Abstract
The underutilized fishery resources, such as non-commercial species and by-products generated on board and in land (e.g. heads, trimmings, skin and others), are an opportunity to obtain food products for human consumption, in line with the Sustainable Development Goals. Fish contains high-quality protein, with a content on a wet basis normally ranging from 15 to 25%, an adequate amino acid profile and good digestibility. Enzymatic hydrolysis of proteins releases peptides that may exert several physiological effects by interacting with human metabolism. Fish waste materials have been reported as a compelling source of antidiabetic peptides, which could be employed as ingredients to fortify foodstuffs intended for nutraceutical applications. This chapter provides a review of the production and characterization of antidiabetic peptides from fish waste protein, the mechanisms explaining their antidiabetic effect and their potential incorporation in food matrices as functional ingredients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Fish by-products
- Fish discards
- Enzymatic hydrolysis
- Amylase
- Diabetes
- Dipeptidyl peptidase IV
- DPP-IV
- Food matrix
- Glucose
- Glucosidase
- Inhibitory peptides
- Insulin
1 Introduction
1.1 Fish Waste
The estimated population increase for the next decades would require a complete restructuring of the food system, including the search for new sources of macronutrients that supply the food demand in a sustainable way, without compromising the health of human beings, and ideally, preventing the development of diseases. A sustainable management of all the available natural resources should become a priority in modern societies (Thirukumaran et al. 2022), including the valorization of fish waste in agreement with the circular economy model. Aquaculture and capture fisheries account for production of almost 180 million tonnes in 2018, showing an increasing compounded annual growth rate of 2–3.5% (FAO 2020). Most of these aquatic resources are dedicated to food (87.2%) but only 50–60% of these materials are finally consumed by humans (Wu et al. 2021). Therefore, a considerable amount of by-products is generated, which currently end up as low-value products or waste. The latter implies the need to develop upgrading processes for the production of high-value compounds (e.g. bioactive compounds) (Abdollahi et al. 2021). Nevertheless, the potential use of fish waste as a source for the production of bioactive compounds requires a safety assessment due to different risks associated with fish consumption. This evaluation should be based on a case-by-case analysis, searching for specific analytes prone to accumulate in fish flesh such as heavy metals or polychlorinated biphenyl (Castro-González and Méndez-Armenta 2008; Judd et al. 2004).
In line with the principles of circular economy, fish waste is regarded as a sustainable source to obtain high-value products. The use of fish waste, including bones, head, skin, scales, fins, tails, gut, and viscera, as raw material to produce bioactive compounds has been proposed as a strategy to provide added value to fish waste materials. Similarly, non-targeted fish species, which are commonly discarded or underutilized due to their low commercial value, can be an adequate source of bioactive compounds (Despoti et al. 2021; Jawad 2021; Więcaszek et al. 2015). Nevertheless, the use of non-commercial species is constrained by the lack of knowledge on their compositional, safety and distribution, as well as the environmental impact that its exploitation would entail. In general, fishes are a source of macronutrients, where proteins are of special interest. Several factors influence the protein content of a species such as sex, age, sexual maturity, nutritive conditions and others. In addition, the variation of protein content can be rhythmic, periodic or non-periodic (Lozano and Hardisson 2003; Maschmeyer et al. 2020). Overall, the protein content does not present wide seasonal variations, as is the case of lipids. In previous studies covering both non-targeted and commercial undersized fish species from the Mediterranean Sea, the proximate composition of the species was monitored within the period 2011–2013, reporting a protein content from 15.5 to 23.1 wt% (Table 1) (García-Moreno et al. 2013; Morales-Medina et al. 2015). Protein content presented minor variations throughout the seasons even for non-fatty and semi-fatty fish species (e.g. axillary seabream, small-spotted catshark and bogue), where lipids may not be sufficient to provide the required energy. It is worth noting that small-spotted catshark showed the highest protein values (>20%), which is attributed to the higher presence of non-protein nitrogen compounds in elasmobranchs such as trimethylamine oxide (TMAO), ammonia and urea (García-Moreno et al. 2013; Morales-Medina et al. 2015; Morales-Medina et al. 2016a).
The use of fish waste for human consumption is aligned with the Sustainable Development Goals (SDGs). Among these SGDs listed by the United Nations, the number 14 is “Life Below Water”. The aim of this goal is the conservation and responsible use of marine resources, which can be split into different objectives such as control of marine pollution, sustainable management of marine ecosystems or regulations on fishing exploitation. The oceans are crucial for maintaining life on Earth. Therefore, marine protected areas must be managed effectively, establishing conservation measures on at least 10% of coastal and marine areas.
In this framework, the valorization of fish by-products and waste materials is needed to achieve this goal (Nirmal et al. 2022a). In fisheries and aquaculture, it is estimated that 35% of the catch is lost or wasted each year worldwide and 12% of total fishery production is used for non-food purposes; in spite of the continuous efforts and the legislative measures taken in different areas. The amount of by-products generated by fishing and fish processing industry presents large variations among species. According to the estimation of by-products generated by commercial trade, fish trade and fish transformation activities in France (Fig. 1) represent between 33 and 64% of the total mass of fish catches (Perez-Galvez and Berge 2008).
This situation poses an economic and environmental problem that has to be tackled from different approaches. Reducing fish loss and waste can lead to reduced pressure on fish stocks and help improve resource sustainability as well as food security. As an example, in Europe, “Regulation (EU) 2020/852 on the establishment of a framework to facilitate sustainable investment” establishes the criteria for determining whether an economic activity qualifies as environmentally sustainable. Marine resources can address other social, economic and environmental challenges currently affecting our food system, by (i) providing high-quality proteins and fatty acids sources, in order to combat poverty (SDG1); (ii) provide safe and healthy foodstuff, ensuring food security and improved health (SDG2 and SGD3); (iii) to address climate change by introducing into the food chain products initially considered worthless (SDG13), among others.
On the other hand, the twenty-first century has brought about a change in the lifestyle of human beings, with new consumption habits due to the development of products thanks to the technological revolution that has occurred in the food industry. This has led to a large part of the population consuming high levels of substances such as sugar or salts which may cause health disorders. This situation, added to the sedentary habits established in modern western society, has increased the prevalence of diseases related to the cardiovascular system or metabolic syndrome, with forecasts of even greater growth in the near future. To avoid this, current research in nutrition and food technology tries to develop products that meet the needs of consumers, at the same time, they produce a health beneficial effect. It has been shown that the consumption of certain foods can help prevent or pre-treat certain diseases, and this strategy could imply a reduction of economic cost, compared to those associated with the treatment of the disease, as well as the welfare of humans (Hewage et al. 2020; Li et al. 2010; Mata-Cases et al. 2020).
Consequently, the use of fish waste as a source of high-value products should be regarded as a strategy with a double purpose: (i) to make use of these currently wasted resources and (ii) to contribute to the development of a healthier society by turning this waste into products suitable for human consumption that can exert beneficial physiological effects on the human.
1.2 Prevention and Pre-treatment of Diabetes
Diabetes mellitus type II is a metabolic disease causing elevated blood glucose levels in humans (impaired glucose homeostasis). It is characterized by insulin resistance, which is an insufficient or inefficient production of this hormone—responsible for the glucose uptake in the muscle, or the lack of activity of it. There are several risk factors for prediabetes and type II diabetes, including sedentary behaviours or food consumption patterns. The consequences of this hyperglycaemia can lead to several health problems of different degrees of severity (e.g. from renal or kidney failure to nerve damage and cardiovascular disorders) (“IDF Diabetes Atlas” 2017; Rivero-Pino et al. 2020a).
There are numerous strategies currently available to overcome this metabolic disorder. Insulin injection is the most commonly known, but it cannot be orally administered. Medicaments with different targets are also available to treat the disease, such as dipeptidyl peptidase IV (DPP-IV) inhibitors, sodium-glucose cotransporter 2 (SGLT2) inhibitors, glucosidase inhibitors, amylases inhibitors, insulin sensitizers, insulin secretagogues, glucagon-like peptide (GLP-1) mimetics or glizofins. Nonetheless, these synthetic drugs may pose a risk for patients due to the side effects that can occur upon its consumption (Hira et al. 2021; Patil et al. 2015; Tahrani et al. 2011).
These adverse side effects have promoted the search for antidiabetic compounds of natural origin, such as bioactive peptides. Peptides and amino acids from food proteins play an essential role in the regulation of glucose homeostasis. These molecules can exert several activities, such as GLP-1 regulation, the inhibition of digestion-related enzymes (as described for the medicaments above), enhancement of cholecystokinin levels, a gut hormone regulating food intake, or as signalling molecules in enteroendocrine cells, among others. Thus, protein intake has been correlated with body fat loss, insulin secretion and glycaemia reduction. However, the mechanisms behind these physiological changes are still under research (Patil et al. 2015; Rivero-Pino et al. 2020a; Tahrani et al. 2011).
The digestion of carbohydrates (i.e. mainly consisting of polysaccharide molecules) releases glucose molecules, which increase their concentration in the bloodstream. A potential approach could be limiting the hydrolysis of such polymers by enzyme inhibitors i.e. amylase and glucosidase, that can occur at different levels of digestion, effectively avoiding postprandial hyperglycaemia (Ibrahim et al. 2017; Patil et al. 2015). In addition, delayed carbohydrate absorption can help stimulate GLP-1 secretion, leading to the incretin effect. Examples of compounds inhibiting these enzymes are acarbose, miglitol and voglibose, correlated with numerous side effects, such as diarrhoea or abdominal pain between others (Inzucchi et al. 2012). In this regard, some food-derived peptides have shown inhibitory activity against digestive enzymes, being an adequate strategy for diabetes prevention as well as for complementing the traditional treatments without negative effects.
Other treatments to tackle diabetes are based on the modulation of incretins. Food intake causes the release of hormones called incretins (GLP-1 and GIP), in concentrations ranging based on physiological needs and regulated by the enzyme DPP-IV, being this latter enzyme responsible for their degradation (e.g. DPP-IV inactivates more than 95% of GLP-1). Thus, it was discovered that the inhibition of DPP-IV would have an impact on the concentrations of incretins, increasing the half-life of these, and consequently, their incretin effect (Curran et al. 2019). Examples of these inhibitors, commonly known as gliptins, are sitagliptin or metformin, which have been showed to exert some adverse effects upon its consumption such as headache, dermatitis and the risk of acute pancreatitis, and long-term effects are not well characterized. Similarly, for the hydrolase inhibitors, the ability of food-derived peptides to interact with this enzyme, and exert the same inhibitory effect avoiding the negative effects is currently seen as an adequate strategy to prevent the development of diabetes.
Currently, in literature, the information regarding antidiabetic peptides from food-derived sources is related to the ability of these sequences to inhibit amylase, glucosidase and/or DPP-IV. At a physiological level, the most relevant would be the inhibition of DPP-IV, considering the mechanism of action of this enzyme.
1.3 Protein, Protein Hydrolysates and Peptides
Proteins are one of the most important components of the human diet, consisting of chains of amino acids linked (Nelson and Cox 2008). The intake of these macromolecules leads to the release of peptides during digestion that can have beneficial effects due to their similarity to the structure of human regulatory peptides, since they can interact with some enzymes and receptors involved in human metabolism. Furthermore, protein hydrolysis provides the amino acids necessary for the synthesis of endogenous proteins in ribosomes. Protein content can vary depending on fish species but, as mentioned before, within the same species the level of protein is considerably stable throughout the year. Fish waste can encompass diverse parts, including muscles, showing high digestibility and well-balanced amino acid composition (Table 2) and according to their solubility index, they can be classified as myofibrillar, sarcoplasmic, and alkali-soluble proteins. Fish skins, which account for 30% w/w of by-products, are a source of collagen, from which gelatin can be obtained by thermal denaturation or partial hydrolysis (Nirmal et al. 2022b).
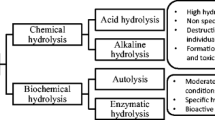
Fish protein can be processed to manufacture protein hydrolysates, which are the resulting pool of peptides obtained by cleaving the native protein, by means of different techniques. The most common techniques to obtain peptides from protein are enzymatic hydrolysis, fermentation or acid/alkali hydrolysis (Kristinsson and Rasco 2000; Marti-Quijal et al. 2020; Nongonierma and FitzGerald 2017).
Enzymatic hydrolysis is the most studied technique in literature to obtain protein hydrolysates and peptides, showing technological advantages compared to the other techniques. Indeed, according to a simple search in the scientific database Scopus, in the last 10 years, the number of studies dealing with protein hydrolysates was around 3500 studies of which 68% would be related to enzymatic hydrolysis.
The enzymatic hydrolysis reaction works under moderate conditions of temperature and pH, leading to a controllable reaction, and thus, a specific product, due to the action of proteases. It is widely reported that peptides exerting bioactivity usually contain from two to twenty amino acid residues. Different hydrolysates have been produced at a pilot or semi-pilot plant scale from fish discards (Harnedy-Rothwell et al. 2020; Remme et al. 2022; Vázquez et al. 2020) for their valorization, where the authors also carried out a sustainability assessment (Vázquez et al. 2020), and concluded that fish protein hydrolysates technology minimizes impact when compared with operating fish meal plants. The enzymes able to cleave proteins to produce the hydrolysates are called proteases (EC 3.4.X.X), classified either as endo- or exopeptidases. Their active site determines its substrate specificity and therefore the length and composition of the peptides released (Tavano et al. 2018). Further information on the proteases employed to produce specific types of antidiabetic peptides can be found elsewhere (Rivero-Pino et al. 2020a).
The enzymatic hydrolysis of protein can be conducted employing the fish waste directly or stabilized by different pre-treatments. Commonly, a high-temperature thermal pretreatment is applied in order to deactivate the endogenous enzymes contained in the material, to avoid the uncontrolled hydrolysis of the material, obtaining a potentially uncharacterized substrate (Derouiche Ben Maiz et al. 2019). In addition, defatting the raw material, in order to obtain a high-concentrated protein product, allows for optimizing the release of peptides as well as avoiding the potential lipid oxidation of the fat content contained in the fish.
It has been also described the application of non-thermal pre-treatments to modify the structure of the protein, aiming to improve the release of bioactive peptides (Rivero-Pino et al. 2020e; Thirukumaran et al. 2022). One such example is the application of microwave before the hydrolysis carried out by Ketnawa et al. (2018a, b) on rainbow trout (Oncorhynchus mykiss) by-products, aiming to obtain DPP-IV inhibitory peptides. Similarly, ultrasound (Petcharat et al. 2021) or high pressure (Hemker et al. 2020; Rivero-Pino et al. 2020b) treatments have been employed on fish waste, assessing the effect over biological properties (e.g. antioxidant) or technologies properties (water holding capacity, oil binding capacity, emulsification, among others). This behaviour is caused by the modification of the tertiary and quaternary protein structures, denaturation, aggregation or precipitation.
The use of fish products as raw material to manufacture protein hydrolysates encompass several challenges, such as: (i) the reproducibility when manufacturing the final product on an industrial scale; (ii) the manufacturing of a product odourless, flavourless and visually appealing to the consumer (Harnedy‐Rothwell et al. 2021a, b); (iii) microbiological contamination and lipid peroxidation; (iv) the feed of the fishes can affect the content of amino acids to some extent and (v) the effect of endogenous enzymes in releasing peptides before the enzymatic treatment has to be taken into consideration.
Furthermore, the safety of the manufactured product has to be evaluated, always considering hygiene measures, quality control, labelling requirements and relevant legislation according to the region where the product wants to be marketed.
2 Bioactivity of Peptides
Several peptide sequences have been identified in fish protein hydrolysates exerting inhibitory activity linked to an antidiabetic effect. The hierarchy of assays currently used to assess peptide activity begins with in vitro assays. These analyses should be completed with further studies on in vivo models, able to evaluate the interaction of bioactive compounds with other food components and their bioavailability after ingestion.
The inclusion of bioactive peptides within nutraceutical preparations should be accompanied by scientific statements on their potential health benefits. Health claims refer to how the intake of a food component implies a reduction of disease risk. A comprehensive and specific regulation applies to health-related claims in the scope of food, including their labelling, advertising, or any kind of communication activity. Each country/region has its own regulatory framework (Domínguez Díaz et al. 2020). Nowadays, there is no standard method to state a health claim for foodstuff but overall, the requirements in scientific terms are similar (Chalamaiah et al. 2019).
In the United States, food companies must make a request to the Food and Drug Administration, and the health claim depends on a Significant Scientific Agreement or on an authoritative statement from an appropriate scientific body of the US Government or the National Academy of Sciences or any of its subdivisions. In Europe, food business operators must submit their request to the European Commission, since health claims are regulated by Regulation (EC) No 1924/2006 on nutrition and health claims made on food, and the scientific evaluation is carried out by the European Food Safety. In Japan, the Minister of Consumer Affairs Agency of the Government of Japan is in charge of categorizing the products as Food for Specified Health Use (FOSHU).
2.1 In Vitro Studies
As mentioned above, antidiabetic peptides from food-derived proteins act as inhibitors of some of the proteases involved in carbohydrate metabolism (e.g. α-amylase, α-glucosidase and dipeptidyl peptidase IV). The effect of α-amylase and α-glucosidase inhibitors obtained from proteins has been reported to a lesser extent, compared to DPP-IV inhibition. A report evaluating the amylase inhibitory activity of fish water-soluble protein hydrolysates was conducted by Liu et al. (2013), using Response Surface Methodology to optimize the hydrolysis conditions for maximal release of amylase inhibitory peptides. Medenieks and Vasiljevic (2008) evaluated three species of underutilized fish (i.e. silver warehou, barracouta and Australian salmon) subjected to different enzymatic treatments as sources for bioactive peptides. Overall, no α-glucosidase inhibition and low amylase inhibition were observed. Many studies employed collagen as a substrate for obtaining inhibitory peptides. Kumar et al. (2019) carried out the hydrolysis of unicorn Leatherjacket (Aluterus Monoceros) collagen, aiming to unravel the potential of the peptides released in inhibiting α-amylase, reporting average half-maximal inhibitory concentration (IC50) values ranging from 1.17 to 2.65 mg mL−1, depending on the reaction temperature. Natsir et al. (2019) reported α-glucosidase inhibition of 24.47% as the highest activity exerted by the different samples analysed, from a collagen hydrolysate from yellowfin tuna (Thunnus albacares) bone hydrolysed using a bacterial collagenase. Similarly, Nur et al. (2021) evaluated the α-glucosidase inhibitory properties of a collagen hydrolysate from Lamuru (Caranx ignobilis) fishbone hydrolysed by collagenase enzyme from Clostridium histolyticum, reporting an IC50 value of 0.574 mg mL−1. To the author’s knowledge, there are no studies to date identifying α-amylase inhibiting peptides obtained from marine waste. However, Nguyen et al. (2022) were able to produce and identify hemi-pyocyanin, a potent α-amylase inhibitor, by microbial fermentation of fish-derived chitinous discards. Regarding α-glucosidase inhibitors, Matsui et al. (1999) identified two peptides with glucosidase inhibitory activity, VW (IC50 = 22.6 mM) and YYPL (IC50 = 3.7 mM) from sardine muscle hydrolysed with alkaline protease from Bacillus licheniformis.
DPP-IV inhibitory activity of peptides has been widely studied and identified in fish waste (Table 3) as well as other by-products from animal origin (Henriques et al. 2021; Rivero-Pino et al. 2020d). Most of the DPP-IV inhibitory peptides reported in the literature were produced by commercial proteases such as subtilisin, trypsin, papain or Flavourzyme. A common feature of DPP-IV inhibiting peptides is their short chain length. In this regard, Ketnawa et al. (2018a, b) hydrolysed rainbow trout (Oncorhynchus mykiss) frames and found that the most potent inhibitors were peptides with molecular sizes between 300 and 500 Da. Gelatin from skin and bones are one of the main fish waste substrate employed for the production of DPP-IV inhibitors. Jin et al. (2020) used several proteases to hydrolyse salmon skin, identifying the potent DPP-IV inhibitor, LDKVFR, in the fraction of molecular weight below 3 kDa of a trypsin hydrolysate. Similarly, Neves et al. (2017) hydrolysed salmon gelatin using different enzymes (Alcalase 2.4L, Flavourzyme 500L, Corolase PP, Promod 144MG and Brewer’s Clarex). The authors found that Corolase PP and the combination of Alcalase and Flavourzyme yielded hydrolysates with high DPP-IV inhibitory activity (IC50 = 0.8 mg mL−1). Both hydrolysates presented a significant proportion of peptides with molecular weight below 1000 Da. Moreover, this fraction allowed the identification of a number of active sequences in the Corolase PP hydrolysate (Table 3). The use of Flavourzyme as the sole enzyme is uncommon, since it is an enzyme mixture with predominant exopeptidase activity, which leads to a low extent of hydrolysis. Nevertheless, several studies confirm its capacity for releasing DPP-IV inhibitors from protein substrates. Atma et al. (2019) found that 4-h hydrolysis of bone gelatin using Flavourzyme can produce DPP-IV inhibitory hydrolysates, reporting maximal activity for the peptide fraction above 3 kDa. In this line, Sila et al. (2016) and Gui et al. (2022) obtained different sequences from fish skin, where the shorter peptides (GPAE and GPGA) showed very potent DPP-IV inhibitory capacity. Other studies identified sequences of inhibitory peptides in the hydrolysates of fish discards and fish cooking juice (Huang et al. 2012; Rivero-Pino et al. 2020d).
2.2 Cell-Based Studies
In situ cell-based and/or ex vivo studies are useful to reduce the gap between the in vitro assays and the in vivo studies, since they provide a higher quality analysis concerning the potential effect these compounds may have. Thus, cell culture assays are a strong tool to evaluate the antidiabetic effects of protein hydrolysates by evaluating different parameters such as: (i) insulin-secreting cells (pancreatic β cells) following glucose exposition; (ii) release of GLP-1 (intestinal hormone dependant on the DPP-IV enzyme); (iii) glucose absorption by fluorimetry; (iv) expression and secretion of gut hormones following a physiological stimuli. Beyond that, studies in animals provide scientific rationale on the mechanisms that these peptides can have, because it allows to evaluate the stability during gastrointestinal digestion, absorption in the lumen and their reaching to the target organ and the actual effect exerted.
In the last years, some authors have reported the efficacy of salmon and boarfish by-products as a source of antidiabetic peptides, by means of cell culture analysis and animal studies (Harnedy et al. 2018; Parthasarathy et al. 2018). In these reports, authors employed protein hydrolysates obtained with food-grade proteases (e.g. Alcalase and Flavourzyme) to obtain a product that can be resistant to gastrointestinal digestion and that exerts an effect on the models employed to evaluate the antidiabetic activity.
Zhang et al. (2020) analysed the potential of peptides derived from common carp (Cyprinus carpio) roe protein, employing four different proteases. The papain-hydrolysed with sample effectively inhibited DPP-IV, maintaining the activity after digestion. Analyses were carried out in cell models, and the identified peptide (IPNVAVD) had no cytotoxicity and reduced the amount of DPP-IV that Caco-2 cells released, as well as increased the absorption of glucose by insulin-resistant HepG2 cells.
Harnedy-Rothwell et al. (2020) identified that the boarfish-derived peptide reported in vitro as DPP-IV inhibitor with an IC50 value of 0.022 mM, showed an IC50 value of 0.004 mM in an in situ DPP-IV inhibition assay employing Caco-2 cells, highlighting the challenges in comparing and extrapolating the results among different types of analyses. In addition, this peptide was also responsible for a potent insulin secretory activity reported in pancreatic BRIN-BD11 cells. Moreover, recently, Heffernan et al. (2022) also demonstrated that blue whiting (Micromesistius poutassou) hydrolysates obtained by enzymatic hydrolysis were able to promote GLP-1 secretion and proglucagon production in murine enteroendocrine STC-1 cells. Theysgeur et al. (2020) recently identified peptides obtained from Tilapia (Oreochromis niloticus) able to exert inhibition over the enzyme DPP-IV, as well as able to regulate intestinal hormones (stimulation of CCK and GLP-1 secretion) on the enteroendocrine cells in an in vitro canine gastrointestinal simulated digestion model.
In terms of ex-vivo analysis, Hjorth et al. (2022) compared how the consumption of whey or salmon protein would affect cultured HepG2 cells by evaluating the transcriptomic profiling by using RNA sequencing, by exposing them to the serum of healthy male subjects, having intake 5.2 g of these samples. Authors concluded no difference in the nutrigenomic effects of the fishmeal and whey, though considering the whole scenario of food sustainability, fish by-products account for a more environmentally friendly source that could be used as a replacer.
On top of that, different reports employing animals can be also found concerning fish-derived peptides exerting antidiabetic effects. For instance, Siala et al. (2016) reported an inhibition of α-amylase exerted by grey triggerfish (Balistes capriscus) protein hydrolysates, obtained with different proteases, in alloxan-induced diabetic rats (AIDR), along with a decrease of blood glucose and glycated haemoglobin levels in the rats. In addition, other lipid-related parameters were affected by the intake of these peptides. Ben Slama-Ben Salem et al. (2018) evaluated the effect of peptides obtained from octopus (Octopus vulgaris) muscle proteins on AIDRs, showing increased α-amylase activity (in plasma, pancreas and intestine) and blood glucose, as well as a notable reduction in the levels of plasma insulin, among other modified parameters. The daily administration of the testing material for one month improved the glucose tolerance test, the glycaemic status of the animals and corrected the lipid profiles, including an attenuation of the increased activities of the plasma enzymes produced by diabetes.
Recently, Takahashi et al. (2021a) proved that a salmon milt peptide (Oncorhynchus keta), considered as an unused fish-processing by-product, is able to inhibit DPP-IV and has an antidiabetic effect on Sprague Dawley rats. Authors were able to identify 12 peptides and to determine that the one highly contributing to the inhibitory action was the sequence Ile-Pro, accounting for 1.3% of the total activity.
2.3 Human Studies
Regarding human studies, there has been recently an increase in the number of reports in order to clearly demonstrate the potential employment of these protein hydrolysates as a nutritional, health-promoting ingredient for human consumption. As evidence to really define whether a peptide (or protein hydrolysate) can exert a real effect in a population, well-defined human studies with a significant population size and with physiological changes clearly proved are needed. Nonetheless, these clinical trials are usually very expensive, and sometimes, conclusions are hard to draw. In this sense, randomized and controlled clinical intervention trials have been determined as the most reliable, and the number needed is variable. The reports must be of high quality in terms of methodology and reporting.
Dale et al. (2018) employed a sustainable marine resource (Atlantic cod (Gadus morhua)) as testing material in a double-blind cross-over trial to evaluate the glucose metabolism after meals in healthy subjects (n = 41, mean age 51). The study consisted of two study days including a wash-out in between, where the dose ingested was 20 mg kg−1 bw. The main parameter analysed was post-prandial response in glucose metabolism and GLP-1 concentrations, although no differences were observed between the testing item and the control. On the other hand, the insulin concentration after meals was significantly lower when the fish hydrolysate was ingested compared with the control. However, the limitations of the study make it impossible to draw any conclusions on the real effect of these protein hydrolysates as antidiabetic agents. Further studies in order to unravel the mechanistic behind are required to understand the physiological changes observed.
Crowe et al. (2018) reported the assessment of how peptides obtained from wasted boarfish (Capros aper) can affect postprandial glycaemic management in human participants. In a randomized controlled intervention crossover study, healthy subjects (n = 20) ingested 3.5 g of the sample, leading to an area under the curve (AUC) for insulin or glucose not different from the control, for 3 h. The design of this study is limited by the criteria assessed as well as the short time during which the metabolic-related parameters were evaluated.
Jensen et al. (2019) evaluated what effects the supplementation with cod protein peptides in older adults has on postprandial glucose metabolism. The experimental design consisted of a double-blind cross-over trial, where subjects (n = 31) received, during 1 week, a dose of the sample. Doses were different among groups, from 10 to 40 mg kg−1 bw. The measured postprandial response in glucose metabolism and in plasma, GLP-1 led to no significant differences among different dose groups; whereas, serum glucose and insulin levels decreased as the dose was increased. Later on, these authors also reported the effects of cod protein hydrolysate supplementation (dose of 4 g) during 8 weeks on glucose metabolism, lipid profile and body composition of Adults with Metabolic Syndrome (n = 30). The experimental design was a double-blind, randomized intervention study with a parallel-group design. Among the different parameters measured, no significant differences were found in postprandial insulin, post-prandial glucose of GLP-1, thus, not implying any physiological difference for the parameters assessed (Jensen et al. 2020).
Hovland et al. (2020) evaluated the efficacy of fish peptides (from fresh rest residual materials of herring (Clupea harengus), salmon (Salmo salar) or cod (Gadus morhua), a dose of 2.5 g per day, during 8 weeks) in a randomized, double-blind study (n = 77 overweight adults). Parameters analysed including assessing how it would affect glucose regulation and insulin sensitivity. The intake of the different samples affected diverse parameters in different extents, and authors concluded that effects were most pronounced following the supplementation with cod and herring samples, compared to salmon.
Vildmyren et al. (2020) reported how the ingestion of cod residual protein would have an impact on markers of glucose regulation in lean adults (n = 50, mean age 28) by a randomized double-blind study. The dose (8.1 g per day of protein) was ingested during 8 weeks. The intake of the protein did not have an effect on fasting glucose or insulin concentrations, but affected the levels of ketone bodies negatively in the subjects as well as an increase of trimethylamine N-oxide concentration was observed in plasma and urine. These are parameters related to impaired glucose metabolism, thus, further studies are needed to unravel the interactions occurring in the metabolism of the ingested protein.
Hustad et al. (2021) in a randomized controlled trial during 8 weeks with adults prone to suffer from T2DM, evaluating the effect of salmon protein supplement obtained from by-products (5.2 g of protein per day) on 2-h glucose, leading to no significant modifications compared to the control. However, it should be noted that in this study, the native protein was employed as a test item, and not a protein hydrolysate, even though peptides are expected to be in the material ingested.
Takahashi et al. (2021a, b) evaluated the effect of the administration of DPP-IV inhibitory peptides during 7 days (dose of 500 mg per day) from chum salmon milt (unused processing byproduct) on postprandial blood glucose level. The experimental design was a randomized, placebo-controlled, double-blind, crossover, pilot clinical trial with healthy Japanese subjects (n = 15). The main parameters evaluated were reduced blood glucose and insulin levels. According to the authors, no clear suppressive effect of the peptides on elevated postprandial blood glucose levels was observed. However, authors indicate the limitations from the study and the need of continuing investigating the effects, since positive results were observed in the animal studies.
Overall, the studies aiming to demonstrate the effect of fish-derived peptides on potential antidiabetic effects are lacking quality experimental design. A more in-depth analysis of the toxicokinetics parameters, as well as glucose-metabolism-related parameters are required in order to have an overview of the physiological changes occurring. In addition, further studies with human subjects in a state of pre-diabetes (exhibiting a result of 100–125 mg dL−1 on the Fasting Plasma Glucose Test; or a level of 5.7–6.4% on the A1c test) are required in order to establish (always on a case-by-case basis) the functionality of these food-derived peptides in real patients. The impaired metabolism of patients is not necessarily representative of the status of healthy patients. Furthermore, differences among populations due to metabotyping have to be considered, because intervariability can occur.
3 Stability and Functionality in Food Matrices
Concerning the structure of the peptides, its maintenance after being included in food matrices and during processing and storage is essential to state them as bioactive ingredients (Rivero-Pino 2023). Firstly, the food matrix composition where fish-derived products are intended to be used has to be deeply characterized, considering that chemical reactions could lead to bioactivity changes (Capuano et al. 2017). Food matrix might also have an impact on the stability through and after the processing, while at the same time, it can be responsible for masking the bitterness that characterize the peptides (Aguilera 2018). During processing, heat treatments are commonly employed with several objectives, including the elimination of microbial activity or the modification of food structure, for instance, by producing Maillard compounds. These treatments may denature or provoke aggregation of proteins and peptides, depending on different parameters (López-Sánchez et al. 2016). Therefore, it would be necessary to investigate how thermal treatment (taking into account the time of the process) can imply modifications in terms of safety and health-promoting properties of fish-derived products. On the other hand, non-thermal treatments currently employed in the food industry are ultrasound, high hydrostatic pressure, pulsed electric field, ultraviolet application, etc. These treatments are also usually employed to change the structure of the components, eliminate microorganisms or deactivate enzymes, or promote the liberation of bioactive peptides. Overall, these processes strengthen food safety while not implying loss of the nutritional and sensorial characteristics of food, which is undoubtedly an adequate alternative with several applications and opportunities in the valorization of fish waste in the upcoming years.
Apart from processing, the chemical interactions with matrix compounds should be also evaluated during storage time, considering the temperature and the time (Rivero-Pino 2023). There are different studies investigating, for example, how heat treatment and long-term storage of a fish soup fortified with peptides can modify the structure, correlated with antioxidant and antihypertensive peptides (Rivero-Pino et al. 2020c). However, further research with diverse food matrices has to be done, including the investigation of how thermal and non-thermal treatments would have an impact on the bioactivity of peptides.
There are not many studies about food fortification with peptides in the literature. For example, Ayati et al. (2022) sought to create a yoghurt enhanced with three fish collagen-derived bioactive peptides, including GPLGAAGP, GRDGEP and MTGTQGEAGR at various doses (up to 1.0 mg mL−1). The samples’ DPP-IV inhibitory activity was assessed together with other factors, and the results showed high bioactive values at the concentrations examined. When compared to the control sample, the yoghurts enriched with bioactive peptides did not exhibit any physico-chemical or sensory modifications. On the other hand, Harnedy‐Rothwell et al. (2021a, b) investigated two different treatments (conditions being (i) 90 °C during 1 min; (ii) 121 °C during 42 s) and subsequently, storage of tomato-based products fortified with protein hydrolysates derived from boarfish (Capros aper) on the antidiabetic activity. The DPP-IV inhibitory activity of the peptides was maintained after heat treatments, and in addition, no changes were observed during storage.
The effects of fish protein hydrolysate made from Sind sardine interacting with pistachio green hull extract were recently assessed by Amini Sarteshnizi et al. (2021). Due to the high concentration of polyphenols in the pistachio green hull extract, several interactions could take place and change the effect. These interactions would depend on the chemical groups exposed and the structure of the polyphenols. The inhibition of the enzymes α-glucosidase, α-amylase and DPP-IV was examined among other bioactivities. While the addition of the pistachio extract had no effect on the bioactivity for the inhibition of DPP-IV, it did result in a reduction in the inhibition of the other two enzymes related to antidiabetic effects.
There is very little information in literature on whether and to which extent heat treatments might modify the properties of α-glucosidase inhibitory peptides derived from fish waste or from other sources. Only Rivero-Pino (2021) reported that a commercial soup supplemented with the insect Tenebrio molitor peptides led to a significant loss of bioactivity after a heat treatment of 121 °C for 21 min, but maintained during storage.
As it has been indicated before, the interactions of peptides with different chemical structures can modify their potential bioactivity as well as their bioavailability. Wu et al. (2015) investigated the formation of stable colloidal complex nanoparticles by self-assembling tannic acid with fish scale gelatin hydrolysates. The tannic acid shows amylase inhibitory activity, but after the nanoparticle formation, this bioactivity decreased compared to that of free tannic acid. The author also evaluated how the interaction created would affect the bioavailability of the complex, indicating a capacity to inhibit Cu2+ ion-induced barrier impairment and hyperpermeability of Caco-2 intestinal epithelial tight junction by the nanoparticles.
Recently, Wu et al. (2022) aimed to convert inorganic chromium into organic chromium—claimed to have higher bioavailability and to act as antidiabetic agent—by bonding to the amine group of a fish scale collagen (from Oreochromis niloticus) extracted by enzymatic hydrolysis. Authors reported that the final structure was obtained and properly characterized, though no formal analysis on the antidiabetic activity was performed.
Overall, there is an evident lack of research concerning the interaction of fish waste-peptides with different food matrices, thermal and non-thermal processing consequences, and employment as ingredients that will need to be addressed in the following years.
4 Bioavailability of Peptides
The bioavailability and safety of fish peptides have to be analysed by means of animal and human studies specifically designed for that purpose, ideally according to harmonized guidelines of the Organisation for Economic Co-operation and Development (OECD). The analysis of toxicokinetics parameters could help define the actual bioavailability of these peptides and how these can modulate specific metabolic pathways, and thus, resulting in a modification of the physiological status of the individual.
The studies available reporting these parameters are scarce, and thus, there is not an exact reporting about how peptides can achieve target organs. Considering the complexity of the hydrolysates, if used as such as an ingredient, it is a challenge to establish the fate of each peptide, and whether the gastrointestinal digestion cleaves the peptides and leads to a modification of the bioactivity. Furthermore, as indicated previously, the food matrix can impact the bioavailability of the peptides. For instance, there are studies reporting how fish waste-derived peptides with proven antioxidant and antihypertensive activity are incorporated into fish-based liquid matrices, and the bioactivity was maintained after simulated gastrointestinal digestion (Rivero-Pino et al. 2020c).
Regarding antidiabetic peptides, Karimi et al. (2021) investigated whether maize herm peptides would increase the bioactive potential of bread before and after digestion, as well as the inhibition of the enzyme α-amylase during digestion. However, there is a lack of studies reporting fish-derived peptides with antidiabetic peptides used as functional ingredients. Harnedy‐Rothwell et al. (2021a, b) fortified tomato-based products with peptides derived from boarfish (Capros aper), reporting maintained or enhanced bioactivity after simulated gastrointestinal digestion.
Given the low likelihood of chemical interactions occurring and the fact that it would prevent the bitter taste of peptides, boosting the sensory acceptance, high-fibre food matrices appear to be an appropriate vehicle to transport bioactive peptides (Sun et al. 2020). To encourage their employment in the food business for human consumption and stop the emergence of certain diseases, further study is required.
Zhang et al. (2018) demonstrated in vitro that IADHFL, a DPP-IV inhibitory peptide identified from bighead carp (Hypophthalmichthys nobilis) remained stable after simulated gastrointestinal digestion and inhibited DPP-IV enzyme in vitro, in cell cultures analysis. However, it should be noted that these analyses done with a purified peptide under in vitro conditions are not representative of the physiological conditions of the humans and of the products intended to be marketed. Purification of peptides in a sufficient amount extracted from fish-by products is still a technological challenge that needs to be addressed.
Ayati et al. (2022) as reported previously, manufactured a functional yoghurt with fish collagen-derived bioactive peptides. These samples were also subjected to in vitro gastrointestinal digestion, showing no significant difference in the bioactivity of two of the three samples analysed, indicating that these two peptides are not hydrolysed by digestive proteases.
In fact, the food matrix in which the antidiabetic food-derived peptides are included would have an impact on bioavailability, based on the pH value modifications. These parameters will influence the net charge, structure, and ability of them to interact with different targets, such as enzymes active sites (Rivero-Pino 2023), thus modulating their capacity of inhibiting them (Marcolini et al. 2015). During digestion, the amino acid content and sequence would affect the resistance to gastrointestinal digestion, with special attention to the terminal sites (Karaś 2019). In addition, it has been suggested that low steric hindrance values and high amphipathicity are correlated with an improved bioactivity exerted by the peptide, since it would stabilize the enzyme conformation (Mora et al. 2020).
One strategy that is currently being studied to overcome the main limitation of peptides, which is a low bioavailability as they are prone to be cleaved during digestion, is their encapsulation (Aguilar-Toala et al. 2022). The encapsulation of bioactive compounds is a way to ensure they reach the target organ and can exert their function. Although encapsulation of antidiabetic peptides has not been extensively studied, Cian et al. (2019) reported that encapsulation by spray drying prevented the reduction in DPP-IV inhibitory activity during the digestion of Lima bean (Phaseolus lunatus) protein hydrolysate.
Furthermore, Li et al. (2015) encapsulated antidiabetic Atlantic salmon peptides (molecular weight <1000 Da) in chitosan-coated liposomes obtained from milk fat globule membrane derived phospholipids. The physical stability of the nanocapsules during freeze-drying, freeze-thawing, and long-term storage was analysed, resulting in a prolonged release of the peptides in simulated biological fluids because of the chitosan coating.
Other authors have also reported the encapsulation of fish-derived peptides, without evaluating the antidiabetic potential of these, but other biological properties and bioavailability (Hanachi et al. 2022; Lima et al. 2021; Subara et al. 2018).
5 Fish Protein Hydrolysates Market
The valorization potential of fish waste is high. However, the number of patents focusing on the production of antidiabetic peptides from fish is limited. There are no patents describing the production of α-amylase or α-glucosidase inhibitory peptides from fish proteins. Only a few patents deal with the production of DPP-IV inhibitory peptides from fish species such as hairtail, silver carp, salmon, herring, cod, bonito (Jin and Y 2013; Shangwu and Ying 2014; Takahashi et al. 2014). The process usually consists of obtaining a uniform fish flesh pulp prior to carrying out enzymatic hydrolysis by proteases (Alcalase, trypsin, bromelain, Flavourzyme, papain among others). Afterwards, the hydrolysate produced is centrifuged and filtered through ultrafiltration membranes for purifying the most active fraction.
The patents regarding the use of fish waste as a source for peptides with DPP-IV inhibitory activity are even more limited. All the current patents employ fish skin as substrate. Particularly, the use of Atlantic salmon (Salmo salar) skin (Kuo-Chiang et al. 2013) implies a preliminary process to extract gelatin. Then, gelatin is hydrolysed by Alcalase, bromelain and Flavourzyme. Hydrolysates should be ultrafiltrated to recover the <1 kDa fraction, where the most potent DPP-IV inhibitors are found. Some of the active peptides identified in that fraction include four amino acid residues having an amino acid sequence of Gly-Pro-X3-X4, in which X3 is alanine or glycine, and X4 is glutamate or alanine. A similar process is proposed for the production of DPP-IV inhibitors from the skin of silver carp (Hypophthalmichthys molitrix) and tilapia (Oreochromis niloticus) (Yongkang 2017). In this process, after cleaning the skin, a protein slurry is generated using hot water and ultrasound treatment. After centrifugation, skin proteins (obtained in supernatant) are subjected to two sequential hydrolysis steps. Initially, skin proteins are hydrolysed by alkaline protease and trypsin during 1–2 h and then bromelain and Flavourzyme are added and kept hydrolysing for 1–2 additional hours. After hydrolysis, enzymes are deactivated thermally and the product is ultrafiltered by ceramic membranes. The filtration is carried out in two steps, first filtration through 5 kDa and then by 3 kDa membranes. The resulting permeate is purified by gel filtration (Sephadex G-25 gel). The resulting fractions are concentrated by freeze-drying. The invention also provides the application of the fish skin protein peptide in medicines, health-care foods and food additives. A slightly different process is described in CN109182435A (Yunping 2018), where croaker (Micropogonias undulatus) skin is cleaned, freeze-dried and crushed to a partial size is 25–50 μm. Then, the fish skin powder is hydrolysed with pepsin at pH 3–5 to extract collagen. Collagen is hydrolysed with photoresponse gelator and papain under ultraviolet lamp to obtain the active peptides. In all cases, fish waste hydrolysates, purified fractions or isolated peptides with DPP-IV inhibitory activity could be potentially added to medicines, health-care foods and food additives.
According to Global Market Insights, the Fish Protein Hydrolysates Market size is estimated to grow at over 4.5% compound annual growth rate between 2020 and 2026. The report indicates that the industry of enzymatically produced hydrolysate will achieve a value of USD 475 million by 2026, highlighting sources such as, tilapia, tuna, sardine, Atlantic salmon and cod fish, the same sources as those mostly under research as stated in the previous sections (Global Market Insight 2020).
Considering antidiabetic products, one example of commercially available preparation based on fish-derived protein hydrolysates is Nutripeptin™, a fine powder obtained from cod, claimed to reduce blood sugar levels after meals. Similarly, a white fish autolysate (Molva Molva) branded as Fortidium Liquamen®, is claimed to reduce the glycemic index, among other potential bioactivities. Similarly, Hydro MN Peptide is a marine cartilage extract with a mix of hydrolysed proteins (mainly Collagen) and polysaccharides, claimed by the company to reduce the glycaemic index and subsequently, alleviating symptoms of type II diabetes. In addition, Wellnex®, food-grade type-D collagen is a hydrolysate obtained from Tilapia (Oreochromis sp.) scale, claimed to exert antidiabetic effect. (Abachi et al. 2022; Nirmal et al. 2022a).
However, as indicated before, the health claims stated for these products vary depending on the country or region intended to be marketed on, and depend on the managers evaluating the data supporting these evidences. Therefore, and based on the scientific requirements mandatory in each region, a product can be considered to exert an effect or not, depending on the market.
6 Conclusions and Perspectives
A redefinition of our current food system is urgently needed, following the global situation affected by climate change and the increase of disease rate in humans. The close relationship that exists between diet and health makes human consumption habits participate in the prevention of the development of diseases. On the other hand, the full use of the resources available in nature is a key element to achieve a circular economy model. In this framework, fish-derived peptides obtained from waste (including non-commercial species and by-products from the industry) have proved not only to be safe, but also nutritionally advantageous upon its consumption, potentially exerting a beneficial effect in different biomarkers. The amount of high-quality protein, as well as the peptide sequences embedded in the native protein, that can be released by enzymatic hydrolysis processing, makes this raw material an adequate source of antidiabetic peptides. There are still some technological challenges that need to be addressed in the manufacturing of these products, from the obtaining of the peptides (unravelling the effect of novel technologies prior or during the release of peptides) to the formulation of the final product (stability, maintained bioactivity, consumer’s acceptance). Furthermore, well-designed human studies, where the physiological effect is demonstrated, are essential to be carried out for each product intended to be marketed with potential health claims, in order not to mislead the consumer.
In addition, standardization of regulatory requirements would help companies aiming to sell fish protein hydrolysates products with health claims, to expand more rapidly, and to achieve a larger audience.
References
Abachi S, Pilon G, Marette A, Bazinet L, Beaulieu L (2022) Beneficial effects of fish and fish peptides on main metabolic syndrome associated risk factors: diabetes, obesity and lipemia. Crit Rev Food Sci Nutr 1–49. https://doi.org/10.1080/10408398.2022.2052261
Abdollahi M, Wu H, Undeland I (2021) Impact of processing technology on macro- and micronutrient profile of protein-enriched products from fish backbones. Foods 10(5). https://doi.org/10.3390/foods10050950
Aguilar-Toala JE, Quintanar-Guerrero D, Liceaga AM, Zambrano-Zaragoza ML (2022) Encapsulation of bioactive peptides: a strategy to improve the stability, protect the nutraceutical bioactivity and support their food applications. RSC Adv 12(11):6449–6458. https://doi.org/10.1039/d1ra08590e
Aguilera JM (2018) The food matrix: implications in processing, nutrition and health. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2018.1502743
AminiSarteshnizi R, Sahari MA, Ahmadi Gavlighi H, Regenstein JM, Nikoo M, Udenigwe CC (2021) Influence of fish protein hydrolysate-pistachio green hull extract interactions on antioxidant activity and inhibition of α-glucosidase, α-amylase, and DPP-IV enzymes. LWT 142:111019. https://doi.org/10.1016/j.lwt.2021.111019
Atma Y, Lioe HN, Prangdimurti E, Seftiono H, Taufik M, Mustopa AZ (2019) Dipeptidyl peptidase IV (DPP-IV) inhibitory activity of ultrafiltration and gel filtration fraction of gelatin hydrolyaste derived from bone of fish for antidiabetes. Int J Adv Sci Eng Inf Technol 9(6):2096–2103. https://doi.org/10.18517/ijaseit.9.6.10658
Ayati S, Eun JB, Atoub N, Mirzapour-Kouhdasht A (2022) Functional yogurt fortified with fish collagen-derived bioactive peptides: antioxidant capacity, ACE and DPP-IV inhibitory. J Food Process Preserv 46(1):1–10. https://doi.org/10.1111/jfpp.16208
Capuano E, Oliviero T, van Boekel MAJ (2017) Modelling food matrix effects on chemical reactivity: challenges and perspectives. Crit Rev Food Sci Nutr 58:2814–2828. https://doi.org/10.1080/10408398.2017.1342595
Castro-González MI, Méndez-Armenta M (2008) Heavy metals: implications associated to fish consumption. Environ Toxicol Pharmacol 26(3):263–271. https://doi.org/10.1016/j.etap.2008.06.001
Chalamaiah M, Keskin Ulug S, Hong H, Wu J (2019) Regulatory requirements of bioactive peptides (protein hydrolysates) from food proteins. J Funct Foods 58:123–129. https://doi.org/10.1016/J.JFF.2019.04.050
Cian R, Campos-Soldini A, Guerrero L, Drago S, Betancur D (2019) Bioactive Phaseolus lunatus peptides release from maltodextrin/gum Arabic microcapsules obtained by spray drying after simulated gastrointestinal digestion. Int J Food Sci Technol 54:2002–2009. https://doi.org/10.1111/ijfs.14031
Crowe W, McLaughlin CM, Allsopp PJ, Slevin MM, Harnedy PA, Cassidy Y, Baird J, Devaney M, Fitzgerald RJ, O’Harte FPM, McSorley EM (2018) The effect of boarfish protein hydrolysate on postprandial glycaemic response and satiety in healthy adults. Proc Nutr Soc 77(OCE3). https://doi.org/10.1017/s002966511800109x
Curran AM, Horner K, O’Sullivan V, Nongonierma AB, Le Maux S, Murphy E, Kelly P, Fitzgerald RJ, Brennan L (2019) Variable glycemic responses to intact and hydrolyzed milk proteins in overweight and obese adults reveal the need for precision nutrition. J Nutr 149(1):88–97. https://doi.org/10.1093/jn/nxy226
Dale HF, Jensen C, Hausken T, Lied E, Hatlebakk JG, Brønstad I, Hoff DAL, Lied GA (2018) Effect of a cod protein hydrolysate on postprandial glucose metabolism in healthy subjects: a double-blind cross-over trial. J Nutr Sci 7:1–9. https://doi.org/10.1017/jns.2018.23
Derouiche Ben Maiz H, Guadix EM, Guadix A, Gargouri M (2019) Valorisation of tuna viscera by endogenous enzymatic treatment. Int J Food Sci Technol 54(4):1100–1108. https://doi.org/10.1111/ijfs.14009
Despoti S, Stergiou KI, Machias A, Vassilopoulou V, Tsagarakis K, Valavanis V, Adamidou A, Giannoulaki M (2021) Assessing the spatial distribution of five non-commercial fish species in the Aegean Sea (Greece, eastern Mediterranean Sea) based on discards data. Reg Stud Mar Sci 44:101736. https://doi.org/10.1016/J.RSMA.2021.101736
Domínguez Díaz L, Fernández-Ruiz V, Cámara M (2020) An international regulatory review of food health-related claims in functional food products labeling. J Funct Foods 68:103896. https://doi.org/10.1016/j.jff.2020.103896
FAO (2020) The state of world fisheries and aquaculture 2020. In brief. Sustainability in action. https://doi.org/10.4060/ca9231en
García-Moreno PJ, Pérez-Gálvez R, Morales-Medina R, Guadix A, Guadix EM (2013) Discarded species in the west Mediterranean sea as sources of omega-3 PUFA. Eur J Lipid Sci Technol 115(9):982–989. https://doi.org/10.1002/ejlt.201300021
García-Moreno PJ, Guadix A, Guadix EM, Jacobsen C (2016) Physical and oxidative stability of fish oil-in-water emulsions stabilized with fish protein hydrolysates. Food Chem 203:124–135. https://doi.org/10.1016/j.foodchem.2016.02.073
Global Market Insight (2020) Fish protein hydrolysate market. https://www.gminsights.com/industry-analysis/fish-protein-hydrolysate-market
Gui M, Gao L, Rao L, Li P, Zhang Y, Han J-W, Li J (2022) Bioactive peptides identified from enzymatic hydrolysates of sturgeon skin. J Sci Food Agric 102:1948–1957. https://doi.org/10.1002/jsfa.11532
Hanachi A, Bianchi A, Kahn CJF, Velot E, Arab-Tehrany E, Cakir-Kiefer C, Linder M (2022) Encapsulation of salmon peptides in marine liposomes: physico-chemical properties, antiradical activities and biocompatibility assays. Mar Drugs 20(4). https://doi.org/10.3390/md20040249
Harnedy PA, Parthsarathy V, McLaughlin CM, O’Keeffe MB, Allsopp PJ, McSorley EM, O’Harte FPM, FitzGerald RJ (2018) Blue whiting (Micromesistius poutassou) muscle protein hydrolysate with in vitro and in vivo antidiabetic properties. J Funct Foods 40:137–145. https://doi.org/10.1016/j.jff.2017.10.045
Harnedy-Rothwell PA, McLaughlin CM, O’Keeffe MB, Le Gouic AV, Allsopp PJ, Mcsorley EM, Sharkey S, Whooley J, McGovern B, O’Harte FPM, Fitzgerald RJ (2020) Identification and characterisation of peptides from a boarfish (Capros aper) protein hydrolysate displaying in vitro dipeptidyl peptidase-IV (DPP-IV) inhibitory and insulinotropic activity. Food Res Int 131:108989. https://doi.org/10.1016/j.foodres.2020.108989
Harnedy-Rothwell PA, Khatib N, Sharkey S, Lafferty RA, Gite S, Whooley J, O’harte FPM, Fitzgerald RJ (2021a) Physicochemical, nutritional and in vitro antidiabetic characterisation of blue whiting (Micromesistius poutassou) protein hydrolysates. Mar Drugs 19(7). https://doi.org/10.3390/md19070383
Harnedy-Rothwell PA, McLaughlin CM, Crowe W, Allsopp PJ, McSorley EM, Devaney M, Whooley J, McGovern B, Parthsarathy V, O’Harte FPM, FitzGerald RJ (2021b) Stability to thermal treatment of dipeptidyl peptidase IV (DPP-IV) inhibitory activity of a boarfish (Capros aper) protein hydrolysate when incorporated into tomato-based products. Int J Food Sci Technol 56:158–165. https://doi.org/10.1111/ijfs.14615
Heffernan S, Nunn L, Gite S, Whooley J, Giblin L, Fitzgerald RJ, Brien NMO (2022) Blue whiting (Micromesistius poutassou) protein hydrolysates co-culture integrity. Mar Drugs 20:112
Hemker AK, Nguyen LT, Karwe M, Salvi D (2020) Effects of pressure-assisted enzymatic hydrolysis on functional and bioactive properties of tilapia (Oreochromis niloticus) by-product protein hydrolysates. LWT 122:109003. https://doi.org/10.1016/j.lwt.2019.109003
Henriques A, Vázquez JA, Valcarcel J, Mendes R, Bandarra NM, Pires C (2021) Characterization of protein hydrolysates from fish discards and by-products from the north-west spain fishing fleet as potential sources of bioactive peptides. Mar Drugs 19(6). https://doi.org/10.3390/md19060338
Hewage SS, Wu S, Neelakantan N, Yoong J (2020) Systematic review of effectiveness and cost-effectiveness of lifestyle interventions to improve clinical diabetes outcome measures in women with a history of GDM. Clin Nutr ESPEN 35:20–29. https://doi.org/10.1016/j.clnesp.2019.10.011
Hira T, Trakooncharoenvit A, Taguchi H, Hara H (2021) Improvement of glucose tolerance by food factors having glucagon-like peptide-1 releasing activity. Int J Mol Sci 22(12). https://doi.org/10.3390/ijms22126623
Hjorth M, Galigniana NM, Ween O, Ulven SM, Holven KB, Dalen KT, Sæther T (2022) Postprandial effects of Salmon fishmeal and whey on metabolic markers in serum and gene expression in liver cells. Nutrients 14(8). https://doi.org/10.3390/nu14081593
Hovland IH, Leikanger IS, Stokkeland O, Waage KH, Mjøs SA, Brokstad KA, McCann A, Ueland PM, Slizyte R, Carvajal A, Mellgren G, Remman T, Høgøy I, Gudbrandsen OA (2020) Effects of low doses of fish and milk proteins on glucose regulation and markers of insulin sensitivity in overweight adults: a randomised, double blind study. Eur J Nutr 59(3):1013–1029. https://doi.org/10.1007/s00394-019-01963-0
Huang SL, Jao CL, Ho KP, Hsu KC (2012) Dipeptidyl-peptidase IV inhibitory activity of peptides derived from tuna cooking juice hydrolysates. Peptides 35:114–121. https://doi.org/10.1016/j.peptides.2012.03.006
Hustad KS, Ottestad I, Hjorth M, Dalen KT, Sæther T, Sheikh NA, Strømnes M, Ulven SM, Holven KB (2021) No effect of salmon fish protein on 2-h glucose in adults with increased risk of type 2 diabetes: a randomised controlled trial. Br J Nutr 126(9):1304–1313. https://doi.org/10.1017/S0007114521000040
Ibrahim MA, Bester MJ, Neitz AWH, Gaspar ARM (2017) Structural properties of bioactive peptides with α-glucosidase inhibitory activity. Chem Biol Drug Des 91:370–379. https://doi.org/10.1111/cbdd.13105
IDF Diabetes Atlas (2017) Federación Internacional de Diabetes, vol 8. International Diabetes Federation
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Diabetes Care 35(6):1364–1379. https://doi.org/10.2337/dc12-0413
Jawad LA (2021) The importance of non-commercial and small-sized fish species: a proposal for an additional revenue to Iraq BT—Tigris and Euphrates Rivers: their environment from headwaters to mouth. In: Jawad LA (Ed) Tigris and Euphrates Rivers: their environment from headwaters to mouth. Springer International Publishing, pp 1611–1623. https://doi.org/10.1007/978-3-030-57570-0_84
Jensen C, Dale HF, Hausken T, Hatlebakk JG, Brønstad I, Lied GA, Hoff DAL (2020) Supplementation with low doses of a cod protein hydrolysate on glucose regulation and lipid metabolism in adults with metabolic syndrome: a randomized, double-blind study. Nutrients 12(7):1–15. https://doi.org/10.3390/nu12071991
Jensen C, Dale HF, Hausken T, Lied E, Hatlebakk JG, Brønstad I, Lied GA, Hoff DAL (2019) Supplementation with cod protein hydrolysate in older adults: a dose range cross-over study. J Nutr Sci 4–11. https://doi.org/10.1017/jns.2019.37
Jin R, Teng X, Shang J, Wang D, Liu N (2020) Identification of novel DPP–IV inhibitory peptides from Atlantic salmon (Salmo salar) skin. Food Res Int 133:109161. https://doi.org/10.1016/j.foodres.2020.109161
Jin T, Y W (2013) Method for preparing dipeptidyl peptidase IV (DPP-IV) inhibitory peptide through using hairtail. (Patent No CN103333940B). https://patents.google.com/patent/CN103333940B/en
Judd N, Griffith WC, Faustman EM (2004) Contribution of PCB exposure from fish consumption to total dioxin-like dietary exposure. Regul Toxicol Pharmacol 40(2):125–135. https://doi.org/10.1016/J.YRTPH.2004.06.004
Karaś M (2019) Influence of physiological and chemical factors on the absorption of bioactive peptides. Int J Food Sci Technol 54:1486–1496. https://doi.org/10.1111/ijfs.14054
Karimi A, Ahmadi Gavlighi H, Amini Sarteshnizi R, Udenigwe CC (2021) Effect of maize germ protein hydrolysate addition on digestion, in vitro antioxidant activity and quality characteristics of bread. J Cereal Sci 97:103148. https://doi.org/10.1016/j.jcs.2020.103148
Ketnawa S, Suwal S, Huang J-Y, Liceaga, AM (2018a) Selective separation and characterisation of dual ACE and DPP-IV inhibitory peptides from rainbow trout (Oncorhynchus mykiss) protein hydrolysates. In J Food Sci Technol 1–12. https://doi.org/10.1111/ijfs.13939
Ketnawa S, Wickramathilaka M, Liceaga AM (2018b) Changes on antioxidant activity of microwave-treated protein hydrolysates after simulated gastrointestinal digestion: purification and identification. Food Chem 254:36–46. https://doi.org/10.1016/J.FOODCHEM.2018.01.133
Kristinsson HG, Rasco BA (2000) Fish protein hydrolysates: production, biochemical, and functional properties. Crit Rev Food Sci Nutr 40:43–81. https://doi.org/10.1080/10408690091189266
Kumar LV, Shakila RJ, Jeyasekaran G (2019) In vitro anti-cancer, anti-diabetic, anti-inflammation and wound healing properties of collagen peptides derived from unicorn leatherjacket (Aluterus monoceros) at different hydrolysis. Turk J Fish Aquat Sci 19(7):551–560. https://doi.org/10.4194/1303-2712-v19_7_02
Kuo-Chiang H, Kit-Pan H, Shih-Li H, Chia-Ling J (2013) Peptide for inhibiting dipeptidyl-peptidase iv. https://patents.google.com/patent/US20140193463
Li R, Zhang P, Barker LE, Chowdhury FM, Zhang X (2010) Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care 33(8):1872–1894. https://doi.org/10.2337/dc10-0843
Li Z, Paulson AT, Gill TA (2015) Encapsulation of bioactive salmon protein hydrolysates with chitosan-coated liposomes. J Funct Foods 19:733–743. https://doi.org/10.1016/j.jff.2015.09.058
Li-Chan ECY, Hunag SL, Jao CL, Ho KP, Hsu KC (2012) Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J Agric Food Chem 60(4):973–978. https://doi.org/10.1021/jf204720q
Lima KO, Pinilla CMB, Alemán A, López-Caballero ME, Gómez-Guillén MC, Montero P, Prentice C (2021) Characterization, bioactivity and application of chitosan-based nanoparticles in a food emulsion model. Polymers 13(19). https://doi.org/10.3390/polym13193331
Liu L, Wang Y, Peng C, Wang J (2013) Optimization of the preparation of fish protein anti-obesity hydrolysates using response surface methodology. Int J Mol Sci 14(2):3124–3139. https://doi.org/10.3390/ijms14023124
López-Sánchez J, Ponce-Alquicira E, Pedroza-Islas R, de la Peña-Díaz A, Soriano-Santos J (2016) Effects of heat and pH treatments and in vitro digestion on the biological activity of protein hydrolysates of Amaranthus hypochondriacus L. grain. J Food Sci Technol 53(12):4298–4307. https://doi.org/10.1007/s13197-016-2428-0
Lozano G, Hardisson A (2003) Fish as food. In: Encyclopedia of food sciences and nutrition, 2nd edn, pp 2417–2423. https://doi.org/10.1016/S0140-6736(01)54974-0
Marcolini E, Babini E, Bordoni A, Di Nunzio M, Laghi L, Maczó A, Picone G, Szerdahelyi E, Valli V, Capozzi F (2015) Bioaccessibility of the bioactive peptide carnosine during in vitro digestion of cured beef meat. J Agric Food Chem 63(20):4973–4978. https://doi.org/10.1021/acs.jafc.5b01157
Marti-Quijal FJ, Remize F, Meca G, Ferrer E, Ruiz MJ, Barba FJ (2020) Fermentation in fish and by-products processing: an overview of current research and future prospects. Curr Opin Food Sci 31:9–16. https://doi.org/10.1016/j.cofs.2019.08.001
Maschmeyer T, Luque R, Selva M (2020) Upgrading of marine (fish and crustaceans) biowaste for high added-value molecules and bio(nano)-materials. Chem Soc Rev 49(13):4527–4563. https://doi.org/10.1039/C9CS00653B
Mata-Cases M, Rodríguez-Sánchez B, Mauricio D, Real J, Vlacho B, Franch-Nadal J, Oliva J (2020) The association between poor glycemic control and health care costs in people with diabetes: a population-based study. Diabetes Care 43(4):751–758. https://doi.org/10.2337/dc19-0573
Matsui T, Oki T, Osajima Y (1999) Isolation and identification of peptidic α-glucosidase inhibitors derived from sardine muscle hydrolyzate. Zeitschrift Fur Naturforschung Sect C J Biosci 54(3–4):259–263
Medenieks L, Vasiljevic T (2008) Underutilised fish as sources of bioactive peptides with potential health benefits. Food Aust 60:581–588
Mora L, González-Rogel D, Heres A, Toldrá F (2020) Iberian dry-cured ham as a potential source of α-glucosidase-inhibitory peptides. J Funct Foods 67:103840. https://doi.org/10.1016/j.jff.2020.103840
Morales-Medina R, García-Moreno PJ, Pérez-Gálvez R, Muñío M, Guadix A, Guadix EM (2015) Seasonal variations in the regiodistribution of oil extracted from small-spotted catshark and bogue. Food Funct 6(8):2646–2652. https://doi.org/10.1039/c5fo00448a
Morales-Medina R, García-Moreno PJ, Pérez-Gálvez R, Muñío MM, Guadix A, Guadix EM (2016a) Nutritional indexes, fatty acids profile, and regiodistribution of oil extracted from four discarded species of the Alboran Sea: seasonal effects. Eur J Lipid Sci Technol 118(9):1409–1415. https://doi.org/10.1002/ejlt.201500486
Morales-Medina R, Tamm F, Guadix A, Guadix EM, Drusch S (2016b) Functional and antioxidant properties of hydrolysates of sardine (S. pilchardus) and horse mackerel (T. mediterraneus) for the microencapsulation of fish oil by spray-drying. Food Chem 194:1208–1216. https://doi.org/10.1016/j.foodchem.2015.08.122
Natsir H, Dali S, Arif AR, Sartika, Leliani (2019) Activity and kinetics of α-glucosidase inhibition by collagen hydrolysate from thunnus albacares bone. J Phys Conf Ser 1341(3). https://doi.org/10.1088/1742-6596/1341/3/032015
Nelson DL, Cox MM (2008) Lehninger: principles of biochemistry. W H Freeman & Co. https://doi.org/10.1017/CBO9781107415324.004
Neves AC, Harnedy PA, O’Keeffe MB, Alashi MA, Aluko RE, FitzGerald RJ (2017) Peptide identification in a salmon gelatin hydrolysate with antihypertensive, dipeptidyl peptidase IV inhibitory and antioxidant activities. Food Res Int 100:112–120. https://doi.org/10.1016/j.foodres.2017.06.065
Nguyen TH, Wang SL, Nguyen AD, Doan MD, Tran TN, Doan CT, Nguyen VB (2022) Novel α-amylase inhibitor hemi-pyocyanin produced by microbial conversion of chitinous discards. Mar Drugs 20(5):1–18. https://doi.org/10.3390/md20050283
Nirmal NP, Santivarangkna C, Benjakul S, Maqsood S (2022a) Fish protein hydrolysates as a health-promoting ingredient—recent update. Nutr Rev 80(5):1013–1026. https://doi.org/10.1093/nutrit/nuab065
Nirmal NP, Santivarangkna C, Rajput MS, Benjakul S, Maqsood S (2022b) Valorization of fish byproducts: sources to end-product applications of bioactive protein hydrolysate. Compr Rev Food Sci Food Saf 21(2):1803–1842. https://doi.org/10.1111/1541-4337.12917
Nongonierma AB, FitzGerald RJ (2017) Strategies for the discovery and identification of food protein-derived biologically active peptides. Trends Food Sci Technol 69:289–305. https://doi.org/10.1016/J.TIFS.2017.03.003
Nur S, Wierson Y, Sami FJ, Megawati Gani SA, Aisyah AN, Yulia, Marwati (2021) Characterization, antioxidant and α-glucosidase inhibitory activity of collagen hydrolysate from lamuru (caranx ignobilis) fishbone. Sains Malays 50(8):2329–2341. https://doi.org/10.17576/jsm-2021-5008-16
Parthsarathy V, McLaughlin CM, Harnedy PA, Allsopp PJ, Crowe W, McSorley EM, FitzGerald RJ, O’Harte FPMM (2018) Boarfish (Capros aper) protein hydrolysate has potent insulinotropic and GLP-1 secretory activity in vitro and acute glucose lowering effects in mice. Int J Food Sci Technol 54(1):271–281. https://doi.org/10.1111/ijfs.13975
Patil P, Mandal S, Tomar SK, Anand S (2015) Food protein-derived bioactive peptides in management of type 2 diabetes. Eur J Nutr 54(6):863–880. https://doi.org/10.1007/s00394-015-0974-2
Perez-Galvez R, Berge JP (2008) General introduction about by-products: worlwide situation and French focus. In Bergé J-P (ed) Added value to fisheries waste, pp 1–22
Petcharat T, Benjakul S, Karnjanapratum S, Nalinanon S (2021) Ultrasound-assisted extraction of collagen from clown featherback (Chitala ornata) skin: yield and molecular characteristics. J Sci Food Agric 101(2):648–658. https://doi.org/10.1002/jsfa.10677
Remme J, Tveit GM, Toldnes B, Slizyte R, Carvajal AK (2022) Production of protein hydrolysates from cod (Gadus morhua) heads: lab and pilot scale studies. J Aquat Food Prod Technol 31(2):114–127. https://doi.org/10.1080/10498850.2021.2021341
Rivero-Pino F (2021) Obtención de biopéptidos reguladores del índice glucémico para alimentación funcional. Universidad de Granada, Granada. http://hdl.handle.net/10481/68559
Rivero-Pino F (2023) Bioactive food-derived peptides for functional nutrition: effect of fortification, processing and storage on peptide stability and bioactivity within food matrices. Food Chem 406:135046. https://doi.org/10.1016/j.foodchem.2022.135046
Rivero-Pino F, Espejo-Carpio FJ, Guadix EM (2020a) Antidiabetic food‐derived peptides for functional feeding: production, functionality and in vivo evidences. Foods 9(8):883. https://doi.org/10.3390/foods9080983
Rivero-Pino F, Espejo-Carpio FJ, Guadix EM (2020b) Bioactive fish hydrolysates resistance to food processing. LWT Food Sci Technol 117(108670). https://doi.org/10.1016/j.lwt.2019.108670
Rivero-Pino F, Espejo-Carpio FJ, Guadix EM (2020c) Evaluation of the bioactive potential of foods fortified with fish protein hydrolysates. Food Res Int 137:109572. https://doi.org/10.1016/j.foodres.2020.109572
Rivero-Pino F, Espejo-Carpio FJ, Guadix EM (2020d) Production and identification of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from discarded Sardine pilchardus protein. Food Chem 328:127096. https://doi.org/10.1016/j.foodchem.2020.127096
Rivero-Pino F, Espejo-Carpio FJ, Pérez-Gálvez R, Guadix A, Guadix EM (2020e) Effect of ultrasound pretreatment and sequential hydrolysis on the production of Tenebrio molitor antidiabetic peptides. Food Bioprod Process 123:217–224. https://doi.org/10.1016/j.fbp.2020.07.003
Salem et al., 2018 Salem RBS-B, Ktari N, Bkhairia I, Nasri R, Mora L, Kallel R, Hamdi S, Jamoussi K, Boudaouara T, El-Feki A, Toldrá F, Nasri M (2018) In vitro and in vivo anti-diabetic and anti-hyperlipidemic effects of protein hydrolysates from Octopus vulgaris in alloxanic rats. Food Res Int 106:952–963. https://doi.org/10.1016/J.FOODRES.2018.01.068
Shangwu C, Ying Z (2014) Silver carp DPP-IV inhibits polypeptide and its application. (Patent No CN104480176B). https://patents.google.com/patent/CN104480176B/en
Siala R, Khabir A, Lassoued I, Abdelhedi O, Elfeki A, Vallaeys T, Nasri M (2016) Functional and antioxidant properties of protein hydrolysates from grey triggerfish muscle and in vivo evaluation of hypoglycemic and hypolipidemic activities. J Appl Environ Microbiol 4(6):105–119. https://doi.org/10.12691/jaem-4-6-1
Sila A, Alvarez OM, Haddar A, Frikha F, Dhulster P, Nedjar-Arroume N, Bougatef A (2016) Purification, identification and structural modelling of DPP-IV inhibiting peptides from barbel protein hydrolysate. J Chromatogr B 1008:260–269. https://doi.org/10.1016/J.JCHROMB.2015.11.054
Subara D, Jaswir I, Alkhatib MFR, Noorbatcha IA (2018) Synthesis of fish gelatin nanoparticles and their application for the drug delivery based on response surface methodology. Adv Nat Sci Nanosci Nanotechnol 9(4). https://doi.org/10.1088/2043-6254/aae988
Sun X, Acquah C, Aluko RE, Udenigwe CC (2020) Considering food matrix and gastrointestinal effects in enhancing bioactive peptide absorption and bioavailability. J Funct Foods 64:103680. https://doi.org/10.1016/j.jff.2019.103680
Tahrani AA, Bailey CJ, Del Prato S, Barnett AH (2011) Management of type 2 diabetes: new and future developments in treatment. The Lancet 378(9786):182–197. https://doi.org/10.1016/S0140-6736(11)60207-9
Takahashi Y, Kamata A, Konishi T (2021a) Dipeptidyl peptidase-IV inhibitory peptides derived from salmon milt and their effects on postprandial blood glucose level. Fish Sci 87(4):619–626. https://doi.org/10.1007/s12562-021-01530-9
Takahashi Y, Kamata A, Nishimura M, Nishihira J (2021b) Effect of one-week administration of dipeptidyl peptidase-IV inhibitory peptides from chum salmon milt on postprandial blood glucose level: a randomised, placebo-controlled, double-blind, crossover, pilot clinical trial. Food Funct 12(18):8544–8551. https://doi.org/10.1039/d1fo00592h
Takahashi Y, Kamata A, Konishi T (2014) Peptide compound inhibiting dipeptidyl peptidase-iv (dppiv), composition containing same, and method for producing same. (Patent No KR101771867B1). https://patents.google.com/patent/KR101771867B1/en
Tavano OL, Berenguer-Murcia A, Secundo F, Fernandez-Lafuente R (2018) Biotechnological applications of proteases in food technology. Compr Rev Food Sci Food Saf 17:412–436. https://doi.org/10.1111/1541-4337.12326
Theysgeur S, Cudennec B, Deracinois B, Perrin C, Guiller I, Lepoudère A, Flahaut C, Ravallec R (2020) New bioactive peptides identified from a tilapia byproduct hydrolysate exerting effects on DPP-IV activity and intestinal hormones regulation after canine gastrointestinal simulated digestion. Molecules (Basel, Switzerland) 26(1). https://doi.org/10.3390/molecules26010136
Thirukumaran R, Anu Priya VK, Krishnamoorthy S, Ramakrishnan P, Moses JA, Anandharamakrishnan C (2022) Resource recovery from fish waste: prospects and the usage of intensified extraction technologies. Chemosphere 299(March):134361. https://doi.org/10.1016/j.chemosphere.2022.134361
Vázquez JA, Fraguas J, Mirón J, Valcárcel J, Pérez-Martín RI, Antelo LT (2020) Valorisation of fish discards assisted by enzymatic hydrolysis and microbial bioconversion: lab and pilot plant studies and preliminary sustainability evaluation. J Clean Prod 246:119027. https://doi.org/10.1016/J.JCLEPRO.2019.119027
Vildmyren I, Halstensen A, McCann A, Midttun Ø, Ueland PM, Oterhals Å, Gudbrandsen OA (2020) Effect of cod residual protein supplementation on markers of glucose regulation in lean adults: a randomized double-blind study. Nutrients 12(5). https://doi.org/10.3390/nu12051445
Więcaszek B, Sobecka E, Keszka S, Stepanowska K, Dudko S, Biernaczyk M, Wrzecionkowski K (2015) Studies on endangered and rare non-commercial fish species recorded in the Pomeranian Bay (southern Baltic Sea) in 2010–2013. Helgol Mar Res 69(4):411–416. https://doi.org/10.1007/s10152-015-0442-7
Wu H, Abdollahi M, Undeland I (2021) Effect of recovery technique, antioxidant addition and compositional features on lipid oxidation in protein enriched products from cod-salmon and herring backbones. Food Chem 360:129973. https://doi.org/10.1016/j.foodchem.2021.129973
Wu CH, Guo HR, Patel AK, Singhania RR, Chen YA, Kuo JM, Dong CD (2022) Production and characterization of lucrative hypoglycemic collagen-peptide-chromium from tilapia scale. Process Biochem 115:10–18. https://doi.org/10.1016/j.procbio.2022.02.004
Wu S-J, Ho Y-C, Jiang S-Z, Mi F-L (2015) Effect of tannic acid–fish scale gelatin hydrolysate hybrid nanoparticles on intestinal barrier function and α-amylase activity. Food Funct 6. https://doi.org/10.1039/C4FO01015A
Yongkang L (2017) Fish skin protein peptide with DPP-IV inhibition function and preparation method and application thereof. (Patent No CN107164445B). https://patents.google.com/patent/CN107164445B/en
Yunping E (2018) A kind of preparation method of biological source dipeptidyl peptidase-iv inhibitor. (Patent No CN109182435A). https://patents.google.com/patent/CN109182435A/en
Zhang C, Liu H, Chen S, Luo Y (2018) Evaluating the effects of IADHFL on inhibiting DPP-IV activity and expression in Caco-2 cells and contributing to the amount of insulin released from INS-1 cells: in vitro. Food Funct 9(4):2240–2250. https://doi.org/10.1039/c7fo01950e
Zhang C, Gao S, Zhang C, Zhang Y, Liu H, Hong H, Luo Y (2020) Evaluating in vitro dipeptidyl peptidase IV inhibition by peptides from common carp (Cyprinus carpio) roe in cell culture models. Eur Food Res Technol 246(1):179–191. https://doi.org/10.1007/s00217-019-03399-6
Acknowledgements
This research was funded by the project B-AGR-400-UGR20 (Producción, concentración y estabilización de biopéptidos moduladores del índice glucémico (BioPepGI)) from University of Granada
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rivero-Pino, F., Espejo-Carpio, F.J., García-Moreno, P.J., Pérez-Gálvez, R., Guadix, A., Guadix, E.M. (2024). Production of Antidiabetic Peptides from Fish Waste. In: Maqsood, S., Naseer, M.N., Benjakul, S., Zaidi, A.A. (eds) Fish Waste to Valuable Products. Sustainable Materials and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-99-8593-7_7
Download citation
DOI: https://doi.org/10.1007/978-981-99-8593-7_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-8592-0
Online ISBN: 978-981-99-8593-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)