Abstract
Study of double perovskite compounds of A2BB'O6 type is significant due to the varied properties they exhibit with compositional variation. Structural and magnetic properties of La2CoMnO6 (LCMO) doped with Sr at La site in smaller concentration (La1.88 Sr0.12CoMnO6) are studied in this paper. LCMO and LSCMO (La1.88 Sr0.12CoMnO6) nanoparticles are synthesized by sol–gel method. To investigate its structural properties XRD, FTIR, and Raman techniques have been used. The detailed Rietveld refinement of XRD data shows the formation of rhombohedral type structure (S.G. No. R3c: 161) for the prepared nanoparticles. Estimated crystallite size from Debye Scherrer formula is found to be in the range of ~6–18 nm. The vibration bands obtained by FTIR and Raman study also confirms the formation of Sr doped LCMO nanoparticle. Magnetization (M–H) curve at room temperature shows the paramagnetic behavior of the prepared nanoparticles. M-H curves recorded at 250, 200, 150, and 60 K indicate the paramagnetic to ferromagnetic transition below 200 K. Temperature-dependent magnetization study indicates super-exchange interactions of Co2+–O2−–Mn4+ and Co3+–O2−–Mn4+ in the parent compound. Due to Sr doping in LCMO host lattice the antisite disorder is enhanced and the temperature dependent magnetization study indicates the existence of ferromagnetic short-range correlations similar to the Griffiths like phase.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Nanoparticles
- Double perovskites
- Sol–gel method
- Magnetization (M–H) curve
- super exchange interaction
- Griffith phase
1 Introduction
Oxide-based double perovskite compounds with crystal formula, A2BB'O6 have attracted the attention of researchers in the last two decades due to multifunctional properties exhibited by these compounds [1]. Double perovskite compounds were found to display interesting magnetic properties such as near room temperature ferromagnetism, colossal magneto-resistance, magneto-dielectric, etc., which have potential applications for the spintronic devices [2]. In comparison to oxide-based single perovskites of ABO3 type, the double perovskites have another element at B-site (B′) [3]. This structural modification, i.e., different orders of ions at B-site produce multiple exchange interactions resulting from different oxidation states of B-site ions exhibited in different crystal symmetry and lattice distortions give rise to many of the functional properties for the compound [4, 5]. In the recent past lead-free inorganic perovskite structures are widely studied due to the need of clean and green technological advancements [3,4,5,6,7,8,9].
Among the various studied double perovskite materials, La-based double perovskites La2CoMnO6 (LCMO) and La2NiMnO6 (LNMO) are widely studied by researchers due to the magnetodielectric, magnetoresistance, and magnetocapacitance properties for possible application in spintronic devices [6]. They exhibit interesting magnetic properties owing to their multiple oxidation state in B/Mn ordering [7]. LNMO is a ferromagnetic semiconductor with paramagnetic to ferromagnetic transition near room temperature ~275 K [8]. La2CoMnO6 (LCMO) is known to be a ferromagnetic insulator which in its ordered state shows a ferromagnetic transition about 240 K due to the super-exchange interaction of Co2+−O2−–Mn4+ ions where Mn appears in its high spin state [9]. In its disordered state Co and Mn also appear in +3 charge state due to which the ferromagnetic transition is around 150 K [9]. When the A site of the compound A2BB′O6 is doped with holes, i.e., when Sr2+ replaces La3+ in LCMO, it is interesting to study the effect of anti-site disorder created in an otherwise ordered perovskite on the magnetic properties of the system [10]. In addition to the chemical disorder created by the doping, the manganite’s structural disorder is also there because of Jahn Teller distortions and octahedral tilt [11]. Due to structural distortion, the interaction among the magnetic ions (B-Site ion) at the centre of the octahedra is also affected because the distortion of the structure leads to a change in the degree of overlap of the t2g and eg orbitals of the transition metal ion. As a result of which the occupying electrons take the type of magnetic configuration which saves maximum energy [12]. The exhibited magnetic properties as a result of interplay of structural and chemical disorder are worth studying. Aga Shahee et al has reported the existence of ferromagnetic correlations above the anti-ferromagnetic ordering temperatures at ~368 K for cubic perovskite manganite’s [11]. Such ferromagnetic correlations are important from the point of view of application in the field of spintronics.
In this paper we have studied the magnetic interactions in LCMO and Sr doped LCMO nanoparticles synthesized by sol–gel method through temperature-dependent magnetization study and have observed the ferromagnetic short-range correlations in LSCMO nanoparticles formed in rhombohedral phase.
2 Experimental Details
2.1 Synthesis Details
Pure, La2CoMnO6 (LCMO) and Sr doped La2-xSrxCoMnO6 (LSCMO: x = 0.12) nanoparticles were synthesized by the sol–gel method. In the preparation of nanoparticles, nitrate precursors La(NO3)3 · Co(NO3)2 · 6H2O, Sr(NO3)2 and Mn(NO3)2 were taken in stoichiometric ratio and dissolved in 100 ml of deionized water. Citric acid was added in the solution as the capping agent. The solution was further stirred continuously on a magnetic stirrer by maintaining a constant temperature of 70 °C till a viscous gel is obtained. The obtained gel was dried in a hot air oven at 150 °C for about 6 h to obtain a dry fluffy powder. The powder was ground in an agate mortar to obtain a very fine powder. This powder was kept for calcination in a muffle furnace at 600 °C for 4 h. The obtained black powder was ground and collected for further investigation of the physical properties via different experimental techniques. Figure 1 shows schematically the different steps in the synthesis of Sr doped LCMO nanoparticles by sol–gel method. The corresponding chemical reaction is as follows;
2.2 Characterization Techniques
X-ray diffraction spectra was recorded from Bruker D8 X-ray diffractometer using Cu-Kα source (λ = 1.54060 Å) to analyze the structural properties of the prepared nanoparticles of LCMO and LSCMO. To study the structural phase formation and bonding nature, Fourier transform infrared (FTIR) with attenuated total reflectance (ATR-FTIR, Bruker Tech.) and Raman spectroscopy were used. Raman Spectra was recorded with an excitation source of wavelength 514 nm of air-cooled Argon ion laser. Laser power at the sample was ~4 mW and typical spectral acquisition time was ~50 s with the spectral resolution of 1 cm−1. To investigate the detailed magnetic properties, M–H curve at different temperature was recorded using the Quantum Design SQUID-VSM (VSM, Versa Lab) from 60 to 300 K.
3 Results and Discussion
3.1 Structural Study and Strain Calculation
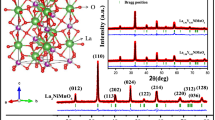
Figure 2 shows the XRD pattern of LCMO and LSCMO nanoparticles. The peaks of XRD are indexed (JCPDS file no #53–1211) with proper (hkl) planes and confirm the formation of the desired nanoparticles. The peak positions in XRD pattern of LSCMO nanoparticles are slightly shifted toward the higher angle side compared to peak positions of LCMO nanoparticles. From Table 1 we can observe widening and shifting of the diffraction peaks with Sr doping indicating the replacement of the La ions with Sr ions in the LCMO host lattice. The crystallite size has also been estimated from Scherrer formula [13] and found to be in the range of 6–18 nm as tabulated in Table 1. It has also been observed that along with the widening and shifting of the diffraction peaks the synthesized materials are within the nanometer range. XRD pattern of the LSCMO shows that the full width at half-maximum (FWHM) of most of the peaks increases with a doping percentage of Sr which can be attributed to a decrease in crystallite size (Table 3).
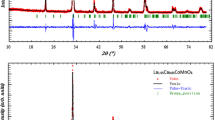
To collect more information regarding the structural phase formed and other lattice parameters, the Rietveld refinement of X-ray diffraction pattern using FullProf software was carried out. Figure 3 shows the Rietveld refinement plots of LCMO and LSCMO nanoparticles. For the refinement procedure, initial input parameters (such as unit cell lattice parameters, space group and atom coordinates) are taken from matching reference patterns [14]. The pseudo-voigt function has been chosen in the present study for peak shapes in the refinement purpose. It has been found from Fig. 3 that observed and calculated patterns are in good agreement to each other. The X-ray diffraction pattern after refinement corresponds to rhombohedral structure (space group R3c (161)). Table 2 lists the Wycoff positions, lattice parameters and bond distances obtained from refinement. The value of parameters for LSCMO varies slightly from LCMO which could be attributed to different ionic radii of Sr (1.13 Å) and La (1.06 Å) [14].
Induced strain \(\left(\varepsilon \right)\) in the prepared LCMO and LSCMO nanoparticles due to some distortions is estimated by plotting Williamson–Hall (W–H) plots as shown in Fig. 4a, b. The values of estimated crystallite size and strain are tabulated in Table 3. The crystallite size obtained from W–H plots is larger compared to values obtained by Debye Scherrer formula as the effect of broadening of peaks due to strain is not considered in it. It has been observed that the value of estimated strain in LSCMO sample is less as compared to the pure LCMO sample and the difference in the crystallite size measurement from Debye Scherrer formula and W–H plots is smaller in this case.
3.2 FTIR Spectroscopy Analysis
FTIR spectra of the material give insights about the attached functional groups and impurities present [15]. Figure 5 shows the absorbance spectra obtained for LCMO and LSCMO nanoparticles in the wavenumber range of 400–4000 cm−1 recorded at room temperature (RT) [16]. A strong band at 586 cm−1 for the LCMO nanoparticles is obtained which is due to Co–O/Mn–O vibrations in LCMO nanoparticles. Some weak bands are also obtained around 1073 cm−1 and 1145 cm−1 which could be due to Mn–O–Co vibration in MnO6 octahedra. Weak bands are also obtained at 1393 cm−1 and about 855 cm−1 which corresponds to vibration of nitrate ions [17]. The bands obtained at 1483 cm−1 could be due to vibration of N–O from the nitrate precursors taken [18]. In the LSCMO nanoparticles a strong band is observed at 581 cm−1, i.e., the bands are shifted to lower wave number side with the doping of Sr in the LCMO lattice.
3.3 Raman Spectroscopy Study
Raman spectra of the material is an efficient way of detecting the phases present. The peaks in the spectrum at different wavelengths are corresponding to specific vibration of molecular bonds and lattices [15]. In double perovskite compounds (A2BB′O6) structure, the vibrational modes are due to BO6 and B′O6 octahedra and A-O bonds [19]. Figure 6 shows the Raman spectra of LCMO and LSCMO nanoparticles recorded at RT at an excitation wavelength of 514 nm. The spectra show a peak at ~662 cm−1 for LCMO and with a slight shifting in case of LSCMO which is due to the incorporation of Sr+2 ions in host LCMO. The observed peak in the spectra is due to A1g symmetric stretching vibration mode of oxygen octahedra around cations [20, 21].
3.4 Magnetic Measurements
Figure 7 plots magnetization as a function of magnetic field for LCMO and LSCMO nanoparticles at RT by varying magnetic field from −30,000 Oe to +30,000 Oe. The inset of Fig. 7 shows the M-H curve in low magnetic field region from −2700 Oe to +2700 Oe. The nature of M-H curves of LCMO and LSCMO nanoparticles indicate dominant paramagnetic nature of the double perovskites at room temperature. The magnetic parameters: maximum magnetization (Ms), coercivity (Hc) and retentivity (Mr) obtained from M-H data (Fig. 7) are tabulated in Table 4. The value of Ms and Mr for LSCMO was found to be increased whereas the value of Hc decreased for the LSCMO nanoparticles as compared to LCMO nanoparticles.
Figure 8a shows M–H curves recorded at various temperatures for LCMO and LSCMO nanoparticles. This also indicates an increase in maximum magnetization and coercivity at low temperature with Sr doping in LCMO host lattice. From Fig. 8a we can see that the LCMO and LSCMO nanoparticles undergo a transition to ferromagnetic behavior below room temperature from paramagnetic behavior shown at room temperature. Figure 8b represents the Zero field cooled (ZFC: black color) and field cooled (FC: red color) magnetization curve of LCMO and LSCMO nanoparticles in the temperature range 60–400 K. To know exactly the transition temperature, the first derivative of the ZFC magnetization as a function of temperature (inset of Fig. 8b), i.e., dM/dT Vs T graph for LCMO nanoparticles was plotted which indicates two transition temperatures at ~220 and ~175 K. Anomaly was observed at further lower temperatures around 100-115 K, which could be attributed to the glassy behavior indicated as Tg [24]. The observed transition at ~220 K and at ~175 K could be due to Co2+–O2−–Mn4+ ferromagnetic super exchange interactions and Co3+–O2−–Mn4+ ferromagnetic super exchange interactions, respectively [22, 23]. The ZFC–FC and dM/dT versus T graph (inset of Fig. 8b) plots for LSCMO(x = 0.12) nanoparticles indicate one prominent transition temperature at 175 K and a small minimum is obtained around ~360 K. This implies that with the doping of Sr in LCMO nanoparticles the effect of ferromagnetic ordering due to Co2+–O2−–Mn4+ super exchange interaction is reduced and due to Co3+–O2−–Mn4+ferromagnetic super exchange interactions are enhanced [10]. The existence of lower transition temperature in case of LSCMO indicates that Co3+ and Mn3+ exist whereas ideally only Co2+ and Mn4+ ions are expected in LCMO system [9].
In order to get more insight about the magnetic transition temperature of the compounds inverse of susceptibility was plotted as a function of temperature (Fig. 9). On extrapolating the linear portion of the inverse susceptibility for LCMO nanoparticles it intersects x-axis at about ~220 K. The linear portion of the graph beyond this temperature indicates paramagnetic nature in accordance with Curie–Weiss law [12]. For LSCMO (x = 0.12) nanoparticles the inverse susceptibility graph has two linear regions which on extrapolating intersect the x-axis at 350 K and ~175 K.
The anomaly observed at ~360 K for Sr doped LCMO could be understood in terms of the effect of hole doping by Sr2+ at A site. Due to hole doping at A site, the valence of the B site ions i.e., Co/Mn can be affected [25]. As reported by Lan and coworkers [26] in their theoretical work that holes introduced by Sr doping in LCMO mainly go to oxygen sites and hence Mn3+ ions are introduced which disrupts the long-range ordering of Co and Mn [10]. Mn3+ ions with valence configuration 3d4, due to Jahn Teller effect spontaneously distorts the octahedron as it is energetically favorable, the increment in elastic energy due to distortion of octahedron is balanced by the energy saved due to lowering of eg orbitals [12]. Mn3+ can interact ferromagnetically with the neighboring cation via double exchange mechanism. The transfer of electrons from Mn3+ to neighboring ion saves kinetic energy and is allowed only if both are ferromagnetically aligned [12]. Many researchers have reported the existence of ferromagnetic correlations above the curie temperature for Sr doped manganites [1, 25]. The inverse susceptibility plots and the dχ−1/dT curves for LSCMO (x = 0.12) (inset of Fig. 9) indicate that above the ferromagnetic to paramagnetic transition temperature the behavior is not purely paramagnetic but ferromagnetic correlations exist and we obtain two peaks in dχ−1/dT versus T graph namely at 368 and 385 K which could be due to varying stoichiometry of oxygen in the compound [26]. The existence of ferromagnetic correlations is evidence of the existence of Griffith like phase [1, 10, 25]
a Inverse susceptibility versus T plot for LCMO nanoparticles, red line indicates linear fit according to Curie Weiss law. b Inverse susceptibility versus T plot for LSCMO nanoparticles, red line indicates linear fit according to Curie Weiss law, inset plots derivative of inverse of susceptibility with respect to temperature
4 Conclusion
Double perovskites La2CoMnO6 (LCMO) and La1.88 Sr0.12CoMnO6 (LSCMO) have been synthesized successfully using the wet chemical synthesis method of sol–gel. The crystallite size estimated from the peaks of X-ray diffraction pattern using Debye Scherrer formula is around 6–18 nm. Rietveld refinement of X-ray diffraction pattern indicates formation of rhombohedral phase (S.G. No. R3c; 161). The Williamson Hall plots indicate that the strain reduces on doping with Sr in LSCMO nanoparticles. The FTIR and Raman spectra show characteristic bands corresponding to Co/MnO6 octahedra further confirming the formation of double perovskite structure. The magnetization study at room temperature shows paramagnetic nature of prepared compounds. From the temperature-dependent magnetization studies, we conclude that multiple interactions between the different oxidation states of B site ions exist. For LSCMO nanoparticles a Griffith like phase above curie temperature exists at 368 K which is useful for spintronics applications.
References
Palakkal JP et al (2015) Observation of high-temperature magnetic transition and existence of ferromagnetic short-range correlations above transition in double perovskite La2FeMnO6. RSC advances 5(128):105531–105536
Kobayashi K-I et al (1998) Room-temperature magnetoresistance in an oxide material with an ordered double-perovskite structure. Nature 395(6703):677–680
Baettig P, Ederer C, Spaldin NA (2005) First principles study of the multiferroics BiFeO3, Bi2FeCrO6, and BiCrO3: structure, polarization, and magnetic ordering temperature. Phys Rev B 72(21):214105
Anderson MT, Greenwood KB, Taylor GA, Poeppelmeier KR (1993) B-cation arrangements in double perovskites. J Solid State Chem 22:197–233
Balcells Ll, Navarro J, Bibes M, Roig A, Martínez B, Fontcuberta J (2001) Appl Phys Lett 78:781
Balcells Ll et al (2001) Cationic ordering control of magnetization in Sr2FeMoO6 double perovskite. Appl Phys Lett 78(6):781–783
Murthy JK et al (2016) Antisite disorder driven spontaneous exchange bias effect in La2−xSrxCoMnO6(0⩽ x⩽ 1). J Phys: Conden Matter 28(8):086003
Mao Y, Parsons J, McCloy JS (2013) Magnetic properties of double perovskite La2BMnO6 (B= Ni or Co) nanoparticles. Nanoscale 5(11):4720–4728
Barrozo P, Albino Aguiar J (2013) Ferromagnetism in Mn half-doped LaCrO3 perovskite. J Appl Phys 113(17):17E309
Khan A et al (2018) Effect of Sr-doping on electronic and magnetic properties of La2–xSrxCoMnO6. In: AIP conference proceedings 1942. No. 1. AIP Publishing LLC
Mandal PR, Nath TK (2015) Evolution of griffith phase in hole doped double perovskite La2−xSrxCoMnO6 (x= 0.0, 0.5, and 1.0). Mater Res Express 2(6):066101
Shahee Aga, et al (2015) Evidence of ferromagnetic short‐range correlations in cubic La1− xSrxMnO3− δ (x= 0.80, 0.85) above antiferromagnetic ordering. Phys Status Solidi (b) 252(8):1832–1838
Blundell S (2003) Magnetism in condensed matter 94–95
Patterson AL (1939) The Scherrer formula for X-ray particle size determination. Phys Rev 56(10):978
Kumar M, Prajapati B, Singh A (2020) B-site ordering effect on structural and magnetic properties of ‘Y’-modified double perovskite La2NiMnO6 nanoparticles. J Mater Sci: Mater Electron 31(10):8099–8105
https://www.taylorfrancis.com/chapters/edit/, https://doi.org/10.1201/9781003197492-13/nanoscale-characterization-arvind-kumar-swati-manish-kumar-neelabh-srivastava-anadi-krishna-atul
Sadhu S, Chowdhury PS, Patra A (2008) Synthesis and time- resolved photoluminescence spectroscopy of capped CdS nanocrystals. J Lumin 128(7):1235–1240
Saberi A et al (2008) Chemical synthesis of nanocrystalline magnesium aluminate spinel via nitrate–citrate combustion route. J Alloy Compd 462(1–2):142–146
Dass RI, Yan J-Q, Goodenough JB (2003) Oxygen stoichiometry, ferromagnetism, and transport properties of La2− x NiMnO6+ δ. Phys Rev B 68(6):064415
Harbi A et al (2021) Tailoring the Griffiths-like cluster formation in the insulator ferromagnet spin-glass Gd2NixCo1− xMnO6 double perovskite. Phys Rev B 104(5):054404
Kumar D, Sathe VG (2015) Raman spectroscopic study of structural transformation in ordered double perovskites La2CoMnO6 bulk and epitaxial film. Solid State Commun 224:10–14
Magray MA, Ikram M (2019) Dielectric and Raman spectroscopy study of structural phase transformation of Sr-doped La2CoMnO6 double perovskite. J Mater Sci: Mater Electron 30(9):8655–8666
Truong KD et al (2007) Impact of Co∕ Mn cation ordering on phonon anomalies in La2Co MnO6 double perovskites: Raman spectroscopy. Phys Rev B 76(13):132413
Dass RI, Goodenough JB (2003) Multiple magnetic phases of La2CoMnO6− δ (0<~ δ<~ 0. 0 5). Phys Rev B 67(1):014401
Eglitis RI (2014) Ab initio calculations of SrTiO3, BaTiO3, PbTiO3, CaTiO3, SrZrO3, PbZrO3 and BaZrO3 (001), (011) and (111) surfaces as well as F centers, polarons, KTN solid solutions and Nb impurities therein. Int J Mod Phys B 28(17):1430009
Lan X, Kong S, Zhang WY (2011) Theoretical study on the electronic and magnetic properties of double perovskite La2− xSrxMnCoO6 (x= 0, 1, 2). Eur Phys J B 84:357–364
Acknowledgements
The financial support received from the UGC in the form of UGC-BSR research start up grant (F.30-374/2017(BSR)), New Delhi, India is acknowledged. The authors are thankful to the Department of Physics, Banasthali Vidyapeeth and IIT Patna for characterization facilities provided.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Jharwal, S., Kumar, A. (2024). Evidence of Ferromagnetic Short-Range Correlations in Sol–gel Synthesized La1.88 Sr0.12CoMnO6 Nanoparticles. In: Krupanidhi, S.B., Sharma, A., Singh, A.K., Tuli, V. (eds) Recent Advances in Functional Materials and Devices. AFMD 2023. Springer Proceedings in Materials, vol 37. Springer, Singapore. https://doi.org/10.1007/978-981-99-6766-7_2
Download citation
DOI: https://doi.org/10.1007/978-981-99-6766-7_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-6765-0
Online ISBN: 978-981-99-6766-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)