Abstract
Developing skin models for dermatological and cosmetic toxicity studies is a relatively challenging area of research, considering the limitations of long-term preservation/maintenance of the phenotypically stable test system (Adler et al. 2011). Global legislation regulating animal usage for cosmetic studies has forcefully impacted the quest for an ideal skin model system for cosmetic toxicity and other dermatological studies. Various in vitro skin models like monolayer cell culture models, co-culture models, organotypic culture models, and 3D reconstructed skin models are available for the said purpose (Randall et al. 2018). However, skin explants are better because their unique characteristics closely reflect the physiological outcome (Eberlin et al. 2020). A comparison of the skin explant model and the reconstructed skin model is given in Table 8.1.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Developing skin models for dermatological and cosmetic toxicity studies is a relatively challenging area of research, considering the limitations of long-term preservation/maintenance of the phenotypically stable test system (Adler et al. 2011). Global legislation regulating animal usage for cosmetic studies has forcefully impacted the quest for an ideal skin model system for cosmetic toxicity and other dermatological studies. Various in vitro skin models like monolayer cell culture models, co-culture models, organotypic culture models, and 3D reconstructed skin models are available for the said purpose (Randall et al. 2018). However, skin explants are better because their unique characteristics closely reflect the physiological outcome (Eberlin et al. 2020). A comparison of the skin explant model and the reconstructed skin model is given in Table 8.1.
Skin explants are derived from excised skin tissue. After removing subcutaneous fat and other contaminants, explant cultures are initiated in a suitable culture medium. This helps in the maintenance of the structure of native skin, including the distinct skin cell types and skin-specific extracellular matrix (Neil et al. 2020). The unique characteristic of this ready-to-use system is that natural stratification of the skin layers is maintained, including skin appendages. In the skin explant model, the entire native skin cell population, including keratinocytes, melanocytes, Langerhans cells, and dermal fibroblast cells, is present in a niche of relevant skin-specific skin extracellular matrix comprising of collagen, elastin, glycosaminoglycans (GAGs), etc. Even though these processed tissues lack blood circulation and innervation, they are still used as an ex vivo model for studying the impact of toxic exposure on the skin. Skin explants serve as organotypic models, providing a 3-dimensional culture environment. This helps in the effective cell–cell interactions by making the model close to the physiological conditions. When cultured at the air–liquid interface (ALI), skin explants can be developed as test systems for understanding the effect of topically applied substances.
8.2 Human Skin Explants—Applications
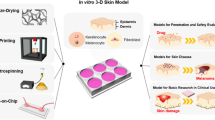
The tremendous usage of cosmetics in the modern world has prompted the need to understand the toxicity issues associated with human exposure in a detailed manner (Mishra and Rahi 2022). The various chemicals widely employed in cosmetic formulation, including active ingredients, preservatives, fragrances, heavy metals, pose a severe threat to consumers and the environment where they are disposed of. Hence, it is imperative to estimate the toxic adverse effects of components of a cosmetic product. Most of the toxicity studies of chemicals performed are in vivo animal studies. However, endorsing the principles of the 3Rs in regulatory toxicity testing has incited the scientific world to seek alternative toxicity methods (Almeida et al. 2017). Skin explants have been an ideal choice for cosmetic toxicity testing from then onwards. Modifying the conventional testing strategies to include additional biological endpoints is possible with such in vitro systems. Human skin explants are particularly interesting as they closely match the subject of interest. Some of the additional endpoints introduced are modeling various skin diseases, studying cutaneous permeation, studying hair follicles, skin infections, dermal and epidermal specific studies, skin resident immune cells and immune responses, melanogenesis and melanocyte permeability studies, etc. (Sutterby et al. 2022). Figure 8.1 summarizes the conventional cosmetic toxicity testing and the prospective applications of skin explants for cosmetic toxicity studies.
8.3 Generation of Skin Explants
Human skin explants are generally obtained from elective plastic surgery/bariatric surgery (weight loss surgery) that involves excess skin removal procedures. Clinical wastes from surgical procedures like panniculectomy, brachioplasty, and abdominoplasty provide sources for skin explants. Skin from the abdominal region is of particular interest. The region’s representative skin has more basal characteristics preserved well because of the low exposure to external toxic perturbances such as solar radiation and pollution. The tissue collection site is cleaned with 10% povidone-iodine solution, and skin with the underlying subcutaneous tissue is harvested. After removing adipose tissue, skin tissue with a thickness of about 0.5 ± 0.1 mm is sliced from the excised skin tissue using an electric dermatome. They are collected in ice-cold physiological saline. Hair appendages are removed, and the skin is soaked in 0.1% benzalkonium bromide for 15 min to sterilize. They are washed extensively with ice-cold saline with antibiotics/antimycotics. All the procedures are carried out under aseptic conditions. From the slices, circular sections of 10–12 mm diameter are cut out and cultured at an air–liquid interface (ALI) in a cell culture insert till further analyses (Fig. 8.2).
8.4 Preservation of Skin Tissue for Explant Generation
Often skin sources are only sometimes available for the generation of explant cultures. For this reason, it is imperative to preserve the clinically sourced skin tissue/explants generated from it as and when available for further use. This could be achieved by skin banking. Skin preservation strategies rely on whether the harvested skin is required to maintain its viability (Kearney 2005). Proper antiseptic measures should be taken for the collection of skin as skin tissue is a rich source of microbial contamination, and sterilization techniques cannot be used to maintain viable cultures. However, they could be collected and stored in solutions with antibiotics and antibiotics to reduce the bioburden.
8.5 Low-Temperature Preservation
Preservation of skin for explant cultures remains a challenge. Even though the tissue is placed in a nutrient-rich medium, ischemic tissue necrosis can occur at the center of the tissue due to insufficient diffusion of oxygen and nutrients from the periphery to the center and inadequate removal of toxic metabolites. Hence, even though the tissue is placed in a nutrient-rich medium, ischemic tissue necrosis can occur at the center of the tissue due to insufficient diffusion of oxygen and nutrients from the periphery to the center and inadequate removal of toxic metabolites. The skin’s moisture content is about 70%, so the quality of skin preserved under low-temperature conditions dramatically depends on the phase transition of water (Mojumdar et al. 2017). Refrigeration at 4 °C is a simple and convenient technique for short-term preservation. However, reports show that cell viability decreases directly proportional to the storage time (Fahmy et al. 1993). Ideally, the preservation time of fresh skin slices should not exceed 72 h when stored in ice-cold physiological saline. It should be used for explant culture generation within a week if stored in tissue culture media with serum.
With the introduction of cryoprotective agents such as DMSO, polyethylene glycol, propylene glycol, and glycerin, it is possible to store at ultra-low temperatures. The cryoprotectants prevent ice crystal formation (Elliott et al. 2017). During the freezing process, two types of damage can occur to the cells. The formation of intracellular ice crystals can damage the cell membrane and subcellular organelles. This type of damage often occurs concerning quick freezing.
On the other hand, the cells will be subjected to solution damage when the water present in the extracellular solution where cells are suspended gets frozen. This type of damage is frequent in the case of slow-freezing methods. Hence, it is possible to overcome the above issues with adequate cryoprotective agents (Karlsson and Toner 1996). So a technique called “vitrification” could be employed in the cryopreservation of skin tissue for long-term storage of skin tissues. Vitrification is the instant solidification of a solution by increasing the solution’s viscosity during the cooling process (Costa et al. 2020). This is achieved by adding anti-freeze or cryoprotective agents that modulate the phase transition process of water. However, revival procedures of this cryopreserved frozen tissue need to be optimized as the incorporated cryoprotective agents can be detrimental at a higher temperature. Also, the chance of getting cryogenic injury to the tissue component cells is higher under cryopreservation. A comparison of different temperature conditions on the preservation of skin is given in Table 8.2.
8.6 Dynamic Culture Conditions
The advantage of an explant culture system is that the skin-specific extracellular matrix (ECM) is not dissociated/disrupted. So the cell–cell interaction and cell–matrix interaction are well preserved so that the functional phenotype of the skin cells is maintained to a more considerable extent (Randall et al. 2011). The ECM provides biomechanical and biochemical cues to modulate the resident cells’ morphogenesis, differentiation, and homeostasis. The ECM proteins and the growth factor receptors on the cells are also well preserved to respond to extraneous supplementation of growth factor, thereby allowing the possibility of manually modulating the cellular response. Three-dimensional (3D) culture methods involving a rotary cell culture system bioreactor benefit the long-term maintenance of explant cultures (Astashkina and Grainger 2014). The rotary cell culture system bioreactor is a rotating culture vessel with a centrally placed co-axial oxygenator. The concurrent rotation of vessel walls and the centrally placed oxygenator creates laminar flow and a minimum shear force so that sufficient diffusion of nutrients and oxygen to the tissue is ensured (Fig. 8.3).
8.7 Culture Techniques for Human Keratinocytes
The human skin implants can be directly used for toxicity studies or as a source for propagating human epithelial cells in culture. However, when used as a source of keratinocytes, the culture conditions need to be optimized to get a good yield of keratinocytes from the explants. Keratinocytes have distinct nutrient requirements, so they are quickly overgrown by other cell populations, such as dermal fibroblasts (Sorg et al. 2017). When attempting to propagate keratinocytes from skin explants, careful separation of the epidermis from the dermis should be tried to avoid contamination with dermal fibroblast. Keratinocytes from the basal layer of the epidermis can proliferate and form colonies. Two methods are currently employed to propagate the keratinocyte cell population from explant culture as follows: (a) culturing in the presence of a feeder layer and serum-containing medium and (b) culturing in the absence of a feeder layer and serum-free medium.
8.7.1 In Serum-Containing Medium with a Feeder Layer
Rheinwald and Green (1975) proposed the method of co-culturing keratinocytes on top of irradiated, non-proliferating fibroblast. Murine fibroblast NH3T3 exposed to gamma rays (6000 rad) or subjected to mitomycin C treatment is shown to give a better result (Rheinwald and Green 1975). The primary explant culture of keratinocytes is plated at a suitable cell density onto the feeder layer. Furthermore, the culture medium for sustaining both cells is provided with 10% fetal bovine serum and special supplements. A typical composition of keratinocyte propagating medium is given in Table 8.3.
Epidermal growth factor (EGF) is added to increase keratinocytes’ proliferation and growth rate. Cholera toxin stimulates adenylate cyclase’s enzymatic activity, thereby increasing intracellular levels of cyclic AMP (Green 1978). In about 10 days, a multilayered sheet of keratinocytes is obtained following this culture method. The secondary culture of keratinocyte is obtained by detaching the confluent layer using proteolytic agents such as dispase and plating into a new flask with feeder culture. As keratinocytes have a limited lifespan, they should be used within 4–5 passages from primary explant culture.
8.7.2 In Serum-Free Media Without a Feeder Layer
Although a considerably better growth rate of keratinocytes is obtained when cultured in serum-containing media and with a feeder layer, it is advisable to use serum-free media to lower the risk of interfering components and to avoid ethical issues. A chemically defined medium can culture the cells under controlled conditions. Determining the ionic concentration, nutrient compositions, and specialized supplements makes it possible to customize according to the growing requirement of keratinocytes. Table 8.4 lists supplements that could be used for keratinocyte propagation.
8.8 Culture at Air–Liquid Interface (ALI)
When the conventional cell culture technique is performed for keratinocytes, the cells are seen attached to the surface of the culture plate submerged in the liquid media. Furthermore, for this reason, the differentiation of the cells is inhibited, so stratification of epithelial layers cannot be achieved. So if the monolayer culture is done in a “lifted manner” so that the basolateral side of the monolayer is in contact with the culture media and the superficial cells are exposed to air which mimics the microenvironment of skin (Green 1978). Various substrates can be used for this purpose, such as membranes made of collagen, fibrin, and laminin floating on a liquid surface medium (Pruniéras et al. 1983). Organotypic skin equivalents could be derived by co-culturing keratinocytes on top of a dermal fibroblast-seeded permeable scaffold and then submerged in suitable media under air–liquid interface conditions (Parenteau et al. 1992). A typical representation of ALI culture is given in Fig. 8.4.
8.9 Animal Component-Free Medium
Animal component-free (ACF) media are defined as a medium that does not contain any primary raw materials derived directly from animal tissue or body fluid. However, it could include secondary or territory raw materials derived from human tissue or proteins produced by recombinant technology (Whitford et al. 2018). The difference between ACF from serum-free (SF) media is that serum-free media is devoid only of serum/plasma/or hemolymph but may contain other primary raw materials of animal origin such as tissue extract, platelet lysate, hormones, and growth factor cocktail. Nevertheless, in the case of animal component-free media, these raw materials of animal origin are not included.
In conventional cell culture methods, fetal bovine serum (FBS) is an essential constituent of cell culture media. However, there are disadvantages to using serum exclusively for culture purposes. The primary disadvantage is its batch-to-batch variability. This variability can affect the performance of culture outcomes. Also, there is a risk of potential viral and other adventitious contaminants, including mycoplasma and endotoxin, present in the serum (Froud 1999). Other animal-derived primary raw materials, such as tissue extract, growth factors, and hormones, can pose the same risk. Media containing animal tissue-derived supplements possess immunogenic potential due to the presence of xenogeneic proteins. Hence, it is not always advisable to use the cells conditioned in such a medium for immunological studies or cell-based therapies. The serum is considered “bio-reactive” and can interfere with many biological cascade pathways (Barnes and Sato 1980). For this reason, there is a potential risk of obtaining unreliable results from in vitro studies using fetal bovine serum. By utilizing an ACF medium, there is an option for a chemically defined medium with traceable ingredients for cell culture. The downstream process can be made simplified using a chemically defined animal component-free (ACF) medium. The essential components replaced in an ACF medium are growth factors and human blood derivatives such as serum albumin, platelet lysate, and protein and lipid supplements derived from non-human/non-animal sources such as plants, bacteria, and yeast. The advantages of using ACF medium are compiled in Fig. 8.5.
8.10 Composition of Animal Component-Free Medium for Skin Explants
With the successful translation and adaptation of KeratinoSens™ as an alternative to animal skin sensitization assay, researchers are in quest of similar animal product-free cell culture systems for the maintenance or preservation of skin explants (Riebeling et al. 2018). The essential components replaced in an ACF medium are growth factors and human blood derivatives such as serum albumin, platelet lysate, and protein and lipid supplements derived from non-human/non-animal sources such as plants, bacteria, and yeast. A typical composition of animal component-free medium for skin explants is given in Table 8.5.
8.11 Future Directions
The prospects of using skin explants and skin explant culture as an alternative to animal testing strategies for cosmetics and other skin disease modeling studies are giving the scientific community promising results. Furthermore, incorporating animal component-free media and better storage modalities would benefit the next-level scope of expansion of cosmetic toxicity studies and enhance the clinical translation potential of skin explants.
References
Adler S, Basketter D, Creton S, Pelkonen O, van Benthem J, Zuang V, Andersen KE, Angers-Loustau A, Aptula A, Bal-Price A et al (2011) Alternative (non-animal) methods for cosmetics testing: current status and future prospects—2010. Arch Toxicol 85(5):367–485. https://doi.org/10.1007/s00204-011-0693-2
Almeida A, Sarmento B, Rodrigues F (2017) Insights on in vitro models for safety and toxicity assessment of cosmetic ingredients. Int J Pharm 519(1):178–185. https://doi.org/10.1016/j.ijpharm.2017.01.024
Astashkina A, Grainger DW (2014) Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev 69-70:1–18. https://doi.org/10.1016/j.addr.2014.02.008
Barnes D, Sato G (1980) Serum-free cell culture: a unifying approach. Cell 22(3):649–655. https://doi.org/10.1016/0092-8674(80)90540-1
Costa CAS, Borges AA, Nascimento MB, Aquino LVC, Silva AR, Oliveira MF, Pereira AF (2020) Effects of vitrification techniques on the somatic tissue preservation of agouti (Dasyprocta leporina Linnaeus, 1758). Biopreserv Biobank 18(3):165–170. https://doi.org/10.1089/bio.2019.0109. Accessed 20 Dec 2022
Eberlin S, Silva MS, Facchini G, Silva GH, Pinheiro AL, Eberlin S, Pinheiro AD (2020) The ex vivo skin model as an alternative tool for the efficacy and safety evaluation of topical products. Altern Lab Anim 48(1):10–22. https://doi.org/10.1177/0261192920914193. Accessed 20 Dec 2022
Elliott GD, Wang S, Fuller BJ (2017) Cryoprotectants: a review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 76:74–91. https://doi.org/10.1016/j.cryobiol.2017.04.004
Fahmy FS, Navsaria HA, Frame JD, Jones CR, Leigh IM (1993) Skin graft storage and keratinocyte viability. Br J Plast Surg 46(4):292–295. https://doi.org/10.1016/0007-1226(93)90005-V
Froud SJ (1999) The development, benefits and disadvantages of serum-free media. Dev Biol Stand 99:157–166. PubMed
Green H (1978) Cyclic AMP in relation to proliferation of the epidermal cell: a new view. Cell 15(3):801–811. https://doi.org/10.1016/0092-8674(78)90265-9
Karlsson JOM, Toner M (1996) Long-term storage of tissues by cryopreservation: critical issues. Biomaterials 17(3):243–256. https://doi.org/10.1016/0142-9612(96)85562-1
Kearney JN (2005) Guidelines on processing and clinical use of skin allografts. Clin Dermatol 23(4):357–364. https://doi.org/10.1016/j.clindermatol.2004.07.018
Mishra G, Rahi S (2022) Need of toxicity studies for cosmetic products and their approaches. Biol Sci 2(1):105–109. https://doi.org/10.55006/biolsciences.2022.0201
Mojumdar EH, Pham QD, Topgaard D, Sparr E (2017) Skin hydration: interplay between molecular dynamics, structure and water uptake in the stratum corneum. Sci Rep 7(1):15712. https://doi.org/10.1038/s41598-017-15921-5
Neil JE, Brown MB, Williams AC (2020) Human skin explant model for the investigation of topical therapeutics. Sci Rep 10(1):21192. https://doi.org/10.1038/s41598-020-78292-4
Parenteau NL, Bilbo P, Nolte CJM, Mason VS, Rosenberg M (1992) The organotypic culture of human skin keratinocytes and fibroblasts to achieve form and function. Cytotechnology 9(1):163–171. https://doi.org/10.1007/bf02521744
Pruniéras M, Régnier M, Woodley D (1983) Methods for cultivation of keratinocytes with an air-liquid interface. J Investig Dermatol 81(1, Supplement):S28–S33. https://doi.org/10.1111/1523-1747.ep12540324
Randall KJ, Turton J, Foster JR (2011) Explant culture of gastrointestinal tissue: a review of methods and applications. Cell Biol Toxicol 27(4):267–284. https://doi.org/10.1007/s10565-011-9187-5
Randall MJ, Jüngel A, Rimann M, Wuertz-Kozak K (2018) Advances in the biofabrication of 3D skin in vitro: healthy and pathological models. Front Bioeng Biotechnol 6:154. https://doi.org/10.3389/fbioe.2018.00154
Rheinwald JG, Green H (1975) Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6:331–343. https://doi.org/10.1016/S0092-8674(75)80001-8
Riebeling C, Luch A, Tralau T (2018) Skin toxicology and 3Rs—current challenges for public health protection. Exp Dermatol 27(5):526–536. https://doi.org/10.1111/exd.13536. Accessed 20 Dec 2022
Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U (2017) Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res 58(1–2):81–94. https://doi.org/10.1159/000454919
Sutterby E, Thurgood P, Baratchi S, Khoshmanesh K, Pirogova E (2022) Evaluation of in vitro human skin models for studying effects of external stressors and stimuli and developing treatment modalities. View 3(2):20210012. https://doi.org/10.1002/VIW.20210012. Accessed 20 Dec 2022
Whitford WG, Lundgren M, Fairbank A (2018) Chapter 8–cell culture media in bioprocessing. In: Jagschies G, Lindskog E, Łącki K, Galliher P (eds) Biopharmaceutical processing. Elsevier, Amsterdam, pp 147–162
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Remya, N.S., Nair, R.P., Bhatt, A. (2023). Animal Component-Free Medium for Long-Term Maintenance of Human Skin Explants and Its Application in Toxicity Studies of Cosmetics. In: Pant, A.B., Dwivedi, A., Ray, R.S., Tripathi, A., Upadhyay, A.K., Poojan, S. (eds) Skin 3-D Models and Cosmetics Toxicity. Springer, Singapore. https://doi.org/10.1007/978-981-99-2804-0_8
Download citation
DOI: https://doi.org/10.1007/978-981-99-2804-0_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2803-3
Online ISBN: 978-981-99-2804-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)