Abstract
The growth of the elderly population and the high prevalence of cardiovascular and orthopedic diseases have increased the demand for biological materials. The combination of additive manufacturing (AM) and biomaterials holds promise, especially for patient-specific applications. Metal biomaterials are used in human medical equipment more than other families of materials. Corrosion resistance of implant materials is the main factor influencing their performance and durability and determining their biocompatibility. The basic paradigm for metallic biomaterials, with the exception of biodegradable metals, is that “the higher the corrosion resistance, the more biocompatible.” This study is a detailed, critical, and analytical review of previous studies and researches in the field of metals and alloys used as implant materials, including magnesium and its alloys, stainless steel, titanium and its alloys, etc. This study focuses on highlighting some of the basic principles for implant material fabrication that underlie the composition, structure, and properties of these materials. The specific conditions associated with the AM process are known to create fine microstructures in these materials with unique directional growth characteristics away from equilibrium. This unique microstructure, along with other special features and microstructural defects caused by the additive manufacturing process, has a significant effect on the corrosion behavior of these materials. Thus, this work provides an in-depth review of the previous work to date investigating the corrosion aspects of additively manufactured metallic biomaterials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Additive manufacturing
- Corrosion performance
- Magnesium alloys
- Titanium alloys
- Stainless steel
- Biomaterials
1 Introduction
AM allows you to build unique and complex structures. Additionally, computer-aided design and direct linking to digital scans enable direct reproducible products. However, choosing the right biomaterials and related AM procedures can be difficult, but it is a vital aspect of success. The aging population, as well as the high prevalence of cardiovascular and orthopedic illnesses, have raised the demand for biological materials [1]. In this chapter, a concise materials’ selection guidance has been provided that will be beneficial for the biomedical AM discipline [2]. Following a general description of biomaterial classes (bio resistant, bio-inert, bioactive, and biodegradable), an overview of common ceramic, polymer, and metal biomaterials is discussed along with their implications, as well as their biomedical and mechanical properties. Since the topic of metal implants is rapidly growing, we devote the major portion of review to this area and present some important directions for future research. The present article delivers a summary of the topic under consideration and also consists of corrosion performance of various biomaterials, their applications, and various AM techniques and resources, so there is also the potential to deepen your knowledge of specific aspects [3].
2 Literature Review
Human bones have excellent mechanical and structural properties that are suitable for bearing the load of the human body. Despite these characteristics, the human body is prone to fractures caused by injury or sudden accident, fractures caused by fatigue or stress caused by repeated loading conditions, pathological fractures caused by bone infection or tumor, etc. Choosing a specific biological material to replace human bone is a very difficult task [4]. Composed of 30% by weight of matrix, 60% by weight of minerals, and 10% by weight of water, human bones often break due to trauma, pathology, erosion, and other reasons. Subsequent surgery and associated medical expenses are required. Apart from that, various health problems have been observed due to previously used non-magnesium permanent metal implants. Table 1 shows the problems encountered with non-degradable implants in the human body [5].
2.1 Magnesium Alloys
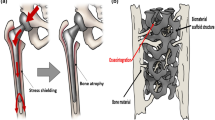
Magnesium (Mg) alloys have developed as encouraging biodegradable materials to be used in orthopedics [6], cardiac [7], respiratory [8], and urology [9]. The major benefit of Mg is that it destroys the organ completely, thus minimizing or preventing long-term complications. Another major benefit in orthopedics is that magnesium has an elastic modulus comparable to bone, by which dangerous effects of stress shielding are reduced. Three firms have so far obtained trial success and regulatory approval. Syntellix got the CE mark for the Magnezix® compression screw in 2013, after selling over 50,000 units [10]. U&i got regulatory approval from the Korean Ministry in 2015 for Resomet, an orthopedic bone screw made of an absorbable MgCa alloy [11]. Biotronik got the CE mark for Magmaris in cardiovascular health in June 2016, making it the first clinically proven bioresorbable magnesium scaffold. [12].
Because of its biocompatibility, magnesium in pure form, a biodegradable and biocompatible metallic substance, has been introduced as a viable material for biodegradable stents. The surrounding environment exposes implants to many corrosive attacks inside the human body, including amino acids in addition to blood, sodium, chlorine, proteins, blood plasma, and mucins in the case of saliva. A wide base of medical fields including various fields like dentistry, orthopedics, plastic surgery, experimental surgery, and veterinary medicine [13, 14].
Table 2 summarizes the uses of Mg in different fields. A new ternary magnesium alloy (Mg–4Li–1Ca (LC41)) adds two elements, Li and Ca, to magnesium to make it more biodegradable and lightweight for hip and knee applications. This alloy was invented by [15]. Using FEM simulations to compare commercial titanium alloys with Mg–REE alloys and by fabricating them as dynamic compression plates (DCPs) in the condition of distal fractures, the analytical results show that Mg–REE justifies the importance of alloys, due to its lightweight and biodegradability [16, 17].
2.1.1 Corrosion Performance of Mg Alloys
According to Wong et al. [18], magnesium and its alloys exhibit increased corrosion rates and H2 gas accumulation, which is irrefutable in terms of biomedical implant performance. They establish that coating a polymer film on the outer surface of a biomaterial (Mg alloy) processed with polycaprolactone and dichloromethane minimized the corrosion rate and also enhanced the mechanical properties. Kuah et al. [19] conducted a study to know how MgO inclusions affected the corrosion performance of additively produced Mg alloys. Results of this study show a significant difference in corrosion rate of binder jet printing (BJP) samples produced by using AM method in comparison with denser Mg sample that are fabricated by using casting. This is because a greater surface area in a porous structure is inadequate to account for the increased corrosion rate of the additively manufactured samples. Liu et al. [20] used the enhanced passivation effect caused by the inclusion of rare earth (RE) elements to design a high-temperature oxidation treatment to improve the corrosion resistance of WE43 alloy manufactured by additive manufacturing. After 30 days of immersion, the oxidation sample lost just 6.87% of its weight after being heated at 525 °C for 4 h. The greater passivation effect of the inclusive RE elements resulted in improved corrosion resistance due to protection from the dense oxide layer as well as the transition layer, where galvanic reactions were prevented due to the lack of precipitates. Hayashi et al. [21] examined silicate electrolyte (MAO)-based micro-arc oxidation treatment on the surface of ZK60 magnesium alloy and discovered that it increased corrosion resistance. Gu et al. [22] examined the MAO behavior of Mg–Ca alloys, which showed significant improvement effects on corrosion resistance. It has been determined that the corrosion resistance is improved in the order of 300 V MAO < 400 V MAO < 360 V MAO. Trinidad [23] studied the corrosion patterns of commercial magnesium alloys (AZ31B, WE43, and ZM21) in phosphate-buffered saline and showed the improvement in fluoride environments. In conclusion, it can be said that corrosion rate increases with the immersion time for these three-phase alloys. For AZ31B and ZM 21 alloys, the coating thickness increases with enhanced fluoride treatment time.
2.2 Titanium Alloys
Alloys of titanium are considered favorable implants due to their small elastic modulus and ability to create pathways for bone development. AM technology is now effectively used in the manufacture of porous alloys of titanium due to the advantages of manageable and accurate manufacturing. For long-term uses in the human body, it is critical to understand the corrosion of porous titanium alloys and the underlying mechanisms [24,25,26,27]. These alloys have piqued the interest of biomedical researchers because of their great biocompatibility, high specific strength, and low elastic modulus [28, 29]. Titanium alloys show very good corrosion resistance. Nevertheless, the risk of tribo-corrosion, ion deposition, and localized corrosion in physical environments is still high in long-term use, which not only alters the surface properties of titanium alloys but also significantly increases the penetration of corrosive materials into the tissue to give because physical harm around titanium alloy implants [30]. In addition, implant failure inevitably occurs under corrosion fatigue [31], which leads to reduced implant service performance and lifespan, and even requires a second surgery.
2.2.1 Corrosion Performance of Titanium Alloys
The sample with isolated pores in the porous CP–Ti, which has a lower electrochemical potential, is more susceptible to corrosion compared to the sample with interconnected pores. To enhance the corrosion resistance of porous CP–Ti, it might be necessary to optimize the powder metallurgy techniques to ensure a more interconnected pore structure, leading to improved electrochemical behavior and better resistance to corrosion [30]. In summary, the confinement of electrolyte and the limited escape of oxygen in the isolated pores of porous CP-Ti contribute to a higher dissolution rate and reduced formation of the protective passivation film, resulting in increased corrosion susceptibility. Moreover, the porosity and pore size of porous materials are critical factors in determining their biocompatibility, making it important to optimize these characteristics for specific applications, especially in the field of biomedicine. In conclusion, higher porosity and larger pore size are generally more favorable for bone growth and tissue integration in porous titanium alloys. Moreover, these characteristics might enhance corrosion resistance by promoting better electrolyte penetration and reducing the likelihood of localized oxygen concentration, which can lead to improved performance in certain environments [32, 33].
The study conducted by Seo and Lee [34] focused on analyzing the corrosion resistance of a heat-treated Ti–6Al–4 V alloy fabricated using additive manufacturing (AM) techniques. The researchers employed various electrochemical techniques, including potentiodynamic polarization, electrochemical impedance spectroscopy, and critical pitting temperature measurements, to evaluate the corrosion behavior of the AM Ti–6Al–4 V alloy at different stages of heat treatment. The findings of the study indicated that the corrosion resistance of the AM Ti–6Al–4 V alloy was significantly reduced compared to the untreated material. This reduction in corrosion resistance is likely attributed to the changes in the alloy’s microstructure and composition resulting from the heat treatment process.
The study conducted by Ettefagh et al. [35] focused on evaluating the corrosion behavior of Ti–6Al–4 V alloy parts produced through laser-based powder bed fusion AM. The researchers aimed to investigate the effect of post-annealing heat treatment on the corrosion resistance of the AM parts by comparing them with cold-rolled commercial titanium alloy samples. The study highlights that the as-fabricated AM parts of Ti–6Al–4 V alloy exhibited significantly worse corrosion resistance compared to cold-rolled commercial titanium alloy samples due to the presence of non-equilibrium phases. However, a proper post-annealing heat treatment process at 800 °C for 2 h ameliorated the corrosion behavior. In summary, the corrosion properties of porous titanium alloys are influenced by a combination of factors, including phase structure, pore morphology, porosity, alloy composition, and surface treatments. The inherent corrosion resistance of titanium alloys, coupled with their favorable properties in biomedical applications, makes them attractive materials for various uses. To thoroughly assess the corrosion behavior of porous titanium alloys, researchers subject them to accelerated corrosion tests, which help predict their long-term performance in challenging environments [33].
2.3 Stainless Steel Alloys
Stainless steel (SS) alloys are widely used in various industrial applications due to their excellent combination of mechanical properties, corrosion resistance, and versatility. Different types of stainless steel alloys, classified based on their microstructure, include austenitic, martensitic, ferritic, and duplex (austenoferritic) stainless steels [31]. The microstructure of stainless steel is determined by its chemical composition, particularly the levels of chromium, nickel, carbon, and other alloying elements [36]. By carefully controlling the composition and content of these alloying elements, stainless steel manufacturers can tailor the properties of the alloy to meet specific application requirements [37]. Stainless steel, particularly grade 316L stainless steel, has gained significant popularity in the medical field for various reasons, including its excellent mechanical properties, wide availability, and outstanding corrosion resistance at a reasonable cost. Due to these favorable attributes, 316L stainless steel is widely used in various medical implant devices, such as orthopedic implants (e.g., hip and knee replacements), vascular stents, bone fixation plates, dental implants, and surgical instruments [38, 39]. Indeed, the electrochemical reactions that occur inside the human body can lead to corrosion of metallic parts, including medical implants and devices. Corrosion is a critical concern in biological applications because it can have several significant implications [40]. For example, Stents made from SS 316L can release metal ions, including molybdenum (Mo), chromium (Cr), and nickel (Ni), into the surrounding tissues due to the electrochemical reactions and corrosion processes. The release of these metal ions can lead to limited immune and inflammatory responses in some individuals [41]. All of these can affect the quality of life of the transplant recipient, and failure can lead to severe pain and postoperative surgery [42].
2.3.1 Corrosion Performance of SS Alloys
In general, pores are a favorable location for corrosive attack, especially pitting. AM as a powder-based manufacturing method involves the inevitable presence of porosity in fabricated parts, which can affect mechanical properties and corrosion performance [33, 43]. Pores usually appear around unmelted powder particles or are created by gases trapped in the powder or molten pool during primary processing such as gas atomization or laser melting processes. In the context of AM parts, elemental mapping of voids involves analyzing the composition of the voids or pores present in the material [44]. Austenitic stainless steels, such as 316L and 304L, are susceptible to pitting corrosion, and the presence of unwanted inclusions, particularly manganese sulfide (MnS), can significantly influence their corrosion performance. These inclusions act as a second phase within the austenitic matrix and play a critical role in initiating and promoting pitting corrosion. By minimizing the presence of MnS inclusions or adjusting their size, the susceptibility to pitting corrosion and other localized forms of corrosion can be reduced [45, 46]. Surface roughness is a critical parameter that significantly influences the corrosion behavior of AM components. As an inherent characteristic of AM processes, the surface roughness of the printed parts can vary based on the printing method, material, and processing parameters. The presence of rough surfaces can accelerate electrochemical reactions between the component’s surface and the surrounding environment, leading to both general and localized corrosion [47, 48]. Grain size is also an essential factor that can significantly affect the corrosion performance of SS, and its influence depends on the specific corrosive environment. The size of the grains in the microstructure of stainless steel can influence the stability of the passive film and the susceptibility to corrosion [49, 50]. At the same time, some studies demonstrate that while reducing the grain size to the nanoscale range can enhance some properties of stainless steel, including mechanical strength and certain types of corrosion resistance, there are also potential challenges associated with nanocrystalline structures, particularly concerning the stability of the passive film. [51]. The effect of grain size on the corrosion performance of additively manufactured SS can indeed be a topic of controversy in the literature. The conflicting results are often attributed to the complexity of the interactions between various factors that influence corrosion behavior in AM SS materials.
3 Conclusions
This chapter reviews the additive manufacturing techniques used for printing magnesium, titanium and stainless steel. The primary emphasis is on biodegradable implants made from magnesium, as well as the challenges associated with its reactivity, high surface energy of the powder, and the rapid corrosion observed in the human body due to the alloy’s high electronegativity. For magnesium implant alloys to be viable, they must possess sufficient mechanical strength, biocompatibility, and corrosion resistance, along with consistent rates of tissue healing. While these challenges initially hindered the development of biodegradable magnesium implants, the field of AM is steadily overcoming these obstacles through various approaches. Regarding titanium alloys, the chapter highlights the significance of additives in influencing their preparation and corrosion properties. The corrosion behavior of biomedical titanium alloys is complex and influenced by several factors, including alloy composition, surface properties, and pore properties. These factors must be carefully considered when designing medical metal implants to ensure optimal performance and long-term functionality. At present, the corrosion performance of titanium alloys is still poor. In addition, stainless steel components are developed with a focus on corrosion performance in a wide range of applications where high corrosion considerations are required, such as the biomedical, nuclear, and fuel cell industries. In summary, the development of metallic biomaterial implants is an area with much room for exploration and innovation.
References
Chen Y, Xu Z, Smith C, Sankar J (2014) Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater 10(1):4561–4573
Staiger AM, Pietak J, Huadmai G, Dias (2008) Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials 27:1728–1734
Witte F, Hort N, Vogt C, Cohen S, Kainer KU, Willumeit R, Feyerabend F (2008) Degradable biomaterials based on magnesium corrosion. Curr Opin Solid State Mater Sci 12:63–72
Xin Y, Hu T, Chu PK (2011) In vitro studies of biomedical magnesium alloys in a simulated physiological environment: a review. Acta Biomater 7:1452–1459
Erdmann N, Bondarenko A, Hewicker-Trautwein M, Angrisani, Reifenrath J, Lucas A, Meyer-Lindenberg A (2010) Evaluation of the soft tissue biocompatibility of MgCa0.8 and surgical steel 316L in vivo: a comparative study in rabbits. Biomed Eng Online 9:63
Castellani C, Lindtner RA, Hausbrandt P, Tschegg E, Stanzl-Tschegg SE, Zanoni G, Beck S, Weinberg A (2011) Bone–implant interface strength and osseointegration: biodegradable magnesium alloy versus standard titanium control. Acta Biomater 7:432–440
Henderson SE, Verdelis K, Maiti S, Pal S, Chung WL, Chou D, Kumta PN, Almarza AJ (2014) Magnesium alloys as a biomaterial for degradable craniofacial screws. Acta Biomater 10:2323–2332
Waizy H, Diekmann J, Weizbauer A, Reifenrath J, Bartsch I, Neubert V, Schavan R, Windhagen H (2014) In vivo study of a biodegradable orthopedic screw (MgYREZr-alloy) in a rabbit model for up to 12 months. J Biomater Appl 28:667–675
Huehnerschulte TA, Reifenrath J, Rechenberg BV, Dziuba D, Seitz J, Bormann D, Windhagen H, Meyer-Lindenberg A (2012) In vivo assessment of the host reactions to the biodegradation of the two novel magnesium alloys ZEK100 and AX30 in an animal model. Biomed Eng Online 11:14
Sealy MP, Guo YB, Liu JF, Li C (2016) Pulsed laser cutting of magnesium-calcium for biodegradable stents. Procedia CIRP 42:67–72
Charpentier E, Barna A, Guillevin L, Juliard J (2015) Fully bioresorbable drug-eluting coronary scaffolds: a review. Arch Cardiovasc Dis 108:385–397
Iqbal J, Onuma Y, Ormiston J, Abizaid A, Waksman R, Serruys P (2014) Bioresorbable scaffolds: rationale, current status, challenges, and future. Eur Heart J 35:765–776
Di Mario C, Griffiths H, Goktekin O, Peeters N, Verbist J, Bosier M, Deloose K, Heublein B, Rohde R, Kasese V, Ilsley C, Erbel R (2004) Drug-Eluting bioabsorbable magnesium stent. J Interv Cardiol 17:391–395
Peeters P, Bosiers M, Verbist J, Deloose K, Heublein B (2005) Preliminary results after application of absorbable metal stents in patients with critical limb ischemia. J Endovasc Ther 12:1–5
Chalisgaonkar R (2020) Insight in applications, manufacturing and corrosion behaviour of magnesium and its alloys—a review. Mater Today Proc 26:1060–1071
Waksman R, Erbel R, Di Mario C, Bartunek J, de Bruyne B, Eberli FR, Erne P, Haude M, Horrigan M, Ilsley C, Böse D, Bonnier H, Koolen J, Lüscher TF, Weissman NJ (2009) Early- and long-term intravascular ultrasound and angiographic findings after bioabsorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc Interv 2:312–320
Hermawan H, Dubé D, Mantovani D (2010) Degradable metallic biomaterials: design and development of Fe-Mn alloys for stents. J Biomed Mater Res 1–11 Part A 93A
Wong HM, Yeung KWK, Lam KO, Tam V, Chu PK, Luk KDK, Cheung KMC (2010) A biodegradable polymer-based coating to control the performance of magnesium alloy orthopedic implants. Biomaterials 31:2084–2096
Kuah KX, Salehi M, Ong WK, Seet HL, Nai MLS, Wijesinghe S, Blackwood DJ (2022) Insights into the influence of oxide inclusions on corrosion performance of additive manufactured magnesium alloys. NPJ Mater Degradation 36:1–10
Liu J, Yin B, Song F, Liu B, Peng B, Wen P, Tian Y, Zheng Y, Ma X, Wang C (2022) Improving corrosion resistance of additively manufactured WE43 magnesium alloy by high temperature oxidation for biodegradable applications. J Magnesium Alloys, 1–14
Lin X, Tan L, Zhang Q (2013) The in vitro degradation process and biocompatibility of a ZK60 magnesium alloy with a forsterite-containing micro-arc oxidation coating. Acta Biomater 9:8631–8642
Gu XN, Li N, Zhou WR (2011) Corrosion resistance and surface biocompatibility of a microarc oxidationcoating on a Mg–Ca alloy. Acta Biomater 7:1880–1889
Trinidad J, Arruebarrena G, Marco I, Hurtado I, Argandoña E (2013) Effectivity of fluoride treatment on hydrogen and corrosion product generation in temporal implants for different magnesium alloys. Proc IMechE. Part H: J Eng Med 227(12):1301–1311
Luffy SA, Chou D, Waterman J, Wearden PD, Kumta PN, Gilbert TW (2014) Evaluation of magnesium-yttrium alloy as an extraluminal tracheal stent. J Biomed Mater Res A 102:611–620
Jang Y, Owuor D, Waterman JT, White L, Boyce C, Sankar J, Gilbert TW, Yun Y (2014) Effect of mucin and bicarbonate ion on corrosion behavior of AZ31 magnesium alloy for airway stents. Materials 7:5866–5882
Zhang S, Zheng Y, Zhang L, Bi Y, Li J, Liu J, Guo H, Li Y (2016) In vitro and in vivo corrosion and histocompatibility of pure Mg and a Mg-6Zn alloy as urinary implants in rat model. Mater Sci Eng C 68:414–422
Li SJ, Li XK, Hou WT (2018) Fabrication of open cellular (porous) titanium alloy implants: osseointegration, vascularization and preliminary human trials. Sci China Mater 61(4):525–536
Fojt J, Joska L, Malek J (2013) Corrosion behavior of porous Ti-39Nb alloy for biomedical applications. Corros Sci 2013(71):78–83
He XC, Li Y, Bi YJ (2020) Finite element analysis of temperature and residual stress profiles of porous cubic Ti-6Al-4V titanium alloy by electron beam melting. J Mater Sci Technol 44:191–200
Lee JTY, Leng Y, Chow KL (2011) Cell culture medium as an alternative to conventional simulated body fluid. Acta Biomater 7(6):2615–2622
Garcia-Ramirez MJ, Lopez-Sesenes R, Rosales-Cadena I (2018) Corrosion behaviour of Ti–Ni–Al alloys in a simulated human body solution. J Market Res 7(3):223–230
Yehia HM, El-Tantawy A, Ghayad IM (2020) Effect of zirconia content and sintering temperature on the density, microstructure, corrosion, and biocompatibility of the Ti-12Mo matrix for dental applications. J Market Res 9(4):8820–8833
Buciumeanu M, Bagheri A, Shamsaei N (2018) Tribocorrosion behavior of additive manufactured Ti-6Al-4V biomedical alloy. Tribol Int 119:381–388
Seo DI, Lee JB (2020) Influence of heat treatment parameters on the corrosion resistance of additively manufactured Ti–6Al–4V alloy. J Electrochem Soc 167:1–18
Ettefagh AH, Zeng C, Guo S, Raush J (2019) Corrosion behavior of additively manufactured Ti-6Al-4V parts and the effect of post annealing. Addit Manuf 28:252–258
Olsson CO, Landolt D (2003) Passive films on stainless steels—chemistry, structure and growth. Electrochim Acta 48:1093–1104
Sander G, Thomas S, Cruz V, Jurg M, Birbilis N, Gao S, Brameld M, Hutchinson C (2017) On the corrosion and metastable pitting characteristics of 316L stainless steel produced by selective laser melting. J Electrochem Soc 164:C250–C257
Murr LE, Martinez E, Hernandez J, Collins S, Amato KN, Gaytan SM, Shindo PW (2012) Microstructures and properties of 17–4 PH stainless steel fabricated by selective laser melting. J Market Res 1:167–177
Mazumder J, Choi J, Nagarathnam K, Koch J, Hetzner H (1997) The direct metal deposition of H13 tool steel for 3-D components. Jom 49:55–60
Schaller RF, Taylor JM, Rodelas J, Schindelholz EJ (2017) Corrosion properties of powder bed fusion additively manufactured 17–4 PH stainless steel. Corrosion 73:796–807
Sun Y, Moroz A, Alrbaey K (2014) Sliding wear characteristics and corrosion behaviour of selective laser melted 316L stainless steel. J Mater Eng Perform 23:518–526
Laleh M, Hughes AE, Xu W, Cizek P, Tan MY (2020) Unanticipated drastic decline in pitting corrosion resistance of additively manufactured 316L stainless steel after high-temperature post-processing. Corros Sci 165:108412
Trelewicz JR, Halada GP, Donaldson OK, Manogharan G (2016) Microstructure and corrosion resistance of laser additively manufactured 316L stainless steel. Jom 68:850–859
Arifvianto B, Mahardika M, Dewo P, Iswanto P, Salim U (2011) Effect of surface mechanical attrition treatment (SMAT) on microhardness, surface roughness and wettability of AISI 316L. Mater Chem Phys 125:418–426
Roland T, Retraint D, Lu K, Lu L (2006) Fatigue life improvement through surface nanostructuring of stainless steel by means of surface mechanical attrition treatment. Scripta Mater 54:1949–1954
Jirandehi AP, Mehdizadeh M, Khonsari M (2020) Temperature-induced buckling of ductile metals during cyclic loading and the subsequent early fracture. Int J Mech Sci 176:105525
Van Boven G, Chen W, Rogge R (2007) The role of residual stress in neutral pH stress corrosion cracking of pipeline steels. Part I Pitting Cracking Occurrence Acta Materialia 55:29–42
Ornek C (2018) Additive manufacturing–a general corrosion perspective. Corros Eng Sci Technol 53:531–535
Buchbinder D, Meiners W, Pirch N, Wissenbach K, Schrage J (2014) Investigation on reducing distortion by preheating during manufacture of aluminum components using selective laser melting. J Laser Appl 26:012004
Zaeh MF, Branner G (2010) Investigations on residual stresses and deformations in selective laser melting. Prod Eng Res Devel 4:35–45
Zuo Y, Wang H, Xiong J (2002) The aspect ratio of surface grooves and metastable pitting of stainless steel. Corros Sci 44:25–35
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, D., Singh, T., Singh, S. (2024). Corrosion Performance of Additively Manufactured Metallic Biomaterials: A Review. In: Mahajan, A., Devgan, S., Zitoune, R. (eds) Additive Manufacturing of Bio-implants. Biomedical Materials for Multi-functional Applications. Springer, Singapore. https://doi.org/10.1007/978-981-99-6972-2_8
Download citation
DOI: https://doi.org/10.1007/978-981-99-6972-2_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-6904-3
Online ISBN: 978-981-99-6972-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)