Abstract
Pearl millet is one of the most important sources of nutrition for millions of people in arid and semi-arid areas in Africa and Asia. Farmers have had, throughout its domestication, to select cultivars adapted to their environments. So, pearl millet appears as an interesting crop model to study drought adaptation. However, current and future climatic changes pose challenges to its cropping sustainability. Drought and heat are the main factors of climate change. To accelerate pearl millet adaptation and improve its productivity to cope with climate change, its mechanisms of adaptation must be dissected. Here, we review the state of research on the physiological and molecular bases of pearl millet adaptation to drought and heat. However, pearl millet remains a neglected crop, and progress in research remains to be made.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Climate Change
Climate change is a major threat to agriculture and food security (Kang et al. 2009; Godfray et al. 2010; Wang et al. 2018). The most conspicuous climate changes in recent times are the increase in atmospheric temperatures due to increasing levels of greenhouse gases (Solomon et al. 2007; Stott et al. 2010; Christidis et al. 2012; Wang et al. 2018) and associated changes in the water cycle (Bates et al. 2008; Collins et al. 2013; Jung et al. 2002; Balling and Cerveny 2003; Fauchereau et al. 2003; Trenberth et al. 2007). A continued rise in global temperature is predicted if greenhouse gas (GHG) emissions continue unabated, according to the Fifth Assessment Report (AR5) of the Intergovernmental Panel on Climate Change (IPCC). The frequency of warm days, warm nights, and heat waves have increased, while the frequency of cold days and cold nights has decreased (Sillmann et al. 2013). Based on emission scenarios, the IPCC predicts a temperature increase between 0.3 and 0.7 °C by 2035 with a medium degree of confidence (Kirtman et al. 2013). In a study assessing long-term projections of climate change, Collins et al. (2013) showed that increase in global mean surface temperatures for 2081–2100, relative to 1986–2005, will likely be in the 5–95% range of CMIP5 (Coupled Model Intercomparison Project Phase 5) models; 0.3–1.7 °C (Representative Concentration Pathway 2.6 or RCP2.6), 1.1–2.6 °C (RCP4.5), 1.4–3.1 °C (RCP6.0), 2.6–4.8 °C (RCP8.5). As a result, an increase in temperature from 0.3 to 4.8 °C is expected by the end of the twenty-first century. This increase in mean temperatures is expected to be greater in the tropics and subtropics than in the mid-latitudes (high confidence; Kirtman et al. 2013).
Along with the increase in temperature, global warming is associated with changes in the intensity/uncertainties of precipitation. Future climate projections show that precipitation could increase or decrease depending on the latitude of the area. These results corroborate those of Bates et al. (2008), who reported that over the past century, precipitation has mainly increased over northern high-latitude lands, while notable decreases have occurred in recent years from 10°S to 30°N. This observed effect is expected to continue in the coming years as climate model simulations predict an increase in precipitation in high latitudes and parts of the tropics and decreases in some subtropics and lower mid-latitudes by the end of the twenty-first century (Collins et al. 2013).
Arid and semi-arid areas, especially those in Asia and Africa, are very vulnerable to climate change. Temperature and precipitation variability is expected to increase in these areas. In Africa, it is predicted that the temperature will rise faster compared to the rest of the world. This increase could exceed 2 °C by the middle of the twenty-first century and 4 °C by the end of the twenty-first century (Niang et al. 2014; Adhikari et al. 2015; Djanaguiraman et al. 2018) and will lead to reduced crop yields (Fischer et al. 2005a, b; Howden et al. 2007; Liu et al. 2016). Regarding rainfall, significant negative trends are observed in West Africa and the Sahel. The Sahelian region of West Africa experienced a decrease in rainfall between the late 1950s and the late 1980s (Dai et al. 2004). Recently, Sultan and Gaetani (2016) predicted an increase in extreme events and increased variability in precipitation from year to year. Precipitation projections for the twenty-first century are not spatially homogeneous in West Africa. As a result, there is a large dispersion in the representation of rainfall from one regional model to another, both on a seasonal and intra-seasonal scale (Salack et al. 2012b). Beyond the changes in the total amount of rain, precipitation patterns are also predicted to change in the Sahelian area. For instance, Salack et al. (2011) identified two agro-climatic zones where rainfall breaks frequently occur at the start and end of the rainy season (Salack et al. 2012a). These rainfall breaks accompanied by heat stress are likely to affect crop yield. These factors constitute a major agronomic problem that contributes to severe yield losses of up to 10% for pearl millet in arid and semi-arid areas (South Asia and Africa) (Knox et al. 2012). It is therefore urgent to better understand heat and drought stress mechanisms in pearl millet to develop new agricultural practices and varieties adapted to these stresses and ensure food and nutritional security in sub-Saharan Africa.
10.2 Effects of Drought and Heat on Pearl Millet and Annual Plant Growth and Development
Pearl millet [Pennisetum glaucum (L.) R. Br.] is an important food crop grown under hot and dry conditions in arid and semi-arid regions of Africa and Asia (Arya and Yadav 2009; Ullah et al. 2017). Since these areas are characterized by low and irregular rainfall, high temperatures, and low soil fertility, they constitute the main constraints for its production. These stresses often occur simultaneously, making it very difficult to separate the effects of each on plants. However, the combined stresses have a negative impact on plant growth and productivity, which is more pronounced than the individual impacts (Craufurd and Peacock 1993; Prasad et al. 2008; Dreesen et al. 2012).
10.2.1 Drought Effects
Drought stress is one of the major constraints limiting crop production worldwide (Forster et al. 2004; Khan et al. 2010). Drought can be defined as a deficit of adequate moisture necessary for normal plant growth in the complete life cycle (Zhu 2002). Plants are subject to drought when the stock of water in the soil is limited, and the vapor pressure deficit is very low (Anjum et al. 2011b). It has a considerable influence on plant growth and development. Pearl millet is sensitive to drought stress at the vegetative and reproductive stages (Vadez et al. 2012). The effects of drought stress depend on the degree, duration, stage of crop development, and tolerance level of the species. Drought stress during the growth phase affects cell division and elongation, the main processes involved in plant growth. Due to the reduction in turgor pressure, cell elongation appears to be one of the most drought-sensitive processes. Even though cell division is less sensitive than cell elongation, it can be affected by mild drought stress. Tardieu et al. (2000) reported that soil moisture deficit resulted in a reduction in the length of the cell division zone and the relative division rate. According to Alves and Setter (2004), the reduction in leaf area was caused largely by development delay and a reduction in cell division in the youngest meristematic leaves.
However, the effects of drought on the leaves depend on the intensity of the stress. A slight drought causes a reduction in the rate of expansion, number, and size of leaves, while severe stress decreases the rate of leaf elongation, which can even cause leaf growth to stop. The most common negative effect of drought stress on crop plants is the reduction in growth. This has been reported in pearl millet by many authors (Muchow 1989; Winkel et al. 1997; Aparna et al. 2014; Kholová et al. 2016). Drought reduces leaf size, stem extension, and root proliferation, disrupts plant–water relationships, and reduces water use efficiency (Farooq et al. 2009; Anjum et al. 2011b). These results agree with those of Khan et al. (2001) and Anjum et al. (2011a), who reported a significant decrease in the growth of maize under drought in terms of plant height, stem diameter, leaf area, and plant biomass.
In addition, drought has been shown to alter physiological processes (Radhouane 2008, 2013; Pinheiro and Chaves 2010; Anjum et al. 2011a; Ghatak et al. 2016). Photosynthesis, transpiration rate, stomatal conductance, water use efficiency, intrinsic water use efficiency, and intercellular CO2 have been reduced by drought (Anjum et al. 2011a). In millet, severe drought stress leads to a reduction in photosynthesis (Ashraf et al. 2001; Golombek 2003; Radhouane 2009). This reduction has also been reported in C4 cereals such as maize (Boyer and Westgate 2004) and sorghum (Ogbaga et al. 2014). One or more steps in the photosynthetic process can be affected by water deficit, such as the diffusion of CO2 through the stomata and into intercellular spaces (Flexas et al. 2006). For Slatyer (1973), almost all the decrease in photosynthesis must be attributed to stomatal closure. On the other hand, according to Bois (1993) and Golombek (2003), the decrease in photosynthesis of pearl millet is due jointly to stomatal resistance and non-stomatal changes. The imposition of drought stress also resulted in a decrease in chlorophyll content (Ghatak et al. 2021), which could be related to the increase in the activity of the enzyme chlorophyllase (Ashraf et al. 1994).
In terms of production, drought stress strongly affects yield during the grain-filling period (Aparna et al. 2014). In sorghum, the yield can be reduced by more than 36% and 55% when water stress occurs in the vegetative and reproductive phases respectively (Assefa et al. 2010). Stress reduces grain size and weight, resulting in a reduction in grain yield (Arya et al. 2010). Many studies corroborate this result (Barnabás et al. 2008; Alqudah et al. 2011; Aparna et al. 2014; Debieu et al. 2018). A severe reduction in panicle filling occurs under drought (Winkel et al. 2001) due to a reduction in the assimilate partitioning and activities of sucrose and starch synthesis enzymes (Farooq et al. 2009; Anjum et al. 2011b). However, according to Aparna et al. (2014), the decrease in grain yield depends more on the number than the size of the seeds. Therefore, it is due to the combined effect of a reduction in the number of panicles (productive tillers) and seeds. In pearl millet, a strong correlation was found between grain yield and grain number (Bidinger and Raju 2000). In wheat, drought stress reduced yield following tiller abortion and a lower grain number per spike (Izanloo et al. 2008). Thus, drought most often occurs during the vegetative and reproductive stages of pearl millet, causing drastic effects on growth and productivity.
10.2.2 Heat Effects
Heat stress (increase in air temperatures above the optimum) is an agricultural problem in many parts of the world (Wahid et al. 2007). It is a key determinant of crop growth and productivity (Al-Khatib and Paulsen 1999), whose adverse effects on cereals vary with the timing, duration, and intensity (sternness) of stress (Barnabás et al. 2008; Fahad et al. 2016a). In Africa and India, soil temperatures generally exceed 45 °C and sometimes reach 60 °C (Yadav et al. 2010). This explains the important place occupied by pearl millet cultivation in these areas because the optimum temperature for its development fluctuates between 33 and 34 °C (Ashraf and Hafeez 2004).
Indeed, pearl millet is the most heat-tolerant cereal crop and needs high temperatures to grow. This has been illustrated by numerous works showing that the growth of pearl millet is optimal up to a temperature of 35 °C (Arya et al. 2014), but beyond that, inhibition of the normal growth process is noted (Ashraf and Hafeez 2004; Yadav et al. 2010, 2016; Arya et al. 2014; Djanaguiraman et al. 2018).
A temperature above the optimum can delay or prevent germination. This germination delay was reported in pearl millet cultivars subjected to supra-optimal temperatures compared to controls (Khalifa and Ong 1990). In pearl millet and maize, heat stress has been reported to cause a decrease in the final percentage of germinated seeds and the germination rate (Ashraf and Hafeez 2004). Yadav et al. (2016) showed that increasing the temperature can decrease the germination rate or even inhibit germination depending on the tolerance of the genotype, which agrees with the results of Wahid et al. (2007).
In later stages, heat stress can also negatively affect the vegetative growth of plants. It induces a reduction in shoot dry mass, relative growth rate, and net assimilation rate (Ashraf and Hafeez 2004; Wahid 2007). On the roots, temperatures above optimal lead to a decrease in primary root length, number of lateral roots, and their angle of emergence (Calleja-Cabrera et al. 2020). In addition, it causes a decrease in shoots or root’s water status, root’s hydraulic conductivity, or leaf stomatal conductance, which only occurs when the stress is prolonged (Heckathorn et al. 2013).
Reduction in photosynthetic activities by higher temperature has been reported by numerous studies (Al-Khatib and Paulsen 1999; Crafts-Brandner and Salvucci 2000, 2002; Prasad et al. 2004; Fahad et al. 2016b; Yadav et al. 2016). This reduction in photosynthesis was attributed to the damage to chlorophyll pigments, a decline in leaf nitrogen contents, blockage of PSII reaction center and electron flow decreased quantum efficiency (Fv/Fm), and down-regulation of PSII photochemistry (Fahad et al. 2016b, 2017). Heat stress decreases the activation and activity of Rubisco (Prasad et al. 2004) by inhibiting the activity of the enzyme Rubisco activase (Crafts-Brandner and Salvucci 2000, 2002). Heat also reduces the photochemical efficiency of photosystem II (PSII), which appears to be the most heat-sensitive photosynthetic component (Al-Khatib and Paulsen 1999).
On the other hand, despite the multiple negative effects of heat stress at the vegetative stage, plants appear more sensitive at the reproductive stages (Farooq et al. 2011; Prasad et al. 2017). Recently, much work has been done on cereals to identify the growth stage(s) most sensitive to heat stress during reproductive development (Prasad and Djanaguiraman 2014; Prasad et al. 2015; Djanaguiraman et al. 2018). Two periods were identified, ranging from 10 to 12 days and 2 to 0 days before anthesis in pearl millet (Djanaguiraman et al. 2018), 8 to 6 days and 2 to 0 days before anthesis in wheat (Prasad and Djanaguiraman 2014), 10 to 5 days before anthesis and 5 days before and 5 days after anthesis in sorghum (Prasad et al. 2015). During these periods, heat stress causes maximum decreases in pollen germination percentage, seed number (Djanaguiraman et al. 2018), and floret fertility (Prasad and Djanaguiraman 2014; Prasad et al. 2015).
In pearl millet, high temperatures (>40 °C) often coincide with the flowering and grain-filling stages (Gupta et al. 2015). Heat stress during these stages leads to a decrease in grain number and weight, leading to poor crop yield and quality (Bita and Gerats 2013; Djanaguiraman et al. 2018). According to Sultan et al. (2013), raising the temperature to 6 °C would lead to a 41% reduction in pearl millet yield. Several studies have reported a decrease in the yield of pearl millet and other cereals under heat stress (Gupta et al. 2015; Prasad et al. 2017; Djanaguiraman et al. 2018; Qaseem et al. 2019; Jagadish 2020).
According to Fahad et al. (2016a, b), the decrease in pollen germination mainly results from the retention of pollen in dehiscent anthers. However, an increase in the content of reactive oxygen species and a decrease in the activity of antioxidant enzymes in pollen and pistils have been reported in millet (Djanaguiraman et al. 2018). Pistils were more sensitive than pollen grains because they had relatively higher reactive oxygen species and lower antioxidant enzyme activity. Increased production of reactive oxygen species under these conditions may be responsible for decreased germination and pollen viability in sorghum (Prasad and Djanaguiraman 2011).
Heat stress also causes a decrease in spikelet fertility due to a decrease in the production and number of pollens on the stigma (Prasad et al. 2006a) and inhibition of panicle emergence (Prasad et al. 2006b). This decrease in spikelet fertility resulted in fewer filled grains, lower grain weight per panicle, and lower harvest index. According to Gupta et al. (2015), heat stress causes reproductive sterility in pearl millet leading to a drastic reduction in grain yield. In addition, the increase in temperature promotes a higher rate of evapotranspiration, which ultimately reduces soil moisture and available water needed to fill the grains.
10.2.3 Heat and Drought Combined Effects
In arid and semi-arid areas, drought and heat stress often occur simultaneously (Shah and Paulsen 2003; Barnabás et al. 2008). This effect occurs because of a negative correlation between temperature and precipitation on inter-annual scales because dry conditions favor more sunshine and less evaporative cooling (Trenberth and Shea 2005; Zscheischler and Seneviratne 2017). However, few studies examining the impact of the combined effects of both stresses on crops, let alone pearl millet, have been carried out. The few studies carried out on this subject have shown that the combined effects on growth and productivity considerably exceed the simple effects (Savin and Nicolas 1996; Prasad et al. 2008; Dreesen et al. 2012; Qaseem et al. 2019).
Drought and heat stresses, by their intensity and duration, can influence the growth and development of plants. At the leaf scale, the distribution of the relative elongation rate was independently affected by these stresses, which had quasi-additive effects (Tardieu et al. 2000). Additionally, leaf elongation rates were positively correlated with leaf temperatures and negatively with vapor pressure deficit and pre-dawn leaf water potential (Welcker et al. 2007). This shows the strong relationship that exists between leaf elongation rates and various physiological components that can be indicative of drought and heat stress. In terms of growth, drought, and heat affect stem growth and plant height (Katerji et al. 1994; Winkel et al. 1997; Prasad et al. 2006b).
Similarly, these stresses can also impact the transition and duration of the developmental stage. Cooper et al. (2009) reported a reduction in the length of the growing period of plants in dry tropical regions. This reduction is the result of both the rapid development of the leaf canopy and an increase in the overall growth rate of the crop stimulated by heat stress. Higher temperature results in faster development, and therefore shorter, growth phase duration.
Heat stress combined with drought can cause stomatal closure leading to an increase in leaf temperature (Rizhsky et al. 2002), unlike the simple effect of heat stress, which promotes the opening of the stomata, thus leading to cooling of the leaves by transpiration. Consequently, the water status of the plant is partly linked to the temperature, which affects several parameters of the plant. According to Shah and Paulsen (2003), the interactions between stresses were pronounced, and the consequences of drought were more severe at high temperatures than at low temperatures on all physiological parameters. Barnabás et al. (2008) confirm these statements by asserting that the synchronization of the two results in even greater severity of drought stress. Thus, heat increases the intensity of the drought by causing the soil to dry faster. This effect results from the increase in the vapor pressure deficit of the air, which favors a greater demand for evapotranspiration. In addition to warming, the indirect effect of heat on water evapotranspiration from the soil can have a great impact on plants.
On the other hand, antagonistic interactions between heat and drought have been reported on net photosynthesis. In wheat, Lu and Zhang (1999) asserted that drought stress increases the tolerance of PSII to heat stress. However, many authors have argued that drought tends to dramatically exacerbate the effects of heat stress on plant growth and photosynthesis (Shah and Paulsen 2003; Xu and Zhou 2005a, b, 2006).
The effect of drought and heat stresses on root growth depends on the intensity of the stress. Moderate stress results in greater root growth due to increased distribution of carbohydrates to the roots and greater exploration of the soil caused by drought and heat stress, respectively. On the other hand, severe stress leads to a reduction in the number, length, and diameter of the roots, which becomes more important when drought and heat stress are associated (Prasad et al. 2008).
When these constraints occur during grain development, they cause significant yield losses in cereals (Chaves et al. 2003; Bai et al. 2004; Prasad et al. 2008). Knowing that starch represents a major part of the dry weight of cereals, this reduction is due to a decrease in its accumulation. On the other hand, data on the possible variation of this trait in pearl millet are limited.
10.2.3.1 Mechanisms of Adaptation to Climate Variability (Drought and Heat) in Pearl Millet
Faced with environmental constraints, plants must be able to react and adapt to increase their chance of survival. Thus, they are developing different strategies to adapt and resist drought and heat stresses. These strategies are characterized by a strong ability to set up biochemical, molecular, and physiological responses which influence various cellular processes in the plant. Drought survival mechanisms of plants are like those used to cope with heat stress (Wu et al. 2018). Escape, avoidance, and tolerance mechanisms have long been considered important strategies for drought adaptation (Chaves et al. 2003). These mechanisms have also been reported by Kooyers (2015) and Li et al. (2017).
Drought escape allows some plants to cope with stress by completing their full development cycle before the water deficit sets in the soil (Annerose 1990). Drought avoidance, on the other hand, is the ability of plants to maintain high levels of water potential in their tissues by reducing water loss or improving water uptake (Ludlow and Muchow 1990). Escape and avoidance strategies may be the most effective for survival and reproduction when drought stress is mild-tomoderate (Kooyers 2015). Mechanisms of dehydration avoidance include morphological and functional modifications such as leaf area size, leaf rolling, stomatal conductance, and osmotic adjustment (Blum 2011; Kadioglu et al. 2012). However, when drought stress becomes severe, plants must be able to rely on tolerance strategies to avoid desiccation. Thus, drought tolerance appears to be the capacity of plants to resist water deficit while maintaining appropriate physiological activities (Xiong et al. 2006). Therefore, the response of plants to drought depends on the species, duration of the drought as well as the timing of application (Sanchez et al. 2002; Pinheiro and Chaves 2011).
Due to its drought and heat tolerance (Arya et al. 2010), pearl millet is an ideal model for studying the heat and drought resistance mechanism of cereal crops.
10.2.3.1.1 Leaf Rolling and Stomatal Conductance
In plants, many changes occur in the leaf, both in structure and morphology, in response to drought and heat stress. Leaf rolling is an abiotic stress avoidance mechanism (Kadioglu and Terzi 2007; Kadioglu et al. 2012). It occurs when plants are under stress and is caused by folding in the midrib of upper leaves and changes in their leaf’s orientations (Kusaka et al. 2005b). This leads to a reduction in leaf temperature via a decrease in incident radiation (O’toole et al. 1979; Heckathorn and DeLucia 1991) and thus offers protection against the effects of excessive radiation (Kadioglu and Terzi 2007). Leaf rolling effectively reduces light interception, transpiration, and leaf dehydration (Kadioglu and Terzi 2007).
However, reduced transpiration has often been associated with leaf senescence which results in an efficient and rapid decrease in leaf area. It can extend the duration of soil water availability by reducing the plant’s water requirements and losses. According to Wallace et al. (1993) and Soegaard and Boegh (1995), senescence constitutes the main mechanism responsible for the reduction of transpiration. However, it can be a limiting factor for the accumulation of crop biomass and hence grain yield due to its irreversible effect. Unlike senescence, the reversible nature of leaf rolling provides flexibility when drought is temporary and intermittent.
However, sorghum and pearl millet studies have shown that leaf rolling did not occur until after stomatal closure (Blum and Sullivan 1986; Ludlow and Muchow 1990). Therefore, the immediate response of plants under drought stress is the stomatal closure to prevent water loss through transpiration (Cornic and Massacci 1996; Assmann et al. 2000; Ghatak et al. 2016; Buckley 2019). Through the transpiration stream, drought induces root-to-leaf signaling promoted by soil drying, which causes stomatal closure (Farooq et al. 2009; Anjum et al. 2011b). This stomatal closure is higher and faster in tolerant genotypes (Ghatak et al. 2021) and appears to be more effective in reducing water loss than leaf rolling. It has been reported that the decrease in water use by stomata is greater pre-anthesis than post-anthesis because of the ontogenetic decline in the range of stomatal conductance (Winkel et al. 2001).
Under drought conditions, plants tend to have lower stomatal conductance, which decreases as drought stress increases (Ghatak et al. 2021). This decrease in conductance allows the plant to conserve water and maintain an adequate water status of the leaves, hence the close relationship between them. A significant correlation between stomatal conductance and leaf xylem water potential has been reported by Matsuura et al. (1996). However, a low stomatal conductance could be partly related to a difference in stomatal density. According to Slama (2002), the increase in the number of stomata per unit area could be one of the factors of resistance to water deficit in cereals if accompanied by appropriate photosynthesis activity. It can decrease water loss and increase the net uptake of CO2, which allows the plant to maintain photosynthesis. Increased stomatal density can also affect crop yield. The variety of durum wheat with the highest yield and the largest kernels has a higher stomatal density at the beard and flag leaf (Slama 2002). On the other hand, according to Kholová et al. (2010), stomatal regulation is more important than stomatal density in regulating the loss of water in pearl millet. Nevertheless, stomatal conductance and leaf rolling have been shown to be reliable physiological indicators of drought tolerance in plants (Kadioglu and Terzi 2007).
Heat stress, often associated with a high vapor pressure deficit (VPD), also causes leaf rolling in plants (Omarova et al. 1995) and stomatal closure (Maroco et al. 1997). It causes a change in the temperature of the leaves, which can be an important factor in controlling the leaf water status under stress. The study by Kadioglu and Terzi (2007) showed that leaf rolling was linearly correlated with osmotic potential and leaf temperature. Thus, the physiological role of leaf rolling was reported as the maintenance of adaptive potential by increasing the efficiency of water metabolism in wheat flag leaves under heat stress (Sarieva et al. 2010). In fact, this is consistent with the fact that leaf rolling results in more efficient use of water during photosynthesis (Kadioglu and Terzi 2007). In addition, a reduction in leaf area was noted in pearl millet and sorghum under high VPD (Choudhary et al. 2020). According to Choudhary et al. (2020), water conservation when increasing VPD depends primarily on reduced leaf area and somewhat on transpiration restriction in these two crops. This restriction of transpiration has also been reported by Kholová et al. (2010). However, partial stomatal closure is achieved by limiting the transpiration rate under conditions of high VPD. Stomatal sensitivity to VPD was correlated with the hydraulic conductance of leaves relative to the total leaf area (Ocheltree et al. 2014). However, reducing the transpiration of pearl millet under a high vapor pressure deficit has been proposed to be beneficial for crop yield under such conditions (Kholová et al. 2010).
10.2.3.1.2 Root Characteristics
Pearl millet is one of the most abiotic stress-tolerant cereal crops in part due to its strong root system. Rapid root growth at depth can offer a chance for survival in harsh conditions as water uptake requests deep roots because of the quick water drainage of the sandy soils where millet is usually grown. The advantage of deeper root systems was demonstrated in pearl millet (Faye et al. 2019), sorghum (Chopart et al. 2008b), wheat (Kirkegaard et al. 2007; Christopher et al. 2008), maize (Sinclair and Muchow 2001; Hund et al. 2009), and rice (Manschadi et al. 2010; Wasson et al. 2012). In pearl millet, the root system is made up of several types of roots, namely the primary root (emerges from the seed and the mesocotyl connecting the seed and the base of the stem), crown roots (emerges from the base of the stem), lateral roots (appear on primary or crown roots), and secondary roots (ramifications of lateral roots) (Passot et al. 2016). Crown roots form most of the root system, even though the primary root characterizes the root system at the start of pearl millet growth. This primary root will regress from 1 month after sowing (within 2 months after germination) (Maiti and Bidinger 1981; Passot 2016). Passot et al. (2016) showed that the number of central metaxylem vessels constitutes the major difference between the different root types.
Different lateral root types have been reported in pearl millet, rice, and maize (Passot et al. 2016, 2018; Hochholdinger and Tuberosa 2009; Rebouillat et al. 2009). The different types of lateral roots in cereals have been identified through anatomical studies of roots, often based on traits such as root diameters and vascularization (Varney et al. 1991; Watt et al. 2008; Henry et al. 2016; Passot et al. 2016). Recently, Passot et al. (2018) were able to classify roots based on their growth rate profiles. The study found three types of lateral roots with similar characteristics in pearl millet and maize. This revealed three types of lateral roots with similar characteristics in pearl millet and maize.
Under drought stress, pearl millet root growth is reoriented toward deeper soil layers that retain more water. Several studies have argued that root growth orientation was only dependent on soil depth (Chopart and Siband 1999; Chopart et al. 2008a, b; Faye et al. 2019). This dependence differed between thick roots and fine roots. Thick root growth was horizontal in shallow soils and became more and more vertical with increased depth, unlike the growth orientation of fine roots, which was only marginally dependent on soil depth (Faye et al. 2019). This result agrees with those found in sugarcane and sorghum (Chopart et al. 2008a, b). They claimed that fine roots appeared isotropic when thick roots were horizontal near the surface and gradually became vertical in deeper horizons. According to Passot et al. (2016), the thick roots correspond to the seminal or crown roots, while the fine ones probably correspond to the different types of laterals.
Increasing water uptake is a way of avoiding stress. It takes place via the roots, and its transport from the soil to the xylem vessels uses two pathways: the apoplastic pathway and the cell-to-cell pathway, which summarizes the transcellular and the symplastic pathways (Steudle 2001). The type of path depends mainly on environmental conditions. Water flows through the apoplastic path under non-stressful conditions due to hydrostatic forces while it flows through the cell-to-cell path under stressful conditions due to osmotic forces. Aquaporins, water channels present in cell membranes, enable cell-to-cell water transport (Prado and Maurel 2013; Chaumont and Tyerman 2014). They are involved in the physiology of plant growth (Maurel et al. 2015), thus influencing the hydraulics, transpiration, and water conservation of the soil. Their importance has been demonstrated in pearl millet where they contribute up to 84% to the hydraulic conductivity of roots (Grondin et al. 2020). Interestingly, aquaporins contribution was higher in root hydraulic conductivity for a pearl millet line with lower water use efficiency (Grondin et al. 2020). Aquaporins are also well known for their response to drought stress (Alexandersson et al. 2005; Aroca et al. 2012; Grondin et al. 2016). Several types of aquaporin families have been identified with different functions. Many of them are involved in the regulation of water uptake by roots under drought conditions (Aroca et al. 2012).
On the other hand, root length is an important trait for tolerance to drought stress. Root length increased in all genotypes under drought stress (Ghatak et al. 2021). This increase in the root system has been reported by many authors (Kusaka et al. 2005a; Ghatak et al. 2016). It has been reported in cereals that a deep root system allows water uptake from deep layers of the soil in drought-stressed environments (Kondo et al. 2000; Kashiwagi et al. 2006; Manschadi et al. 2010; Wasson et al. 2012; Steele et al. 2013; Wasaya et al. 2018; Faye et al. 2019). In sorghum and pearl millet, there is a positive correlation between drought tolerance and root length (Matsuura et al. 1996), even though the roots of pearl millet were found to be longer than those of sorghum (Rostamza et al. 2013). In addition to length, root density can be a factor in drought tolerance. In a study on maize, Zhan et al. (2015) argue that reduced lateral root density improves drought tolerance. This reduction in density is associated with deeper rooting resulting in lower root length density for thick roots than for fine roots (Chopart et al. 2008b).
Therefore, fine root diameter, specific root length, specific root surface area, root angle, and root length density are considered useful traits for improving plant productivity under drought conditions (Wasaya et al. 2018). Increased root airspaces (aerenchyma) and root xylem diameter have also been linked with greater yield under drought conditions in maize (Chimungu et al. 2015) and the conservation of water resources to laid grain filling in wheat (Richards and Passioura 1989) respectively. Increased root growth can also be helpful for heat stress conditions. It allows plants to maintain their water potential despite significant transpiration. Under these conditions, plants develop strategies to restrict water loss. The reduction of transpiration in pearl millet under high vapor pressure deficit (VPD) was associated with aquaporins function (Reddy et al. 2017b). VPD-insensitive genotypes increased their transpiration rate, which may be since they used more symplastically mediated water transport (aquaporins) pathways than VPD-sensitive genotypes (Reddy et al. 2017b). Thus, water uptake in warmer soil appears to be positively correlated with aquaporin activity in wheat (Carvajal et al. 1996). In mature maize plants, increasing temperature slows lateral root growth to promote the development of long axial roots to reach water in deeper soil layers (Hund et al. 2008). Therefore, leaf and roots hydraulic conductance plays an important role in the response of plants to evaporative demand (Ocheltree et al. 2014).
10.2.3.1.3 Osmotic Adjustment
Osmotic adjustment is considered a major drought adaptation mechanism (Kusaka et al. 2005b; Izanloo et al. 2008; Sanders and Arndt 2012; Blum 2017). It allows the maintenance of water absorption and cell turgor pressure thanks to the accumulation of solutes. As a result, increasing the number of osmotically active substances in the cell leads to a more negative osmotic potential, which can improve the degree of cell hydration, maintaining turgor in leaf tissue and other metabolically active cells (Sanders and Arndt 2012). Osmotically active substances can be either organic solutes (amino acids, glycerol, sugars, and other low molecular weight metabolites) or inorganic ions (Na+, K+, Ca2+, and Cl−). Several studies have reported the important role of these solutes in tolerance to abiotic stresses (Chaves and Oliveira 2004; Ashraf and Foolad 2007; Chen and Jiang 2010; Verslues and Sharma 2010). Depending on the adjustment capacity, the types of solutes accumulated, and their relative contribution to lowering osmotic potential, the osmotic adjustment may vary between species and genotypes (Chen and Jiang 2010).
Variation in the osmotic adjustment of cultivars in response to drought has been reported in many crop plants, including pearl millet. Local varieties from more arid areas exhibited a greater capacity for osmotic adjustment (Blum and Sullivan 1986). However, the latter depends not only on the stage of development of the plant (Chimenti et al. 2006) but also on the degree and duration of the water deficit (Shangguan et al. 1999; Kusaka et al. 2005a, b; Nio et al. 2011). Kusaka et al. (2005b) studied the contribution of several solutes to the osmotic adjustment of two pearl millet cultivars, one susceptible (IP8949) and the other tolerant (IP8210) to drought stress. They reported that, for both accessions, the stem exhibited higher osmotic adjustment than the younger and expanded leaves, while their decrease in relative water content was different. In addition, an increase in the concentration of organic components (sucrose, glucose, proline, and QAC) was noted in both accessions in response to drought stress. This has also been reported in durum wheat, where drought stress increased sugar and proline concentrations and decreased nitrate levels (Bajji et al. 2001). Sugars were the main solutes that contributed to osmotic adjustment, especially in growing leaves, followed by proline and quaternary ammonium compounds (Bajji et al. 2001). However, in pearl millet, accumulation of proline was greater (more than four times) in young leaves of the tolerant genotype than in the susceptible genotype (Kusaka et al. 2005b). This increase has also been reported in maize up to 10 days after stress application and declines when stress becomes severe (Anjum et al. 2011a). A strong accumulation of proline increases the cell solute concentration, resulting in increased water potential in the tissues and decreased cellular damage. As a result, it constitutes the first response of plants exposed to drought stress and contributes to the immediate recovery of plants after stress.
In addition to proline, glycine betaine, and soluble sugars contribute to osmotic adjustment and stress adaptation in pearl millet. However, the accumulation of organic components remained lower than that of K+ and NO3− (Kusaka et al. 2005b). The concentration of K+ increased both in the cell sap of the leaves and the stems of the two accessions at the onset of stress. The accumulation of K+ in the cell sap reached a very high level and was relative to decreasing relative water content. This proves the role of inorganic compounds in contradiction to the conclusion of Bajji et al. (2001). Fischer et al. (2005a, b) found a positive correlation between osmotic adjustment and grain yield under moisture deficit. Likewise, in a critical review examining 26 published studies in which osmotic adjustment and yield were measured under drought stress in variable genotypes from 12 crops, Blum (2017) reported a positive and significant association between osmotic adjustment and performance in 24 published studies. In pearl millet, drought tolerance was more correlated with osmotic adjustment capacity than total root length under severe drought stress (Kusaka et al. 2005a).
Like drought stress, heat stress also leads to changes in the accumulation of compatible osmolytes (Sakamoto and Murata 2002). The early synthesis of these osmolytes (proline, glycine betaine, or soluble sugars) compensates for the effect of the decrease in leaf water potential, which appears as an immediate response to heat. By maintaining the cell water balance and membrane stability and buffering the cellular redox potential, the accumulation of osmolytes regulates osmotic activities and protects cellular structures from high temperatures (Farooq et al. 2008). The role of glycine betaine in photosynthesis in plants under heat stress has been reported by Allakhverdiev et al. (2008). The activation of Rubisco is maintained by the production of glycine betaine in the chloroplasts by sequestering Rubisco activase near thylakoids and preventing its thermal inactivation (Allakhverdiev et al. 2008). However, there is a difference between species in their ability to synthesize glycine betaine under heat-stress conditions (Ashraf and Foolad 2007). In maize and sugarcane, a strong accumulation of glycine betaine has been reported in response to elevated temperatures (Quan et al. 2004; Wahid and Close 2007). At the same time, the increase in temperatures causes the accumulation of proline and soluble carbohydrates in wheat while the levels of valuable proteins are reduced (Qaseem et al. 2019). Thus, despite the lack of studies on pearl millet, work on other crops has shown the role of osmotic adjustment in tolerance to heat stress.
10.2.3.1.4 Transpiration Efficiency
In times of stress, plants need to develop mechanisms to conserve soil water or maximize their water use efficiency to alleviate the effect of stress. Several studies have been carried out in recent years to determine the water use efficiency of crops (Vadez et al. 2011, 2013a, 2014, 2021; Schittenhelm and Schroetter 2014; van Oosterom et al. 2021). Water use efficiency appears to be an important criterion for evaluating the water supply. It provides a simple and quick measure of how the available water can be converted into biomass and grain (Sekhon et al. 2010). Defined as the accumulation of biomass per unit of water transpired (Xin et al. 2009), transpiration efficiency can also be understood at the leaf level as the intrinsic water-use efficiency. It is the ratio of the instantaneous rates of CO2 assimilation and transpiration at the level of the stomata (Condon et al. 2002). Thus, increased biomass or photosynthesis, decreased transpiration, or a combination of both can result in reduced water use reflecting higher water use efficiency of plants. However, Xin et al. (2009) reported that transpiration efficiency based on biomass production was strongly correlated with increased biomass accumulation rather than reduced water use.
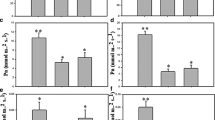
In a recent study comparing the transpiration efficiency of C4 cereals, Vadez et al. (2021) found that transpiration efficiency in maize was higher than in millet and somewhat higher than in sorghum (Fig. 1.12). This difference between species could be explained by differences in the ability to restrict transpiration under high VPD (Vadez et al. 2014). Likewise, Choudhary et al. (2020) reported that maize conserves water by limiting transpiration during increased VPD and under higher soil moisture than sorghum and pearl millet. In addition, the transpiration efficiency seems to depend on the type of soil. It was higher in high clay than in sandy soil under a high VPD (Vadez et al. 2021), while the differences in transpiration efficiency between maize and sorghum were not visible in Alfisol and sandy soil.
However, there is variation in transpiration efficiency within species that has been demonstrated for C4 plant species (Mortlock and Hammer 2000; Xin et al. 2009; Vadez et al. 2011, 2014, 2021). Transpiration efficiency was higher under stress conditions (Mortlock and Hammer 2000). Sorghum genotypes with low internal CO2 concentration and improved photosynthetic capacity may be a factor explaining the high transpiration efficiency in some lines (Xin et al. 2009). Under water deficit conditions, water use efficiency was also increased in sweet sorghum, while for maize, it was reduced (Zegada-Lizarazu et al. 2012). Bhattarai et al. (2020) confirm this result by stating that the water use efficiency was highest for sorghum cultivars, followed by millet and maize. According to Blum (2005), water use efficiency is often associated with drought tolerance and improved crop yields under stress conditions. Thus, genotypes with higher water use efficiency have greater survivability.
However, water extraction also seems to play an important role during grain filling. Differences in yield under terminal drought in sorghum have been reported to be determined by TE, followed by water extraction (Vadez et al. 2013b). Although the amount of water extracted by tolerant and susceptible genotypes was similar under drought stress, tolerant genotypes extracted less water before anthesis and more water after anthesis. This explains the lower yield of sensitive genotypes than that of tolerant lines under drought stress (Vadez et al. 2013a). Thus, the early conservation of water during pre-anthesis increases the yield of pearl millet during terminal drought (Kholová and Vadez 2013; Kholová et al. 2010). The drought tolerance of pearl millet is explained by the higher water use efficiency. Therefore, improving transpiration efficiency can effectively increase pearl millet yield in arid and semi-arid regions.
10.3 Molecular Basis of Stress Tolerance
Pearl millet is an important model for studying the physiological and molecular mechanisms of drought tolerance. Yet, compared to other cereals, the molecular mechanisms of drought stress tolerance in pearl millet remain elusive. Recently, genome-wide association studies (GWAS) have detected some genes associated with the domestication and differentiation of local millet varieties that have adapted to climatic conditions. These genes are related to heading date and plant height (Lakis et al. 2012), flowering time, morphological character, and yield (Saïdou et al. 2009; Vigouroux et al. 2011; Clotault et al. 2012; Diack et al. 2020), fitness under irregular climatic conditions (Ousseini et al. 2017), biomass and stay green (Debieu et al. 2018). Molecular responses involve a set of genes and signal transduction pathways that are highly regulated. The tolerance mechanisms begin with the detection of the stress, which causes a series of signal molecules transported in the leaves via the xylem.
Among the signals, abscisic acid (ABA) is a very important drought response pathway. It is a key root-to-shoot signal of drought stress (Xu et al. 2010) even though it is variable among species (Zhang et al. 2005; Jia and Zhang 2008). However, how the root cells detect the moisture state of the soil remains a mystery. Root tissues synthesize ABA upon detection of stress, which induces stomatal closure, thereby reducing transpiration and photosynthesis and allowing plant adaptation to drought conditions (De Ollas et al. 2013). ABA is also involved in regulating aquaporin activity, which contributes to the maintenance of the favorable water status of the plant (Parent et al. 2009; Reddy et al. 2017a). The ABA-dependent signaling response to stress involves different genes and transcription factors (Tuteja 2007). These genes and transcription factors are either involved in ABA biosynthesis or induced by the presence of ABA. For example, under drought conditions, strong expression of the 9-cis-epoxycarotenoid dioxygenase gene (NCED) is the first step in ABA biosynthesis (Qin and Zeevaart 1999; Behnam et al. 2013), provides evidence of ABA accumulation. In addition, several transcripts such as the WRKY transcription family (Jaiswal et al. 2018; Chanwala et al. 2020), PYL/PYR, PP2C SnRK2, ABRF DREB2A/2B, AREB1, RD22BP1, NAC, and MYC/MYB family (Tuteja 2007; Bhargava and Sawant 2013; Dudhate et al. 2018; Zhang et al. 2021) are known for their role in phytohormone signaling and response to abiotic stresses in plants. Furthermore, during drought stress, reactive oxygen species (ROS) accumulation increases considerably (Farooq et al. 2009; Alam et al. 2010). ROS form a natural by-product of normal oxygen metabolism and plays an important role in cell signaling. ROS include oxygen ions, free radicals, and peroxides, whose common characteristic is their ability to cause oxidative damage to proteins, DNA, and lipids (Apel and Hirt 2004). They target various organelles, including chloroplasts, mitochondria, and peroxisomes, resulting in premature leaf senescence or plant death (Ma et al. 2013). However, a versatile and cooperative antioxidant system tightly controls improved ROS production. It modulates the intracellular concentration of ROS and defines the redox status of the cell. Superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), ascorbate peroxidase (APX), and guaiacol peroxidase (GPOX) constitute the main mechanisms of entrapment (Cruz de Carvalho 2008, Takayuki et al. 2013). An increase in the levels of these antioxidant enzymes (AOX) has been noted in pearl millet in water-deficit stress conditions (Vijayalakshmi et al. 2012). On the other hand, prolonged drought stress leads to the ineffectiveness of the antioxidant system causing cell damage and death. Many genes conferring tolerance to drought stress have been identified in plants. These genes were presented in the study by Kumar et al. (2018) and classified into two groups (Bray 1997; Yamaguchi-Shinozaki and Shinozaki 2005; Kumar et al. 2018). The first group includes functional genes encoding proteins whose catalytic activities are responsible for the protection of cells and organs against stress, regulatory genes encoding proteins necessary for signal transduction and the regulation of expression of genes. The second group comprises several genes and transcription factors responsive to drought, such as the binding gene to elements sensitive to dehydration, aquaporin, abundant proteins of late embryogenesis, and dehydrins (Farooq et al. 2009).
In pearl millet seeds, Ghatak et al. (2016) also identified heat shock proteins (HSP), molecular chaperones, storage proteins, and abundant late embryogenesis (LEA) with increased levels. These proteins help stabilize the folding and conformation of structural proteins and the functionality of enzymes (Wang et al. 2004), hence their protective function.
In recent years, proteomic studies have become increasingly important as proteins are the main drivers of all cellular events. At the proteomic level, several studies have been carried out to understand the effect of drought stress on cereals such as millet (Ghatak et al. 2016, 2021), maize (Riccardi et al. 2004), and wheat (Ford et al. 2011; Komatsu et al. 2014; Ghatak et al. 2021). In pearl millet, a shotgun proteomics approach was used to study protein signatures from different tissues under drought and control conditions (Ghatak et al. 2016). Proteins have been identified and quantified in the root, leaf, and seed tissues. However, there is a pronounced change in the proteome of stressed plants compared to control conditions. Putative drought-sensitive proteins have also been identified in the root (271 proteins), seed (159 proteins), and leaf (292 proteins). Leaf tissue showed the most significant changes, followed by roots and seeds.
A high temperature also triggers important molecular changes in plants. Many transcripts and proteins alter their expression and levels to prevent or reverse the effects of heat on proteins. Under stress, plants synthesize (induce) a set of heat shock proteins (HSPs), unlike in normal conditions where they are almost absent. Based on their molecular weight, HSPs are divided into different families with distinct functions. Numerous studies have shown the role of HSP families in thermotolerance (Gurley 2000; Queitsch et al. 2000; Sun et al. 2002; Hu et al. 2010; Reddy et al. 2010; Nitnavare et al. 2016). Heat stress transcription factors (Hsfs) are the main regulators of heat stress response gene expression (Baniwal et al. 2004; Kotak et al. 2007; Schramm et al. 2008). Since protein aggregation is irreversible, HSPs appear important in the thermo-tolerance reaction and act as molecular chaperones to prevent the denaturation or aggregation of target proteins and facilitate protein refolding (Ahuja et al. 2010; Scharf et al. 2012). In addition, they lead to an improvement in physiological parameters and membrane stability or hydration of cellular structures (Camejo et al. 2005; Ahn and Zimmerman 2006; Wahid and Close 2007). These improvements promote adequate plant growth and development under heat stress. Thus, it has been reported that the expression of genes inducing HSPs may be an important mechanism for increasing tolerance to heat stress (Wahid et al. 2007).
The plant cuticle is a protective layer made of lipids and waxes present on the surface of aerial organs. It forms a barrier against pathogen infection and limits water losses through transpiration. A similar hydrophobic barrier, the Casparian strip, is formed at the periphery of the endodermis’s root vascular bundle and is thought to contribute to the limitation of water loss. Recent results suggest that these lipid-made barriers are important components of water potential and heat stress tolerance in pearl millet. Indeed, sequencing of the pearl millet genome revealed an expansion of gene families involved in the biosynthesis and transport of cut-in, suberin, and wax components of the cuticle and Casparian strip (Varshney et al. 2017; Debieu et al. 2017). Moreover, an association genetics study for tolerance to water stress at the vegetative stage in pearl millet led to the identification of four genetic loci associated with increased biomass production under early drought stress. One of these associations contained a gene encoding an enzyme (3-ketoacyl-CoA synthase or KCS) catalyzes the elongation of C24 fatty acids during wax and suberin biosynthesis (Debieu et al. 2018). Altogether, this suggests that wax and fatty acids biosynthesis could be targeted to increase water and heat stress tolerance in dryland cereals.
10.4 Breeding Pearl Millet for Drought and Heat Tolerance
Current climate models’ prediction showed that more inter- and intra-annual variability in rainfall and temperature are expected in sub-Saharan Africa (Brown and Lall 2006; Sultan and Gaetani 2016). This situation will increasingly threaten pearl millet production in this part of the world, where this cereal is one of the most important sources of nutrition for more than 90 million people (Anuradha et al. 2017). Therefore, breeders should develop efficient breeding strategies to accelerate the development of improved varieties with better tolerance to drought and heat stresses. During the last decades, several attempts to improve phenotypic screening methods, study the genetic variation in breeding lines, and develop high-yielding and tolerant pearl millet varieties to heat and drought stress using conventional and molecular breeding methods have been accomplished.
Drought is considered the primary abiotic constraint for pearl millet production and is caused by the low and erratic rainfall distribution. In breeding, progress has been made in the development of screening techniques, identification of sources of tolerance, development of early maturing varieties, and identification of QTL linked to drought tolerance (Yadav et al. 2017). Screening of 21 genotypes for osmotic stress tolerance (as a proxy for drought) using PEG 6000 revealed three genotypes (TNBH 0538, TNBH 0642, and ICVM 221) tolerant to moisture stress at germination and early growth stages (Govindaraj et al. 2010). However, pearl millet grain yield is more affected by post-flowering and terminal drought stresses (Kholová and Vadez 2013). To overcome these stresses, drought escape mechanisms have been successfully exploited by targeting early maturity (Yadav and Rai 2013). It has been demonstrated that early flowering pearl millet genotypes with low biomass, few basal tillers and high harvest index can tolerate terminal drought stress (Bidinger et al. 2005). Across West African countries, several landraces characterized by earliness, high grain yield, bold grain, and compact and conical panicles have contributed to the development of pearl millet cultivars adapted to drought-prone areas, including ICTP-8203 and GB-8735 (Wilson et al. 2008). These varieties flower within 40–45 days and mature within less than 75 days, making them suitable cultivars for arid zones. Interestingly, recurrent drought in the 1980s led to selection by farmers in Niger for earlier flowering pearl millet varieties (Vigouroux et al. 2011).
Another important research strategy was using molecular markers to identify genomic regions associated with drought tolerance in pearl millet. Several major QTLs with significant effects on pearl millet grain in terminal drought stress environments were identified and successfully used in marker-assisted selection to improve drought-sensitive pearl millet lines (Yadav et al. 2002, 2004; Serraj et al. 2005; Bidinger et al. 2005). Similarly, potential QTLs for tolerance to water stress during the vegetative phase were identified (Debieu et al. 2018).
For heat tolerance, a good amount of work has been accomplished in breeding. Both field and greenhouse heat tolerance screening techniques have been developed and improved at ICRISAT for pearl millet (Gupta et al. 2015). These techniques were widely used in assessing the effect of heat on a large number of hybrid parental lines, germplasm accessions, and improved varieties across several field locations in India for four consecutive years. The field screenings led to the identification of five hybrid parental lines and a germplasm accession as new sources of resistance to heat tolerance that has been used to develop a high-yielding and heat-tolerant composite variety (Gupta et al. 2016).
10.5 Conclusion
Climate change in arid and semi-arid areas is led by heat and drought, often intermittent or terminal. Drought is due to soil water and vapor pressure deficits. The climate models predicted an increase in climate change effects in the near or long term. Heat and drought are distinctive stresses but often occur simultaneously on crops. They are studied separately or jointly to understand their effects on the plant better. Dry spells and heat stress are the factors constituting major agronomic problems that cause severe yield losses for pearl millet in arid and semi-arid areas. Their effects on pearl millet depend on intensity, phase of occurrence, and duration. However, the flowering period is more vulnerable for pearl millet. Drought and heat affect aerial and root development and physiological and molecular performance. This results in a proportional decrease in pearl millet yield.
In general, drought survival mechanisms of plants are similar to those used to cope with heat stress. Escape, avoidance and tolerance mechanisms have long been considered important strategies for drought adaptation. Due to its drought and heat tolerance, pearl millet is an ideal model for studying the heat and drought resistance mechanism of cereal crops. The avoidance mechanism is commonly used by pearl millet. It responds to water deficit with leaf rolling, senescence, and stomatal conductance, leading to reduced water loss when water soil stock becomes limiting. Also, transpiration restriction under high vapor pressure deficit reducing the transpiration of pearl millet is beneficial for crop yield under terminal drought. Increasing water uptake by root morphological and functional modifications reinforces pearl millet avoidance of heat and drought. Few studies on pearl millet mention osmotic adjustment, the leading mechanism of heat and drought tolerance. These tolerance mechanisms led to changes in the accumulation of compatible osmolytes. The water use efficiency also explains the drought tolerance of pearl millet.
The molecular mechanisms of drought stress tolerance in pearl millet remain elusive. However, gene discovery is ongoing with the detection of genes associated with domestication, genes conferring tolerance to drought stress like gene families involved in the biosynthesis and transport of antioxidant and constitutive molecules. The role of these genes has to be precise and validated to be useful for pearl millet improvement. Progress in pearl millet breeding is based essentially on drought escape mechanisms, with the development of early flowering and maturing varieties. However, molecular breeding started to use QTLs to improve terminal drought-sensitive pearl millet. Heat-tolerant varietal sources are also identified and used in pearl millet improvement.
Progress is made in understanding the molecular bases of pearl millet adaptation to drought and heat. However, pearl millet in research is still far from other major cereals and remains a neglected crop. So, it is urgent to address drought and heat adaptation mechanisms to breed pearl millet varieties for the benefit of the dry-land farmers.
References
Adhikari U, Nejadhashemi AP, Woznicki SA (2015) Climate change and eastern Africa: a review of impact on major crops. Food Energy Secur 4:110–132
Ahn Y, Zimmerman JL (2006) Introduction of the carrot HSP17. 7 into potato (Solanum tuberosum L.) enhances cellular membrane stability and tuberization in vitro. Plant Cell Environ 29:95–104
Ahuja I, de Vos RC, Bones AM, Hall RD (2010) Plant molecular stress responses face climate change. Trends Plant Sci 15:664–674
Alam I, Sharmin SA, Kim K-H et al (2010) Proteome analysis of soybean roots subjected to short-term drought stress. Plant and Soil 333:491–505
Alexandersson E, Fraysse L, Sjövall-Larsen S et al (2005) Whole gene family expression and drought stress regulation of aquaporins. Plant Mol Biol 59:469–484
Al-Khatib K, Paulsen GM (1999) High-temperature effects on photosynthetic processes in temperate and tropical cereals. Crop Sci 39:119–125
Allakhverdiev SI, Kreslavski VD, Klimov VV et al (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res 98:541–550
Alqudah AM, Samarah NH, Mullen RE (2011) Drought stress effect on crop pollination, seed set, yield and quality. In: Lichtfocus E (ed) Alternative farming systems, biotechnology, drought stress and ecological fertilisation. Springer, Dordrecht, pp 193–213
Alves AA, Setter TL (2004) Response of cassava leaf area expansion to water deficit: cell proliferation, cell expansion and delayed development. Ann Bot 94:605–613
Anjum S, Wang L, Farooq M et al (2011a) Methyl jasmonate-induced alteration in lipid peroxidation, antioxidative defence system and yield in soybean under drought. J Agron Crop Sci 197:296–301
Anjum S, Wang L, Farooq M et al (2011b) Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J Agron Crop Sci 197:177–185
Annerose D (1990) Recherches sur les mécanismes physiologiques d’adaptation à la sécheresse. Application au cas de l’arachide (Arachis hypogea l.) cultivée au Sénégal, Thèse de doctorat, Université Paris VII
Anuradha N, Satyavathi CT, Bharadwaj C et al (2017) Deciphering genomic regions for high grain iron and zinc content using association mapping in pearl. Front Plant Sci 8:1–17
Aparna K, Hash C, Yadav R, Vadez V (2014) Seed number and 100-seed weight of pearl millet (Pennisetum glaucum L.) respond differently to low soil moisture in genotypes contrasting for drought tolerance. J Agron Crop Sci 200:119–131
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Aroca R, Porcel R, Ruiz-Lozano JM (2012) Regulation of root water uptake under abiotic stress conditions. J Exp Bot 63:43–57
Arya RK, Yadav H (2009) Stability of grain yield and its contributing traits in white and grey grain hybrids of pearl millet (Pennisetum glaucum). Indian J Agric Sci 79:941–944
Arya R, Yadav H, Yadav A, Singh M (2010) Effect of environment on yield and its contributing traits in pearl millet. Forage Res 36:176–180
Arya R, Singh M, Yadav A et al (2014) Advances in pearl millet to mitigate adverse environment conditions emerged due to global warming. Forage Res 40:57–70
Ashraf MY, Azmi AR, Khan AH, Ala S (1994) Effect of water stress on total phenols, peroxidase activity and chlorophyll content in wheat [Triticum aestivum L.]. Acta Physiol Plant 16(3):185–191
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Ashraf M, Hafeez M (2004) Thermotolerance of pearl millet and maize at early growth stages: growth and nutrient relations. Biol Plant 48:81–86
Ashraf M, Ahmad A, McNeilly T (2001) Growth and photosynthetic characteristics in pearl millet under water stress and different potassium supply. Photosynthetica 39:389–394
Assefa Y, Staggenborg SA, Prasad PV (2010) Grain sorghum water requirement and responses to drought stress: a review. Crop Manage 9:1–11
Assmann SM, Snyder JA, Lee YJ (2000) ABA-deficient (aba1) and ABA-insensitive (abi1-1, abi2-1) mutants of Arabidopsis have a wild-type stomatal response to humidity. Plant Cell Environ 23:387–395
Bai Y, Han X, Wu J et al (2004) Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 431:181–184
Bajji M, Lutts S, Kinet J-M (2001) Water deficit effects on solute contribution to osmotic adjustment as a function of leaf ageing in three durum wheat (Triticum durum Desf.) cultivars performing differently in arid conditions. Plant Sci 160:669–681
Balling RC, Cerveny RS (2003) Compilation and discussion of trends in severe storms in the United States: Popular perception v. climate reality. Nat Hazards 29:103–112
Baniwal SK, Bharti K, Chan KY et al (2004) Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. J Biosci 29:471–487
Barnabás B, Jäger K, Fehér A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31:11–38
Bates B, Kundzewicz Z, Wu S, Palutikof J (eds) (2008) Climate change and water. Intergovernmental Panel on Climate Change Secretariat, Geneva
Behnam B, Iuchi S, Fujita M et al (2013) Characterization of the promoter region of an Arabidopsis gene for 9-cis-epoxycarotenoid dioxygenase involved in dehydration-inducible transcription. DNA Res 20:315–324. https://doi.org/10.1093/DNARES/DST012
Bhargava S, Sawant K (2013) Drought stress adaptation: metabolic adjustment and regulation of gene expression. Plant Breed 132:21–32
Bhattarai B, Singh S, West CP et al (2020) Water depletion pattern and water use efficiency of forage sorghum, pearl millet, and corn under water limiting condition. Agric Water Manag 238:106206. https://doi.org/10.1016/j.agwat.2020.106206
Bidinger F, Raju D (2000) Response to selection for increased individual grain mass in pearl millet. Crop Sci 40:68–71
Bidinger FR, Serraj R, Rizvi SMH et al (2005) Field evaluation of drought tolerance QTL effects on phenotype and adaptation in pearl millet [Pennisetum glaucum (L.) R. Br.] topcross hybrids. Field Crop Res 94:14–32. https://doi.org/10.1016/j.fcr.2004.11.006
Bita C, Gerats T (2013) Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci 4:273. https://doi.org/10.3389/fpls.2013.00273
Blum A (2005) Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Aust J Agr Res 56:1159–1168
Blum A (2011) Plant water relations, plant stress and plant production. In: Blum A (ed) Plant breeding for water-limited environments. Springer, New York, pp 11–52
Blum A (2017) Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ 40:4–10
Blum A, Sullivan C (1986) The comparative drought resistance of landraces of sorghum and millet from dry and humid regions. Ann Bot 57:835–846
Bois J-F (1993) Effets de la contrainte hydrique sur la photosynthèse du mil. In: Hamon S (ed) Le mil en Afrique, diversité génétique et agro-physiologique: potentialités et contraintes pour l’amélioration génétique et l’agriculture. Orstom, Paris, pp 191–198
Boyer J, Westgate M (2004) Grain yields with limited water. J Exp Bot 55:2385–2394
Bray EA (1997) Plant responses to water deficit. Trends Plant Sci 2:48–54
Brown C, Lall U (2006) Water and economic development: the role of variability and a framework for resilience. Nat Resour Forum 30(4):306–317
Buckley TN (2019) How do stomata respond to water status? New Phytol 224:21–36
Calleja-Cabrera J, Boter M, Oñate-Sánchez L, Pernas M (2020) Root growth adaptation to climate change in crops. Front Plant Sci 11:544. https://doi.org/10.3389/fpls.2020.00544
Camejo D, Rodríguez P, Morales MA et al (2005) High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J Plant Physiol 162:281–289
Carvajal M, Cooke D, Clarkson D (1996) Plasma membrane fluidity and hydraulic conductance in wheat roots: interactions between root temperature and nitrate or phosphate deprivation. Plant Cell Environ 19:1110–1114
Chanwala J, Satpati S, Dixit A et al (2020) Genome-wide identification and expression analysis of WRKY transcription factors in pearl millet (Pennisetum glaucum) under dehydration and salinity stress. BMC Genomics 21:1–16. https://doi.org/10.1186/s12864-020-6622-0
Chaumont F, Tyerman SD (2014) Aquaporins: highly regulated channels controlling plant water relations. Plant Physiol 164:1600–1618
Chaves M, Oliveira M (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55:2365–2384
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239–264
Chen H, Jiang J-G (2010) Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ Rev 18:309–319
Chimenti CA, Marcantonio M, Hall A (2006) Divergent selection for osmotic adjustment results in improved drought tolerance in maize (Zea mays L.) in both early growth and flowering phases. Field Crop Res 95:305–315
Chimungu JG, Maliro MF, Nalivata PC et al (2015) Utility of root cortical aerenchyma under water limited conditions in tropical maize (Zea mays L.). Field Crop Res 171:86–98
Chopart J-L, Siband P (1999) Development and validation of a model to describe root length density of maize from root counts on soil profiles. Plant and Soil 214:61–74
Chopart J-L, Rodrigues SR, De Azevedo MC, de Conti MC (2008a) Estimating sugarcane root length density through root mapping and orientation modelling. Plant and Soil 313:101–112
Chopart J-L, Sine B, Dao A, Muller B (2008b) Root orientation of four sorghum cultivars: application to estimate root length density from root counts in soil profiles. Plant Root 2:67–75
Choudhary S, Guha A, Kholova J et al (2020) Maize, sorghum, and pearl millet have highly contrasting species strategies to adapt to water stress and climate change-like conditions. Plant Sci 295:110297. https://doi.org/10.1016/j.plantsci.2019.110297
Christidis N, Stott PA, Jones GS et al (2012) Human activity and anomalously warm seasons in Europe. Int J Climatol 32:225–239
Christopher J, Manschadi A, Hammer G, Borrell A (2008) Developmental and physiological traits associated with high yield and stay-green phenotype in wheat. Aust J Agr Res 59:354–364
Clotault J, Thuillet A-C, Buiron M et al (2012) Evolutionary history of pearl millet (Pennisetum glaucum [L.] R. Br.) and selection on flowering genes since its domestication. Mol Biol Evol 29:1199–1212
Collins M, Knutti R, Arblaster J et al (2013) Long-term climate change: projections, commitments and irreversibility. In: Stocker T, Quin D, Plattner G et al (eds) Climate change 2013-the physical science basis: contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 1029–1136
Condon AG, Richards R, Rebetzke G, Farquhar G (2002) Improving intrinsic water-use efficiency and crop yield. Crop Sci 42:122–131
Cooper P, Rao K, Singh P et al (2009) Farming with current and future climate risk: advancing a ‘Hypothesis of Hope’ for rainfed agriculture in the semi-arid tropics. J SAT Agric Res 7:1–19
Cornic G, Massacci A (1996) Leaf photosynthesis under drought stress. In: Baker N (ed) Photosynthesis and the environment. Advances in photosynthesis and respiration, vol 5. Springer, Dordrecht, pp 347–366
Crafts-Brandner SJ, Salvucci ME (2000) Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc Natl Acad Sci 97:13430–13435
Crafts-Brandner SJ, Salvucci ME (2002) Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol 129:1773–1780
Craufurd P, Peacock J (1993) Effect of heat and drought stress on sorghum (Sorghum bicolor). II Grain yield. Exp Agric 29:77–86
Cruz de Carvalho MH (2008) Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav 3:156–165
Dai A, Lamb PJ, Trenberth KE et al (2004) The recent Sahel drought is real. Int J Climatol 24:1323–1331
De Ollas C, Hernando B, Arbona V, Gómez-Cadenas A (2013) Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol Plant 147:296–306
Debieu M, Kanfany G, Laplaze L (2017) Pearl millet genome: lessons from a tough crop. Trends Plant Sci 22(11):911–913
Debieu M, Sine B, Passot S et al (2018) Response to early drought stress and identification of QTLs controlling biomass production under drought in pearl millet. PloS One 13:1–19. https://doi.org/10.1371/journal.pone.0201635
Diack O, Kanfany G, Gueye MC et al (2020) GWAS unveils features between early-and late-flowering pearl millets. BMC Genomics 21:1–11. https://doi.org/10.21203/rs.3.rs-25381/v1
Djanaguiraman M, Perumal R, Ciampitti I et al (2018) Quantifying pearl millet response to high temperature stress: thresholds, sensitive stages, genetic variability and relative sensitivity of pollen and pistil. Plant Cell Environ 41:993–1007
Dreesen FE, De Boeck HJ, Janssens IA, Nijs I (2012) Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community. Environ Exp Bot 79:21–30
Dudhate A, Shinde H, Tsugama D et al (2018) Transcriptomic analysis reveals the differentially expressed genes and pathways involved in drought tolerance in pearl millet [Pennisetum glaucum (L.) R. Br]. PLoS One 13:1–14. https://doi.org/10.1371/journal.pone.0195908
Fahad S, Hussain S, Saud S et al (2016a) Exogenously applied plant growth regulators affect heat-stressed rice pollens. J Agron Crop Sci 202:139–150
Fahad S, Hussain S, Saud S et al (2016b) A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol Biochem 103:191–198
Fahad S, Bajwa AA, Nazir U et al (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147. https://doi.org/10.3389/fpls.2017.01147
Farooq M, Basra S, Wahid A et al (2008) Physiological role of exogenously applied glycine betaine to improve drought tolerance in fine grain aromatic rice (Oryza sativa L.). J Agron Crop Sci 194:325–333
Farooq M, Wahid A, Kobayashi N et al (2009) Plant drought stress: effects, mechanisms and management. Sustain Agric 29:185–212
Farooq M, Bramley H, Palta JA, Siddique KH (2011) Heat stress in wheat during reproductive and grain-filling phases. Crit Rev Plant Sci 30:491–507
Fauchereau N, Trzaska S, Rouault M, Richard Y (2003) Rainfall variability and changes in southern Africa during the 20th century in the global warming context. Nat Hazards 29:139–154
Faye A, Sine B, Chopart J-L et al (2019) Development of a model estimating root length density from root impacts on a soil profile in pearl millet (Pennisetum glaucum (L.) R. Br). Application to measure root system response to water stress in field conditions. PloS One 14:e0214182. https://doi.org/10.1371/journal.pone.0214182
Fischer G, Shah MN, Tubiello F, Van Velhuizen H (2005a) Socio-economic and climate change impacts on agriculture: an integrated assessment, 1990–2080. Philos Trans R Soc B Biol Sci 360:2067–2083
Fischer R, Sayre K, Reynolds M (2005b) Osmotic adjustment in wheat in relation to grain yield under water deficit environments. Agron J 97:1062–1071
Flexas J, Ribas-Carbó M, Bota J et al (2006) Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol 172:73–82
Ford KL, Cassin A, Bacic AF (2011) Quantitative proteomic analysis of wheat cultivars with differing drought stress tolerance. Front Plant Sci 2:44
Forster B, Ellis R, Moir J et al (2004) Genotype and phenotype associations with drought tolerance in barley tested in North Africa. Ann Appl Biol 144:157–168
Ghatak A, Chaturvedi P, Nagler M et al (2016) Comprehensive tissue-specific proteome analysis of drought stress responses in Pennisetum glaucum (L.) R. Br.(Pearl millet). J Proteomics 143:122–135
Ghatak A, Chaturvedi P, Bachmann G et al (2021) Physiological and proteomic signatures reveal mechanisms of superior drought resilience in pearl millet compared to wheat. Front Plant Sci 11:600278
Godfray HCJ, Beddington JR, Crute IR et al (2010) Food security: the challenge of feeding 9 billion people. Science 327:812–818
Golombek S (2003) Effect of drought on gas exchange and carbon metabolism in pearl millet. Technological and Institutional Innovations for Sustainable Rural Development. Deutshe Tropentag, Göttingen, 8–10 October 2003, pp 339–348
Govindaraj M, Shanmugasundaram P, Sumathi P, Muthiah A (2010) Simple, rapid and cost effective screening method for drought resistant breeding in pearl millet. Electron J Plant Breed 1:590–599
Grondin A, Mauleon R, Vadez V, Henry A (2016) Root aquaporins contribute to whole plant water fluxes under drought stress in rice (Oryza sativa L.). Plant Cell Environ 39:347–365
Grondin A, Affortit P, Tranchant-Dubreuil C et al (2020) Aquaporins are main contributors to root hydraulic conductivity in pearl millet [Pennisetum glaucum (L) R. Br.]. PLoS One 15:e0233481. https://doi.org/10.1371/journal.pone.0233481
Gupta S, Rai K, Singh P et al (2015) Seed set variability under high temperatures during flowering period in pearl millet (Pennisetum glaucum L.(R.) Br.). Field Crop Res 171:41–53
Gupta S, Ameta V, Pareek S, et al. (2016) Genetic enhancement for flowering period heat tolerance in pearl millet (Pennisetum glaucum L.(R.) Br.). In: 7th international crop science congress, Beijing. http://oar.icrisat.org/9757/1/Page1.pdf. Accessed 15 Jul 2021
Gurley WB (2000) HSP101: a key component for the acquisition of thermotolerance in plants. Plant Cell 12:457–460
Heckathorn SA, DeLucia EH (1991) Effect of leaf rolling on gas exchange and leaf temperature of Andropogon gerardii and Spartina pectinata. Bot Gaz 152:263–268
Heckathorn SA, Giri A, Mishra S, Bista D (2013) Heat stress and roots. In: Climate change and plant abiotic stress tolerance. Wiley, Hoboken, pp 109–136
Henry S, Divol F, Bettembourg M et al (2016) Immunoprofiling of rice root cortex reveals two cortical subdomains. Front Plant Sci 6:1139. https://doi.org/10.3389/fpls.2015.01139
Hochholdinger F, Tuberosa R (2009) Genetic and genomic dissection of maize root development and architecture. Curr Opin Plant Biol 12:172–177
Howden SM, Soussana J-F, Tubiello FN et al (2007) Adapting agriculture to climate change. Proc Natl Acad Sci 104:19691–19696
Hu X, Li Y, Li C et al (2010) Characterization of small heat shock proteins associated with maize tolerance to combined drought and heat stress. J Plant Growth Regul 29:455–464
Hund A, Fracheboud Y, Soldati A, Stamp P (2008) Cold tolerance of maize seedlings as determined by root morphology and photosynthetic traits. Eur J Agron 28:178–185
Hund A, Ruta N, Liedgens M (2009) Rooting depth and water use efficiency of tropical maize inbred lines, differing in drought tolerance. Plant and Soil 318:311–325
Izanloo A, Condon AG, Langridge P et al (2008) Different mechanisms of adaptation to cyclic water stress in two South Australian bread wheat cultivars. J Exp Bot 59:3327–3346
Jagadish SK (2020) Heat stress during flowering in cereals–effects and adaptation strategies. New Phytol 226:1567–1572
Jaiswal S, Antala TJ, Mandavia M et al (2018) Transcriptomic signature of drought response in pearl millet (Pennisetum glaucum (L.) and development of web-genomic resources. Sci Rep 8:1–16. https://doi.org/10.1038/s41598-018-21560-1
Jia W, Zhang J (2008) Stomatal movements and long-distance signaling in plants. Plant Signal Behav 3:772–777
Jung H, Choi Y, Oh J, Lim G (2002) Recent trends in temperature and precipitation over South Korea. Int J Climatol 22:1327–1337
Kadioglu A, Terzi R (2007) A dehydration avoidance mechanism: leaf rolling. Bot Rev 73:290–302
Kadioglu A, Terzi R, Saruhan N, Saglam A (2012) Current advances in the investigation of leaf rolling caused by biotic and abiotic stress factors. Plant Sci 182:42–48
Kang Y, Khan S, Ma X (2009) Climate change impacts on crop yield, crop water productivity and food security–a review. Prog Nat Sci 19:1665–1674
Kashiwagi J, Krishnamurthy L, Crouch J, Serraj R (2006) Variability of root length density and its contributions to seed yield in chickpea (Cicer arietinum L.) under terminal drought stress. Field Crop Res 95:171–181
Katerji N, Tardieu F, Bethenod O, Quetin P (1994) Behavior of maize stem diameter during drying cycles: comparison of two methods for detecting water stress. Crop Sci 34:165–169
Khalifa F, Ong C (1990) Effect of supra-optimal temperatures on germination of pearl millet (Pennisetum glaucum (L) R. BR.) hybrids. Ann Arid Zone 29:279–288
Khan MB, Hussain N, Iqbal M (2001) Effect of water stress on growth and yield components of maize variety YHS 202. J Res Sci 12:15–18
Khan H, Paull J, Siddique K, Stoddard F (2010) Faba bean breeding for drought-affected environments: A physiological and agronomic perspective. Field Crop Res 115:279–286
Kholová J, Vadez V (2013) Water extraction under terminal drought explains the genotypic differences in yield, not the antioxidant changes in leaves of pearl millet (Pennisetum glaucum). Funct Plant Biol 40:44–53. https://doi.org/10.1071/FP12181
Kholová J, Hash CT, Kakkera A et al (2010) Constitutive water-conserving mechanisms are correlated with the terminal drought tolerance of pearl millet [Pennisetum glaucum (L.) R. Br.]. J Exp Bot 61:369–377
Kholová J, Zindy P, Malayee S et al (2016) Component traits of plant water use are modulated by vapour pressure deficit in pearl millet (Pennisetum glaucum (L.) R. Br.). Funct Plant Biol 43:423–437
Kirkegaard J, Lilley J, Howe G, Graham J (2007) Impact of subsoil water use on wheat yield. Aust J Agr Res 58:303–315
Kirtman B, Power SB, Adedoyin AJ et al (2013) Near-term climate change: projections and predictability. In: Stocker T, Quin D, Plattner G et al (eds) Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 953–1028
Knox J, Hess T, Daccache A, Wheeler T (2012) Climate change impacts on crop productivity in Africa and South Asia. Environ Res Lett 7:034032. https://doi.org/10.1088/1748-9326/7/3/034032
Komatsu S, Kamal AH, Hossain Z (2014) Wheat proteomics: proteome modulation and abiotic stress acclimation. Front Plant Sci 5:684. https://doi.org/10.3389/fpls.2014.00684
Kondo M, Murty MV, Aragones DV (2000) Characteristics of root growth and water uptake from soil in upland rice and maize under water stress. Soil Sci Plant Nutr 46:721–732
Kooyers NJ (2015) The evolution of drought escape and avoidance in natural herbaceous populations. Plant Sci 234:155–162
Kotak S, Larkindale J, Lee U et al (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10:310–316
Kumar S, Sachdeva S, Bhat K, Vats S (2018) Plant responses to drought stress: physiological, biochemical and molecular basis. In: Vats S (ed) Biotic and abiotic stress tolerance in plants. Springer, India, pp 1–25
Kusaka M, Lalusin AG, Fujimura T (2005a) The maintenance of growth and turgor in pearl millet (Pennisetum glaucum [L.] Leeke) cultivars with different root structures and osmo-regulation under drought stress. Plant Sci 168:1–14
Kusaka M, Ohta M, Fujimura T (2005b) Contribution of inorganic components to osmotic adjustment and leaf folding for drought tolerance in pearl millet. Physiol Plant 125:474–489
Lakis G, Navascués M, Rekima S et al (2012) Evolution of neutral and flowering genes along pearl millet (Pennisetum glaucum) domestication. PloS One 7:e36642. https://doi.org/10.1371/journal.pone.0036642
Li P, Ma B, Xiong Y, Zhang W (2017) Morphological and physiological responses of different wheat genotypes to chilling stress: a cue to explain yield loss. J Sci Food Agric 97:4036–4045
Liu B, Asseng S, Müller C et al (2016) Similar estimates of temperature impacts on global wheat yield by three independent methods. Nat Clim Change 6:1130–1136
Lu C, Zhang J (1999) Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants. J Exp Bot 50:1199–1206
Ludlow M, Muchow R (1990) A critical evaluation of traits for improving crop yields in water-limited environments. Adv Agron 43:107–153
Ma C, Wang Z, Kong B, Lin T (2013) Exogenous trehalose differentially modulate antioxidant defense system in wheat callus during water deficit and subsequent recovery. Plant Growth Regul 70:275–285
Maiti R, Bidinger F (1981) Growth and development of the pearl millet plant. Research Bulletin no. 6. ICRISAT, Patancheru
Manschadi A, Christopher J, Hammer G, Devoil P (2010) Experimental and modelling studies of drought-adaptive root architectural traits in wheat (Triticum aestivum L.). Plant Biosyst 144:458–462
Maroco JP, Pereira JS, Chaves MM (1997) Stomatal responses to leaf-to-air vapour pressure deficit in Sahelian species. Funct Plant Biol 24:381–387
Matsuura A, Inanaga S, Sugimoto Y (1996) Mechanism of interspecific differences among four gramineous crops in growth response to soil drying. Jpn J Crop Sci 65:352–360
Maurel C, Boursiac Y, Luu D-T et al (2015) Aquaporins in plants. Physiol Rev 95:1321–1358
Mortlock MY, Hammer GL (2000) Genotype and water limitation effects on transpiration efficiency in sorghum. J Crop Prod 2:265–286
Muchow R (1989) Comparative productivity of maize, sorghum and pearl millet in a semi-arid tropical environment I. Yield potential. Field Crops Res 20:191–205
Niang I, Ruppel OC, Abdrabo MA et al (2014) Africa climate change 2014: impacts, adaptation, and vulnerability: part B: regional aspects. In: Barros V, Field C, Dokken D et al (eds) Contribution of working group II to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 1199–1266
Nio S, Cawthray G, Wade L, Colmer T (2011) Pattern of solutes accumulated during leaf osmotic adjustment as related to duration of water deficit for wheat at the reproductive stage. Plant Physiol Biochem 49:1126–1137
Nitnavare RB, Yeshvekar RK, Sharma KK et al (2016) Molecular cloning, characterization and expression analysis of a heat shock protein 10 (Hsp10) from Pennisetum glaucum (L.), a C 4 cereal plant from the semi-arid tropics. Mol Biol Rep 43:861–870
O’toole J, Cruz R, Singh T (1979) Leaf rolling and transpiration. Plant Sci Lett 16:111–114
Ocheltree T, Nippert J, Prasad PV (2014) Stomatal responses to changes in vapor pressure deficit reflect tissue-specific differences in hydraulic conductance. Plant Cell Environ 37:132–139
Ogbaga CC, Stepien P, Johnson GN (2014) Sorghum (Sorghum bicolor) varieties adopt strongly contrasting strategies in response to drought. Physiol Plant 152:389–401
Omarova E, Bogdanova E, Polimbetova F (1995) Regulation of water-loss by the leaves of soft winter-wheat with different organization of leaf structure. Russ J Plant Physiol 42:383–385
van Oosterom E, Kulathunga M, Deifel K et al (2021) Dissecting and modelling the comparative adaptation to water limitation of sorghum and maize: role of transpiration efficiency, transpiration rate and height. In Silico Plants 3:diaa012
Ousseini I, Bakasso Y, Kane N et al (2017) Myosin XI is associated with fitness and adaptation to aridity in wild pearl millet. Heredity 119:88–94. https://doi.org/10.1038/hdy.2017.13
Parent B, Hachez C, Redondo E et al (2009) Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant Physiol 149:2000–2012. https://doi.org/10.1104/pp.108.130682
Passot S (2016) Exploring pearl millet root system and its outcome for drought tolerance, Thèse de doctorat, Université Montpellier
Passot S, Gnacko F, Moukouanga D et al (2016) Characterization of pearl millet root architecture and anatomy reveals three types of lateral roots. Front Plant Sci 7:829. https://doi.org/10.3389/fpls.2016.00829
Passot S, Moreno-Ortega B, Moukouanga D et al (2018) A new phenotyping pipeline reveals three types of lateral roots and a random branching pattern in two cereals. Plant Physiol 177:896–910
Pinheiro C, Chaves M (2010) Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot 62:869–882
Pinheiro C, Chaves M (2011) Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot 62:869–882
Prado K, Maurel C (2013) Regulation of leaf hydraulics: from molecular to whole plant levels. Front Plant Sci 4:255
Prasad PV, Djanaguiraman M (2011) High night temperature decreases leaf photosynthesis and pollen function in grain sorghum. Funct Plant Biol 38:993–1003
Prasad PV, Djanaguiraman M (2014) Response of floret fertility and individual grain weight of wheat to high temperature stress: sensitive stages and thresholds for temperature and duration. Funct Plant Biol 41:1261–1269
Prasad PV, Boote KJ, Vu JC, Allen LH Jr (2004) The carbohydrate metabolism enzymes sucrose-P synthase and ADG-pyrophosphorylase in phaseolus bean leaves are up-regulated at elevated growth carbon dioxide and temperature. Plant Sci 166:1565–1573
Prasad PV, Boote K, Allen L Jr et al (2006a) Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crop Res 95:398–411
Prasad PV, Boote KJ, Allen LH Jr (2006b) Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain-sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperatures. Agric For Meteorol 139:237–251
Prasad PV, Staggenborg S, Ristic Z (2008) Impacts of drought and/or heat stress on physiological, developmental, growth, and yield processes of crop plants. In: Response of crops to limited water: Understanding and modeling water stress effects on plant growth processes, vol 1. ASA, CSSA, Madison, pp 301–355
Prasad PV, Djanaguiraman M, Perumal R, Ciampitti IA (2015) Impact of high temperature stress on floret fertility and individual grain weight of grain sorghum: sensitive stages and thresholds for temperature and duration. Front Plant Sci 6:820
Prasad PV, Bheemanahalli R, Jagadish SK (2017) Field crops and the fear of heat stress—opportunities, challenges and future directions. Field Crop Res 200:114–121
Qaseem MF, Qureshi R, Shaheen H (2019) Effects of pre-anthesis drought, heat and their combination on the growth, yield and physiology of diverse wheat (Triticum aestivum L.) genotypes varying in sensitivity to heat and drought stress. Sci Rep 9:1–12
Qin X, Zeevaart JA (1999) The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc Natl Acad Sci 96:15354–15361
Quan R, Shang M, Zhang H et al (2004) Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol J 2:477–486
Queitsch C, Hong S-W, Vierling E, Lindquist S (2000) Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12:479–492
Radhouane L (2008) Caractéristiques hydriques du mil (Pennisetum glaucum (L.) R. Br.) en présence de contraintes hydriques. C R Biol 331:206–214
Radhouane L (2009) La photosynthèse du mil (Pennisetum glaucum (L.) R. Br.) en présence de contrainte hydrique et saline. J Agric Environ Int Devel 103:185–200
Radhouane L (2013) Les échanges gazeux d’un écotype autochtone de mil (Pennisetum glaucum LR Br.) au cours d’un dessèchement hydrique. J Anim Plants Sci 2:2552–2566
Rebouillat J, Dievart A, Verdeil J-L et al (2009) Molecular genetics of rice root development. Rice 2:15–34
Reddy PS, Mallikarjuna G, Kaul T et al (2010) Molecular cloning and characterization of gene encoding for cytoplasmic Hsc70 from Pennisetum glaucum may play a protective role against abiotic stresses. Mol Genet Genomics 283:243–254
Reddy KS, Sekhar KM, Reddy AR (2017a) Genotypic variation in tolerance to drought stress is highly coordinated with hydraulic conductivity–photosynthesis interplay and aquaporin expression in field-grown mulberry (Morus spp.). Tree Physiol 37:926–937. https://doi.org/10.1093/treephys/tpx051
Reddy PS, Tharanya M, Sivasakthi K et al (2017b) Molecular cloning and expression analysis of aquaporin genes in pearl millet [Pennisetum glaucum (L) R. Br.] genotypes contrasting in their transpiration response to high vapour pressure deficits. Plant Sci 265:167–176
Riccardi F, Gazeau P, Jacquemot M-P et al (2004) Deciphering genetic variations of proteome responses to water deficit in maize leaves. Plant Physiol Biochem 42:1003–1011
Richards R, Passioura J (1989) A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Aust J Agr Res 40:943–950
Rizhsky L, Liang H, Mittler R (2002) The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 130:1143–1151
Rostamza M, Richards R, Watt M (2013) Response of millet and sorghum to a varying water supply around the primary and nodal roots. Ann Bot 112:439–446
Saidou AA, Mariac C, Luong V et al (2009) Association studies identify natural variation at PHYC linked to flowering time and morphological variation in pearl millet. Genetics 182(3):899–910
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25:163–171
Salack S, Muller B, Gaye AT (2011) Rain-based factors of high agricultural impacts over Senegal. Part I: integration of local to sub-regional trends and variability. Theor Appl Climatol 106:1–22
Salack S, Muller B, Gaye AT et al (2012a) Analyses multi-échelles des pauses pluviométriques au Niger et au Sénégal. Sécheresse 23:3–13. https://doi.org/10.1684/sec.2012.0335
Salack S, Sultan B, Oettli P et al (2012b) Representation of rainfall in regional climate models and application to millet yield estimations in Senegal. Science and changements planétaires/Sécheresse 23:14–23. https://doi.org/10.1684/sec.2012.0332
Sanchez A, Subudhi P, Rosenow D, Nguyen H (2002) Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor L. Moench). Plant Mol Biol 48:713–726
Sanders GJ, Arndt SK (2012) Osmotic adjustment under drought conditions. In: Aroca R (ed) Plant responses to drought stress: from morphological to molecular features. Springer, Berlin, pp 199–229
Sarieva G, Kenzhebaeva S, Lichtenthaler H (2010) Adaptation potential of photosynthesis in wheat cultivars with a capability of leaf rolling under high temperature conditions. Russ J Plant Physiol 57:28–36
Savin R, Nicolas ME (1996) Effects of short periods of drought and high temperature on grain growth and starch accumulation of two malting barley cultivars. Funct Plant Biol 23:201–210
Scharf K-D, Berberich T, Ebersberger I, Nover L (2012) The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim Biophys Acta 1819:104–119
Schittenhelm S, Schroetter S (2014) Comparison of drought tolerance of maize, sweet sorghum and sorghum-sudangrass hybrids. J Agron Crop Sci 200:46–53
Schramm F, Larkindale J, Kiehlmann E et al (2008) A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J 53:264–274
Sekhon H, Singh G, Sharma P, Bains T (2010) Water use efficiency under stress environments. In: Yadav S, Mc Neil D, Redden R et al (eds) Climate change and management of cool season grain legume crops. Springer, New York, pp 207–227
Serraj R, Hash CT, Rizvi SMH et al (2005) Recent advances in marker-assisted selection for drought tolerance in pearl millet. Plant Prod Sci 8:334–337. https://doi.org/10.1626/pps.8.334
Shah N, Paulsen G (2003) Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant Soil 257:219–226
Shangguan Z, Shao M, Dyckmans J (1999) Interaction of osmotic adjustment and photosynthesis in winter wheat under soil drought. J Plant Physiol 154:753–758
Sillmann J, Kharin VV, Zwiers F et al (2013) Climate extremes indices in the CMIP5 multimodel ensemble: Part 2. Future climate projections. J Geophys Res Atmos 118:2473–2493
Sinclair TR, Muchow RC (2001) System analysis of plant traits to increase grain yield on limited water supplies. Agron J 93:263–270
Slama A (2002) Étude comparative de la contribution des différentes parties du plant du blé dur dans la contribution du rendement en grains en irrigué et en conditions de déficit hydrique. Thèse de doctorat en biologie, faculté des sciences de Tunis
Slatyer R (1973) Effects of short periods of water stress on leaf photosynthesis. In: Slatyer R (ed) Plant response to climatic factors proceedings of theuppsala symposium. UNESCO, Paris, pp 271–276
Soegaard H, Boegh E (1995) Estimation of evapotranspiration from a millet crop in the Sahel combining sap flow, leaf area index and eddy correlation technique. J Hydrol 166:265–282
Solomon S, Manning M, Marquis M et al (eds) (2007) Climate change 2007-the physical science basis: Working group I, contribution to the fourth assessment report of the IPCC. Cambridge University Press, Cambridge
Steele K, Price A, Witcombe J et al (2013) QTLs associated with root traits increase yield in upland rice when transferred through marker-assisted selection. Theor Appl Genet 126:101–108
Steudle E (2001) The cohesion-tension mechanism and the acquisition of water by plant roots. Annu Rev Plant Biol 52:847–875
Stott PA, Gillett NP, Hegerl GC et al (2010) Detection and attribution of climate change: a regional perspective. Wiley Interdiscip Rev Clim Change 1:192–211
Sultan B, Gaetani M (2016) Agriculture in West Africa in the twenty-first century: climate change and impacts scenarios, and potential for adaptation. Front Plant Sci 7:1262
Sultan B, Roudier P, Quirion P et al (2013) Assessing climate change impacts on sorghum and millet yields in the Sudanian and Sahelian savannas of West Africa. Environ Res Lett 8(1):014040
Sun W, Van Montagu M, Verbruggen N (2002) Small heat shock proteins and stress tolerance in plants. Biochim Biophys Acta 1577:1–9
Takayuki Y, Hiroyuki K, Takashi M, Hiroshi M (2013) Simultaneous Transcriptome Analysis of Sorghum and Bipolaris sorghicola by Using RNA-seq in Combination with De Novo Transcriptome Assembly. PLoS ONE 8(4):e62460. https://doi.org/10.1371/journal.pone.0062460
Tardieu F, Reymond M, Hamard P et al (2000) Spatial distributions of expansion rate, cell division rate and cell size in maize leaves: a synthesis of the effects of soil water status, evaporative demand and temperature. J Exp Bot 51:1505–1514
Trenberth KE, Shea DJ (2005) Relationships between precipitation and surface temperature. Geophys Res Lett 32:L14703. https://doi.org/10.1029/2005GL022760
Trenberth KE, Jones PD, Ambenje P et al (2007) Observations. Surface and atmospheric climate change. In: Solomon S, Qin M, Manning Z et al (eds) Climate change 2007: the physical science basis. Cambridge University Press, Cambridge, pp 235–336
Tuteja N (2007) Abscisic acid and abiotic stress signaling. Plant Signal Behav 2:135–138
Ullah A, Ahmad A, Khaliq T, Akhtar J (2017) Recognizing production options for pearl millet in Pakistan under changing climate scenarios. J Integr Agric 16:762–773
Vadez V, Krishnamurthy L, Hash C et al (2011) Yield, transpiration efficiency, and water-use variations and their interrelationships in the sorghum reference collection. Crop Pasture Sci 62:645–655
Vadez V, Hash T, Kholova J (2012) II. 1.5 Phenotyping pearl millet for adaptation to drought. Front Physiol 3:386
Vadez V, Kholová J, Yadav RS, Hash CT (2013a) Small temporal differences in water uptake among varieties of pearl millet (Pennisetum glaucum (L.) R. Br.) are critical for grain yield under terminal drought. Plant and Soil 371:447–462
Vadez V, Kholova J, Zaman-Allah M, Belko N (2013b) Water: the most important ‘molecular’ component of water stress tolerance research. Funct Plant Biol 40:1310–1322
Vadez V, Kholova J, Medina S et al (2014) Transpiration efficiency: new insights into an old story. J Exp Bot 65:6141–6153
Vadez V, Choudhary S, Kholová J et al (2021) Transpiration efficiency: insights from comparisons of C4 cereal species. J Exp Bot 72:5221–5234
Varney GT, Canny MJ, Mccully ME (1991) The branch roots of Zea. I. First order branches, their number, sizes and division into classes. Ann Bot 67:357–364
Varshney RK, Shi C, Thudi M et al (2017) Pearl millet genome sequence provides a resource to improve agronomic traits in arid environments. Nat Biotechnol 35:969–976
Verslues PE, Sharma S (2010) Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book 8:1–23
Vigouroux Y, Cédric M, de Mita S et al (2011) Selection for earlier flowering crop associated with climatic variations in the Sahel. PloS One 6:1–9. https://doi.org/10.1371/journal.pone.0019563
Vijayalakshmi T, Varalaxmi Y, Jainender S et al (2012) Physiological and biochemical basis of water-deficit stress tolerance in pearl millet hybrid and parents. Am J Plant Sci 3(12):1730–1740
Wahid A (2007) Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J Plant Res 120:219–228
Wahid A, Close T (2007) Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol Plant 51:104–109
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wallace J, Lloyd C, Sivakumar M (1993) Measurements of soil, plant and total evaporation from millet in Niger. Agric For Meteorol 63:149–169
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Wang J, Vanga SK, Saxena R et al (2018) Effect of climate change on the yield of cereal crops: a review. Climate 6:41
Wasaya A, Zhang X, Fang Q, Yan Z (2018) Root phenotyping for drought tolerance: a review. Agronomy 8:24. https://doi.org/10.3390/agronomy8110241
Wasson AP, Richards R, Chatrath R et al (2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot 63:3485–3498
Watt M, Magee LJ, McCully ME (2008) Types, structure and potential for axial water flow in the deepest roots of field-grown cereals. New Phytol 178:135–146
Welcker C, Boussuge B, Bencivenni C et al (2007) Are source and sink strengths genetically linked in maize plants subjected to water deficit? A QTL study of the responses of leaf growth and of anthesis-silking interval to water deficit. J Exp Bot 58:339–349
Wilson J, Sanogo M, Nutsugah S et al (2008) Evaluation of pearl millet for yield and downy mildew resistance across seven countries in sub-Saharan Africa. Afr J Agric Res 3(5):371–378
Winkel T, Renno J-F, Payne W (1997) Effect of the timing of water deficit on growth, phenology and yield of pearl millet (Pennisetum glaucum (L.) R. Br.) grown in Sahelian conditions. J Exp Bot 48:1001–1009
Winkel T, Payne W, Renno J (2001) Ontogeny modifies the effects of water stress on stomatal control, leaf area duration and biomass partitioning of Pennisetum glaucum. New Phytol 149:71–82
Wu W, Ma B, Whalen JK (2018) Enhancing rapeseed tolerance to heat and drought stresses in a changing climate: perspectives for stress adaptation from root system architecture. Adv Agron 151:87–157
Xin Z, Aiken R, Burke J (2009) Genetic diversity of transpiration efficiency in sorghum. Field Crop Res 111:74–80
Xiong L, Wang R-G, Mao G, Koczan JM (2006) Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol 142:1065–1074
Xu Z, Zhou G (2005a) Effects of water stress and nocturnal temperature on carbon allocation in the perennial grass, Leymus chinensis. Physiol Plant 123:272–280
Xu Z-Z, Zhou G-S (2005b) Effects of water stress and high nocturnal temperature on photosynthesis and nitrogen level of a perennial grass Leymus chinensis. Plant and Soil 269:131–139
Xu ZZ, Zhou GS (2006) Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta 224:1080–1090
Xu Z, Zhou G, Shimizu H (2010) Plant responses to drought and rewatering. Plant Signal Behav 5:649–654
Yadav RS, Hash C, Bidinger FR et al (2002) Quantitative trait loci associated with traits determining grain and stover yield in pearl millet under terminal drought stress conditions. Theor Appl Genet 104:67–83
Yadav RS, Hash CT, Bidinger FR et al (2004) Genomic regions associated with grain yield and aspects of post-flowering drought tolerance in pearl millet across stress environments and tester background. Euphytica 136:265–277
Yadav A, Narwal M, Arya R (2010) Stability studies for seedling traits with supra-optimal temperature exposure at seedling stage in pearl millet [Pennisetum glaucum (L.) R. Br.]. Forage Res 32:65–70
Yadav OP, Rai KN (2013) Genetic improvement of pearl millet in India. Agric Res 2:275–292
Yadav A, Arya R, Singh M et al (2016) Heat tolerance in pearl millet: a review. Forage Res 42:65–81
Yadav OP, Singh DV, Vadez V et al (2017) Improving pearl millet for drought tolerance-retrospect and prospects. Indian J Genet Plant Breed 77:464–474. https://doi.org/10.5958/0975-6906.2017.00062.1
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic-and cold-stress-responsive promoters. Trends Plant Sci 10:88–94
Zegada-Lizarazu W, Zatta A, Monti A (2012) Water uptake efficiency and above-and belowground biomass development of sweet sorghum and maize under different water regimes. Plant Soil 351:47–60
Zhan A, Schneider H, Lynch JP (2015) Reduced lateral root branching density improves drought tolerance in maize. Plant Physiol 168:1603–1615
Zhang X, Wang T, Li C (2005) Different responses of two contrasting wheat genotypes to abscisic acid application. Biol Plant 49:613–616
Zhang A, Ji Y, Sun M et al (2021) Research on the drought tolerance mechanism of Pennisetum glaucum (L.) in the root during the seedling stage. BMC Genomics 22:1–14. https://doi.org/10.1186/S12864-021-07888-5
Zhu J-K (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Zscheischler J, Seneviratne SI (2017) Dependence of drivers affects risks associated with compound events. Sci Adv 3:e1700263
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sine, B. et al. (2024). Physiological and Molecular Bases of Drought and Heat Tolerance in Pearl Millet. In: Tonapi, V.A., Thirunavukkarasu, N., Gupta, S., Gangashetty, P.I., Yadav, O. (eds) Pearl Millet in the 21st Century. Springer, Singapore. https://doi.org/10.1007/978-981-99-5890-0_10
Download citation
DOI: https://doi.org/10.1007/978-981-99-5890-0_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-5889-4
Online ISBN: 978-981-99-5890-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)