Abstract
Probiotics are live microorganisms that enhance the health benefits of the host. They are capable of colonizing the gastrointestinal tract by surviving gastric and bile acids. It strengthens the host’s immunity and lowers the risk of urogenital infections, allergies, cancer, and other intestinal issues that are common among people. The mechanism of their action is through the production of antimicrobial substances, and increased adhesion to the intestinal mucosa. It has several immunomodulatory properties which enhance humoral and cell-mediated immunity. In this chapter, we will learn about the health benefits of probiotics and their potential therapeutic application.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Probiotics are live bacteria that provide health benefits to the host when administered in appropriate doses (FAO/WHO 2002). Probiotic bacteria are safe for human consumption and provide health benefits to the host, such as improving gut health, boosting immunity, reducing urogenital infections, allergies, cancer and other intestinal problems that affect a majority of the world’s population. Although there is limited data to support their health benefits in humans, probiotics are an essential component of functional foods. To maintain a healthy gut, specific beneficial bacterial strains must be introduced into the gastrointestinal tract (GIT) to prevent disease. In the early 1900s, Elle Metchnikoff hypothesized that beneficial bacteria may be administered to replace harmful microorganisms with healthy ones. In the 1960s, Lilly and Stillwell coined the phrase probiotic, which means ‘for life’. Probiotics, on the other hand, contain live microbial supplements that improve the microbial balance in the intestine of the host (Fuller 1989). Lactobacillus, Bifidobacterium, Escherichia coli, Enterococcus, Bacillus, Streptococcus and Saccharomyces are some of the most often used probiotic microbes.
Microorganisms present on the skin, in the mouth and in the gastrointestinal tract coexist with humans. The GIT, covering more than 400 square metres of surface area, has the highest concentration of commensal microorganisms. Probiotic bacteria present in the GIT and establish themselves rapidly after birth, are relatively stable throughout life and are essential for human homeostasis. Interactions between the intestinal microflora and the host during the establishment of the microbiota result in the evolution of a unique and distinct intestinal immune system. Probiotic foods account for 65% of the global functional food market, resulting in a wide range of probiotic food products available in supermarkets and health food stores. Probiotic supplements have been introduced to the market since they protect against infections, reduce lactose intolerance, lower blood cholesterol levels and activate the immune system. This has prompted more research into the identification of foods and food components that provide unique consumer benefits. Prebiotic, for example, is a non-digestible food component that benefits the body by activating one or more probiotic bacteria in the colon. When a product has both probiotics and prebiotics, it is referred to as a synbiotic.
The first probiotic to gain widespread clinical attention was Lactobacillus rhamnosus GG strain (LGG). It was discovered in 1985, and used in the dairy sector in the fermentation process. LGG boosts intestinal immunity by increasing the number of cells that secrete IgA and other immunoglobulins in the intestinal mucosa. It stimulates the local interferon release and facilitates antigen transport to underlying lymphoid cells, resulting in increased antigen uptake in Peyer’s patches. Bile, hydrochloric acid and pancreatic juice have no effect on it and it possesses anti-carcinogenic properties (Gupta and Garg 2009).
2 Probiotic Microorganisms and Their Characteristics
Lactobacillus and Bifidobacterium, two major gram-positive bacteria species, are widely utilized as probiotics. Other genera, such as Escherichia, Enterococcus, and Saccharomyces, have been promoted as probiotics, while safety concerns persist (Holzapfel et al. 2001). Probiotics are strain-specific, therapeutic benefits attributed to one strain cannot be expected to be delivered by another, even if they are from the same species.
According to the Food and Agriculture Organization (FAO) and the World Health Organization (WHO), probiotics have the following characteristics (FAO/WHO, 2001):
-
1.
It must be able to survive passage through the gut by withstanding gastric acids and bile exposure.
-
2.
It should be able to survive both conjugated and deconjugated bile acids, as well as low pH and high bile acid concentrations.
-
3.
It should be able to colonize and grow in the gastrointestinal tract.
-
4.
The immune system should be able to withstand it.
-
5.
It must be free of any infectious, allergic or mutagenic/carcinogenic properties.
-
6.
It must have the administration sites that are desired.
-
7.
They must be safe as well as effective.
-
8.
It should remain effective and potent for the duration of the product’s shelf life.
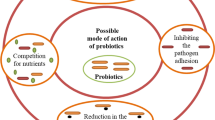
3 Mechanism of Action
Probiotics compete with pathogens for nutrition by inhibiting bacterial growth, as a result, infections in the gastrointestinal tract become less common. Probiotic protective mechanisms include epithelial barrier improvement, immunomodulatory properties, antibacterial activity and competitive adhesion to the intestinal mucosa (Bermudez-Brito et al. 2012). A recent study finds that introducing probiotics to the intestine may prevent the spread of potential infections by lowering luminal pH, producing bacteriocins, releasing antimicrobial defensins in the intestinal crypts and preventing bacterial adhesion to epithelial cells (Plaza-Diaz et al. 2019).
3.1 Production of Antimicrobial Substances
A good example of probiotic antibacterial action is the effect of Lactobacillus species on Helicobacter pylori infection of the gastric mucosa (Homan and Orel 2015). Acid-resistant Lactobacillus species and commensal organisms persist longer in the stomach than other bacteria. Lactobacillus acidophilus produces two bacteriocin compounds: lactacin B and acidolin, which may interfere with H. pylori infection or inflammation. An in vitro study found that lactacin and acidolin inhibit the growth of H. pylori and enteropathogenic bacteria, respectively. De-conjugated bile acids, which are bile salt derivatives produced by probiotic bacteria, have better antibacterial properties than the bile salts produced by the human host (Ridlon et al. 2016). Gut bacteria produce a wide range of health-promoting fatty acids (Conlon and Bird 2014). Bifidobacterium and Lactobacillus in the intestine have been found to produce conjugated linoleic acid (CLA), a potent anti-carcinogenic agent (Den Hartigh 2019). CLA-producing L. plantarum was reported to have an anti-obesity effect in rats having diet-induced obesity (Lee et al. 2007). In mouse models, CLA-producing Bifidobacterium and Lactobacilli were shown to regulate the fatty acid content of the host’s liver and adipose tissue (Rosberg-Cody et al. 2011). Lactobacillus produces fungicidal compounds like benzoic acid, methyl hydantoin, mevalolactone and short-chain fatty acids. Lactobacillus coryniformis, for example, produces antifungal proteinaceous compounds (Magnusson and Schnürer 2001).
3.2 Increased Adhesion to the Intestinal Mucosa
Inflammatory bowel disease is caused whenever a pathogen or foodborne antigen breaches the intestinal barrier and penetrates the intestinal sub-mucosa. Hence, the effectiveness of the probiotics relies on their ability to adhere and colonize the human stomach, which in turn strengthens the host immune system (Chichlowski and Hale 2008). Many Lactobacillus bacteria maintain intestinal barrier integrity by regulating and increasing the expression of genes, such as E-cadherin/β-catenin, involved in tight junction signalling (Hummel et al. 2012). Several lactobacillus proteins have been found to improve surface and mucosal adhesion by integrating with glycoproteins secreted by intestinal epithelial cells, such as mucin, resulting in the competitive exclusion of mucosal pathogens (Wang et al. 2016). Attachment to the intestinal mucosa is required for colonization in host–probiotic interaction. Lactobacillus possesses a mannose-specific adhesion mechanism that allows it to adhere to human intestinal epithelial cells. When the probiotic adheres to the cell, it triggers a cascade of biological events, including the production of cytokines and chemokines. As a result, secondary functions in the host, such as activating mucosal and systemic immune responses, were activated (Plaza-Díaz et al. 2020). Probiotics increase gut mucin levels, which inhibit pathogen binding and induce epithelial cells to release defensins. These small proteins help to maintain the integrity of the intestinal barrier by exhibiting antibacterial, antifungal and antiviral properties (Gong et al. 2021).
3.3 Probiotics and the Immune System
Probiotic bacteria have long been known to have immunomodulating properties. These bacteria can communicate with epithelial cells, dendritic cells (DCS), monocytes/macrophages and lymphocytes. It can regulate the immune system by increasing endogenous host defense mechanisms, including humoral, cellular and non-specific immunity (Belkaid and Hand 2014). According to a recent study, probiotics improve the natural killer cell activity and alter non-specific host defense mechanisms in the elderly (Aziz and Bonavida 2016). Probiotics play important roles in mucus production, macrophage activation, stimulation of secretory IgA and neutrophil, suppression of inflammatory cytokines and stimulation of peripheral immunoglobulins (Cristofori et al. 2021). Probiotics have different immune-regulatory effects in healthy and diseased persons (Yan and Polk 2011). Probiotics enhance phagocytosis in healthy persons, while they reduce it in allergy patients (Huang et al. 2022).
4 Health Benefits of Probiotics
Probiotics promote gastrointestinal health and immunity; prevent urogenital infections, allergies, cancer and certain bowel disorders (Binnendijk and Rijkers 2013). Several well-characterized Lactobacillus and Bifidobacterium strains are now available for human use to prevent and manage gastrointestinal (GI) infections. Probiotics provide a number of health benefits, including:
-
1.
Improved gut health through microbiota control and immune system stimulation and development.
-
2.
Increasing nutrient bioavailability by synthesizing and improving their absorption.
-
3.
Alleviating lactose intolerance symptoms and lowering the risk of certain diseases.
-
4.
It restores the normal gut flora, improving gut barrier function.
4.1 Disease Prevention and Treatment
Probiotic LGG, Bifidobacterium lactis BB-12 and Lactobacillus reuteri SD2222 were used to prevent and treat rotavirus diarrhoea in children (Shornikova et al. 1997). In children, the usage of LGG resulted in a significant reduction in diarrhoea symptoms as well as a shorter hospital stay (Li et al. 2019). For the treatment of rotavirus diarrhoea, LGG in milk or capsule form was used as an adjuvant to oral rehydration therapy (Guandalini et al. 2000).
Necrotizing enterocolitis is a leading cause of morbidity and mortality in premature infants. In premature babies, a combination of two probiotic strains, Lactobacillus acidophilus and Bifidobacterium infantis, was reported to reduce the risk of necrotizing enterocolitis and death (Patel and Underwood 2018).
Probiotics play an effective role in the prevention and treatment of Helicobacter pylori infection and other inflammatory bowel diseases, such as Crohn’s disease and irritable bowel syndrome (Verna and Lucak 2010). Intestinal microbiota plays a critical role in the pathogenesis of bowel inflammatory disease, and the use of a combination probiotic strain proves effective in alleviating the symptoms (Hold et al. 2014)
Probiotics reduce cholesterol levels by deconjugating the bile salt. The bile salt is deconjugated, which makes it less soluble and absorbable in the intestines and excreted in the stool. Cholesterol is then used to make new bile acids, reducing blood cholesterol levels in a homeostatic reaction. In vitro studies proved that Lactobacillus gasseric reduce cholesterol in the laboratory media by adhering to its cellular surfaces (Kumar et al. 2012). Probiotics, both living and dead, work in a similar way to reduce cholesterol levels. Growing cells, on the other hand, eliminated more cholesterol than dead cells. During the developmental phase, some probiotics Lactococcus strain lower cholesterol levels in the blood through the absorption and integration of cholesterol into their cellular membranes. Cholesterol incorporation into the cellular membrane benefits the bacterial strain by improving the membrane strength, which increases cellular resistance to lysis (Ooi and Liong 2010).
The presence of Neisseria gonorrhoeae and Chlamydia trachomatis is indicated by the absence of Lactobacilli in the vagina (Wiesenfeld et al. 2003). Direct instillation of Lactobacilli resulted in a significant reduction of Escherichia coli growth, as well as a reduction in the severity of inflammation and risk of recurrent urinary tract infection (Asahara et al. 2001). Probiotic Lactobacilli taken orally have been shown to reduce the incidence of urinary tract infection, bacterial vaginosis and candidiasis (Reid et al. 2003).
5 Role of Probiotics in Healthy Individuals
Recent studies have shown that probiotics can modestly decrease the incidence and duration of common upper respiratory tract infections in children and adults (King et al. 2014). People who have a low amount of the intestinal enzyme i8-galactosidase, the disaccharide lactose can cause severe intestinal irritation, including bloating, gas and stomach pain. It restricts the use of calcium-rich dairy products at a time when the elderly are in desperate need of them owing to bone loss. Lactose digestion in lactose-intolerant people can be improved by probiotics, with the majority of data suggesting that lactose taken in yoghurt containing alive cultures is more easily digested than lactose consumed without live cultures (Oak and Jha 2019). Lactobacilli produce lactase, which hydrolyses lactose in dairy products to glucose and galactose during fermentation, potentially benefiting lactose-intolerant persons (Saqib et al. 2017).
The animals were given probiotics to help them gain weight on a liquid diet of milk, yoghurt or milk fermented with S. thermophilus. The animal gut microbiota was altered, resulting in a significant increase in weight gain (Goldin 1998).
The composition of the microbiota can influence the development of mucosal and systemic immunity. Probiotics colonize the oral cavity naturally, reducing the negative effects of bacterial infections, while also improving the inflammatory cytokine system. Plaque growth can be inhibited by neutralizing free electrons, affecting the systemic immune system and controlling mucosal permeability. Lactobacillus bulgaricus, L. acidophilus, L. casei, L. Helveticus and L. lactis are the most commonly used oral probiotics (Parul et al. 2020). Probiotics that stabilize the oral flora are used to treat gingivitis (Gupta 2011). Acidic probiotic bacteria like Lactobacilli, Streptococcus and Bifidobacterium produce antimicrobial substances that suppress pathogens by aggregation, toxic by-product release and competition for substrates (Markowiak and Śliżewska 2017). Probiotics contribute to the treatment of periodontitis by maintaining the oral microbiota. The majority of Lactobacillus strains inhibit the spread of dermabrasion periodontal diseases (Haukioja 2010). Recent studies have shown that the use of beneficial bacteria in addition to root resurfacing inhibits the re-colonization of periodontal pathogens, which reduces overall pocket depth and improve clinical adhesion (Retamal-Valdes et al. 2021).
Microbiota in the intestine produce enzymes such as glycosidase, nitroreductase, azoreductase and β-glucuronidase, which control the onset of carcinogenesis and protect against carcinogenic activity (Molska and Reguła 2019). L. casei strain shirota was found to decrease the recurrence rate of superficial bladder cancer, indicating that probiotic consumption had a positive influence on cancer risk (Mutoh et al. 2020).
The composition of a mother’s vaginal microbiota affects a baby’s asthmatic condition. The administration of LGG to pregnant women and newborns resulted in a significant reduction in the prevalence of early atopic illness in neonates (Sestito et al. 2020).
The administration of LGG increased the levels of rotavirus-specific IgM-secreting cells than the control and placebo groups (Maidens et al. 2013). Similarly, the LGG group had significantly increased the IgA and IgM levels in the control group. As a result, LGG is used as an adjuvant in a rotavirus vaccination for children.
Acute liver diseases sometimes lead to liver failure. Probiotics significantly reduce acute liver injury by up-regulating the tight junction protein gene, increasing microbial flora, lowering bacterial translocation, minimizing endotoxin infiltration and reducing pro-inflammatory cytokines (Xu et al. 2021).
6 Drawbacks
Probiotics are living microorganisms that can infect the host. The risk of probiotic-induced sepsis should be evaluated against the risk of pathogenic-bacterial-induced sepsis. Future studies should validate the benefits of probiotics such as the selection of a suitable probiotic agent, dosage standardization, a complete understanding of their therapeutic effects and long-term storage. Although more promising potential health effects of probiotics are being observed in ongoing research, probiotic strains should be examined for antibiotic susceptibility patterns, toxin production, metabolic and haemolytic activities, infectivity in immunocompromised animal models and adverse events in humans.
References
Asahara T, Nomoto K, Watanuki M, Yokokura T (2001) Antimicrobial activity of intraurethrally administered probiotic Lactobacillus casei in a murine model of Escherichia coli urinary tract infection. Antimicrob Agents Chemother 45(6):1751–1760
Aziz N, Bonavida B (2016) Activation of natural killer cells by probiotics. For Immunopathol Dis Therap 7(1–2):41–55
Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation. Cell 157(1):121–141
Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A (2012) Probiotic mechanisms of action. Ann Nutr Metab 61(2):160–174
Binnendijk KH, Rijkers GT (2013) What is a health benefit? An evaluation of EFSA opinions on health benefits with reference to probiotics. Benef Microbes 4(3):223–230
Chichlowski M, Hale LP (2008) Bacterial-mucosal interactions in inflammatory bowel disease: an alliance gone bad. Am J Physiol Gastrointest Liver Physiol 295(6):G1139–G1149
Conlon MA, Bird AR (2014) The impact of diet and lifestyle on gut microbiota and human health. Nutrients 7(1):17–44
Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R (2021) Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol 12:578386
den Hartigh LJ (2019) Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: a review of pre-clinical and human trials with current perspectives. Nutrients 11(2):370
FAO/WHO (2001) Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Report from FAO/WHO Expert Consultation, pp 1–4
Fuller R (1989) Probiotics in man and animals. J Appl Bacteriol 66(5):365–378
Goldin BR (1998) Health benefits of probiotics. Br J Nutr 80(4):S203–S207
Gong T, Fu J, Shi L, Chen X, Zong X (2021) Antimicrobial peptides in gut health: a review. Front Nutr 8:751010
Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, Kolacek S, Massar K, Micetic-Turk D, Papadopoulou A, de Sousa JS, Sandhu B, Szajewska H, Weizman Z (2000) Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr 30(1):54–60
Gupta G (2011) Probiotics and periodontal health. J Med Life 4(4):387–394
Gupta V, Garg R (2009) Probiotics. Indian J Med Microbiol 27(3):202–209
Haukioja A (2010) Probiotics and oral health. Eur J Dent 4(3):348–355
Hold GL, Smith M, Grange C, Watt ER, El-Omar EM, Mukhopadhya I (2014) Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol 20(5):1192–1210
Holzapfel WH, Haberer P, Geisen R, Björkroth J, Schillinger U (2001) Taxonomy and important features of probiotic microorganisms in food and nutrition. Am J Clin Nutr 73(2 Suppl):365S–373S. https://doi.org/10.1093/ajcn/73.2.365s. PMID: 11157343
Homan M, Orel R (2015) Are probiotics useful in Helicobacter pylori eradication? World J Gastroenterol 21(37):10644–10653
Huang J, Zhang J, Wang X, Jin Z, Zhang P, Su H, Sun X (2022) Effect of probiotics on respiratory tract allergic disease and gut microbiota. Front Nutr 9:821900
Hummel S, Veltman K, Cichon C, Sonnenborn U, Schmidt MA (2012) Differential targeting of the E-cadherin/β-catenin complex by gram-positive probiotic lactobacilli improves epithelial barrier function. Appl Environ Microbiol 78(4):1140–1147
Joint FAO/WHO Working Group and Joint FAO/WHO Working Group (2002) Guidelines for the evaluation of probiotics in food. World Health Organization/Food and Agriculture Organization, London/Ottawa
King S, Glanville J, Sanders ME, Fitzgerald A, Varley D (2014) Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr 112(1):41–54
Kumar M, Nagpal R, Kumar R, Hemalatha R, Verma V, Kumar A, Chakraborty C, Singh B, Marotta F, Jain S, Yadav H (2012) Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res 2012:902917
Lee K, Paek K, Lee HY, Park JH, Lee Y (2007) Antiobesity effect of trans-10,cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J Appl Microbiol 103(4):1140–1146
Li YT, Xu H, Ye JZ, Wu WR, Shi D, Fang DQ, Liu Y, Li LJ (2019) Efficacy of Lactobacillus rhamnosus GG in treatment of acute pediatric diarrhea: a systematic review with meta-analysis. World J Gastroenterol 25(33):4999–5016
Magnusson J, Schnürer J (2001) Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl Environ Microbiol 67(1):1–5
Maidens C, Childs C, Przemska A, Dayel IB, Yaqoob P (2013) Modulation of vaccine response by concomitant probiotic administration. Br J Clin Pharmacol 75(3):663–670
Markowiak P, Śliżewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9(9):1021
Molska M, Reguła J (2019) Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients 11(10):2453
Mutoh M, Yoshimura K, Fujii G, Nakamura T, Takeshita T, Wakabayashi K, Sakai T, Ishikawa H (2020) Very long-term treatment with a Lactobacillus probiotic preparation, Lactobacillus casei strain shirota, suppresses weight loss in the elderly. Nutrients 12(6):1599
Oak SJ, Jha R (2019) The effects of probiotics in lactose intolerance: a systematic review. Crit Rev Food Sci Nutr 59(11):1675–1683
Ooi LG, Liong MT (2010) Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci 11(6):2499–2522
Parul C, Renuka D, Anuradha S, Neeru B, Mahesh SD (2020) Critical appraisal of the effects of probiotics on oral health. J Funct Foods 70:103985
Patel RM, Underwood MA (2018) Probiotics and necrotizing enterocolitis. Semin Pediatr Surg 27(1):39–46
Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A (2019) Mechanisms of action of probiotics. Adv Nutr 10(Suppl 1):S49–S66
Plaza-Díaz J, Solís-Urra P, Rodríguez-Rodríguez F, Olivares-Arancibia J, Navarro-Oliveros M, Abadía-Molina F, Álvarez-Mercado AI (2020) The gut barrier, intestinal microbiota, and liver disease: molecular mechanisms and strategies to manage. Int J Mol Sci 21(21):8351
Reid G, Jass J, Sebulsky MT, McCormick JK (2003) Potential uses of probiotics in clinical practice. Clin Microbiol Rev 16(4):658–672
Retamal-Valdes B, Teughels W, Oliveira LM, da Silva RN, Fritoli A, Gomes P, Soares GMS, Temporão N, Furquim CP, Duarte PM, Doyle H, Faveri M, Figueiredo LC, Feres M (2021) Clinical, microbiological, and immunological effects of systemic probiotics in periodontal treatment: study protocol for a randomized controlled trial. Trials 22(1):283
Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB (2016) Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 7(1):22–39
Rosberg-Cody E, Stanton C, O’Mahony L, Wall R, Shanahan F, Quigley EM, Fitzgerald GF, Ross RP (2011) Recombinant lactobacilli expressing linoleic acid isomerase can modulate the fatty acid composition of host adipose tissue in mice. Microbiology (Reading) 157(Pt 2):609–615
Saqib S, Akram A, Halim SA, Tassaduq R (2017) Sources of β-galactosidase and its applications in food industry. 3 Biotech 7(1):79. https://doi.org/10.1007/s13205-017-0645-5
Sestito S, D’Auria E, Baldassarre ME, Salvatore S, Tallarico V, Stefanelli E, Tarsitano F, Concolino D, Pensabene L (2020) The role of prebiotics and probiotics in prevention of allergic diseases in infants. Front Pediatr 8:583946
Shornikova AV, Casas IA, Mykkänen H, Salo E, Vesikari T (1997) Bacteriotherapy with Lactobacillus reuteri in rotavirus gastroenteritis. Pediatr Infect Dis J 16(12):1103–1107
Verna EC, Lucak S (2010) Use of probiotics in gastrointestinal disorders: what to recommend? Ther Adv Gastroenterol 3(5):307–319
Wang G, Xia Y, Song X, Ai L (2016) Common non-classically secreted bacterial proteins with experimental evidence. Curr Microbiol 72(1):102–111
Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL (2003) Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis 36(5):663–668
Xu S, Zhao M, Wang Q, Xu Z, Pan B, Xue Y, Dai Z, Wang S, Xue Z, Wang F, Xu C (2021) Effectiveness of probiotics and prebiotics against acute liver injury: a meta-analysis. Front Med (Lausanne) 8:739337
Yan F, Polk DB (2011) Probiotics and immune health. Curr Opin Gastroenterol 27(6):496–501
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ganapathy, Y., Muthusamy Sridhar, N., Dhandapani, P. (2023). Probiotics: A Healthy Treasure. In: Sobti, R., Kuhad, R.C., Lal, R., Rishi, P. (eds) Role of Microbes in Sustainable Development. Springer, Singapore. https://doi.org/10.1007/978-981-99-3126-2_4
Download citation
DOI: https://doi.org/10.1007/978-981-99-3126-2_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-3125-5
Online ISBN: 978-981-99-3126-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)