Abstract
The use of cosmetic items has increased significantly across all age groups globally without knowing their hazardous impact in long-term exposure. Additionally, these cosmetics have some ingredients, especially preservatives, colorant, and fragrances that promote inflammation and allergy in users’ skin under exposure of UVR/sunlight. Therefore, identification and development of predictive molecular signatures for cosmetics toxicity via use of omics approaches are important for safety of consumers. OMICS includes biological techniques that aid in the detection of potential adverse outcome pathways (AOPs) of any toxicological response. These AOPs elucidate underlying molecular principles and affirm key molecular events that take place at various levels of biological organizations. This chapter will demonstrate how the omics method has been integrated into the toxicological evaluation of cosmetics and help in development of safe and quality cosmetic products.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Skin is an important organ that is mainly exposed with cosmetics. Their use triggers a chain of chemical reactions that alter the expression of several genes and protein synthesis, significantly altering cellular responses and resulting in skin allergies, phototoxicity, aging, and skin cancer (Khan and Alam 2019). Alternative test models and methods after the animal use banned for cosmetics toxicity testing by EU in 2013 and Indian in 2014 will be desperately needed for the detection of harmful effects upon the usage of cosmetic compounds.
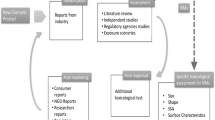
Molecular alterations at various levels of biological organization have been quantified as a result of improvements in traditional toxicological testing. The strategy that was based on the conventional method of animal testing was superseded by the new paradigm of “system toxicology.” The “omics” method used by this system technology encompasses genomics, transcriptomics, proteomics, and metabolomics (Fig. 12.1).
Omics is a vast system of biological methods, which assists in the identification of potential adverse outcome pathways (AOPs) (Wang et al. 2021), which confirms significant molecular events occurring at different levels of biological organizations and thus aids in the elucidation of underlying molecular principles. Omics techniques offer mechanistic analysis for the discovery of important targets, indicators, and toxicity pathways in toxicological evaluation (Gouveia et al. 2019). With the aid of these techniques, it is possible to identify adaptive reactions to low toxicant concentrations that do not cause toxicity but instead subject cells to oxidative stress, which is known to be a damaging action mechanism. Proteomics, the study of proteins at the systems level, and metabolomics, the study of cellular metabolic processes, are some of the omics approaches that are being used in the study of various biological responses that involve thousands of genes, proteins, and metabolites, respectively. Genomics and transcriptomics currently refer to the study of alterations in gene expression at the genome-wide level (Reay and Cairns 2021).
In order to evaluate the pertinent outcomes or endpoints that would aid in the development of customized skin care products, this article focuses on the function of omics technology as a holistic approach, its application, and its significance in the toxicity assessment of the cosmetics.
12.2 Cosmetic Toxicology
Our skin is exposed to a variety of chemicals that are found in many cosmetic items almost every day. As a result, people of all ages continue to experience a steady increase in demand for cosmetic items worldwide. Cosmetic preservatives and fragrances have been connected to a number of serious health problems, including endocrine disruption, cancer, mutation, and reproductive damage (Mishra and Rahi 2022). Many heavy metals are utilized in cosmetics, and even in very little amounts, they can impair the body’s vital organs. These negative effects of metals in cosmetics have been linked to a wide range of problems, including organ failure, cancer, respiratory disorders, and intellectual disability (Paithankar et al. 2021).
12.3 Need to Study Cosmetic Toxicity
Safety and toxicological testing is one of the most significant and crucial tests in the field of cosmetic toxicology. In order to conduct numerous toxicity and safety tests on the various cosmetic products and their effects on the skin when applied topically, safety and toxicological assessment of the raw chemicals used in cosmetics is necessary. The study of cosmetic toxicology enables the prediction of potential hazards connected to the use of cosmetic products, risks that, if a person is exposed to them, may result in undesirable effects such as skin redness, a burning sensation, inflammation, and a wide range of allergic reactions (Okereke et al. 2015). To identify any potentially detrimental effects related to the use of cosmetics, cosmetic toxicology needs in-depth study of the biological processes that are triggered by the ingredients in the formulations used in cosmetics. A person may encounter a number of pertinent outcomes while frequently utilizing cosmetic components, including skin or eye irritation, corrosion, allergy, blisters, and inflammation. It is necessary to assess the risks associated with these endpoints and ensure their safety. Cosmetic ingredients may develop phototoxic properties when exposed to UV light, which might result in a range of dermatological responses (Tomankova et al. 2011).
12.4 The Way to Omics Approaches
In an early investigation, it was discovered that skin senses and reacts to even the smallest environmental changes in order to preserve homeostasis. The use of cosmetics and solar radiation sets off a chain of events that change gene expression. This modification catalyzes chemical processes at the protein level that results in cellular response. Metabolic byproducts as a result reveal the chemical reactions that had occurred. To fully understand the biological response, it is necessary to view these complex events holistically, which involve many different genes, proteins, and metabolites. Only a few methods were available to researchers in the previous decade for the analysis of these substances. However, advances in technology have transformed the study of skin’s responses to the environment.
Omics approaches have evolved in the present day with the development of high-throughput technology for the analysis of diseases and online datasets of various biological samples (Table 12.1). With the study of connections between biochemical, molecular, and environmental factors, omics studies—which comprise genomes, transcriptomics, proteomics, metabolomics, and epigenomics—provide comprehensive knowledge of system biology (Manzoni et al. 2018).
12.4.1 Genomics
There are two ways that genomics can be used to investigate the toxicity of cosmetics. First, it can support the persistence and detectability of chemicals in humans. Second, it can be used to seek new toxicity pathways. qRT-PCR, DNA chips, Serial Analysis of Gene Expression (SAGE), genome-wide association study (GWAS), and CRISPR/Cas are examples of genomic technology (Jinek et al. 2012). Following the use of cosmetic goods, prolonged exposure to UV-R causes photosensitization, which results in burning, inflammatory, and painful sensations. As a result, a person develops various dermatological problems like psoriasis, acne, and dermatitis. The gene expression profile changes with time. The identification of genes involved in skin disorders should provide a better mechanistic understanding given recent advances in genomics. A small variation known as an SNP is connected to a certain disease through GWAS, allowing researchers to investigate and identify the main gene responsible for the disease’s development (Ober and Yao 2011). This might be a useful approach to investigate population genetic variants that contribute to skin disorders. A researcher may be able to accurately anticipate treatment techniques in precision skincare or cosmetic products using data from GWAS investigations. In a single experiment, gene chips or microarrays detect the expression levels of almost all human genes. They can be used to examine how the skin responds to treatments like topical application of personal care products and to external factors like sun exposure. They have been used, for example, to identify molecular distinctions between the skin of different ages (young skin and older skin) (Fig. 12.2) (Robinson et al. 2008). This information might be used to develop newer skin beneficial products and treatments that will eventually improve skin health.
12.4.2 Transcriptomics
The use of a molecular method called transcriptomics, which examines the expression levels of genes with well-known biological activity, can help us to better understand how toxicological mechanisms work (Cui and Paules 2010). Better sequencing-based technologies are now available, and they promise to teach us more about how skin cells function (Fig. 12.3). Technologies like RNA sequencing (RNA-Seq) and digital gene expression profiling (DGE) leverage new advancements in next-generation sequencing to assess the genes being expressed in a sample in a more accurate and thorough manner (Kimball et al. 2012).
In RNA-Seq/DGE, the quantity of a particular transcript is calculated without taking into account the intensity of the fluorescent signal. Instead, it concentrates on counting the number of times each gene occurs while sequencing several genes that are expressed in a sample. Additionally, RNA-Seq/DGE gives a more accurate overview of all the RNAs in a sample, including mRNAs, microRNAs, and other ncRNAs (noncoding RNA) species (Kimball et al. 2012).
Primitive monolayer keratinocytes produced from damaged and non-psoriatic tissue slices were studied using RNA-seq (Swindell et al. 2017). Additionally, a whole skin biopsy from the same individual was examined. Compared to the transcriptome of the entire skin, there is a greater difference between the damaged and normal psoriasis keratinocytes. Significant gene overlap around disease-related SNPs may indicate that the keratinocytes of people with psoriasis are less differentiated. Skin aging is a result of both genetic and environmental factors. Sun-protected epidermis, sun-damaged skin before the ear, and sun-protected skin behind the ear were all subjected to a sequence comparison analysis of gene expression (Urschitz et al. 2002). Genes expressed in human skin and genes with differential expression in response to UV exposure were found using SAGE. In skin that had been exposed to the sun, before the ear, 19 distinct labels were diminished (at least four times lower), whereas 15 labels were increased. Numerous genes whose transcription levels change in response to sunlight are expressed by epidermal keratinocytes that might act as a novel biomarker.
12.4.3 Proteomics
Proteomics is a study of proteins that quantifies and qualitatively analyses the numerous types of protein content in a cell, including protein–protein interactions, protein–ligand interactions, and post-translational modifications (Jensen 2006). The proteomic method aids in the protein characterization of skin biology (Fig. 12.4). Atopic skin disease develops and worsens as a result of numerous factors. In contrast to a control group, the skin proteome of an individual with atopic dermatitis (AD) showed higher and more significant upregulation than blood protein, demonstrating inflammation and cardiovascular features in the skin proteome of the AD patient and their interaction with the blood proteome and skin genome (Pavel et al. 2020).
The quantity of cytokines and structural proteins can be measured using a clinical sampling technique (noninvasive) like tape stripping to isolate and collect protein from skin surface in order to learn more about skin irritation and inflammation and the condition of the skin barrier in healthy and damaged skin (like dandruff) (Keurentjes et al. 2021). Using particular antibody-based ELISA techniques, many proteins can be measured and evaluated simultaneously (Kerr et al. 2011).
12.4.4 Metabolomics
Analysis of the biochemical, physiological, and chemical changes in a biological system is provided by metabolomics. A wide range of fields use the analysis of metabolites to determine safety. In the past few years, metabolomics has become a novel method for evaluating the safety profile of chemical compounds in regulatory toxicology. Metabolomics is similar to analyzing disrupted metabolic pathways in terms of toxicity (Ramirez et al. 2013). It simplifies the process of determining potentially harmful substances and their target. The metabolomic method also elucidated a compound’s mode of action and its impact on the target organ. The endogenous compounds that are altered during cellular metabolism include nucleotides, amino acids, steroids, phospholipids, carbohydrates, and their derivatives (Patti et al. 2012). These metabolites are byproducts of proteins, mRNA, and genes and have the ability to control the expression and function of other biomolecules (Ramirez et al. 2013). Metabolites provide a clear reflection of the metabolic processes taking place in a system, allowing for analysis of the role that particular biochemical pathway plays in the production of a given metabolite profile and the effects of perturbations to those pathways (Fig. 12.5). Metabolomics facilitates detection since any slight alteration in gene or protein expression directly reflects metabolite changes.
Continuous exposure to UV-R causes erythema, wrinkles, and loss of skin moisture (Amaro-Ortiz et al. 2014). Mice treated with green tea catechin and those exposed to UV-B had their skin metabolites examined using the MS method. The findings demonstrated that UV-B exposure in ECGT-treated mice reduced alterations in metabolites including ceramide, amino acid, and lysophospholipid, but purine bases, lactoses, and ascorbic acid were most affected (Jung et al. 2015). These modifications in skin metabolite can also be utilized as a biomarker to determine how a specific substance with a hazardous potential affects the skin, which could offer a theoretical framework for formulations and creams for the cosmetic industry.
When metabolic pathways are dysregulated in specific diseases, such as psoriasis, GC-MS is employed to find potential biomarkers that are associated with psoriatic patients as opposed to healthy people (Kang et al. 2017). Understanding the diseases connected to the various skin allergies better may aid in the discovery of possible biomarkers. Skin metabolites to some extent reflect the state of the skin. Analyzing different skin metabolites through metabolomics can help us understand different skin illnesses better while also improving formulations for skin care products.
12.4.5 Lipidomics
The outermost skin layer, or epidermis, is a lipid-rich area that provides structural support and prevents chemical access. Lipids, particularly ceramides, acyl ceramides, cholesterol, cholesterol esters, and non-esterified fatty acids (NEFA, also known as free fatty acids), are the main constituents of the extracellular space of the epidermis. These lipids are arranged across many bilayers (Knox and O’Boyle 2021). For the integrity and functionality of the skin, an active lipid metabolism and fatty acid profile are essential (Kendall and Nicolaou 2013).
Identification and measurement of cellular lipid profiles in biological samples are the focus of the subfield of lipidomics, which falls under metabolomics (Ahluwalia et al. 2022; Lydic and Goo 2018). The skin lipid profile can be determined quickly, accurately, and non-invasively via skin lipidomic analysis (Li et al. 2016). The identification of many bioactive lipid mediators that are involved in immune function is made possible by lipidomic analysis. Research funded by the (skincare) business is increasingly finding lipidomics to be helpful, and it may be used to evaluate the effectiveness of both cosmetic formulations and skincare active ingredients. Oils, fats, waxes, and lipid antioxidants including carotenoids, retinoids, and tocopherols are used as active ingredients in cosmetic and personal care formulations, and lipidomic analysis is necessary for both quality assurance and efficacy testing (Ahmad and Ahsan 2020). A biological specimen may be subjected to lipidomic profiling directly or after being extracted with an organic solvent (Yang and Han 2016). Shotgun or LC-based lipidomics were typically the two kinds of sophisticated mass spectrometry techniques (MS) used for lipid profiling (Han and Ye 2021). Liquid chromatography–mass spectrometry (LC-MS or LC-MS/MS), MALDI-MS, ion mobility MS, high mass accuracy MS, and tandem MS were used for LC-based lipidomic analysis, whereas multidimensional MS, MALDI-MS, and DESI-MS were used for shotgun-based lipidomic investigations. The steps involved in lipidomic analysis of biological samples include sample collection, sample processing, data capture, and data processing (Fig. 12.6) (Hyötyläinen and Orešič 2015).
Healthy skin requires a particular lipid composition to maintain a barrier that offers defense and prevents excessive water loss, facilitates cell–cell communication, and controls epidermal homeostasis (Murakami et al. 2018). Lipidomic study improves our knowledge of how skin lipid composition can be altered by cosmetic products, which can lead to a number of dermatological issues. An important chronic inflammatory skin condition known as atopic dermatitis is frequently characterized by a compromised skin barrier and increased trans-epidermal water loss (TEWL) (Williamson et al. 2020). Changes in stratum corneum (SC) lipids have been extensively studied. Patients with atopic dermatitis (AD) had different SC lipid profiles, according to retrospective investigations. Furthermore, lipidomic study indicates that ceramide levels are significantly lower in AD patients compared to healthy people (Emmert et al. 2021). Through lipidomic research, ceramidase overexpression in psoriatic patients was discovered (Łuczaj et al. 2021). Smeden and his colleagues conducted a thorough LC-MS-based lipidomic investigation that identified significant changes in the SC lipid profile in NTS (Netherton syndrome) patients (van Smeden et al. 2020).
As a result, lipidomics offers fresh perspectives on how cosmetic ingredients affect skin lipid profiles or the skin microbiome–lipidome relationship. Based on these findings, there is also significant potential for customized cosmetics by segmenting consumer groups according to skin lipid composition.
12.4.6 Microbiomics
Microorganisms such as bacteria, viruses, yeast, fungus, and archaea all live on human skin. These skin-dwelling microorganisms make up the skin barrier, which facilitates the maintenance of healthy skin (Byrd et al. 2018). The skin microbiota, which makes up our top layer of skin, is continually in contact with outside factors including UV radiation, pollution, and cosmetic additives (Skowron et al. 2021). It is hypothesized that the use of synthetic chemical ingredients in modern cosmetics has an impact on skin bacteria (Wallen-Russell 2018). Application of cosmetic products to the skin, including soaps, shampoos, lotions, moisturizers, anti-aging, and hygiene items, might alter the lipid layer that protects the skin and affect the diversity of resident microflora (Pinto et al. 2021). The active ingredients in cosmetics may promote the growth of some microbial species or may hinder them. In the future, skin microbiomics may be used as a comprehensive strategy to assess whether cosmetic compounds are good for the skin or harm it.
The study of the entire microbiota, or bacterial population, is the focus of the discipline of microbiomics, which is constantly expanding. The goal of the field of microbiomics is to comprehend a specific microbial community’s makeup and how it could shift over time or in response to a specified pressure. The composition of a particular microbial community is examined using high-throughput sequencing of the 16S rRNA gene (Kim et al. 2021).
Genomic DNA from the targeted sample was collected, and PCR was used to amplify the 16S rRNA gene (PCR). The amplified 16S rRNA is sequenced utilizing the standard sequencing methods. The collected sequence must be matched to widely available online databases in order to identify the bacterium (Zemb et al. 2020). Analysis has advanced from kingdom to strain level with the development of shotgun metagenomic technology in recent years since it simultaneously captures all genetic material in sample and provides sufficient resolution for identifying species and strains (Fig. 12.7) (Quince et al. 2021) Numerous dermatoses pathogenesis has been demonstrated to involve changes to the cutaneous microbiota, according to studies’ reference. Higher microbial community biodiversity was a sign of healthier skin. In terms of microbial diversity, the synthetic and “natural” product categories have shown the slowest growth during periods of 2 and 4 weeks. Face wash demonstrated the fastest average growth rate because it has no artificial components (Wallen-Russell 2018). It was further concluded that cosmetic product, preservatives, can remain active on the skin and, if used frequently, alter the local microbiota over time (Holland and Bojar 2002). In this essence, the pool of resistance genes basically increases, and these genes can spread to other microorganisms (transients) that are infectious agents and spread around the neighborhood through local microflora. One of the elements that stimulate the skin microbiota is N-acetylglucosamine, a component typically presents in skincare products and a precursor to hyaluronic acid (Skowron et al. 2021). Instead of getting rid of microorganisms, antiperspirants and foot powders increased the variety of microbial flora in armpits and between toes (Bouslimani et al. 2019). Lipids included in moisturizers promote the growth of lipophilic microorganisms including staphylococcus and propionium bacteria (Diaz and Ditre 2020). Companies are working to grow beneficial microorganisms to treat both more severe and less serious conditions like eczema and acne and minor ailments like dryness and wrinkles. Cosmetic manufacturers have begun looking into the relationship between a healthy microbiome and healthy skin (Reisch 2017). In conclusion, skin microbiota is important to the cosmetic industry. The microbiome is where the future of cosmetics is taking place, and how skin care products affect the microbial makeup of the skin is crucial. A precise skincare strategy should be developed using the microbiomic approach and relevant cosmetic ingredients based on microbiological and chemical evidence that are important players in host defense.
12.5 Conclusion
Omics technology is currently widely employed in all scientific disciplines, including biology, medicine, and nutrition. The development of omics techniques in the field of cosmetic toxicity will advance knowledge of the etiology and disorders associated with the skin. It is anticipated that the development of novel techniques for examining molecular mechanisms and related signaling pathways in the diagnosis, treatment, and creation of personalized precision medicine will lead to the production of more relevant scientific data in the identification and management of various skin diseases, their prognosis, and the identification of new biomarkers.
References
Ahluwalia K, Ebright B, Chow K, Dave P, Mead A, Poblete R, Louie SG, Asante I (2022) Lipidomics in understanding pathophysiology and pharmacologic effects in inflammatory diseases: considerations for drug development. Meta 12(4):333
Ahmad A, Ahsan H (2020) Lipid-based formulations in cosmeceuticals and biopharmaceuticals. Biomed Dermatol 4(1):1–10
Amaro-Ortiz A, Yan B, D'Orazio JA (2014) Ultraviolet radiation, aging and the skin: prevention of damage by topical cAMP manipulation. Molecules 19(5):6202–6219
Bouslimani A, da Silva R, Kosciolek T, Janssen S, Callewaert C, Amir A, Dorrestein K, Melnik AV, Zaramela LS, Kim JN, Humphrey G (2019) The impact of skin care products on skin chemistry and microbiome dynamics. BMC Biol 17(1):1–20
Byrd AL, Belkaid Y, Segre JA (2018) The human skin microbiome. Nat Rev Microbiol 16(3):143–155
Cui Y, Paules RS (2010) Use of transcriptomics in understanding mechanisms of drug-induced toxicity. Pharmacogenomics 11(4):573–585
Diaz D, Ditre CM (2020) The effect of cleansers on the skin microbiome. Pract Dermatol:62–65
Emmert H, Baurecht H, Thielking F, Stölzl D, Rodriguez E, Harder I, Proksch E, Weidinger S (2021) Stratum corneum lipidomics analysis reveals altered ceramide profile in atopic dermatitis patients across body sites with correlated changes in skin microbiome. Exp Dermatol 30(10):1398–1408
Gouveia D, Almunia C, Cogne Y, Pible O, Degli-Esposti D, Salvador A, Cristobal S, Sheehan D, Chaumot A, Geffard O, Armengaud J (2019) Ecotoxicoproteomics: a decade of progress in our understanding of anthropogenic impact on the environment. J Proteome 198:66–77
Han X, Ye H (2021) Overview of lipidomic analysis of triglyceride molecular species in biological lipid extracts. J Agric Food Chem 69(32):8895–8909
He J, Jia Y (2022) Application of omics technologies in dermatological research and skin management. J Cosmet Dermatol 21(2):451–460
Holland KT, Bojar RA (2002) Cosmetics. Am J Clin Dermatol 3(7):445–449
Hyötyläinen T, Orešič M (2015) Optimizing the lipidomics workflow for clinical studies—practical considerations. Anal Bioanal Chem 407(17):4973–4993
Jensen ON (2006) Interpreting the protein language using proteomics. Nat Rev Mol Cell Biol 7(6):391–403
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 337(6096):816–821
Jung ES, Park HM, Lee KE, Shin JH, Mun S, Kim JK, Lee SJ, Liu KH, Hwang JK, Lee CH (2015) A metabolomics approach shows that catechin-enriched green tea attenuates ultraviolet B-induced skin metabolite alterations in mice. Metabolomics 11(4):861–871
Kang H, Li X, Zhou Q, Quan C, Xue F, Zheng J, Yu Y (2017) Exploration of candidate biomarkers for human psoriasis based on gas chromatography-mass spectrometry serum metabolomics. Br J Dermatol 176(3):713–722
Kendall AC, Nicolaou A (2013) Bioactive lipid mediators in skin inflammation and immunity. Prog Lipid Res 52(1):141–164
Kerr K, Darcy T, Henry J, Mizoguchi H, Schwartz JR, Morrall S, Filloon T, Wimalasena R, Fadayel G, Mills KJ (2011) Epidermal changes associated with symptomatic resolution of dandruff: biomarkers of scalp health. Int J Dermatol 50(1):102–113
Keurentjes AJ, Jakasa I, Kezic S (2021) Research techniques made simple: stratum corneum tape stripping. J Investig Dermatol 141(5):1129–1133
Khan AD, Alam MN (2019) Cosmetics and their associated adverse effects: a review. J Appl Pharm Sci Res:1–6
Kim KB, Kwack SJ, Lee JY, Kacew S, Lee BM (2021) Current opinion on risk assessment of cosmetics. J Toxicol Environ Health Part B Crit Rev 24(4):137–161
Kimball AB, Grant RA, Wang F, Osborne R, Tiesman JP (2012) Beyond the blot: cutting edge tools for genomics, proteomics and metabolomics analyses and previous successes. Br J Dermatol 166:1–8
Knox S, O’Boyle NM (2021) Skin lipids in health and disease: a review. Chem Phys Lipids 236:105055
Li S, Ganguli-Indra G, Indra AK (2016) Lipidomic analysis of epidermal lipids: a tool to predict progression of inflammatory skin disease in humans. Expert Rev Proteomics 13(5):451–456
Łuczaj W, Gęgotek A, Skrzydlewska E (2021) Analytical approaches to assess metabolic changes in psoriasis. J Pharm Biomed Anal 205:114359
Lydic TA, Goo YH (2018) Lipidomics unveils the complexity of the lipidome in metabolic diseases. Clin Transl Med 7(1):1–13
Manzoni C, Kia DA, Vandrovcova J, Hardy J, Wood NW, Lewis PA, Ferrari R (2018) Genome, transcriptome and proteome: the rise of omics data and their integration in biomedical sciences. Brief Bioinform 19(2):286–302
Mishra G, Rahi S (2022) Need of toxicity studies for cosmetic products and their approaches. Biol Sci 2(1):105–109
Murakami M, Yamamoto K, Taketomi Y (2018) Phospholipase A2 in skin biology: new insights from gene-manipulated mice and lipidomics. Inflamm Regen 38(1):1–10
Ober C, Yao TC (2011) The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev 242(1):10–30
Okereke JN, Udebuani AC, Ezeji EU, Obasi KO, Nnoli MC (2015) Possible health implications associated with cosmetics: a. Science 3(5–1):58–63
Paithankar JG, Saini S, Dwivedi S, Sharma A, Chowdhuri DK (2021) Heavy metal associated health hazards: an interplay of oxidative stress and signal transduction. Chemosphere 262:128350
Patti GJ, Yanes O, Siuzdak G (2012) Metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol 13(4):263–269
Pavel AB, Zhou L, Diaz A, Ungar B, Dan J, He H, Estrada YD, Xu H, Fernandes M, Renert-Yuval Y, Krueger JG (2020) The proteomic skin profile of moderate-to-severe atopic dermatitis patients shows an inflammatory signature. J Am Acad Dermatol 82(3):690–699
Pinto D, Ciardiello T, Franzoni M, Pasini F, Giuliani G, Rinaldi F (2021) Effect of commonly used cosmetic preservatives on skin resident microflora dynamics. Sci Rep 11(1):1–7
Quince C, Nurk S, Raguideau S, James R, Soyer OS, Summers JK, Limasset A, Eren AM, Chikhi R, Darling AE (2021) STRONG: metagenomics strain resolution on assembly graphs. Genome Biol 22(1):1–34
Ramirez T, Daneshian M, Kamp H, Bois FY, Clench MR, Coen M, Donley B, Fischer SM, Ekman DR, Fabian E, Guillou C (2013) Metabolomics in toxicology and preclinical research. ALTEX 30(2):209
Reay WR, Cairns MJ (2021) Advancing the use of genome-wide association studies for drug repurposing. Nat Rev Genet 22(10):658–671
Reisch MS (2017) Cosmetics: the next microbiome frontier. Mitsui Chemicals Catalysis Science Award 95: 30–34
Robinson M, Tiesman J, Binder R, Juhlin K (2008) Immune and inflammatory gene expression profiles of chronological skin aging and photoaging. J Am Acad Dermatol 58(2):AB34
Skowron K, Bauza-Kaszewska J, Kraszewska Z, Wiktorczyk-Kapischke N, Grudlewska-Buda K, Kwiecińska-Piróg J, Wałecka-Zacharska E, Radtke L, Gospodarek-Komkowska E (2021) Human skin microbiome: impact of intrinsic and extrinsic factors on skin microbiota. Microorganisms 9(3):543
Swindell WR, Sarkar MK, Liang Y, Xing X, Baliwag J, Elder JT, Johnston A, Ward NL, Gudjonsson JE (2017) RNA-seq identifies a diminished differentiation gene signature in primary monolayer keratinocytes grown from lesional and uninvolved psoriatic skin. Sci Rep 7(1):1–13
Tomankova K, Kejlova K, Binder S, Daskova A, Zapletalova J, Bendova H, Kolarova H, Jirova D (2011) In vitro cytotoxicity and phototoxicity study of cosmetics colorants. Toxicol in Vitro 25(6):1242–1250
Urschitz J, Urban Z, Granda C, Souza KA, Lupp C, Csiszar K, Boyd CD, Iobst S, Schilling K, Scott I (2002) A serial analysis of gene expression in sun-damaged human skin. J Investig Dermatol 119(1):3–13
van Smeden J, Al-Khakany H, Wang Y, Visscher D, Stephens N, Absalah S, Overkleeft HS, Aerts JM, Hovnanian A, Bouwstra JA (2020) Skin barrier lipid enzyme activity in Netherton patients is associated with protease activity and ceramide abnormalities [S]. J Lipid Res 61(6):859–869
Wallen-Russell C (2018) The role of every-day cosmetics in altering the skin microbiome: a study using biodiversity. Cosmetics 6(1):2
Wang X, Li F, Chen J, Ji C, Wu H (2021) Integration of computational toxicology, toxicogenomics data mining, and omics techniques to unveil toxicity pathways. ACS Sustain Chem Eng 9(11):4130–4138
Williamson S, Merritt J, De Benedetto A (2020) Atopic dermatitis in the elderly: a review of clinical and pathophysiological hallmarks. Br J Dermatol 182(1):47–54
Yang K, Han X (2016) Lipidomics: techniques, applications, and outcomes related to biomedical sciences. Trends Biochem Sci 41(11):954–969
Zemb O, Achard CS, Hamelin J, De Almeida ML, Gabinaud B, Cauquil L, Verschuren LM, Godon JJ (2020) Absolute quantitation of microbes using 16S rRNA gene metabarcoding: a rapid normalization of relative abundances by quantitative PCR targeting a 16S rRNA gene spike-in standard. Microbiologyopen 9(3):e977
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gaur, P. et al. (2023). Role of Omics Approach in the Toxicity/Safety Study of Cosmetics. In: Pant, A.B., Dwivedi, A., Ray, R.S., Tripathi, A., Upadhyay, A.K., Poojan, S. (eds) Skin 3-D Models and Cosmetics Toxicity. Springer, Singapore. https://doi.org/10.1007/978-981-99-2804-0_12
Download citation
DOI: https://doi.org/10.1007/978-981-99-2804-0_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2803-3
Online ISBN: 978-981-99-2804-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)