Smith VL, Karakousis PC, Schorey JS (2012) Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo. J Immunol Baltim Md 1950(189):777–785. doi:10.4049/jimmunol.1103638
Skokos D, Le Panse S, Villa I, Rousselle JC, Peronet R, David B, Namane A, Mécheri S (2001) Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J Immunol Baltim Md 1950(166):868–876
Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, Boudaly S, Mécheri S (2003) Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol 170:3037–3045. doi:10.4049/jimmunol.170.6.3037
Song J, Chen X, Wang M, Xing Y, Zheng Z, Hu S (2014) Cardiac endothelial cell-derived exosomes induce specific regulatory B cells. Sci Rep 4:7583. doi:10.1038/srep07583
Sotillo J, Pearson M, Potriquet J, Becker L, Pickering D, Mulvenna J, Loukas A (2015) Extracellular vesicles secreted by Schistosoma mansoni contain protein vaccine candidates. Int J Parasitol. doi:10.1016/j.ijpara.2015.09.002
Stoorvogel W, Strous GJ, Geuze HJ, Oorschot V, Schwartz AL (1991) Late endosomes derive from early endosomes by maturation. Cell 65:417–427
Stuffers S, Sem Wegner C, Stenmark H, Brech A (2009) Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic Cph Den 10:925–937. doi:10.1111/j.1600-0854.2009.00920.x
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang H-G (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther J Am Soc Gene Ther 18:1606–1614. doi:10.1038/mt.2010.105
Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M, Lochs H (2002) Mucosal flora in inflammatory bowel disease. Gastroenterology 122:44–54. doi:10.1053/gast.2002.30294
Szajnik M, Derbis M, Lach M, Patalas P, Michalak M, Drzewiecka H, Szpurek D, Nowakowski A, Spaczynski M, Baranowski W, Whiteside TL (2013) Exosomes in plasma of patients with ovarian carcinoma: potential biomarkers of tumor progression and response to therapy. Gynecol Obstet Sunnyvale Calif Suppl 4:3. doi:10.4172/2161-0932.S4-003
Tamai K, Tanaka N, Nakano T, Kakazu E, Kondo Y, Inoue J, Shiina M, Fukushima K, Hoshino T, Sano K, Ueno Y, Shimosegawa T, Sugamura K (2010) Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun 399:384–390. doi:10.1016/j.bbrc.2010.07.083
Tan SS, Yin Y, Lee T, Lai RC, Yeo RWY, Zhang B, Choo A, Lim SK (2013) Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. J. Extracell Vesicles 2. doi:10.3402/jev.v2i0.22614
Taylor DD, Gercel-Taylor C (2008) MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 110:13–21. doi:10.1016/j.ygyno.2008.04.033
Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1999) Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 147:599–610
Théry C, Duban L, Segura E, Véron P, Lantz O, Amigorena S (2002) Indirect activation of naïve CD4+ T cells by dendritic cell–derived exosomes. Nat Immunol 3:1156–1162. doi:10.1038/ni854
Théry C, Amigorena S., Raposo, G., Clayton, A., (2006). Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Editor. Board Juan Bonifacino Al Chapter 3, Unit 3.22. doi:10.1002/0471143030.cb0322s30
Théry C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593. doi:10.1038/nri2567
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G (2014) A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35:2383–2390. doi:10.1016/j.biomaterials.2013.11.083
Tokuhisa M, Ichikawa Y, Kosaka N, Ochiya T, Yashiro M, Hirakawa K, Kosaka T, Makino H, Akiyama H, Kunisaki C, Endo I (2015) Exosomal miRNAs from peritoneum lavage fluid as potential prognostic biomarkers of peritoneal metastasis in gastric cancer. PLoS One 10, e0130472. doi:10.1371/journal.pone.0130472
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247. doi:10.1126/science.1153124
Usami Y, Popov S, Popova E, Inoue M, Weissenhorn WG, Göttlinger H (2009) The ESCRT pathway and HIV-1 budding. Biochem Soc Trans. 37:181–184. doi:10.1042/BST0370181
Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M, Minami M (2003) MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol Lett 89:125–131. doi:10.1016/S0165-2478(03)00128-7
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659. doi:10.1038/ncb1596
van den Boorn JG, Daßler J, Coch C, Schlee M, Hartmann G (2013) Exosomes as nucleic acid nanocarriers. Adv Drug Deliv Rev 65:331–335. doi:10.1016/j.addr.2012.06.011
Van Giau V, An SSA (2016) Emergence of exosomal miRNAs as a diagnostic biomarker for Alzheimer’s disease. J Neurol Sci 360:141–152. doi:10.1016/j.jns.2015.12.005
van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G (2011) The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell 21:708–721. doi:10.1016/j.devcel.2011.08.019
Verweij FJ, van Eijndhoven MAJ, Hopmans ES, Vendrig T, Wurdinger T, Cahir-McFarland E, Kieff E, Geerts D, van der Kant R, Neefjes J, Middeldorp JM, Pegtel DM (2011) LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-kB activation. EMBO J 30:2115–2129. doi:10.1038/emboj.2011.123
Viaud S, Terme M, Flament C, Taieb J, André F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T, Zitvogel L, Chaput N (2009) Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One 4, e4942. doi:10.1371/journal.pone.0004942
Viaud S, Théry C, Ploix S, Tursz T, Lapierre V, Lantz O, Zitvogel L, Chaput N (2010) Dendritic cell-derived exosomes for cancer immunotherapy: what’s next? Cancer Res 70:1281–1285. doi:10.1158/0008-5472.CAN-09-3276
Wakim LM, Bevan MJ (2011) Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature 471:629–632. doi:10.1038/nature09863
Wang J, Chen C, Xie P, Pan Y, Tan Y, Tang L (2014) Proteomic analysis and immune properties of exosomes released by macrophages infected with Mycobacterium avium. Microbes Infect Inst Pasteur 16:283–291. doi:10.1016/j.micinf.2013.12.001
Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L (2001) Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7:297–303. doi:10.1038/85438
Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH (2009) Membrane scission by the ESCRT-III complex. Nature 458:172–177. doi:10.1038/nature07836
Woodburn JR (1999) The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther 82:241–250
Woodman PG, Futter CE (2008) Multivesicular bodies: co-ordinated progression to maturity. Curr Opin Cell Biol 20:408–414. doi:10.1016/j.ceb.2008.04.001
Wubbolts R, Leckie RS, Veenhuizen PTM, Schwarzmann G, Möbius W, Hoernschemeyer J, Slot J-W, Geuze HJ, Stoorvogel W (2003) Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem 278:10963–10972. doi:10.1074/jbc.M207550200
Yáñez-Mó M, Siljander PR-M, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NHH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers E-M, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen ENM, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MHM, De Wever O (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066
Yang C, Chalasani G, Ng Y-H, Robbins PD (2012) Exosomes released from Mycoplasma infected tumor cells activate inhibitory B cells. PLoS One 7, e36138. doi:10.1371/journal.pone.0036138
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S (2015) Exosome delivered anticancer drugs across the blood–brain barrier for brain cancer therapy in Danio rerio. Pharm Res 32:2003–2014. doi:10.1007/s11095-014-1593-y
Yewdell JW, Dolan BP (2011) Cross–dressers turn on T cells. Nature 471:581–582. doi:10.1038/471581a
Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, Wang K, Ba Y, Wang Q, Wang D, Yang J, Liu P, Xu T, Yan Q, Zhang J, Zen K, Zhang C-Y (2010) Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 39:133–144. doi:10.1016/j.molcel.2010.06.010
Zhang J, Basher F, Wu JD (2015) NKG2D ligands in tumor immunity: two sides of a coin. Front Immunol 6. doi:10.3389/fimmu.2015.00097
Zhao Y, Haney MJ, Gupta R, Bohnsack JP, He Z, Kabanov AV, Batrakova EV (2014) GDNF-transfected macrophages produce potent neuroprotective effects in Parkinson’s disease mouse model. PLoS One 9, e106867. doi:10.1371/journal.pone.0106867
Zhao Z, Yang Y, Zeng Y, He M (2015) A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. doi:10.1039/c5lc01117e
Zhou H, Pisitkun T, Aponte A, Yuen PST, Hoffert JD, Yasuda H, Hu X, Chawla L, Shen R-F, Knepper MA, Star RA (2006) Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int 70:1847–1857. doi:10.1038/sj.ki.5001874
Zhou X, Zhu W, Li H, Wen W, Cheng W, Wang F, Wu Y, Qi L, Fan Y, Chen Y, Ding Y, Xu J, Qian J, Huang Z, Wang T, Zhu D, Shu Y, Liu P (2015) Diagnostic value of a plasma microRNA signature in gastric cancer: a microRNA expression analysis. Sci Rep 5:11251. doi:10.1038/srep11251
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang H-G (2011) Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther J Am Soc Gene Ther 19:1769–1779. doi:10.1038/mt.2011.164
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4:594–600
Zöller M (2016) Exosomes in cancer disease. Methods Mol Biol Clifton NJ 1381:111–149. doi:10.1007/978-1-4939-3204-7_7
Zylbersztejn K, Galli T (2011) Vesicular traffic in cell navigation. FEBS J 278:4497–4505. doi:10.1111/j.1742-4658.2011.08168.x
Acknowledgments
This work was supported by the Ministère de la Recherche et de la Technologie, Inserm (UMR1071), INRA (USC 2018), the European Union FP7 People Marie Curie International Incoming Fellowship (to H.N.), and grants from the Association F. Aupetit (AFA), Région Auvergne (“Nouveau chercheur” grant to H.N.) and Agence Nationale de la Recherche (ANR “Jeune Chercheuse Jeune Chercheur” Nutribiote to N.B).
No competing interest exists.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Carrière, J., Barnich, N., Nguyen, H.T.T. (2016). Exosomes: From Functions in Host-Pathogen Interactions and Immunity to Diagnostic and Therapeutic Opportunities. In: Nilius, B., de Tombe, P., Gudermann, T., Jahn, R., Lill, R., Petersen, O. (eds) Reviews of Physiology, Biochemistry and Pharmacology, Vol. 172. Reviews of Physiology, Biochemistry and Pharmacology, vol 172. Springer, Cham. https://doi.org/10.1007/112_2016_7
Download citation
DOI: https://doi.org/10.1007/112_2016_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49901-7
Online ISBN: 978-3-319-49902-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)