Abstract
Filamentous fungi are often identified as deadly human pathogens. They can cause invasive diseases in immunocompromised hosts, especially in those with hematological malignancies and transplant recipients. Aspergillus species are the most common fungi linked to invasive mold disease. Other molds of medical importance are Fusarium, Mucorales, and Scedosporium. This chapter exclusively addresses the most commonly encountered hyaline and dematiaceous molds responsible for central nervous system (CNS) mycoses. The primary insult is almost always associated with the paranasal sinuses and/or the lungs, wherefrom the infection disseminates. Hematogenous spread or direct invasion from adjoining sinuses lead to the involvement of the CNS. Mucor, Rhizopus, Rhizomucor and other genera under the order Mucorales tend to present as rapidly progressive paranasal sinus or rhino-orbito-cerebral infection, mostly in diabetics and high-dose steroid recipients. The diagnosis of CNS mold infections is challenging. While serological markers, such as galactomannan and (1,3)-β-D-Glucan (BDG) are of value in diagnosing many filamentous mold infections, there are exceptions, such as Mucorales, which lack BDG entirely or produce it in very low amounts. Culture-based methods still form the cornerstone for diagnosis of CNS mold diseases. In mucormycosis, the presence of broad, aseptate, ribbon-like hyphae with right angle branching in direct microscopy and histopathological examination of necrotic tissue is considered diagnostic. Treatment of CNS mold infections encompasses an early aggressive multidisciplinary approach with surgical debridement, antifungal therapy and correction of underlying disease, if any.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Molds
- Filamentous fungi
- Aspergillus
- Invasive mold disease
- Fusarium
- Mucorales
- Scedosporium
- Non-dematiaceous molds
- Central nervous system
1 Introduction
Central nervous system (CNS) mold infections are rare but serious conditions that can cause significant morbidity and mortality. These infections are caused by a variety of molds, most commonly, Aspergillus, Fusarium, and Mucorales. CNS infection caused by dematiaceous or melanized fungus is known as cerebral phaeohyphomycosis, often referred as black molds or phaeoid fungi. Most of the causative agents of cerebral phaeohyphomycosis belong to the order Chaetothyriales and include Cladophialophora bantiana, Rhinocladiella mackenziei, Exophiala dermatitidis, and Fonsecaea monophora. Other less common causes include Verruconis gallopava, Acrophialophora fusispora, Neoscytalidium dimidiatum, Curvularia spp., Exserohilum spp., Chaetomium spp., and Nodulisporium sp. C. bantiana is the most common (48%) cause of cerebral phaeohyphomycosis worldwide (Revankar and Sutton 2010).
The incidence of CNS mold infections has increased in recent years, primarily due to the growing population of immunocompromised patients. The risk factors for CNS mold infections include HIV infection, organ transplantation, malignancy, chemotherapy, prolonged neutropenia, long term use of systemic steroids, uncontrolled diabetes, severe renal failure, and intravenous drug usage. Additionally, patients are more likely to develop CNS mold infections if they have a history of prior fungal diseases like aspergillosis or mucormycosis. A high level of suspicion is essential for the diagnosis of CNS mold infections, especially in patients with risk factors.
CNS mold infections are more common in developing countries, where access to healthcare and antifungal medications is limited. In these countries, CNS mold infections are often associated with tuberculosis and other opportunistic infections.
2 Etiologic Agents
The causative agents of CNS mold infections include hyaline and dematiaceous fungi. Filamentous fungi encompass moniliaceous molds (light-colored) with septate hyphae, such as Aspergillus spp., Fusarium spp., and Mucorales (Rhizopus, Mucor, Lichtheimia, Syncephalestrum, and Rhizomucor) (Murthy and Sundaram 2014; Bongomin et al. 2017; McCarthy et al. 2014). Melanized fungi, although less common, are also implicated in some cases, albeit rarely and include Cladophialophora bantiana (Asian countries, particularly in India), Rhinocladiella mackenziei (Middle East), Exophiala dermatitidis (East Asia), Fonsecaea monophora, and Verruconis gallopava (worldwide). These molds can cause a variety of clinical syndromes, which are classified according to the site of infection and the extent of the disease (Table 14.1). A proper identification of the causative agent is crucial for appropriate management.
3 Burden of CNS Fungal Infections
CNS fungal infections impose a significant burden on global healthcare systems and public health. These infections are linked to high morbidity and mortality, with outcomes influenced by factors such as the patient’s underlying immune status, the severity and duration of the infection, and the specific fungal pathogen involved. The incidence of CNS fungal infections varies across regions and populations studied. Generally, these infections are more prevalent among immunocompromised individuals, including those with HIV, hematologic malignancies, solid organ and hematopoietic stem cell transplantation, and prolonged neutropenia. The occurrence of CNS fungal infections is also on the rise in terminally diseased patients, such as those receiving mechanical ventilation and prolonged ICU stay.
Cerebral aspergillosis is the most common form of CNS mold infection. The increasing use of immunosuppressive medications and the expanding population of immunocompromised hosts in recent years have contributed to the rise in the incidence of cerebral aspergillosis. Mortality rates for cerebral aspergillosis can reach as high as 90% in some individuals, making it a substantial concern in both clinical and public health contexts.
Cerebral mucormycosis and disseminated fusariosis are less common than cerebral aspergillosis, but are associated with a higher mortality rate. Cerebral mucormycosis typically affects patients with uncontrolled diabetes, and its incidence is increasing globally due to the rising prevalence of diabetes mellitus and indiscriminate use of high-dose steroid medications.
The impact of CNS fungal infections extends beyond high morbidity and mortality rates. These infections pose a significant economic burden on healthcare systems due to prolonged hospital stays, increased diagnostic testing, and expensive antifungal treatments. Additionally, CNS fungal infections can have long-term sequelae, including cognitive impairment and neurologic deficits.
4 Epidemiology
The epidemiology of CNS mold infections displays notable variations worldwide, encompassing differences in incidence rates, risk factors, and the specific causative agents involved. This section aims to offer a comprehensive overview of the epidemiology of CNS mold infections across various regions, drawing upon relevant studies and references.
North America: Invasive mold infections contribute to increased levels of suffering and death among immunosuppressed patients (Patterson et al. 2016). Cerebral aspergillosis is particularly prevalent among individuals with compromised immune system, including those with hematologic malignancies, solid organ or bone marrow transplantation, and prolonged neutropenia. Furthermore, the use of immunosuppressive medications, such as corticosteroids, presents a significant risk factor for cerebral aspergillosis. In North America, the incidence of cerebral aspergillosis appears to be on the rise, likely due to the use of immunosuppressive therapies and the rising number of immunocompromised hosts.
Cerebral mucormycosis is less common than cerebral aspergillosis in North America, but is associated with similar high mortality rates. Malignancy is currently the main risk factor for mucormycosis in developed nations like the USA, whereas in developing countries, uncontrolled diabetes and overuse of steroids remain the predominant causes. (Ruping et al. 2010; Prakash et al. 2018; Chakrabarti et al. 2009).
Disseminated fusariosis is less common than cerebral aspergillosis in North America. It can affect multiple organs, including the CNS, and is associated with prolonged neutropenia and immunosuppression.
Europe: The epidemiology of CNS mold infections in Europe shares similarities with North America. Cerebral aspergillosis stands out as the predominant CNS mold infection, especially among immunocompromised patients. In Europe, mortality rates due to invasive fungal diseases (IFDs) vary depending on several factors, such as the specific pathogen, geographical location, and underlying patient characteristics. The mortality rates for invasive Aspergillus infections vary between 38% and 80% (Lass-Flörl 2009).
Though cerebral mucormycosis is less prevalent than cerebral aspergillosis in Europe, an increasing trend is being observed in recent years. The frequency of cerebral mucormycosis is rising globally as a result of rising cases of diabetes, and is particularly prevalent among individuals with uncontrolled diabetes.
Asia: In Asia, the epidemiology of CNS mold infections differs from that in North America and Europe. While cerebral aspergillosis remains the most common CNS mold infection in Asia, its incidence is comparatively lower than in North America and Europe. In Asia, cerebral aspergillosis is particularly prevalent among immunocompromised patients, including those with hematologic malignancies and individuals who have undergone solid organ or bone marrow transplantation.
Cerebral mucormycosis, on the other hand, is more frequently encountered in Asia compared to North America and Europe. This difference is likely attributed to the higher prevalence of uncontrolled diabetes in the region. In the general population, mucormycosis is considered a rare disease with an estimated incidence of 0.005 to 1.7 cases per million individuals (Jeong et al. 2019). Nevertheless, reports indicate that in India, the prevalence of mucormycosis among diabetic patients is approximately 0.14 per 1000, which is significantly higher (80 times) compared to other regions of the world (Chander et al. 2018). This incidence is also higher than the estimated rate in the general population based on computational modeling (Ray et al. 2022).
Cladophialophora bantiana, a dematiaceous fungus with global distribution, is particularly prevalent in Asian countries such as India. It is highly neurotropic and is a major cause of fungal brain abscess both in immunocompetent and immunocompromised individuals. Other CNS mold infections, including disseminated fusariosis, are less frequently reported from Asia.
Africa: In Africa, the epidemiology of CNS mold infections is poorly understood. However, the existing evidence from case reports and small case series highlights a significant occurrence of cerebral aspergillosis and cerebral mucormycosis among immunocompromised patients in the region, including individuals with HIV and hematologic malignancies.
The epidemiology of CNS mold infections, including aspergillosis, mucormycosis, and fusariosis, varies significantly around the globe.
4.1 CNS Aspergillosis
Aspergillosis, the most common CNS mold infection globally, has shown an increasing incidence in recent years. The highest rates of cerebral aspergillosis are observed in immunocompromised patients, including those with HIV, hematologic malignancies, and solid organ or bone marrow transplantation. Furthermore, critically ill patients, particularly those in intensive care units and receiving mechanical ventilation, are at higher risk of developing invasive aspergillosis.
The most common invasive mold infection, especially among individuals with hematopoietic stem cell transplants and hematological malignancies, is invasive aspergillosis (Patterson et al. 2016). Nearly 90% of human infections are attributed to Aspergillus fumigatus. However, infections caused by non-fumigatus species, such as A. niger, A. terreus, A. flavus, A. nidulans, A. oryzae, and A. ustus, are also being observed. Tropical and subtropical regions like North America, the Middle East, and Southeast Asia have higher incidences of non-fumigatus Aspergillus spp. (Zanganeh et al. 2018; Lionakis et al. 2005). In immunosuppressed hosts, Aspergillus fumigatus is the primary cause of CNS infections, whereas Aspergillus flavus is more prevalent in immunocompetent individuals (Candoni et al. 2019).
4.2 Rhino-Orbito-Cerebral Mucormycosis
Rhizopus arrhizus is the most common cause of mucormycosis worldwide, while Lichtheimia species is predominant in Europe and Apophysomyces variabilis in Asia (Roden et al. 2005; Prakash and Chakrabarti 2019). Rhino-orbital cerebral mucormycosis is the commonest clinical manifestation, with Rhizopus oryzae being the most common etiologic agent. Uncontrolled diabetes mellitus is the leading risk factor, accounting for majority of cases (36%), followed by hematologic malignancies (17%) and hematopoietic stem cell or solid organ transplantation (12%) (Roden et al. 2005). Environmental factors like high humidity and temperature, may also contribute to the increased incidence of mucormycosis.
The current survival rate of mucormycosis patients without brain involvement ranges from 50% to 80%, which reduces to 20% when brain is affected. The prognosis and fatality associated with rhino-orbito-cerebral mucormycosis depend on several factors, such as the early identification of underlying disease, aggressive surgical debridement, and the timely administration of appropriate antifungal therapy. (Prakash and Chakrabarti 2019; Tooley et al. 2022).
4.3 CNS Fusariosis
Despite being less common than aspergillosis and mucormycosis, disseminated fusariosis, nevertheless, is a serious concern due to high morbidity and mortality. The incidence of fusariosis is highest in patients with prolonged neutropenia, T-cell immunodeficiency, hematologic malignancies, and those undergoing immunosuppressive therapy. The infection has a trimodal distribution in the allogeneic hematopoietic stem cell transplantation (HSCT) population with three peaks. The first peak occurs during neutropenia in the initial post-transplant phase. Patients receiving corticosteroids for acute graft-versus-host disease (GvHD) experience the second peak, and the third peak appears during treatment for chronic severe GvHD more than a year following transplant (Nucci et al. 2004). Notably, the main risk factor for fusariosis in these patients is severe T-cell immunodeficiency, not neutropenia. Fusariosis occurs in about 6 cases for every 1000 HSCTs, with autologous recipients experiencing the lowest incidence (1.5 to 2/1000), matched related and unrelated allogeneic recipients reporting intermediate incidence (2.5 to 5/1000), and mismatched related donor allogeneic recipients having the highest incidence (20/1000) (Nucci et al. 2004).
The epidemiology of CNS mold infections varies significantly around the globe, with incidence rates influenced by various factors, including population demographics, underlying medical conditions, environmental factors, and healthcare infrastructure. To improve the outcomes and alleviate the burden of these infections, it is crucial to raise awareness, facilitate early diagnosis, and ensure appropriate treatment.
5 Pathogenesis
CNS mold infections are uncommon, yet highly fatal conditions caused by fungi that invade the brain. The pathogenesis of CNS mold infections involves a complex interplay between the fungal virulence factors, host immune response and the structural and functional characteristics of the CNS.
Fungi can enter the CNS through different routes, including direct extension from a nearby infected site, hematogenous dissemination, or accidental inoculation during neurosurgical procedures or trauma. Once inside the CNS, the fungi can cause a range of pathologic changes, including tissue necrosis, inflammation, hemorrhage, and edema. Some fungi, such as Aspergillus and Rhizopus, can produce angioinvasive hyphae that penetrate blood vessels and cause thrombosis and infarction, leading to ischemic injury and further tissue damage (Fig. 14.1).
The pathogenesis of CNS mold infection involves a series of events, as depicted in the diagram (1) the portal of entry, typically the respiratory or in few cases extra-pulmonary site, extension from the ear or paranasal sinuses, or direct inoculation through cranial trauma or neurosurgery. (2) The host immune response involving secretion of cytokines and chemokines to clear the fungal infection. (3) Fungal elements multiply and disseminate via the bloodstream. (4) The fungi invade the central nervous system by disrupting the blood-brain barrier. Mycotoxins may contribute to this process, leading to damage to neurons and astrocytes
The outcome of CNS mold infections greatly relies on the host immune response. Immunosuppressed patients, such as those with HIV/AIDS, organ transplants, or hematologic malignancies, are particularly vulnerable to fungal infections because of their compromised immune function. Conversely, immunocompetent patients possess the ability to mount an effective immune response, which helps contain the infection and facilitate fungal clearance. The immune response to fungi involves innate, as well as adaptive mechanisms, which includes phagocytic cell-activation (neutrophils and macrophages), in addition to the cytokine production and chemokines that recruit and stimulate immune cells.
The pathogenesis of CNS mold infections is complex and involves several steps. These include:
-
Direct inoculation or inhalation of fungal spores: The fungi enter the body either through inhalation or by accidental inoculation during surgery or trauma. In certain instances, the infection may originate from a primary site of infection located elsewhere in the body, which then spreads to other areas. There may be contiguous spread from nearby sites such as sinuses, mastoid or orbit. Other sources, such as intravenous drug use, or contaminated medical supplies have been reported in patients infected with Exophiala dermatitidis and Exserohilum rostratum.
-
Adherence and invasion: The fungal cells adhere to the host tissue and form a biofilm. The biofilm allows the fungi to evade host defenses and invade the host tissue, and also acts as permeability barrier to antifungal drugs.
-
Host immune response: The effective containment of fungal infection depends substantially on the host immunological response. However, in immunocompromised patients, the immune response is often inadequate allowing the fungi to multiply and disseminate rapidly.
-
Fungal growth and dissemination: Fungal growth can cause tissue damage and may lead to the dissemination of the fungi to other organs, including the CNS.
-
CNS invasion: In CNS mold infections, the fungi invade the brain, spinal cord, or meninges. The invasion can lead to inflammation, necrosis, and hemorrhage.
Immunocompromised state causes the BBB to become more permeable, which makes it easier for fungi to invade the brain. Penetration of the fungal pathogen is facilitated but increased permeability of the blood brain barrier and the breach of the barrier occurs by one of the following mechanisms:
-
Trans-cellular migration,
-
Para-cellular migration, and
-
Trojan Horse Mechanism (inside infected phagocytes)
Once inside the brain parenchyma, the pathogens begin to multiply and cause inflammation. Fungal invasion is typically linked to immunocompromised states because the pathogens must breach the effective defenses encircling the brain. Once the BBB, cerebral and subarachnoid spaces are crossed, the CNS is involved by the invading fungus. Hence, the development of CNS fungal infections is influenced by the interaction between fungal cells and nerve cells, as well as the production of immune-suppressing and immune-enhancing chemokines and cytokines (Sharma et al. 2012). The blood-brain barrier, subarachnoid space, and brain parenchyma are affected by fungal invasion (Koutsouras et al. 2017). Various factors, such as surgical intervention, trauma, cytokine release, and microglial activation can disrupt blood-brain barrier, facilitating this process.
6 Pathogenesis by Specific Fungi
6.1 Aspergillus species
CNS infections due to Aspergillus species are intricate and exhibit variations depending on the specific species involved. Risk factors, such as prolonged neutropenia and corticosteroid usage increase the likelihood of Aspergillus brain infection. Moreover, individuals with cytomegalovirus infection or cerebral trauma, immunocompromised patients, hematological malignancies and organ transplant recipents are at increased risk of neuroaspergillosis. CARD-9 deficiencies are associated with impaired neutrophil accumulation and predispose to neuroaspergillosis.
CNS infection may occur either through hematogenous dissemination of the fungus from a primary pulmonary focus or through contiguous spread from adjoining regions, such as ear, paranasal sinuses or mastoids (Candoni et al. 2019). Immunocompromised individuals are more prone to develop CNS infection through hematogenous route, whereas direct extension from sinuses, following trauma and neurosurgical procedures are more likely to occur in the immunocompetent (Candoni et al. 2019; Pasqualotto and Denning 2006; Jensen et al. 2010; Gonzales Zamora et al. 2018). The exact mechanism by which Aspergillus species cross the blood-brain barrier and enter the CNS is unclear. Studies have demonstrated that mycotoxins produced by Aspergillus species, such as gliotoxins and aflatoxins, prevent phagocytosis and decrease opsonization of conidia during invasion (Lewis et al. 2005a, b; Eichner et al. 1986; Murayama et al. 1996). These mycotoxins possess the ability to disrupt the integrity of the blood-brain barrier, leading to neuronal, astrocytic and microglial damage or apoptosis (Patel et al. 2018). Aspergillus angioinvasion in the brain can cause hemorrhage, cerebral infarction, meningitis, and mycotic aneurysms in patients with severe immunosuppression, with mortality rates as high as 90% (Economides et al. 2017). Aspergillus spp. is the most common cause of intracranial mycotic aneurysms, with the internal carotid artery being the most common site involved. Aneurysm formation is proposed to occur by (a) direct invasion of the arterial wall from within the lumen, (b) invasion of the arterial wall from adjacent structures (sinus, bone, and meninges), (c) immune complex deposition leading to vascular injury, and (d) embolic occlusion of the vasa vasorum. In immunocompetent individuals, granulomas, brain abscess and meningitis may develop with mortality rates ranging from 40–80% (Nadkarni and Goel 2005).
6.2 Mucorales
Fungi belonging to the order Mucorales can cause various infections in humans, including CNS infections. The pathogenesis of rhino-orbito-cerebral mucormycosis is complex and is greatly influenced by underlying host factors. Majority (~80%) of the patients have uncontrolled diabetes. Other risk factors include hematological malignancy (13-22%), solid-organ transplantation (7-14%), and chronic kidney disease (~10%) (Sundaram et al. 2005; Abdollahi et al. 2016; Vironneau et al. 2014). During the recent Covid-19 pandemic, India encountered several cases of rhino-orbito-cerebral mucormycosis, predominantly associated with indiscriminate use of corticosteroids, poor glycemic control, COVID-19-associated immunosuppression and blanket use of broad-spectrum antibiotics.
Individuals with uncontrolled diabetes exhibit a sluggish immune response, impaired functioning of neutrophils, monocytes and macrophages, and suppressed inflammatory response. In addition, elevated serum levels of free iron in the background of diabetic ketoacidosis favors fungal growth, leading to angioinvasion and ischaemic tissue necrosis (Koutsouras et al. 2017; Sundaram et al. 2005; Gen et al. 2013).
Mucorales reach the brain through the cerebral vasculature (Bannykh et al. 2018). The internal elastic lamina of the vessel wall is invaded by fungal hyphae, leading to obliteration of vessel lumen, intravascular thrombosis and intimal hyperplasia (Bannykh et al. 2018; Higo et al. 2015). Hemorrhagic necrosis and cerebral infarction are caused by vascular occlusion. In advanced stages, hyphal invasion of the brain parenchyma occurs, often as a preterminal event (Malik et al. 2014). This is attributed to larger morphology of hyphal elements, which prevents access to the meningeal microcirculation.
The outcome of rhinocerebral mucormycosis depends significantly on the host immune status. In immunocompromised patients, the immune response is often insufficient to contain fungal invasion, allowing rapid fungal proliferation, tissue damage and dissemination to the CNS. Giant cell infiltration and the development of granulomas suggest a substantially unaltered immune response, and these features are linked to improved outcomes (Economides et al. 2017).
6.3 Fusarium species
Invasive infections caused by Fusarium spp. have become more common in immunocompromised individuals in recent years. Fusarium is the second leading etiologic agent of invasive mold infections in immunocompromised patients after Aspergillus spp. (Peterson et al. 2014; Garcia et al. 2015; Kleinschmidt-Demasters 2009). In patients with prolonged neutropenia, recipients of stem cell transplants, and those with hematological malignancies, Fusarium solani is the predominant species responsible for invasive infections (Dignani and Anaissie 2004; Koutsouras et al. 2017; Nucci et al. 2015). Like Aspergillus spp., Fusarium possesses the ability to produce mycotoxins, such as fumonisins and trichothecenes, which primarily impede cellular and humoral immunity. Fumonisin B1, the predominant mycotoxin produced by Fusarium spp., has been linked to cerebral invasion, resulting in impaired mitochondrial function and axonal degeneration (Bertero et al. 2018).
Cerebral fusariosis is usually a manifestation of disseminated infection (Góralska et al. 2018). Patients may present with single or multiple cerebral abscesses, cutaneous nodules, endophthalmitis, chorioretinitis, meningitis, and fungemia (McCarthy et al. 2014). Among the medically relevant Fusarium species, F. oxysporum, F. solani, and F. moliniforme complexes are most commonly encountered in clinical settings (Peterson et al. 2014). As for other invasive mold diseases, a definitive diagnosis of CNS fusariosis requires direct microscopy and culture of CSF and brain biopsies to identify the etiologic agent. In some cases, positive blood cultures can aid in the diagnosis, as disseminated fusariosis is often associated with fungemia (Nucci et al. 2015). Fusarium spp. can often be isolated from blood cultures in up to 40% of invasive fusariosis cases, with faster detection of growth in fungal blood culture bottles compared with standard aerobic bottles.
6.4 Scedosporium species
Lomentospora prolificans (formerly, Scedosporium prolificans) is an emerging fungal pathogen that is commonly found in soil, polluted water, and plant sources worldwide. Individuals at a higher risk include those who are immunocompromised or have hematological malignancies (Cortez et al. 2008; Guarro et al. 2006). There is a particular risk of CNS infections with Scedosporium apiospermum (Pseudallescheria boydii) in individuals who have experienced near-drowning incidents in water contaminated with fungal propagules (Guarro et al. 2006). The Scedosporium genus consists of three medically significant species: Scedosporium apiospermum, Scedosporium boydii (formerly known as Pseudoallescheria boydii), and Scedosporium aurantiacum (Lackner et al. 2014).
Scedosporium species are among the pathogenic fungi capable of causing infections in both immunocompetent and immunocompromised individuals, acting as primary or opportunistic pathogens (Lackner et al. 2014; Richardson and Lass-Flörl 2008). Immunocompromised patients have been reported to develop CNS infection by L. prolificans, which commonly results in rapid hematogenous dissemination with high mortality rate.
Colonization by Scedosporium species can progress to invasive and disseminated infections involving the CNS, especially following organ transplantation, resulting in poor outcomes (Husain et al. 2005). The outcome of CNS infections caused by Scedosporium species is significantly influenced by the host immune status. In immunocompromised individuals, the immunologic response is often inadequate, allowing rapid fungal multiplication and dissemination to the CNS.
The infection is acquired by inhalation of airborne conidia. In healthy individuals, the inhaled conidia are effectively cleared by mucociliary action of the airways and phagocytic action of alveolar macrophages. However, individuals with one or more defects in the terminal airways and innate immunity, fail to expel the inhaled conidia, leading to colonization and infection. The conidia germinate into hyphal morphotypes, the tissue-invading form of the fungus, which penetrate through the endothelial layer into the vascular lumen, leading to angioinvasion and subsequent hematogenous dissemination to the CNS (Ortoneda et al. 2002; Riddell 4th et al. 2004).
Other potential mediators of pathogenesis are:
-
1.
Extracellular protease peptidases produced by Scedosporium apiospermum and Pseudoallescheria boydii have been identified as metallo-proteases that depend on zinc and exhibit their highest activity in an acidic pH of 5.5. This specific pH environment may function as a mechanism to evade host effector cells, such as fibronectin-activated monocytes and macrophages. For instance, S. apiospermum secretes a 33-kDa extracellular serine protease (Larcher et al. 1996), while P. boydii releases two extracellular peptidases, one of 28 kDa and another of 35 kDa (Silva et al. 2006). These peptidases are capable of degrading human fibrinogen, contributing to tissue damage and inflammation. Moreover, the degradation of matrix proteins by these peptidases may facilitate the invasion of fungal cells into adjacent tissues and dissemination throughout the body.
-
2.
Melanin is thought to play an important role in the virulence of fungi and the ability to defend against host immune response. It allows fungi to tolerate environmental stress and evade host defenses (Rosas et al. 2002). One of the functions of melanin is to scavenge nitrogen and oxygen free radicals, which are generated by phagocytic cells in the course of the oxidative burst. Additionally, it imparts heat resistance by securing host defence proteins, cross-linking, and protecting the components of the cell wall from hydrolytic enzymes (Dixon et al. 1987; Sutton et al. 1998). These mechanisms help fungi, such as Scedosporium prolificans, to evade host immune responses and contribute to the high level of fungemia observed in patients with these infections. Moreover, the ability of the fungus to undergo in vivo adventitious sporulation may further enhance its survival and dissemination within the host.
-
3.
Siderophore activity: S. apiospermum and L. prolificans have been found to exhibit siderophore activity, indicating their dependence on iron. This iron dependency may contribute to their neurotropism, as the central nervous system (CNS) contains more free iron compared to the bloodstream (de Hoog et al. 1994). Multiple pathways allow S. apiospermum to enter the central nervous system (CNS), which include:
-
(a)
direct inoculation resulting from trauma (Pérez et al. 1988),
-
(b)
hematogenous spread from a primary pulmonary source (such as, inhalation of spores following near-drowning) (Fisher et al. 1982),
-
(c)
introduction of intravenous catheter (Yoo et al. 1985), and,
-
(d)
direct spread from infected paranasal sinuses (Bryan et al. 1980).
-
(a)
6.5 Cladophialophora bantiana
The pathogenesis of CNS infections caused by the neurotropic dematiaceous fungus Cladophialophora bantiana is not fully understood. However, several factors have been identified that contribute to the pathogenesis of these infections. C. bantiana is the most common etiological agent of cerebral phaeohyphomycosis, accounting for nearly 48% of the cases (Góralska et al. 2018). About half of the patients with cerebral phaeohyphomycosis have no underlying risk factors. The infection is more common in men (male : female ratio for C. bantiana infection is 3:1 worldwide, whereas India presents a much higher ratio of 14:1).
The virulence of C. bantiana involves the ability of the fungus to produce melanin, which can contribute to tissue damage and evasion of host defenses. Melanin is a pigment that protects the fungal cells from phagocytosis, oxidative stress, ultraviolet radiation, and antifungal agents. Additionally, C. bantiana secretes extracellular enzymes, such as carbohydrate-active enzymes, proteases and phospholipases, which contribute to tissue damage and facilitate CNS invasion. The host immune status is also critical in determining the outcome of the infection, as immunocompromised patients are more likely to have invasive disease and poor prognosis.
6.6 Verruconis gallopava
Verruconis gallopava (formerly, Ochroconis gallopava) are another group of neurotropic dematiaceous fungi widely found in the environment and are capable of causing various infections in humans, including pulmonary and CNS diseases. It primarily infects the respiratory system and specifically targets the CNS in solid organ transplant recipients and those with underlying immunodeficiency, such as chronic granulomatous disease (Shoham et al. 2008; Meriden et al. 2012).
7 Clinical Presentation
The manifestations of CNS mold infections vary depending on the particular mold species, the location and extent of the lesion and the presence of underlying conditions. However, some common clinical features include:
-
1.
Headache: One of the most commonly observed signs of CNS mold infections, which may be accompanied by fever and other symptoms of a systemic illness.
-
2.
Seizures: Seizures are a common presentation of CNS mold infections and can occur even in the absence of other neurological symptoms.
-
3.
Focal neurologic deficits: Patients may experience focal neurologic deficits, in the form of weakness or paresthesia, visual disturbances, unsteady gait or dysphasia, depending on the location and severity of the infection.
-
4.
Altered mental status: Patients with CNS mold infections may experience confusion, disorientation and altered mental status.
-
5.
Meningismus: It refers to the triad of fever, headache, and nuchal rigidity, which can be present in some cases of CNS mold infections.
-
6.
Cranial nerve involvement: Certain types of CNS mold infections, such as aspergillosis, may involve the cranial nerves, leading to symptoms such as diplopia or facial palsy.
Children with CNS invasive mold infection often present with non-specific symptoms like fever and failure to respond to antibiotic treatment. They may present with cerebritis or brain abscess (Luckowitsch et al. 2021).
The clinical symptoms of CNS mold infections are non-specific and may overlap with other neurological disorders. Therefore, maintaining a high level of suspicion and performing appropriate diagnostic investigations are vital for achieving an accurate diagnosis. The clinical presentation of some important mold infections of the CNS are as follows:
7.1 CNS Aspergillosis
Fungal infections of the CNS are often missed as they lack classic remarkable clinical features, and are often erroneously diagnosed as brain tumors, pyogenic abscesses, or tuberculous meningitis. The clinical presentation of cerebral aspergillosis includes recurring headaches, altered mental status, localized neurologic impairments, and loss of consciousness. Immunosuppressed patients are more likely to get CNS infections due to granulocytopenia and defective cellular and humoral immune responses. The most common Aspergillus species infecting humans is Aspergillus fumigatus. The lungs and maxillary sinuses are the most common sites for primary Aspergillus infection. The infection may reach the brain by contiguous spread from the adjecent paranasal sinuses or through the hematogenous route from a primary focus in the lungs. A characteristic feature of neuroaspergillosis is the presence of one or more abscesses, with evidence of angioinvasion and thrombosis on histopathologic examination of brain biopsies. Neuro aspergillosis should be considered in the differential diagnosis for immunocompromised patients who develop new onset focal neurologic deficits and focal seizures, especially those presenting with intracranial space-occupying lesions and characteristic pulmonary infiltrates (McCarthy et al. 2014).
7.2 Rhino-Orbito-Cerebral Mucormycosis
The clinical manifestations of mucormycosis are categorized on the basis of site of involvement. The most common clinical presentation is rhino-orbito-cerebral mucormycosis (ROCM) that characteristically involves the nose, paranasal sinuses, orbit, and brain. The characteristic “Red flag” signs of ROCM include:
-
Altered sensorium.
-
Epistaxis.
-
Eyelid/periocular/facial—edema and/or discoloration.
-
Facial pain and/or palsy.
-
Facial paresthesia.
-
Fever.
-
Focal seizures.
-
Foul smell.
-
Nasal discharge—mucoid/purulent/bloody.
-
Nasal mucosal erythema/ inflammation/purple or blue discoloration/ulceration/ischemia/eschar.
-
Nasal stuffiness.
-
Ptosis, proptosis.
-
Regional pain—orbit, paranasal sinus, or dental pain.
-
Restriction of ocular motility, diplopia.
-
Sudden loss of vision.
-
Worsening headache.
Based on the anatomical progression of the disease, ROCM is categorized into four stages:
-
Stage I: Involvement of nasal mucosa
-
Stage II: Involvement of paranasal sinuses
-
Stage III: Involvement of the orbit
-
Stage IV: Involvement of central nervous system
7.3 CNS Fusariosis
The clinical presentation of fusariosis varies depending on the site of infection, and the extent and duration of immune suppression. In comparison to aspergillosis, fusariosis is characterized by a notably high rate of positive blood cultures, primarily observed in cases of disseminated disease. The most prevalent and fatal clinical form of fusariosis in immunocompromised patients is disseminated disease, accounting for over 70% of cases in individuals with protracted neutropenia, acute leukemia, and hematopoietic stem cell transplant recipients (Nucci and Anaissie 2007; Vadhan et al. 2022).
Other mold infections of the central nervous system are mentioned in Table 14.2.
8 Diagnosis
Diagnosis of CNS mold infections can be challenging due to the non-specific and diverse clinical presentation and limitation of diagnostic tests. Definitive diagnosis requires a combination of clinical, radiological, microbiological, and histopathological findings.
The clinical samples obtained from patients need to be processed promptly, and if immediate processing is not possible, they should be stored at 4–5 °C. It is important to keep biopsy specimens moist and avoid placing them in formalin. Fungal growth obtained in culture should be identified to the species level, as significant variations in antifungal susceptibility exist between different species of fungi. Antifungal susceptibility testing is recommended. Culture is gold-standard but lacks sensitivity and a negative culture report does not exclude fungal infection.
Histopathological analysis of the affected brain tissue demonstrates the presence of hyphal angioinvasion, characterized by the penetration of fungal hyphae into small and large blood vessels, leading to thrombosis. This invasive process is accompanied by hemorrhagic infarction, coagulative necrosis, vasculitis, and the formation of granulomas. Moreover, the susceptibility to angioinvasion increases the likelihood of developing mycotic aneurysms (McCarthy et al. 2014).
In pediatric cases of invasive mold infections, the MRI findings exhibit non-specific characteristics, usually appearing hyperintense with varying enhancement on T2-weighted and FLAIR imaging (Fig. 14.2). It might be challenging to differentiate perilesional edema cerebritis set on by an abscess from hemorrhage in both adults and children (Fig. 14.3). It is extremely unusual for children to develop isolated cerebritis without CNS hemorrhage or an abscess that shows little to no enhancement. Differentiating fungal abscesses from pyogenic and tubercular abscesses based on MRI features is difficult as they exhibit hypointense ring enhancement and restricted diffusion in T2-weighted images.
Diffusion-weighted image (DWI) showing restricted diffusion in the region of frontal cerebritis (Luckowitsch et al. 2021)
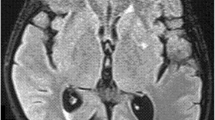
Axial T2-weighted MRI showing a mass with low signal intensity in the left central region, surrounded by hyperintense perilesional edema and a well-defined ring-like hypointense area (arrow) (Luckowitsch et al. 2021)
Fungal abscesses, unlike bacterial abscesses, tend to be multiple, affecting deep gray matter nuclei and are situated close to the grey-white matter junction, resulting from the hematogenous spread of the fungal pathogen (Figs. 14.4 and 14.5) (Luckowitsch et al. 2021). The diagnostic approaches are summarized in Table 14.3.
T1-weighted image showing a robust ring enhancement (arrow) (Luckowitsch et al. 2021)
Enhanced T1-weighted image showing multiple ring-enhancing fungal abscesses on the right (indicated by star), while no enhancement is observed on the left side (Luckowitsch et al. 2021)
8.1 CNS Aspergillosis
Diagnosis of CNS aspergillosis is challenging and is often a complex task. Aspergillosis is diagnosed on direct examination by Calcofluor-potassium hydroxide stain demonstrating thin, hyaline, septate hyphae, along with fungal growth in culture. The diagnostic modalities include:
Culture: The gold standard for diagnosis of CNS aspergillosis is growth of Aspergillus species in CSF culture. However, it has low sensitivity and takes a long time to provide results.
Histopathology: Histopathology of brain tissue can be diagnostic, but it requires invasive procedures, which can be risky in some patients.
Imaging studies: Magnetic resonance imaging (MRI) and computed tomography (CT) scan of brain, with or without intravenous contrast, can be used for detecting the size, location and extent of CNS lesion(s) (Fig. 14.6), but they are non-specific and should always be correlated with direct microscopy, histopathology and culture results.
MRI brain showing hyperintense T2 and FLAIR signal in the cerebral white matter and deep gray nuclei, with discrete ring enhancing lesions and perilesional edema in the left occipital lobe and right cerebellum (arrows). FLAIR, Fluid attenuated inversion recovery (Miceli 2019)
PCR assay: The utilization of Aspergillus PCR testing on blood samples has the potential to assist in diagnosing or ruling out invasive aspergillosis (IA). For neutropenic patients who are at a high risk of developing IA and are not getting mold-active prophylaxis, this test is particularly valuable as a screening tool, given its high negative predictive value. (Egger et al. 2020).
Detection of biomarkers: Detection of Aspergillus-specific biomarkers, such as galactomannan antigen and (1,3)-β-D-Glucan in CSF or serum can provide early and specific diagnosis of CNS aspergillosis.
8.2 Rhino-Orbito-Cerebral Mucormycosis
Direct Microscopy: Direct microscopy is of utmost importance for an evidence-based diagnosis and also, for deciding the significance of a positive culture. The sensitivity of direct microscopy can be enhanced by employing optical brighteners like Calcofluor white. The presence of hyaline, broad (6–16 μm), non-septate (or pauci-septate) haphae, having branching at right angles is suggestive of Mucorales infection.
Culture: Culture of Mucorales from biopsies is the gold standard for diagnosis and, in addition, enables fungal speciation and antifungal susceptibility testing. However, culture lacks sensitivity and is positive only in 50% of the microscopy positive cases.
Histopathology: When mucormycosis is suspected, it is important to avoid homogenization of tissue specimens as nonseptate Mucorales hyphae have a high risk of being damaged by shear stress. When compared to other fungi, Mucorales may require longer staining durations to be detected in histopathological investigation. Grocott Gomori methenamine silver (GMS) and periodic acid Schiff (PAS) stains are quite effective in delineating fungal hyphae and can aid in the detection of Mucorales. Using immunohistochemical staining on tissue samples has demonstrated high levels of accuracy in detecting Mucorales species, which improves the ability to distinguish between mucormycosis and aspergillosis based on morphology.
Due to the potential for neurological complications associated with brain biopsy, CNS mucormycosis diagnosis is frequently made by determining the primary focus in the lungs or sinuses. Patients who have symptoms suggestive of rhino-orbito-cerebral mucormycosis should be assessed by an experienced ENT surgeon. Fiberoptic endoscopy of the nasal cavity and paranasal sinuses can help in detection of necrotic or ischemic lesions that may require biopsy for further evaluation.
Imaging studies: Imaging modalities, such as MRI and CT can be employed to identify brain lesions (Fig. 14.7), which may exhibit nonspecific nodular mucosal thickening attributing to small blood vessel occlusion, leading to mucosal ischemia.
T1-weighted MRI (a) and CT scan (b) of brain showing mucormycosis with cerebral extension, involving the right frontal lobe (Chikley et al. 2019)
Cavernous sinus thrombosis, internal carotid artery blockage, and brain infarction are the most frequent imaging findings in intracranial mucormycosis. Brain imaging on T2-weighted sequences typically reveals involvement of the lower regions of the frontal lobes, with the lesions appearing either hyperintense or hypointense. Diffusion-weighted imaging (DWI) demonstrates notable restriction of diffusion. The potential utility of magnetic resonance spectroscopy in differentiating bacterial causes of cerebritis from CNS mucormycosis has been proposed. However, more research is necessary to confirm or validate this finding. Cavernous sinus syndrome can also be caused by metastatic cancer or Tolosa-Hunt syndrome, and therefore, these conditions should be considered as potential differential diagnosis.
PCR assay: Early and specific diagnosis of CNS mucormycosis can be achieved by detecting fungal DNA in CSF through PCR. PCR-based tests utilizing universal fungal primers or primers specific to Mucorales, such as CotH, offer promising results in accurately detecting the infection. Unfortunately, there are presently no biomarkers for screening or diagnosing mucormycosis available for clinical use (Dadwal and Kontoyiannis 2018).
8.3 CNS Fusariosis
CNS fusariosis can be diagnosed using different methods, including culture, histopathology, imaging studies, and PCR-based detection of fungal DNA. When a hyaline mold is identified from the blood culture of a patient with prolonged neutropenia, it strongly indicates the possibility of Fusarium infection.
Culture: The gold standard for diagnosis is CSF culture, but it has low sensitivity. Fusarium species tend to grow rapidlyin a broad range of media and typically do not require the presence of cycloheximide. The fungus can be recognized by its ability to generate multicellular macroconidia that are hyaline and banana-shaped and contain a foot cell at their base. Severely immunocompromised patients with disseminated fusariosis may exhibit skin lesions, such as cellulitis or metastatic lesions, along with positive blood cultures for the mold. Unlike aspergillosis, fusariosis frequently results in positive blood cultures. This happens because Fusarium species create yeast-like structures which help them spread and thrive in the blood, a process known as adventitious sporulation (Liu et al. 1998).
Histopathology: Histopathology of brain tissue may help in establishing a confirmatory diagnosis of fusariosis. In the absence of fungal growth, it may be difficult to differentiate fusariosis from other types of hyalohyphomycosis. In such cases, the diagnosis of fusariosis can be achieved by in situ hybridization in paraffin-embedded tissue specimens. In tissue, the hyphae of Fusarium species resemble those of Aspergillus, with hyaline septate filaments having dichotomous acute or right angle branching. However, the presence of adventitious sporulation within tissue and the simultaneous presence of both hyphae and yeast-like structures are highly suggestive of fusariosis in high-risk individuals.
Imaging studies: CT scan and MRI are employed to identify brain lesions, although their findings are not specific to a particular condition. The cerebral cortex is the most commonly affected region within the brain (Fig. 14.8), but intracranial fusariosis can involve any part of the brain, without predilection to any particular region.
MRI brain showing enhancing intraparenchymal lesion in the right frontoparietal region with mild diffusion restriction and surrounding hyperintense FLAIR, suggesting vasogenic edema (Vadhan et al. 2022)
Detection of fungal DNA: PCR-based detection of fungal DNA in CSF and brain tissues enables early and precise diagnosis of Fusarium infection.
Detection of biomarkers: Among at-risk individuals with mold infections, positive (1,3)-β-D-Glucan and negative galactomannan in CSF is strongly suggestive of CNS fusariosis. However, the (1,3)-β-D-Glucan test cannot distinguish between Fusarium and other fungal infections. Hence, biomarker tests should be correlated with clinical, radiological and culture results.
8.4 Other Mold Infections
-
Scedosporiosis: The diagnosis of CNS scedosporiosis is routinely done through a combination of imaging studies, CSF analysis, and fungal cultures. Intracranial lesions revealed through imaging studies like CT or MRI can suggest invasive scedosporiosis, while CSF analysis can show elevated protein levels, low glucose levels, and fungal elements. The presence of Scedosporium species can be confirmed by fungal culture of CSF or tissue samples. Unlike S. apiospermum, Lomentospora prolificans (formerly, S. prolificans) may be recovered from blood cultures. Molecular methods like PCR or sequencing can provide rapid and accurate identification. Diagnosis can also be achieved by directly observing the fungal elements in histological samples or isolating it in cultures. Though real-time PCR-based assays can detect Scedosporiosis from clinical samples quickly and accurately, culture remains the most useful tool for diagnosis.
-
Cladophialophora infection: Diagnosing CNS infections caused by Cladophialophora bantiana can be difficult since these infections are uncommon and may show nonspecific symptoms. Imaging techniques such as MRI or CT may show intracranial space-occupying lesions, mimicking tuberculosis or malignancy. Fungal culture of CSF or brain biopsies can confirm the presence of C. bantiana. However, culture can be time-consuming and pose safety risks for laboratory workers. Molecular techniques, such as PCR or sequencing can provide rapid and accurate identification of C. bantiana. While culture is still considered as the gold standard for diagnosing CNS fungal infections, in recent years, advanced techniques, such as real-time PCR, amplified fragment length polymorphism analysis, matrix-assisted laser desorption/ionization-time of flight, and rolling circle amplification have emerged as alternative methods.
9 Management
There are four critical components in the management of CNS mold infections: prompt diagnosis, treatment with antifungal medication, assessment and intervention by healthcare providers, and addressing underlying immunodeficiency. Failure to address these components adequately can result in unfavorable outcomes, particularly in immunocompromised individuals who may exhibit solitary brain abscesses resembling CNS tumors (McCarthy et al. 2014). The approach to management may vary depending on the specific mold involved.
The traditional approach for the treatment of CNS mold infections includes:
9.1 Antifungal Therapy
Antifungal therapy is the cornerstone of treatment for CNS mold infections. The choice of antifungal drug depends on the type of mold causing the infection, the patient’s medical history and overall health status. Voriconazole is the recommended first-line treatment for CNS aspergillosis due to its extensive distribution in the cerebrospinal fluid (Walsh et al. 2008; Ullmann et al. 2018). Therapeutic drug monitoring is crucial for optimizing its efficacy and safety. Voriconazole, fluconazole, and flucytosine have good CNS penetration, but fluconazole and flucytosine have a limited spectrum of activity. Voriconazole is the preferred treatment option for CNS aspergillosis and may also be considered for CNS infections caused by other fungi that demonstrate susceptibility, including Lomentospora and Scedosporium species.
Isavuconazole is a viable option for treating invasive aspergillosis, including cerebral aspergillosis, as it exhibits excellent CNS penetration and distributes effectively in infected brain tissue. Clinical evidence indicates that isavuconazole demonstrates satisfactory efficacy against invasive aspergillosis and disseminated mucormycosis, particularly when they affect the CNS. Itraconazole and posaconazole have limited CNS penetration, but can be considered for patients who do not tolerate or respond to voriconazole. Echinocandins are not advised for CNS aspergillosis due to limited distribution, although there have been cases where caspofungin and micafungin have been promising.
Amphotericin B liposomal formulations are better tolerated and show promising results in CNS aspergillosis and mucormycosis, while conventional amphotericin B has limited CNS penetration and associated toxicities.
Echinocandins have a higher molecular mass (1140 to 1292 daltons) and therefore, have poor penetration of blood–CSF and blood–brain barrier. In comparison, voriconazole is a relatively small (349-dalton), moderately lipophilic molecule with a CSF : plasma concentration ratio of 1:2. For CNS aspergillosis, voriconazole is recommended as primary therapy, with a serum trough concentration of 2–5 μg/ml, while liposomal amphotericin B (L-AmB) is reserved for intolerant or refractory cases. Studies have shown that use of voriconazole improves survival rates to about 35%. Isavuconazole has been proved in recent trials to be non-inferior to voriconazole. For patients with severe disease, addition of echinocandin is recommended. For CNS fusariosis, pending susceptibility testing results, voriconazole plus a lipid formulation of amphotericin B is advised.
9.2 Surgery
Surgical intervention may be necessary to remove infected tissues and relieve pressure on the brain caused by swelling or abscesses. The decision to perform surgery is made on a case-by-case basis and depends on the severity and location of the infection. Whenever feasible, it is recommended to consider surgical excision of lesions and infected adjoining structures. Several studies have indicated that surgical debridement combined with early effective antifungal therapy may improve survival rates.
9.3 Adjunctive Therapies
Reversal of neutropenia and underlying immunodeficiency, glycemic control and discontinuing glucocorticoids should be employed.
9.4 Supportive Care
Patients with CNS mold infections may require supportive care to manage symptoms such as fever, headache, and seizures. This may include medications to control pain and reduce inflammation, as well as anticonvulsants to prevent seizures.
9.5 Monitoring
Patients with CNS mold infections require close monitoring to track their response to treatment and to watch for any complications. This may include regular imaging studies, such as CT or MRI scans to evaluate the progression of the infection and the effectiveness of treatment.
It is important to note that the management of CNS mold infections is complex and requires a multidisciplinary approach involving infectious disease specialists, neurologists, neurosurgeons, and critical care physicians. The treatment plan should be tailored based-on the patient’s needs and should be regularly re-evaluated to assess the response to therapy. Fungal neuroinfections exhibit a dismal prognosis and higher mortality rate compared to viral, bacterial and parasitic infections. An early diagnosis and aggressive treatment are essential for improving the outcomes. The recommended treatment guidelines for CNS mold infections are stated in Table 14.4 (Góralska et al. 2018; McCarthy et al. 2014; Miceli 2019).
10 Conclusion
CNS mold infections present significant challenges in diagnosis and treatment, with high morbidity and mortality rates. CNS mold infections exhibit variable presentations, which make them difficult to differentiate for other common space occupying lesions, such as malignancy or tuberculoma, causing delay in appropriate treatment. Fungal culture is specific but not sensitive, and a negative result does not exclude fungal infection. Fungal biomarkers have the potential to be an effective diagnostic tool; however, standardization is necessary. The prognosis is favorable in patients when surgical debridement is combined with at least one antifungal medication. For neuroaspergillosis, voriconazole is the preferred medication. It is also the preferred drug for infections caused by dematiaceous molds, as it offers broad-spectrum activity and has excellent CNS penetration. Moreover, as immunodeficiency is the predominant risk factor, the management of these conditions should have a strong emphasis on reversal of immune suppression. An increased awareness with a high index of suspicion may help in establishing a timely diagnosis and initiating appropriate treatment, which can improve the survival in such cases.
References
Abdollahi A, Shokohi T, Amirrajab N et al (2016) Clinical features, diagnosis, and outcomes of rhino-orbito-cerebral mucormycosis—a retrospective analysis. Curr Med Mycol 2:15–23
Bannykh SI, Hunt B, Moser F (2018) Intra-arterial spread of Mucormycetes mediates early ischemic necrosis of brain and suggests new venues for prophylactic therapy. Neuropathology 38:539–541
Bertero A, Spicer LJ, Caloni F (2018) Fusarium mycotoxins and in vitro species-specific approach with porcine intestinal and brain in vitro barriers: a review. Food Chem Toxicol 121:666–675
Bongomin F, Gago S, Oladele RO, Denning DW (2017) Global and multi-national prevalence of fungal diseases—estimate precision. J Fungi 3:57
Bryan CS, DiSalvo AF, Kaufman L, Kaplan W, Brill AH, Abbott DC (1980) Petriellidium boydii infection of the sphenoid sinus. Am J Clin Pathol 74:846–851
Candoni A, Klimko N, Busca A et al (2019) Fungal infections of the central nervous system and paranasal sinuses in onco-haematologic patients. Epidemiological study reporting the diagnostic-therapeutic approach and outcome in 89 cases. Mycoses 62:252–260
Chakrabarti A, Chatterjee SS, Das A et al (2009) Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J 85:573–581
Chander J, Kaur M, Singla N et al (2018) Mucormycosis: battle with the deadly enemy over a five-year period in India. J Fungi 4:46
Chikley A, Ben-Ami R, Kontoyiannis DP (2019) Mucormycosis of the central nervous system. J Fungi 5:59
Cortez KJ, Roilides E, Quiroz-Telles F et al (2008) Infections caused by Scedosporium spp. Clin Microbiol Rev 21:157–197
Dadwal SS, Kontoyiannis DP (2018) Recent advances in the molecular diagnosis of mucormycosis. Expert Rev Mol Diagn 18:845–854
de Hoog GS, Marvin-Sikkema FD, Lahpoor GA, Gottschall JC, Prins RA, Guého E (1994) Ecology and physiology of the emerging opportunistic fungi Pseudallescheria boydii and Scedosporium prolificans. Mycoses 37:71–78
Dignani MC, Anaissie E (2004) Human fusariosis. Clin Microbiol Infect 10:67–75
Dixon DM, Polak A, Szaniszlo PJ (1987) Pathogenicity and virulence of wild-type and melanin-deficient Wangiella dermatitidis. J Med Vet Mycol 25:97–106
Economides MP, Ballester LY, Kumar VA et al (2017) Invasive mold infections of the central nervous system in patients with hematologic cancer or stem cell transplantation (2000–2016): uncommon, with improved survival but still deadly often. J Infect 75:572–580
Egger M, Jenks JD, Hoenigl M, Prattes J (2020) Blood aspergillus PCR: the good, the bad, and the ugly. J Fungi 6:18
Eichner RD, Al Salami M, Wood PR, Müllbacher A (1986) The effect of gliotoxin upon macrophage function. Int J Immunopharmacol 8:789–797
Fisher JF, Shadomy S, Teabeaut JR, Woodward J, Michaels GE, Newman MA, White E, Cook P, Seagraves A, Yaghmai F, Rissing JP (1982) Near-drowning complicated by brain abscess due to Petriellidium boydii. Arch Neurol 39:511–513
Garcia RR, Min Z, Narasimhan S, Bhanot N (2015) Fusarium brain abscess: case report and literature review. Mycoses 58:22–26
Gen R, Horasan ES, Vaytsoglu Y, Arpaci RB, Esoz G, Ozcan C (2013) Rhino-orbito-cerebral mucormycosis in patients with diabetic ketoacidosis. J Craniofac Surg 24:e144–e147
Gonzales Zamora JA, Henry Z, Gultekin SH (2018) Central nervous system aspergillosis: an unexpected complication following neurosurgery. Diseases 6:46
Góralska K, Blaszkowska J, Dzikowiec M (2018) Neuroinfections caused by fungi. Infection 46:443–459
Guarro J, Kantarcioglu AS, Horré R et al (2006) Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med Mycol 44:295–327
Higo T, Kobayashi T, Yamazaki S et al (2015) Cerebral embolism through hematogenous dissemination of pulmonary mucormycosis complicating relapsed leukemia. Int J Clin Exp Pathol 8:13639–13642
Husain S, Muñoz P, Forrest G et al (2005) Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin Infect Dis 40:89–99
Jensen J, Guinea J, Torres-Narbona M, Muñoz P, Peláez T, Bouza E (2010) Post-surgical invasive aspergillosis: an uncommon and under-appreciated entity. J Infect 60:162–167
Jeong W, Keighley C, Wolfe R et al (2019) The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect 25:26–34
Kleinschmidt-Demasters BK (2009) Disseminated fusarium infection with brain abscesses in a lung transplant recipient. Clin Neuropathol 28:417–421
Koutsouras GW, Ramos RL, Martinez LR (2017) Role of microglia in fungal infections of the central nervous system. Virulence 8:705–718
Lackner M, de Hoog GS, Yang L et al (2014) Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Divers 67:1–10
Larcher G, Cimon B, Symoens F, Tronchin G, Chabasse D, Bouchara JP (1996) A 33 kDa serine proteinase from Scedosporium apiospermum. Biochem J 315:119–126
Lass-Flörl C (2009) The changing face of epidemiology of invasive fungal disease in Europe. Mycoses 52:197–205
Lewis RE, Wiederhold NP, Chi J et al (2005a) Detection of gliotoxin in experimental and human aspergillosis. Infect Immun 73:635–637
Lewis RE, Wiederhold NP, Lionakis MS, Prince RA, Kontoyiannis DP (2005b) Frequency and species distribution of gliotoxin-producing aspergillus isolates recovered from patients at a tertiary-care cancer center. J Clin Microbiol 43:6120–6122
Lionakis MS, Lewis RE, Torres HA, Albert ND, Raad II, Kontoyiannis DP (2005) Increased frequency of non-fumigatus aspergillus species in amphotericin B- or triazole-pre-exposed cancer patients with positive cultures for aspergilli. Diagn Microbiol Infect Dis 52:15–20
Liu K, Howell DN, Perfect JR, Schell WA (1998) Morphologic criteria for the preliminary identification of fusarium, Paecilomyces, and Acremonium species by histopathology. Am J Clin Pathol 109:45–54
Luckowitsch M, Rudolph H, Bochennek K, Porto L, Lehrnbecher T (2021) Central nervous system mold infections in children with hematological malignancies: advances in diagnosis and treatment. J Fungi 7:168
Malik AN, Bi WL, McCray B, Abedalthagafi M, Vaitkevicius H, Dunn IF (2014) Isolated cerebral mucormycosis of the basal ganglia. Clin Neurol Neurosurg 124:102–105
McCarthy M, Rosengart A, Schuetz AN, Kontoyiannis DP, Walsh TJ (2014) Mold infections of the central nervous system. N Engl J Med 371:150–160
Meriden Z, Marr KA, Lederman HM et al (2012) Ochroconis gallopava infection in a patient with chronic granulomatous disease: case report and review of the literature. Med Mycol 50:883–889
Miceli MH (2019) Central nervous system infections due to aspergillus and other hyaline molds. J Fungi 5:79
Murayama T, Amitani R, Ikegami Y, Nawada R, Lee WJ, Kuze F (1996) Suppressive effects of aspergillus fumigatus culture filtrates on human alveolar macrophages and polymorphonuclear leucocytes. Eur Respir J 9:293–300
Murthy JMK, Sundaram C (2014) Fungal infections of the central nervous system. In: Biller J, Ferro JM (eds) Handbook of clinical neurology. Elsevier, New York, NY, pp 1383–1401
Nadkarni T, Goel A (2005) Aspergilloma of the brain: an overview. J Postgrad Med 51:S37–S41
Nucci M, Anaissie E (2007) Fusarium infections in immunocompromised patients. Clin Microbiol Rev 20(4):695–704. https://doi.org/10.1128/CMR.00014-07; PMID: 17934079; PMCID: PMC2176050
Nucci M, Marr KA, Queiroz-Telles F et al (2004) Fusarium infection in hematopoietic stem cell transplant recipients. Clin Infect Dis 38:1237–1242
Nucci F, Nouér SA, Capone D, Anaissie E, Nucci M (2015) Fusariosis. Sem Res Crit Care Med 36:706–714
Ortoneda M, Pastor FJ, Mayayo E, Guarro J (2002) Comparison of the virulence of Scedosporium prolificans strains from different origins in a murine model. J Med Microbiol 51:924–928
Pasqualotto AC, Denning DW (2006) Post-operative aspergillosis. Clin Microbiol Infect 12:1060–1076
Patel R, Hossain MA, German N, Al-Ahmad AJ (2018) Gliotoxin penetrates and impairs the integrity of the human blood-brain barrier in vitro. Mycotoxin Res 34:257–268
Patterson TF, Thompson GR 3rd, Denning DW et al (2016) Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60
Pérez RE, Smith M, McClendon J, Kim J, Eugenio N (1988) Pseudallescheria boydii brain abscess. Complication of an intravenous catheter. Am J Med 84:359–362
Peterson A, Pham MH, Lee B et al (2014) Intracranial fusarium fungal abscess in an immunocompetent patient: case report and review of the literature. J Neurol Surg Rep 75:e241–e245
Prakash H, Chakrabarti A (2019) Global epidemiology of mucormycosis. J Fungi 5:26
Prakash H, Ghosh AK, Rudramurthy SM et al (2018) A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol 57:395–402
Ray A, Aayilliath KA, Banerjee S, Chakrabarti A, Denning DW (2022) Burden of serious fungal infections in India. Open Forum Infect Dis 9:ofac603
Revankar SG, Sutton DA (2010) Melanized fungi in human disease. Clin Microbiol Rev 23:884–928
Richardson M, Lass-Flörl C (2008) Changing epidemiology of systemic fungal infections. Clin Microbiol Infect 14(Suppl 4):5–24
Riddell J 4th, Chenoweth CE, Kauffman CA (2004) Disseminated Scedosporium apiospermum infection in a previously healthy woman with HELLP syndrome. Mycoses 47:442–446
Roden MM, Zaoutis TE, Buchanan WL et al (2005) Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 41:634–653
Rosas AL, MacGill RS, Nosanchuk JD, Kozel TR, Casadevall A (2002) Activation of the alternative complement pathway by fungal melanins. Clin Diagn Lab Immunol 9:144–148
Ruping MJ, Heinz WJ, Kindo AJ et al (2010) Forty-one recent cases of invasive zygomycosis from a global clinical registry. J Antimicrob Chemother 65:296–302
Sharma RR, Pawar SJ, Lad SD, Mishra GP, Netalkar AS, Rege S (2012) Fungal infections of the central nervous system. In: Schmidek and sweet: operative neurosurgical techniques, pp 1691–1732
Shoham S, Pic-Aluas L, Taylor J et al (2008) Transplant-associated Ochroconis gallopava infections. Transpl Infect Dis 10:442–448
Silva BA, Pinto MR, Soares RM, Barreto-Bergter E, Santos AL (2006) Pseudallescheria boydii releases metallopeptidases capable of cleaving several proteinaceous compounds. Res Microbiol 157:425–432
Sundaram C, Mahadevan A, Laxmi V et al (2005) Cerebral zygomycosis. Mycoses 48:396–407
Sutton DA, Fothergill AW, Rinaldi MG (1998) Guide to clinically significant fungi, 1st edn. Williams & Wilkins, Baltimore, MD
Tooley AA, Bradley EA, Woog JJ (2022) Rhino-orbital-cerebral Mucormycosis-another deadly complication of COVID-19 infection. JAMA Ophthalmol 140:73–74
Ullmann AJ, Aguado JM, Arikan-Akdagli S et al (2018) Diagnosis and management of aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 24:e1–e38
Vadhan JD, Melo AJ, Shogan JC, Singh V, Carrillo M (2022) Fast and Fusariosis: a systematic review and case report of a rapidly fatal central nervous system infection. J Emerg Crit Care Med 6:24
Vironneau P, Kania R, Morizot G, Elie C, Garcia-Hermoso D, Herman P et al (2014) Local control of rhino-orbito-cerebral mucor- mycosis dramatically impacts survival. Clin Microbiol Infect 20:O336–O339
Walsh TJ, Anaissie EJ, Denning DW et al (2008) Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 46:327–360
Yoo D, Lee WH, Kwon-Chung KJ (1985) Brain abscesses due to Pseudallescheria boydii associated with primary non-Hodgkin’s lymphoma of the central nervous system: a case report and literature review. Rev Infect Dis 7:272–277
Zanganeh E, Zarrinfar H, Rezaeetalab F et al (2018) Predominance of non-fumigatus aspergillus species among patients suspected to pulmonary aspergillosis in a tropical and subtropical region of the middle east. Microb Pathog 116:296–300
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Choudhary, S., Thakker, R., Samaddar, A. (2023). Mold Infections of the Central Nervous System. In: Sami, H., Firoze, S., Khan, P.A. (eds) Viral and Fungal Infections of the Central Nervous System: A Microbiological Perspective . Springer, Singapore. https://doi.org/10.1007/978-981-99-6445-1_14
Download citation
DOI: https://doi.org/10.1007/978-981-99-6445-1_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-6444-4
Online ISBN: 978-981-99-6445-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)