Abstract
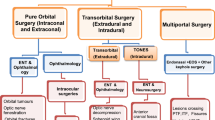
Endoscopic transorbital approach (ETOA) to the skull base has gained popularity among skull base surgeons over the last decade to access orbital and intracranial lesions. Given its minimal morbidity, better cosmetic results, and minimal brain retraction, it provided an alternative minimally invasive approach to resecting orbital and intracranial lesions. Kris Moe et al. first described and published in 2010 (Moe KS et al. Oper Neurosurg 67:ons16–ons28, 2010). It serves to provide a distinct corridor for more laterally placed anterior skull base lesions or those which cross neurovascular structures that cannot be effectively addressed with endonasal approaches. The surgery can be performed with or without orbitotomy to increase surgical freedom for the deeply seated intracranial lesions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
With the development of microscopic and endoscopic instruments for use in neurosurgery, neurosurgeons are attempting to reduce morbidity and invasiveness during skull base procedures. Endoscopic transorbital approach (ETOA) provides a direct anterior surgical option through, which a corridor can be utilized laterally to the cavernous segment of the internal carotid artery (ICA) and cavernous sinus. This technique represents a minimally invasive and versatile means of addressing deep-seated skull base lesions. Kris Moe et al. initially introduced this method in June 2007 at the Pacific Coast Otolaryngology-Ophthalmology Society Annual Meeting, with his first case series being published 3 years later [1]. The aim is to give an additional path for tackling anterior skull base lesions or those that extend across neurovascular structures, which cannot be reached by endonasal surgeries alone. It is estimated that the central portion of the ventral anterior cranial fossa bounded laterally by orbits occupies around 20% of the anterior skull base, which is accessible via the endonasal route without crossing critical neurovascular structures [1]. With its minimal bone removal techniques and cosmetic advantages ETOA with the application of modern-day illumination and magnification capabilities via microscopic and endoscopic tools prove advantageous.
2 Surgical Methods
2.1 Step 1: Skin Incision
The skin incision is designed and created jointly with oculoplastic surgeons for optimal planning and minimizing wound complications. The orbit can be using superior, inferior, lateral, and medial approaches; an example of a superior lid crease incision is illustrated in Fig. 23.1a. Upon completion of this step, division of the orbicularis oculi muscle and stripping of the periosteum (Fig. 23.1b) ensue. Lateral orbital rim can be removed to increase the working angles and surgical freedom up to sixfold from our investigational studies (Fig. 23.1c).
(a–f) (reprinted with permission, original from the last author): (a) Left lid crease incision. (b) Periosteum stripped and exposure of superolateral orbital rim. (c) Periorbita stripped to reach superior orbital fissure (SOF). (d) View after stripping of periobita from greater sphenoid wing (GSW) with exposure of superior orbital fissure (SOF). (e) Drilling of GSW to expose temporal dura (TD). (f) Temporal dura was elevated from middle cranial fossa floor. (reprinted with permission)
2.2 Step 2: Drilling of Greater Sphenoid Wing
The high-speed drill with a self-irrigation system was utilized to remove the greater sphenoid wing following the introduction of the endoscope. Maintaining copious irrigation throughout this process is vital in order to avoid thermal injuries to the orbital contents. It was shown that orbital compression of more than 1.5 cm was associated with a dramatic increase in intraocular pressure [2]. After the sphenoid wing has been removed, the meningo-orbital band is exposed and divided, thus establishing access to both the anterior and middle cranial fossa to unlock the anterior and middle cranial fossa (Fig. 23.1d–f).
2.3 Step 3: Extradural Dissection-Peeling of the Cavernous Sinus
The dura mater of the anterior and middle cranial fossa were separated, and the lateral wall of the cavernous sinus is then exposed via an extradural approach (Fig. 23.1f). This technique is analogous to extradural peeling of the cavernous sinus wall during craniotomy. The anteromedial triangle, created between the ophthalmic division (V1) and maxillary division (V2) of the trigeminal nerve, and anterolateral triangle formed between V2 and mandibular division (V3), can be identified (Fig. 23.2b). These two triangles are vital in guiding entrance into adjacent anatomical spaces such as petrous apex, infratemporal fossa, and cavernous sinus (Fig. 23.2a–d). Figure 23.2 shows the dissection via the left endoscopic transorbital approach.
(a–d) (reprinted with permission, original from the last author): Left endoscopic transorbital view, with 30-degree endoscope. (a) Periorbita (PO) and temporal dura (TD) were split with dissector to expose the anterior cavernous sinus wall. (b) Anteromedial (Ant Med) and anterolateral (Ant Lat) triangles, which were bounded by ophthalmic (V1), maxillary (V2) and mandibular (V3) divisions of the trigeminal nerves were exposed. V2 exited via the foramen rotundum (FR) and V3 exited via the foramen ovale (FO). (c) Exposure of cavernous sinus content between V1 and V2, with temporal dura (TD) elevated. Abducens nerve (V1) travelled lateral to the cavernous segment of internal carotid artery (ICA(Ca)). (d) Exposure of infratemporal fossa between V2 and V3, the anterolateral triangle. Temporal dura was further elevated till Meckel’s cave (MC) was seen. A pneumatized lateral recess of sphenoid sinus (Sph sinus) can be identified. Vidian nerve (Vidian n.) travelled superolaterally and served as a landmark to identify the petrous ICA and laceral segment of ICA (ICA Lac). Pterygoid muscles were removed to expose the Eustachian tube (Eus tube), sphenopalatine artery (sphenopalatine a), and lateral pterygoid plate (lat pterygoid plate)
3 Indications
The two most commonly described indications include repairing of cerebrospinal fluid (CSF) leaks and excision of skull base tumors.
The occurrence of iatrogenic and traumatic cerebrospinal fluid (CSF) leaks is a common cause for repair via a transorbital route. In most cases, the leak site was determined to be in the anterior cranial fossa, successfully addressed endoscopically from a transorbital approach [3].
For tumor excision, the most common indications are excision of spheno-orbital meningiomas and middle fossa schwannomas. The majority of the surgeries utilized only a transorbital approach while combined transorbital and endonasal approaches were used in some of the cases. The typical amount of time spent in the hospital afterward was approximately 3 days. Other indications described include cavernous hemangioma, intracranial abscesses, fibrous dysplasia, frontal mucocele, and fibroxanthoma [3, 4].
In our experience of over 30 cases, ETOA was used, either alone or in adjunct to other routes, to excise orbital tumor or vascular malformation, spheno-orbital meningiomata, trigeminal schwannomas, and infratemporal fossa tumors. Dura defects were repaired using artificial dura substitute and tissue glue. Abdominal fat graft was also used to repair large dura defects. We so far have not encountered any cases of CSF leakage nor pseudomeningocele.
Kong et al. recently conducted a case series to compare the efficacy of endoscopic transorbital approach (ETOA) and endoscopic transorbital approach combined with lateral orbitotomy in spheno-orbital meningioma resections. The results showed that ETOA with lateral orbitotomy had a higher gross total excision rate than ETOA alone. Our experience also suggests that this improved rate could be attributed to the improved surgical freedom from lateral orbitotomy, which can increase the surgical freedom from threefold to sixfold depending on the level of removal.
4 Complications
A systematic review of (ETOA) conducted by the Barrow Neurological Institute reported a complication rate of 13%, with permanent proptosis in one case [3]. CSF leaks were the most frequent complication, with most being transient. Other reported complications included diplopia, facial numbness, ptosis, levator muscle dysfunction, meningitis, periorbital hematoma, epiphora, superficial surgical site infection, and orbital pseudomeningocele [5].
5 Surgical Outcomes
Endoscopic transorbital approach (ETOA) has been demonstrated to be quite successful in treating cerebrospinal fluid leaks, ranging in success rate from 83 to 100%, with a recurrence rate of 7%. On the other hand, the rate of gross total excision of tumors surgically managed by this method is approximately 70%. Generally, patients presented with neurological deficits such as impaired visual acuity, ptosis, and reduced extraocular eye movement (EOM); most of these deficits were ameliorated postoperatively [3].
6 Discussion
Since its first description, the transorbital approach for endoscopic transorbital cranial surgery (ETOA) has been limitedly evaluated in contrast to traditional approaches such as craniotomies. Most existing studies are composed primarily of anatomical studies and case series. In 2020, our center initiated the utilization of this method; close collaborations between neurosurgeons and oculoplastic surgeons enabled a better aesthetic outcome through a minimal incision along natural creases of the skin. Satisfactory proptosis correction also provides good cosmetic outcome. Compared to craniotomies, there is no risk of temporalis muscle atrophy or injury to the facial nerve’s frontal branch, and lower rates of cerebrospinal fluid leak than reported via the endonasal route. Moreover, less blood loss and quicker postoperative recovery is possible due to the minimal amount of brain retraction during surgery.
ETOA has the capability to be combined with other operative pathways, including endonasal and transoral routes. Through operating along multiple passages, ETOA provides an excellent view of important neurovascular structures using multi-angled endoscopes. Important cranial nerves and great vessels can be protected to facilitate the removal of lesions via ETOA alone, or in combination with other endoscopic corridors [6, 7].
Cadaveric dissection and the formation of a multidisciplinary surgical team are important components for the successful development of endoscopic transorbital approaches. While this surgical method may be unfamiliar to neurosurgeons and skull base, cadaveric dissection and anatomical studies facilitate a solid understanding of operative anatomy. Furthermore, oculoplastic surgeons and otolaryngologists contribute to mitigating morbidities and enhancing safety when treating complex head and neck tumors, to provide the best care and outcome to the patients.
Case 1
A 64-year-old Chinese woman exhibited decreased vision in her right eye (visual acuity of 0.6) with a relative afferent pupillary defect (RAPD) [8]. Fundoscopic examination indicated no swelling of the right optic disc, and automated visual field testing showed a deficit affecting the superior and infratemporal regions. Imaging with optical coherence tomography revealed subtle thinning of the retinal nerve fiber layer on the right side. Magnetic resonance imaging uncovered an oval mass that was hypointense on T1-weighted images and hyperintense on T2-weighted images with gradual enhancement, which was compatible with cavernous hemangioma.
Endoscopic transorbital excision of the tumor was performed with an indocyanine green (ICG) -assisted endoscope, through a lateral skin crease incision. A crescent-shaped superolateral orbital rim was removed to gain more surgical freedom. Periorbita was stripped to locate the superior orbital fissure. The meningo-orbital band was divided, and the greater wing of sphenoid bone was removed using a 3 mm high-speed diamond burr under irrigation. Lateral rectus muscle showed a rapid enhancement at 30 s after venous injection of ICG, while the lesion demonstrated delayed enhancement at 90 s. The tumor was then dissected away from the lateral rectus and the superior division of the oculomotor nerve, after which it was removed in an en bloc fashion. The supraorbital rim was replaced using two mini plates. Postoperatively, the patient enjoyed a good recovery, with right eye visual acuity of 0.8 and resolution of relative afferent pupillary defect (Fig. 23.3a–f).
(a): Contrast enhanced T1-weighted axial scan of right orbit showed a right orbital apex lesion with heterogenous enhancement, (b): Endoscopic view of meningio-orbital band at superior orbital fissure after stripping the periorbita from the sphenoid wing. (c): Indocyanine green (ICG) was injected showing early enhancement of the lateral rectus muscle at around 30 s. (d) Delayed ICG enhancement of cavernous hemangioma at around 1 min 30 s help to differentiate the cavernous hemangioma from the lateral rectus muscle. (e) The cavernous hemangioma was dissected from superior division of third nerve. (f) Lateral orbitotomy was reconstructed with miniplates
Case 2
A 55-year-old man presented with progressive right eye proptosis. There is no diplopia with full extraocular muscle movement. Preoperative visual acuity of the right eye was 20/30. Serial MRI of the orbit showed an enlarging right intraconal lesion. Endoscopic transorbital surgery was performed to remove the lesion. Lateral canthotomy was performed, followed by a superomedial forniceal incision. Orbital fat was dissected away to expose the lesion. ICG was used, showing delayed enhancement at around 1 min 30 s of the lesion. The lesion was removed en bloc with a cryoprobe assisting the dissection. The advantages of using a cryoprobe are shrinking the lesion and attaching to the lesion firmly to facilitate dissection. The wound was closed by oculoplastic surgeons. Postoperative visual acuity was 1.0, with a resolution of proptosis (Fig. 23.4a–h).
(a) Coronal T1-weighted MRI showed a right intraconal lesion superior to optic nerve. (b) Axial T2-weighted MRI of the lesion. (c) Lateral canthotomy. (d) Superomedial forniceal incision(dotted line). (e) Exposure of the cavernous hemangioma after division of fat. (f) Delayed enhancement of the lesion (outline by the blue circle) helped to differentiate it from the surrounding. (g) Cryoprobe-assisted removal of the cavernous hemangioma (h) Excellent cosmetic outcome of the patient with resolution of proptosis
7 Conclusion
ETOA offered a direct anterior approach to the orbital apex and skull base pathologies lateral to the cavernous sinus and at the infratemporal fossa. Clinical outcomes with tumor excision and CSF leak repair are satisfactory, with acceptable transient morbidities. This minimally invasive approach also opens up potential for biportal or multiportal endoscopic skull base surgeries.
References
Moe KS, Bergeron CM, Ellenbogen RG. Transorbital neuroendoscopic surgery. Oper Neurosurg. 2010;67(3):ons16–28. https://doi.org/10.1227/01.neu.0000373431.08464.43.
Kim W, Moon JH, Kim EH, Hong CK, Han J, Hong JB. Optimization of orbital retraction during endoscopic transorbital approach via quantitative measurement of the intraocular pressure—[SevEN 006]. BMC Ophthalmol. 2021;21(1):76. https://doi.org/10.1186/s12886-021-01834-5.
Houlihan LM, Staudinger Knoll AJ, Kakodkar P, Zhao X, O’Sullivan MG, Lawton MT, Preul MC. Transorbital neuroendoscopic surgery as a mainstream neurosurgical corridor: a systematic review. World Neurosurg. 2021;152:167–179.e4. https://doi.org/10.1016/j.wneu.2021.04.104.
Ramakrishna R, Kim LJ, Bly RA, Moe K, Ferreira M. Transorbital neuroendoscopic surgery for the treatment of skull base lesions. J Clin Neurosci. 2016;24:99–104. https://doi.org/10.1016/j.jocn.2015.07.021.
Chen HI, Bohman LE, Emery L, Martinez-Lage M, Richardson AG, Davis KA, Pollard JR, Litt B, Gausas RE, Lucas TH. Lateral transorbital endoscopic access to the hippocampus, amygdala, and entorhinal cortex: initial clinical experience. ORL J Otorhinolaryngol Relat Spec. 2015;77(6):321–32. https://doi.org/10.1159/000438762.
Dallan I, Castelnuovo P, Locatelli D, Turri-Zanoni M, AlQahtani A, Battaglia P, Hirt B, Sellari-Franceschini S. Multiportal combined transorbital transnasal endoscopic approach for the management of selected skull base lesions: preliminary experience. World Neurosurg. 2015;84(1):97–107. https://doi.org/10.1016/j.wneu.2015.02.034.
di Somma A, Langdon C, de Notaris M, Reyes L, Ortiz-Perez S, Alobid I, Enseñat J. Combined and simultaneous endoscopic endonasal and transorbital surgery for a Meckel’s cave schwannoma: technical nuances of a mini-invasive, multiportal approach. J Neurosurg. 2021;134(6):1836–45. https://doi.org/10.3171/2020.4.jns20707.
Fong Ng BC, Kwan Mak CH, Chan NL, Lam CW, Yuen HK, Poon TL. Indocyanine green−assisted endoscopic transorbital excision of lateral orbital apex cavernous hemangioma. World Neurosurg. 2022;158:167. https://doi.org/10.1016/j.wneu.2021.11.060.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ng, B., Park, H.H., MAK, C. (2023). Endoscopic Transorbital Approach to the Optic Canal and Orbital Apex. In: POON, T.L., MAK, C., YUEN, H.K.L. (eds) Orbital Apex and Periorbital Skull Base Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-99-2989-4_23
Download citation
DOI: https://doi.org/10.1007/978-981-99-2989-4_23
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2988-7
Online ISBN: 978-981-99-2989-4
eBook Packages: MedicineMedicine (R0)