Abstract
Because of the unique features of a derivative of allotropic carbon graphite that has been known as graphene oxide, a number of unique optical, electrical and thermal breakthroughs has come into limelight. This has made graphene oxide as the material having the most intriguing nature which is still under investigation. Furthermore, apart from just a precursor for the manufacturing of graphene, researchers have discovered a plethora of unique optical, electrical, and chemical characteristics of graphene oxide that may be used in a variety of applications. The synthesis of GO, its structure and characterisation along with its functionalization and GO applications are the subject of this chapter. Additionally, we have discussed the use of GO in environmental, medicinal, and biological applications, freestanding membranes, and diverse composite systems. The synthesis of graphene oxide and its nanocomposite based on novel nanoparticles will be covered in this chapter. A brief overview has also been provided, with a focus on the use of graphene oxide and its nanocomposite in various fields, particularly waste water treatment of water.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Graphene oxide is the oxidised form of graphene with disrupted lattice as compared to the lattice of the pristine graphene. For the first time oxidation of graphite has been studied in the mid-nineteenth century by Brodie in 1860 when graphite was treated with potassium chlorate (KClO3) and fuming nitric acid (HNO3) [5]. Later in 1898 Staudenmaier improved this approach for oxidising graphite by slowly adding the potassium chlorate to a solution containing concentrated sulphuric acid, concentrated nitric acid (63%) and graphite [59]. Researchers, however, deemed this approach unsafe as the mass ratio of potassium chlorate with respect to graphite is quite high, along with that the method is also time consuming. After more than half-century Hummers and Offeman in 1958 gave a safe method of graphene oxide synthesis, based on a water-free mixture of concentrated sulphuric acid, sodium nitrate, and potassium permanganate known as the Hummers method [49]. Temperatures of only 45 °C are necessary for this approach, and the entire reaction took only two hours to finish. Literature-based on graphene oxide states that Brodie, Staudenmaier, and Hummer proposed three methodologies which were later considered to be the main approaches for the synthesis of graphene oxide via the oxidation of graphite, resulting in the formation of exfoliated graphite sheets with oxygen based functional groups attached to it. The structure of graphene oxide constitutes nonstoichiometric geometry having various oxygen based functional groups such as hydroxyl, epoxy, carboxyl groups and their bonding to carbon atoms in the graphene layer. Since the functional groups are having electron pairs which makes graphene oxide as negatively charged in nature. Thus, graphene oxide sheets disperse well in water due to the negative surface charges on the sheets that arise from the phenols, epoxy, hydroxyl, carbonyl and carboxylic acid groups present on graphene oxide and keep it from aggregating [23]. Due to the hydrophilic nature of graphene oxide, intersheet separations range from 0.6 nm to 1.2 nm [6]. Not only in water, but Graphene oxide is also dispersible in a wide range of organic solvents, such as N,N dimethylformamide, N-methyl-2-pyrrolidone, Tetrahydrofuran and ethylene glycol [45].
Initially there have been five proposed models of Graphene oxide but at last the final model of graphene oxide comes out to be the collective model of Scholz-Boehm’s and Ruess’ models. Graphene oxide on reduction gives rise to graphene thus it is used to synthesise graphene by means of a reducing agent like sodium borohydride (NaBH4), hydrazine and dimethylhydrazine [55, 65]. In pure anhydrous hydrazine, maximum percentile carboxyl groups attached with the lattice carbon atoms of graphene oxide are reduced which makes graphene formation by anhydrous hydrazine an effective method. However, the former use of pure hydrazine for the formation of graphene shows effective and impressive results but the nature of hydrazine in this methodology should be anhydrous which requires a dry-box that act as a limitation that hinders the large-scale production. Graphene oxide can also be used to form graphene by reducing graphene oxide via electrochemical method in which a sharp increase in current is employed that give rise to electrochemical reduction of the graphene oxide.
Exfoliation of graphite is not only done by chemical means but also by thermal means. In case of thermal expansion of graphite oxide, the decomposition rate of epoxy and hydroxyl sites of graphene oxide becomes larger than the rate of diffusion of the evolved gases and this causes a build-up of pressure which overcomes the van der Waals forces that bind the graphite sheets together and exfoliation occurs resulting in the formation of graphene oxide [40]. The carbon atom has different hybridization due to which graphene and graphene oxide exhibit different electrical nature. The functionalised graphene sheets are electrically conductive due to sp2 hybridization while graphene oxide act as an insulator because of sp3 hybridization [52].
Graphene oxide is a two-dimensional crystal structure formed by a flat monolayer of carbon atoms arranged in a hexagonal lattice [42]. Graphene oxide has been a source of contention in recent years, as it represents a reasonable starting material for the mass manufacture of graphene. Additionally, Graphene oxide Nano sheets have recently attracted a great deal of attention due to their unique chemical and physical properties that highlight its potential as a promising material for biological applications such as bio functionalization [74]. Graphene oxide nanosheets are interesting materials for enzyme immobilization due to their large specific surface area and abundant functional groups [37]. Depending on the degree of oxidation Graphene oxide acts as a semiconductor or insulator. This behaviour of graphene oxide attracted attention towards its chemical structure [47], electronic properties [74], reductive nature [46] and chemical modification or functionalization [7]. The electronic properties of graphene oxide mainly depend on the oxidation level and chemical composition which can be tailored by removal or addition of certain oxygen groups to adjust the proportion of sp2 and sp3 carbon [30]. The electrical, mechanical, and electrochemical characteristics of graphene oxide are all influenced by the oxygenated groups present in it. Because of the polar oxygen functional groups, graphene oxide exhibit hydrophilic nature due to which graphene oxide shows dispensability in water and hence form stable graphene oxide dispersion, making it possible to make thin conductive films by means of drop-casting, spraying, or spin-coating [31]. Additionally, these functional groups act as sites responsible for functionalization of graphene oxide. Thus, the chemical composition of graphene oxide makes it possible to tune its physicochemical properties and makes graphene oxide a promising nanomaterial to fabricate electrochemical and electro analysis sensors.
Graphene consists of a one-atom-thick planar sheet comprising sp2 bonded carbon structure with exceptionally high crystal and electronic quality [20]. Graphene has been making a profound impact in many areas of science and technology due to its remarkable physicochemical properties like high specific surface area [64] strong mechanical strength and excellent thermal and electrical conductivities [4]. These unique physicochemical properties make graphene useful for critical improvements in the field of electrochemistry.
Apart from graphene oxide and graphene there exist a partially reduced form of graphene oxide that has layered lattice of carbon atoms similar to that of graphene oxide but the all the carbon atoms of lattice plane in reduced graphene oxide are not attached with oxygen-containing groups, rather of all the carbon atoms of lattice plane, some of them are sp2 hybridised while some of them are sp3 hybridised. Therefore, based on the observations it has been concluded that graphene oxide is a single atomic layered nanomaterial synthesized by the oxidation of graphite [18], and has property of water dispersibility. However, the partly reduced graphene oxide consist of carbon atoms having sp3 as well as sp2 hybridisation. Some other names have also been given to reduced graphene oxide, such as functionalized graphene, chemically modified graphene, chemically converted graphene, or reduced graphene [16].
Graphene oxide can be synthesized using graphite, an allotrope of carbon that act as raw material through cost effective chemical methods with a high yield. Since, graphene oxide is hydrophilic in nature therefore it forms stable water colloids and makes it possible for the construction of macroscopic structures by economical processes. The graphene oxide sheets consist of sp3 carbon atoms which are attached with oxygen based functional groups that are oriented either above or below the lattice plane. Because of the structure deformation and the presence of covalently bonded functional groups, graphene oxide sheets are atomically rough. Several researchers have studied the surface of graphene oxide and observed highly defective regions, probably due to the presence of oxygen, and other areas are nearly intact [11]. According to a report the carbon atoms of graphene oxide attached to functional groups are slightly displaced, but the overall size of the unit cell in graphene oxide remains similar to that of graphene [44].
2 Synthesis of Graphene Oxide
The synthesis methods of graphene oxide include two main techniques that are termed as, Top-down approach & Bottom-up approach.
2.1 Top-Down Approach
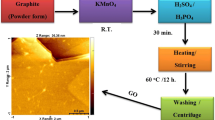
Synthesis of graphene oxide based on top-down approach includes the principle of separation of various graphite layers by overcoming the van der Walls forces of attraction between them. A schematic representation showing top down approach is depicted here in Fig. 1.
The major synthesis routes which come under this category are chemical exfoliation and electrochemical exfoliation. Chemical exfoliation employs appropriate liquid environment and temperature to exfoliate graphite into individual layers via oxidation. This technique can be considered to be divided into three steps:
-
(a)
Dispersion of graphite
-
(b)
Exfoliation of graphite into single and multi-layer graphene oxide
-
(c)
Purification which separates single and multilayer graphene oxide generally by centrifugation.
The graphite exfoliation by chemical means is economical and appropriate for the large scale synthesis. Further for applications like composites [24] thin films [22] and ink [64], this method gained wide popularity. The major limitations of this technique are the limited size of synthesized graphene oxide sheets and the presence of defects and non-exfoliated graphene oxide sheets [43]. Based on the principle of chemical exfoliation, graphene oxide can be synthesized by using methods developed by Brodie, Park and Hummers. The quality of graphene oxide can be improved by means of thermal exfoliation under constant pressure [40]. Graphene oxide synthesized by chemical oxidation method contains many functional groups which make them ideal for making composites [58]. This technique produces large size flakes, controllable solution processing and can be scaled up for industrial synthesis. Graphene oxide synthesised from top down approach is used in different applications in its actual as well as in its reduced form like electrochemical glucose sensor, transparent conducting films, electrodes, solar cells, and ultrafast lasers [17, 66]. The time-consuming nature of this technique and implication of hazardous chemicals like hydrazine hydrate, sulphuric acid during the reduction and oxidation process makes this approach non eco-friendly.
2.2 Bottom-Up Approach
This type of approach refers to the building of material from constituent particle by constituent particle where, constituent particle includes atom, molecule or cluster. In the bottom-up approach, one starts with employing carbon molecules usually obtained from different sources as precursors [68]. The schematic representation showing concept of bottom up approach is shown below in Fig. 2.
Graphene oxide is usually prepared by various methods that are based on this approach, and are known as Hummers’ method or modified Hummers’ methods. Since, top-down approaches employs strong oxidizing agents, to overcome this drawback, synthesis of graphene oxide is done by the bottom-up approach in such a way that graphene oxide so formed will be of required thickness (as per the need) ranging from 1 to 1500 nm. The lateral sizes of the monolayer and few-layer graphene oxide are about 20 and 100 mm respectively [27].
In bottom up approach the graphene oxide is prepared by a hydrothermal method. In this method glucose is used as a sole reagent, that utilizes the bottom-up approach to synthesize graphene oxide with controllable number of layers ranging from monolayer to multilayers, having tuneable properties that have been achieved by thermal annealing of sample using Rapid Thermal Processor so that the amount of oxygen present in graphene oxide can be controlled. This method is advantageous over other methods of top-down approaches as it is environmentally friendly, facile, low-cost as well as capable of scaling up for mass production [63].
Since graphene oxide is an emerging area of research, a large number of articles as well as reviews have been published exploring different synthesis approaches to graphene oxide. Each synthesis technique specializes in various properties of graphene oxide like dimensions, layers, conductivity, quality, cost-effectiveness, and so on. The methods used for the synthesis of graphene oxide include chemical and electrochemical exfoliation of graphene oxide and by means of a hydrothermal method based on bottom-up technique using glucose as a sole reagent [33, 63].
Since, functionalisation of graphene oxide has a risk on specificity, greater loading capacity, solubility, stability, and biocompatibility. Therefore, researchers have implemented a various level of interactions like covalent bonding of the functional molecules with the graphene oxide basal planes, defects and edges of graphene oxide sheets, noncovalent adsorption via hydrogen bonding, π-π stacking, electrostatic interactions, and van der Waals attractions in order to make graphene oxide synthesis more efficient [12]. Considering the above factors various chemical and electrochemical methods have been proposed for the synthesis of graphene oxide which have been discussed in the following section below.
2.2.1 Chemical Methods
There are various chemical approaches reported to extract graphene oxide from graphite. The possibility of producing graphene oxide from natural graphite by chemical approach was first pointed out by Horiuchi et al. during their attempt to produce carbon Nano films [25]. The method consists of the oxidation process, dilution in methanol and centrifugation. Another chemical method was developed using sulphuric acid and nitric acid which introduce intercalate into the layers of graphite. Then the graphite is followed by rapid heating at 1000 °C which results in the formation of the thin oxidised graphitic sheet. Chemical exfoliation from graphite crystal is a widely accepted technique to produce graphene oxide. This method is capable of producing low-cost graphene oxide in large quantities. Typically, the method includes oxidation followed by thermal expansion of oxidised graphite [39]. This results in the formation of graphene oxide having covalently bonded functional groups namely hydroxyl, epoxide groups, that are bonded to the basal plane of the graphene oxide sheets, and carboxyl groups that occupy the edges of the lattice planes. Due to the presence of such functional groups graphene oxide sheets exhibit hydrophilic nature and hence form negatively charged colloidal suspension.
Reduction of graphene oxide can be reduced to form graphene. The method is advantageous because of its scalability, large volume of production and effectiveness in multipurpose functionalization, but the higher number of defects is the main limitation of the method. In the chemical methodology, one of the reliable techniques is used which is called a modified Hummers’ method. Hummers’ method is a chemical process in which graphite is subjected to oxidation, with strong oxidising agents namely nitric acid, potassium permanganate and sulphuric acid, which results in an increment of spacing between two consecutive layers of graphite from 0.335 nm for to more than 0.625, nm resulting in the synthesis of large quantities of one atom thick graphene oxide. B. C. Brodie for the very first time synthesized graphene oxide in 1859 by oxidising slurry of graphite in the presence of fuming nitric acid and potassium chlorate. Later, in 1898, Staudenmaier developed this method by introducing concentrated sulphuric acid to the above-mentioned reaction system and this work opened a new systematic approach towards graphene oxide synthesis. In 1958, the scientist William S. Hummers reported an alternative method for the synthesis of graphene oxide by using KMnO4 and NaNO3 in concentrated H2SO4, which came to be known as modified Hummers’ method [55]. This method is economically favourable, but a significant number of defects is the limitation of the method. The typical experimental procedure of modified Hummer’s method is as follows:
-
1.
Five grams of graphite powder and 2.5 g of NaNO3 dispersed in 115 ml H2SO4 (98%) as an intercalating agent and stirred for 2 h.
-
2.
Then 15 gm of ground KMnO4 added gradually to the above solution. The temperature of the solution was kept less than 20 °C by keeping it in the ice bath then the solution is a deep oily green colour.
-
3.
Next, the mixture was stirred at 35–37 °C for 2 h. The solution colour in the medium temperature stage is like thick brown paste.
-
4.
The resulting solution is diluted gradually by adding 200 ml of deionized water under vigorous stirring to get a reddish-brown suspension of graphite oxide solution with the temperature at this stage being 95 °C keeping constant stirring for 1 h.
-
5.
The suspension is then treated further by adding 30 ml of 30% H2O2 solution and 350 ml of distilled water. The bright orange colour of graphene oxide suspension will appear after adding the peroxide solution (H2O2 convert the residual permanganate to soluble manganese sulphate and the solution colour changed to yellowish-brown indicating the oxidation of graphite).
-
6.
The suspension was first washed with 5% hydrochloric acid (HCl) in order to remove the sulphate ions attached with the graphene oxide and then with DI until the pH of suspension became neutral by removing the attached salt impurities.
-
7.
After maintaining the pH at 7 the thick brown sticky precipitate is dried in temperature not exceeding the limit of 60 °C.
-
8.
After drying, the precipitate is ground and finally, the silver-brown powder of graphene oxide is ready to use.
Transmission Electron Microscopy is used to determine the structure of graphene oxide that has been found similar to that of the structure discussed in the former. Figure 3 exhibits TEM image of graphene oxide along with SAED pattern representing its crystalline nature.
2.2.2 Electrochemical Methods
Electrolysis is an important phenomenon occurring due to the chemical effect of electric current. The effect produced on metallic conductors due to the flow of electric current heavily differs from the effect produced by the electric current in an ionic solution called electrolyte. The conduction of electric current through a metallic conductor is due to the drifting of free electrons and hence chemically or physically no change occurs except the generation of heat. At the same time, the conduction of electric current through an electrolyte is due to the movement of ions and hence that will be associated with chemical changes. In a typical electrolytic experiment, two metal rods or graphite rods are used as electrodes which are connected to the positive and negative terminal of the battery and are known as anode and cathode respectively. These two electrodes are immersed in an ionic solution called electrolyte. An appropriate dc voltage is provided across the electrodes by connecting the battery. The dissociation process of an electrolyte occurs and the dissociated ions namely cation and anion will move towards the electrodes.
Electrolytic synthesis is a reliable, cost-effective and eco-friendly method for the synthesis of nanomaterials. A wide range of nanomaterials can be synthesized using this technique. An electrochemical approach for the synthesis of nanomaterials was first effectively introduced and studied in detail by Reetz and Helbig in 1994. For the formation of metallic particles, Reetz et al. dissolved a metal sheet (anodic reaction) to form an intermediate metal salt which was reduced at the cathode. In the reaction, Tetraalkylammonium salts were used as a stabilizer. Later as motivation from above work, Rodigrigous Sanchez et al. successfully synthesized silver nanoparticles using acetonitrile containing tetrabutylammonium salt. The synthesis of high purity nanoparticles with controlled size without requiring any expensive equipment or vacuum is considered as the main advantage of the electrochemical route. The features like reliable operation, possibly in size and shape control, high yield and absence of unwanted side products, etc. increased the acceptance of electrolysis method among researchers. The method is especially advanced in the synthesis of metallic nanostructured materials and is also used for the synthesis of graphene sheets [34]. The electrochemical synthesis of graphene oxide includes two graphite rods as electrodes, the ionic solution as an electrolyte and a constant dc voltage as a power supply. When a voltage is applied across the graphite rods, the anode gets structural expansion due to the intercalation of anions and hence exfoliated graphene oxide sheets form by this oxidation process [57]. Thus, on electrolysis, the graphene oxide sheets are obtained at graphite made anode electrode.
3 Properties of Graphene Oxide
Graphene oxide sheets disperse well in water due to the negative surface charges on the sheets and keep it from aggregating [23]. Also due to the hydrophilic nature of graphene oxide, it shows variable inter sheet separations ranging from 0.6 to 1.2 nm [6]. Inspite of having hydrophilic nature, graphene oxide can dissolve in a wide range of organic solvents such as N-methyl-2-pyrrolidone, THF, N, N dimethylformamide and ethylene glycol. Graphene oxide can act as a semiconductor or in some cases as an insulator depending upon the parameters of the system and their optical as well as electronic properties, as per their requirement, can be modified.
The structure of graphene oxide is of nonstoichiometric nature [28]. Graphene oxide has a carbon network consisting of two kinds of regions i.e. trans-linked cyclohexane chairs and ribbons of flat hexagons with C–C double bonds as well as functional groups [62]. Being a 2D structure with various oxygenated functional groups, graphene oxide exhibits various excellent qualities in the electrochemical, optical, mechanical, thermal and chemical reactivity fields. Depending on the chemical & atomic structures, structural defects and presence of sp3 hybridised carbon atoms, graphene oxide shows electronic properties such as conductivity. Graphene oxide shows insulating nature with a sheet resistance of 1012 Ω sq−1 or higher due to the sp3 hybridised C–O bonding [54]. However, the reduction of graphene oxide results in a decrease in sheet resistance by several orders of magnitude and hence transforms the material into a semiconductor and ultimately into a carbonaceous semimetal [21]. The conductivity of the reduced graphene oxide is approximately equal to 1000 S/m and the activation energy of the graphene oxide has been estimated to be 32 ± 5 kcal/mol. In addition to these interesting electronic properties, graphene oxide also exhibit optical properties due to which it shows photoluminescence in Uv–Visible wavelength range which changes with reduction of graphene oxide and makes it useful for biosensing, and optoelectronic applications [14]. Graphene oxide shows electrochemical properties due to its electron mobility and unique surface properties which make it be used efficiently in electrical wires of the redox centres of several heme-containing metalloproteins [56]. Graphene oxide also possesses excellent electrocatalytic properties toward oxygen reduction and certain biomolecules [38]. Graphene oxide exhibits high electrochemical capacitance with excellent cycle performance and hence has potential application in ultra-capacitors [8]. Due to the presence of a large number of oxygen-containing functional groups and structural defects, graphene oxide exhibits enhanced chemical activity [15]. One of the most important reactions of graphene oxide is its reduction which can be done using various reducing agents like hydrazine, sodium borohydride or hydroquinone in liquid medium [50]. One more significant chemical reactivity of graphene oxide is its capacity regarding chemical functionalization that involves introducing other groups to graphene oxide sheets via chemical reactions based on in-situ technique and ex-situ technique. For chemical functionalization, various oxygen-based groups attached to graphene oxide are subjected to orthogonal reactions. Typically, graphene oxide can be covalently functionalized using specially selected small molecules or polymers through activation and amidation/esterification of either the carboxyls or hydroxyls in graphene oxide via coupling reactions [69]. The noncovalent functionalization of graphene oxide is achieved by π–π stacking, van der Waals interactions, or hydrogen bonding graphene oxide can also be used as carbocatalyst for facilitating oxidation and hydration reactions and hence can be used to catalyse the oxidation of alcohols and alkenes and for the hydration of various alkynes into their respective aldehydes and ketones [48, 19]. Also graphene oxide shows strong oxidizing properties which makes it liable to use for oxidation in a broad range of reactions like oxidation of olefins to diones, methyl benzenes to aldehydes, diarylethenes to ketones, and various dehydrogenations [29].
4 Applications of Graphene Oxide
Due to antimicrobial properties and low toxicity graphene oxide has been proposed for the use as antimicrobial agents. The functionalized graphene oxide could inhibit Herpes Simplex Virus Type-1 infection through cell attachment inhibition at low concentrations [51]. A graphene oxide/carbon nanotube-based nanocomposite has been created and is being used in the treatment of trichomoniasis sickness. This due to the fact that nanocomposite formed a stable colloidal solution and interacted with the cell membrane of the protozoan trichomonas foetus strongly. Therefore, the hybrid nanocomposite might be an excellent candidate for a drug carrier, which could be used to deliver therapeutic agents to treat trichomoniasis disease [71]. Graphene oxide sheets has high potential for biological applications which makes it possible to use graphene oxide biofunctionalization like enzyme immobilization. For example, cross-linking of amino groups on pectinase and functional groups and site-specific interactions can be used to immobilize acid pectinase and Chloroperoxidase on graphene oxide Nanosheets acting as the matrix. The system showed higher activity, better thermal stability, and remained active in basic medium and at higher concentrations of oxidant in comparison with the free enzyme [14]. α-Amylase immobilized on graphene oxide sheets also exhibits enhanced thermal and storage stability. The application fields of graphene oxide are mainly focused on sensor and drug delivery [56]. A number of graphene oxide based electronic devices have been fabricated that are now implemented in the medicinal field for diagnosis, sensing and monitoring of drugs. One such device is a graphene-based field-effect transistor (FET) [26]. Another example is fluorescent-based biosensors that have been employed for the detection of DNA and proteins, with the potential for improved HIV diagnosis. One more remarkable application of graphene oxide is that folic acid-functionalized graphene oxide helps in detecting human cervical cancer and human breast cancer cells [69]. Graphene oxide is also used to make glucose sensors that use dc power. One of the primary ways by which graphene oxide is anticipated to be used is to create conductive transparent films that can be put on any surface. Such coatings could be used in flexible electronics, solar cells, liquid crystal devices, chemical sensors, and touch screen devices [8]. One such example is the transparent electrode of graphene oxide that has been used as a hole transport layer in polymer solar cells and LEDs [50]. Graphene oxide has an extremely high surface area which makes it useful as an electrode in batteries and double-layered capacitors, as well as in fuel cells and solar cells [2]. Due to its ability to store hydrogen, graphene oxide based nanocomposite can also be employed in fabricating high-capacity energy storage devices like lithium ion batteries. Anticancer medications have been delivered using functionalized graphene oxide. Furthermore, since, the interlayer distance of dried Hummers graphite oxide was 6.35 A°, but it rose to 11.6 A° in liquid water. This swelling of graphene oxide structures allows a water penetration channel between individual graphene oxide layers, which is attributed to water permeation and water diffusion across the membrane at a rate of 0.1 mg/min/cm2 and 1 cm/h respectively. These property of graphene oxide makes it suitable for using as a potentially active nanomaterial for water desalination in 1960, as the membranes of graphene oxide in a reverse osmosis process shows 90% retention rate for NaCl solutions, and as the cation exchange membrane especially for the case of massive alkaloid ions. Graphene oxide based polymer nanocomposites exhibit enhanced properties like elasticity, tenacity, thermal stability and conductivity as compared to the properties of the original polymer that is used as the matrix for nanocomposite. Keeping in view of this extraordinary behaviour of nanocomposite, it is used in the form of stabilized paper-like structures specifically for hydrogen storage applications, ion conductors and Nanofiltration membranes [48].
5 Economical Aspects of Graphene Oxide
Due to the unique and exceptional electrical, optical, mechanical, and chemical capabilities of graphene and graphene oxide, these materials have received a lot of attention in the last decade [70]. Keeping in view of their applications the economical aspect of graphene oxide has been a great centre of concern. Graphene Oxide used in high-performance energy generation and storage technologies. Researchers have harnessed graphene oxide’s unusual capabilities to build revolutionary electronic materials such as transparent conductors and ultrafast transistors. Understanding graphene oxide’s numerous chemical characteristics has recently facilitated its use in high-performance energy generation and storage technologies [13]. The excellent 2D planar structure, large surface area, mechanical as well as chemical stability, superconductivity and biocompatibility of graphene oxide, have been extensively investigated as some of the most promising in biomedical applications. These characteristics suggest that they could be useful in the development of sophisticated drug delivery systems and the delivery of a wide range of treatments [35]. A graphene oxide based drug delivery system can also achieve co-distribution of numerous drugs or genes with improved chemotherapeutic efficacy, thanks to the specialised design of molecular structure of matrices. It was discovered when two anticancer medicines, doxorubicin (DOX) and camptothecin (CPT), were simultaneously loaded onto the Folic Acid-conjugated graphene oxide, via p–p stacking and hydrophobic interactions. Folic acid—graphene oxide delivered two medicines simultaneously that had superior targeting efficacy and cytotoxicity than graphene oxide that simply delivered DOX or CPT [72]. It indicates that the usage of functionalized graphene oxide for controlled loading and targeted delivery of multiple drugs, can improve the therapeutic efficacy because of the increased surface area and surface functionality of graphene oxide. Also, the pi-pi stacking interactions of graphene oxide GO could be useful in successfully creating reactive oxygen species (ROS) that can destroy cancer cells when exposed to radiation [41].
The graphene oxide nano-walls generated by a chemical exfoliation technique by electrophoretic deposition of Mg2+ on graphene oxide nanosheets are found to have antibacterial activity. The cell membrane injury of the bacteria generated by direct contact of the bacteria with the extremely sharp edges of the nanowalls was discovered to be the effective mechanism in the bacterial inactivation based on monitoring the outflow of cytoplasmic components of the bacteria. Gram-negative Escherichia coli bacteria with an outer membrane were more resistant to nanowall-induced cell membrane disruption than Gram-positive Staphylococcus aureus bacteria without an outside membrane. Furthermore, hydrazine-reduced graphene oxide nanowalls were more hazardous to bacteria than unreduced graphene oxide nanowalls. The better charge transfer between the bacteria and the more sharpened edges of the reduced nanowalls during the contact interaction was attributed to the reduced nanowall’s enhanced antibacterial activity [1]. An experiment conducted on antibacterial activity of four types of graphene-based materials graphite, graphite oxide, graphene oxide, and reduced graphene oxide against Escherichia coli as a bacterial model. Graphene Oxide dispersion had the strongest antibacterial activity (under similar concentration and incubation circumstances), followed by reduced graphene oxide, graphite and graphite oxide. Both membrane and oxidative stress may play a role in bacterial cytotoxicity as graphene materials with a higher density of functional groups and smaller sizes have a greater likelihood of interacting with bacteria, leading in cell deposition. Graphene oxide nanosheets can cause membrane stress by disrupting and destroying cell membranes, resulting in cell death when they come into close contact [36].
Graphene oxide can be used as a catalyst as it has an extraordinary catalytic capability on its own and in combination with another substance. It's an ideal environment for molecular engineering. Graphene oxide can act as an oxidant during anaerobic oxidation and is decreased at the end of the first catalytic cycle due to its many oxygen atoms. During aerobic oxidation, however, partly reduced graphene oxide can continue to activate molecular oxygen. Organic dyes or organo-catalysts can also be used to hybridise graphene oxide. Dye-induced photosensitization and easy charge transfer across the graphene contact have a synergistic effect that improves catalytic conversion [60].
The single-layer graphene oxide sheets sized down to a few nanometres in lateral width have been used to develop techniques for cell imaging in biological systems. The functionalization chemistry is implemented in order to impart solubility and compatibility of nano-graphene oxide in biological environments. The obtained size separated pegylated nano-graphene oxide sheets are soluble in buffers and serum without agglomeration. The nano-graphene oxide sheets are found to be photoluminescent in the visible and infrared regions. The intrinsic photoluminescence (PL) of nano-graphene oxide is used for live cell imaging in the near-infrared (NIR). One technique for producing photoluminescence in graphene materials is by the induction of energy band gaps by reducing graphene to finite sizes or by forming sp2 islands, while the other involves the production of defects [61]. In the photoluminescence emission of nanoscale graphene materials, both processes are usually active at the same time. This is due to the fact that these materials are often made by extensive oxidising graphene cutting, followed by reduction to reduced graphene oxide. Chemical treatments invariably result in structural defects. Any sites that are not excellent sp2 domains are referred to as defects. The defect-derived photoluminescence is similar to that of other carbon materials, such as carbon dots, in terms of mechanism [10]. Owing to its small size, intrinsic optical properties, large specific surface area, low cost, and useful non-covalent interaction they are effective in imaging [61].
Due to the unique properties of graphene oxide, the graphene oxide based materials have been used to generate a number of biosensors using a variety of sensing modes, including electrochemical and optical signalling. The electrochemical approach has received a lot of interest for biomolecule detection as one of the best methods due to its ease of operation, quick response, low cost, and high sensitivity. Graphene oxide based electrodes for detection fields can produce high-sensitivity electrochemical sensors because graphene has shown significant efficiency [3].
6 Functionalisation of Graphene Oxide with Metal Nanoparticles
The synthesis of graphene oxide/nanoparticle hybrids is categorized into two basic categories i.e. In-situ technique and Ex-situ technique. In the former approach, the crystalline nanoparticles are formed in the presence of graphene oxide and direct development or growth of nanostructures on graphene oxide sheets is obtained. On the other hand, in the ex-situ technique, nanostructures with desired shape and size are first synthesised, followed by the functionalisation of the graphene oxide sheets with nanoparticles to form a graphene oxide-based nanocomposite [34]. Figure 4 represents the TEM image of graphene oxide nanocomposite which is functionalised by gold nanoparticles.
There are many synthesis techniques reported under these approaches. However, the two main synthesis techniques that have been used for the functionalisation of graphene oxide to form graphene oxide-based nanocomposites are; hydrothermal method and electrochemical method.
The hydrothermal technique adapted from mineral formation occurring in nature has been used as an efficient method for the synthesis of inorganic nanomaterials. In nature generally, minerals are formed under specific pressure and temperature in the presence of water. The possibilities of a hydrothermal method for the artificial synthesis of inorganic compounds were first commercially explored by Karl Josef Bayer in 1892. Bayer converted aluminium hydroxide to Al2O3 by hydrothermal method. Today, the hydrothermal method has conquered the major fields of science such as nanotechnology, medical research, etc. due to its attractive properties like reduced contamination, the low temperature of synthesis, etc. The hydrothermal method has found its application in the field of crystal growth, thin-film preparation, material processing, etc.
Typically, hydrothermal reactions are carried out in an autoclave. The simple scale type autoclave uses a 50–100 ml Teflon lined vessel for carrying the precursors and is designed to work at low pressure and low temperature (around 300 °C and 1000 bars).
The hydrothermal method is capable of reducing graphene oxide. Moreover, in the hydrothermal method, formation of nanoparticles from its precursors and functionalization of the nanoparticles on reduced form of graphene oxide, both are simultaneously possible. Superheated H2O facilitates acid-catalyzed reactions during the hydrothermal process, which results in an increase in the restoration of the -conjugation network inside the graphene oxide sheets owing to the availability of suitably high H+ concentration. This results in the formation of reduced graphene oxide from graphene oxide. The main advantages of the hydrothermal method are that it has high production efficiency and the possibility for synthesising nanoparticles with a high crystalline degree. The requirement of high temperature and need of a long time is the limitations of the hydrothermal method [53, 67].
The electrochemical approach is also used for the functionalization of nanoparticles with graphene oxide by using different electrolytes. This method is considered as advantageous as it is reliable and cost-effective. Although the in-situ functionalization is advantageous because of its efficiency, simplicity and cost-effectiveness, it has certain limitations, one of which is tuning the morphology of the nanoparticles. Keeping in view the above merits and limitations of in situ technique, ex situ technique of graphene oxide functionalization for the fabrication of nanocomposite have been proposed. In ex situ technique the noble metal based nanocomposites are prepared using nanoparticles which are not dispersed directly into the graphene oxide matrix. Although the ex situ approach is an excellent one, its limitation acts as a challenge during nanocomposite synthesis especially for nanoparticles having higher dispersibility in the matrix of graphene oxide. In order to solve these problems sonication methods if needed have been employed as per the requirement, to get complete and stable dispersion of the nanoparticles in the matrix.
7 Graphene Oxide Nanocomposites
Nanotechnology is an emerging technology, which deals with synthesis, characterization, and manipulations in the nanometre scale, which leads to various applications of material science, engineering, and devices. The main attraction of nanotechnology is its tendency to approach material to explore the unique physical and chemical properties of a material. Generally, for nanoparticles, at least one phase of the particle must be in a range of size 1–100 nm. The larger surface-volume ratio compared to bulk materials makes nanoparticles as authentic structures to fascinate the chemical transformations. The nanomaterials are bulkily classified as environmental and engineered. Metal oxides and metal sulphides are commonly found as minerals in nature and are termed as environmental. Artificially synthesized nanoparticles from their precursors and other basic units of the materials are called engineered nanoparticles. Recent trends in research are mainly based on four different types of engineered nanoparticles. They are metal-based nanoparticles, carbon-based and Nano polymers (constructed from pieces of different Nano molecules—also called dendrimers). Today, advanced research is concentrated on a combination of various engineered nanoparticles to form nanocomposites. Basically, nanocomposites are a mixture of different nanoparticles in which one type of nanoparticles (size in a range of 1–100 nm) will be dispersed on or attached to larger, continuous matrices. Hence the nanocomposites contain two parts, one is the continuous phase and the other is discontinuous dispersed phase [32]. An enhancement in the properties of a nanomaterial is indented by the formation of its nanocomposite. Any combinations of materials can be designed for the fabrication of nanocomposites and they are bulkily classified into three basic building blocks; they are metals (metal oxides), polymers and ceramics. The composites can have different dimensions such as core–shell (0D), nanowires (1D), lamellar or sheet-like structures (2D) and metal matrix composites (3D) [9]. The schematic representation of nanocomposite is shown as below in Fig. 5.
To utilize the full potential of unique properties of graphene oxide, one possible route is by making its composites with other materials. The major requirement of making composites is the availability of processable graphene oxide in large amount and the two popular ways to achieve it are:
-
1.
Chemical and Electrochemical exfoliation of graphite.
-
2.
By utilizing the bottom-up assembly technique and glucose as a sole reagent in the hydrothermal method.
These methods have advantages of functionalization and tuning of properties of graphene oxide depending on the desired applications. Graphene oxide composites are broadly classified into two categories:
-
1.
Graphene oxide/inorganic composites
-
2.
Graphene oxide/polymer composites.
Depending on the material other than graphene oxide, graphene oxide/inorganic composites can be further classified into graphene oxide/metal, graphene oxide/carbon and graphene oxide/noble metal nanocomposites.
Noble metal nanoparticles have unique optical and chemical properties which are completely different from their bulk counterparts. These properties are a function of size, shape, composition and structure of nanoparticles. They have found a wide variety of applications viz. catalysis, electronics, sensors and biomedical [73]. Incorporation of noble metal nanoparticles in carbonaceous materials further enhances their chemical reactivity, catalytic behaviour and electrical properties. Among all carbonaceous materials, graphene oxide has emerged as the most promising base matrix. The synthesis of graphene oxide/noble metal nanocomposites generally follows the reduction of metal salt in the presence of graphene oxide Nanosheets. Further graphene oxide, being two dimensional in nature, besides providing a base for nanoparticles also increases the effective surface area, which is helpful in enhancing the properties of noble metal nanostructures. The graphene oxide-based metal nanocomposites have found applications in biosensors, intracellular analysis, bio-distribution, bio-imaging, and gene mapping [2].
Although graphite and its intercalated compounds have been known and studied for more than a century it is only after the experimental discovery of wonder material graphene that carbon-based materials have created a cornucopia for many potential applications and new physics. There are a number of reports describing the utilization of graphene oxide-based composites in many applications. The present status of the ongoing research in the field graphene oxide-based bioinspired nanocomposite has exhibited a great potential. Both top-down and bottom-up approaches are available for the synthesis of noble metal nanoparticles composites but still, the non biocompatibility, self-assembly, aggregation, reproducibility, low through-output, control over size and shape of nanoparticles are major obstacles in their implication with respect to their reliability. Therefore a reliable substrate for the synthesis of nanocomposite is demanded till now. Two-dimensional nature, cost-effectiveness, bio-compatibility and the ability to absorb or form complexes with target molecules make graphene oxide an attractive candidate. So, it would be interesting to study the noble metal nanoparticles decorated with graphene oxide as composite [73].
References
Akhavan O, Ghaderi E (2010) Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 4(10):5731–5736
Ali MA, Singh C, Srivastava S, Admane P, Agrawal VV, Sumana G, John R, Panda A, Dong L, Malhotra BD (2017) Graphene oxide-metal nanocomposites for cancer biomarker detection. RSC Adv 7(57):35982–35991 https://doi.org/10.1039/C7RA05491B
Behbudi G (2020) Mini review of graphene oxide for medical detection and applications. Adv Appl NanoBio-Technol 1(3):63–66
Bonaccorso F, Lombardo A, Hasan T, Sun Z, Colombo L, Ferrari AC (2012) Production and processing of graphene and 2d crystals. Mater Today 15(12):564–589. https://doi.org/10.1016/S1369-7021(13)70014-2
Brodie BC (1860) Sur le poids atomique du graphite. Ann Chim Phys 59(466):e472
Buchsteiner A, Lerf A, Pieper J (2006) Water dynamics in graphite oxide investigated with neutron scattering. J Phys Chem B 110(45):22328–22338
Burress JW, Gadipelli S, Ford J, Simmons JM, Zhou W, Yildirim T (2010) Graphene oxide framework materials: theoretical predictions and experimental results. Angew Chem Int Ed 49(47):8902–8904
Cai B, Wang S, Huang L, Ning Y, Zhang Z, Zhang G-J (2014) Ultrasensitive label-free detection of PNA–DNA hybridization by reduced graphene oxide field-effect transistor biosensor. ACS Nano 8(3):2632–2638
Cury CPH, Gundappa SK, Fernando W (2009) Nanocomposites : synthesis, structure, properties and new application opportunities. Mater Res 12(1):1–39. https://doi.org/10.1590/S1516-14392009000100002
Cao LI, Meziani MJ, Sahu S, Sun Y-P (2013) Photoluminescence properties of graphene versus other carbon nanomaterials. Acc Chem Res 46(1):171–180
Castro Neto AH, Guinea F, Peres NMR, Novoselov KS, Geim AK (2009) The electronic properties of graphene. Rev Mod Phys 81(1):109–162. https://doi.org/10.1103/RevModPhys.81.109
Chen D, Feng H, Li J (2012) Graphene oxide: preparation, functionalization, and electrochemical applications. Chem Rev 112(11):6027–6053 https://doi.org/10.1021/cr300115g
Chung C, Kim Y-K, Shin D, Ryoo S-R, Hong BH, Min D-H (2013) Biomedical applications of graphene and graphene oxide. Acc Chem Res 46(10):2211–2224
Ding Y, Cui R, Hu M, Li S, Zhai Q, Jiang Y (2017) Well-oriented bioarchitecture for immobilization of chloroperoxidase on graphene oxide nanosheets by site-specific interactions and its catalytic performance. J Mater Sci 52(17):10001–10012
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39(1):228–240
Eda G, Chhowalla M (2010) Chemically derived graphene oxide: towards large-area thin-film electronics and optoelectronics. Adv Mater 22(22):2392–2415
Eda G, Lin Y-Y, Mattevi C, Yamaguchi H, Chen H-A, Chen I-S, Chen C-W, Chhowalla M (2010) Blue photoluminescence from chemically derived graphene oxide. Adv Mater 22(4):505–509
Geim AK, Novoselov KS (2010) The rise of graphene. In: Nanoscience and technology: a collection of reviews from nature journals. World Scientific, pp 11–19
Ghorbani M, Abdizadeh H, Golobostanfard MR (2015) reduction of graphene oxide via modified hydrothermal method. Procedia Mater Sci 11:326–330. https://doi.org/10.1016/j.mspro.2015.11.104
Gómez-Navarro C, Meyer JC, Sundaram RS, Chuvilin A, Kurasch S, Burghard M, Kern K, Kaiser U (2010) Atomic structure of reduced graphene oxide. Nano Lett 10(4):1144–1148
Han HJ, Chen YN, Wang ZJ (2015) Effect of microwave irradiation on reduction of graphene oxide films. RSC Adv 5(113):92940–92946
Hasan T, Torrisi F, Sun Z, Popa D, Nicolosi V, Privitera G, Bonaccorso F, Ferrari AC (2010) Solution-phase exfoliation of graphite for ultrafast photonics. Phys Status Solidi (B) Basic Res 247(11–12):2953–2957. https://doi.org/10.1002/pssb.201000339
He H, Klinowski J, Forster M, Lerf A (1998) A new structural model for graphite oxide. Chem Phys Lett 287(1–2):53–56
Hernandez Y, Nicolosi V, Lotya M, Blighe FM, Sun Z, De Sukanta I, McGovern T et al (2008) High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol 3(9):563–568. https://doi.org/10.1038/nnano.2008.215
Horiuchi S, Gotou T, Fujiwara M, Sotoaka R, Hirata M, Kimoto K, Asaka T, Yokosawa T, Matsui Y, Watanabe K (2003) Carbon nanofilm with a new structure and property. Jpn J Appl Phys 42(9A):L1073
Hu H, Onyebueke L, Abatan A (2015) Characterizing and modeling mechanical properties of nanocomposites-review and evaluation. J Miner Mater Charact Eng. https://doi.org/10.4236/jmmce.2010.94022
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339. https://doi.org/10.1021/ja01539a017
Inagaki M, Kang F (2014) Engineering and applications of carbon materials. materials science and engineering of carbon: fundamentals. https://doi.org/10.1016/b978-0-12-800858-4.00003-6
Jia H-P, Dreyer DR, Bielawski CW (2011) C–H oxidation using graphite oxide. Tetrahedron 67(24):4431–4434
Johari P, Shenoy VB (2011) Modulating optical properties of graphene oxide: role of prominent functional groups. ACS Nano 5(9):7640–7647
Kim F, Cote LJ, Huang J (2010) Graphene oxide: surface activity and two-dimensional assembly. Adv Mater 22(17):1954–1958
Lateef A, Nazir R (2017) Metal nanocomposites : synthesis , characterization and their applications. Sci Appl Tailored Nanostruct 239–56
Lee G, Kim KS, Cho K (2011) Theoretical study of the electron transport in graphene with vacancy and residual oxygen defects after high-temperature reduction. J Phys Chem C 115(19):9719–9725
Liu F, Choi JY, Seo TS (2010) Graphene oxide arrays for detecting specific DNA hybridization by fluorescence resonance energy transfer. Biosens Bioelectron 25(10):2361–2365
Liu J, Cui L, Losic D (2013) Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater 9(12):9243–9257
Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, Kong J, Chen Y (2011) Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5(9):6971–6980
Liu Y, Li Q, Feng Y-Y, Ji G-S, Li T-C, Jie T, Gu X-D (2014) Immobilisation of acid pectinase on graphene oxide nanosheets. Chem Pap 68(6):732–738
Liu Y, Yu D, Zeng C, Miao Z, Dai L (2010) Biocompatible graphene oxide-based glucose biosensors. Langmuir 26(9):6158–6160
Mattevi C, Eda G, Agnoli S, Steve Miller K, Mkhoyan A, Celik O, Mastrogiovanni D, Granozzi G, Carfunkel E, Chhowalla M (2009) Evolution of electrical, chemical, and structural properties of transparent and conducting chemically derived graphene thin films. Adv Func Mater 19(16):2577–2583. https://doi.org/10.1002/adfm.200900166
McAllister MJ, Li J-L, Adamson DH, Schniepp HC, Abdala AA, Liu J, Herrera-Alonso M, Milius DL, Car R, Robert K, Prud’homme RK, Aksay IA (2007) Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem Mater 19(18):4396–4404
Mousavi S, Zarei M, Hashemi S (2018) polydopamine for biomedical application and drug delivery system. Med Chem (Los Angeles) 8:218–229
Novoselov KS (2011) Nobel lecture: graphene: materials in the flatland. Rev Mod Phys 83(3):837–849. https://doi.org/10.1103/RevModPhys.83.837
O’Neill A, Khan U, Nirmalraj PN, Boland J, Coleman JN (2011) Graphene dispersion and exfoliation in low boiling point solvents. The J Phys Chem C 115(13):5422–5428
Pandey D, Reifenberger R, Piner R (2008) Scanning probe microscopy study of exfoliated oxidized graphene sheets. Surf Sci 602(9):1607–1613
Paredes JI, Villar-Rodil S, Martínez-Alonso A, Tascon JMD (2008) Graphene oxide dispersions in organic solvents. Langmuir 24(19):10560–10564
Pei S, Cheng H-M (2012) The reduction of graphene oxide. Carbon 50(9):3210–3228
Rasheed M, Shihab S, Wissam Sabah O (2021) An investigation of the structural, electrical and optical properties of graphene-oxide thin films using different solvents. J Phys Conf Ser 1795:12052. IOP Publishing
Ray SC (2015) Application and uses of graphene oxide and reduced graphene oxide. Elsevier Inc., Applications of graphene and graphene-oxide based nanomaterials. https://doi.org/10.1016/b978-0-323-37521-4.00002-9
Sabzevari M, Cree D, Wilson L (2018) Preparation and characterization of graphene oxide cross-linked composites 2014. https://doi.org/10.25071/10315/35427
Saha SK, Bhaumik S, Maji T, Mandal TK, Pal AJ (2014) Solution-processed reduced graphene oxide in light-emitting diodes and photovoltaic devices with the same pair of active materials. RSC Adv 4(67):35493–35499
Sametband M, Kalt I, Gedanken A, Sarid R (2014) Herpes simplex virus type-1 attachment inhibition by functionalized graphene oxide. ACS Appl Mater Interfaces 6(2):1228–1235
Schniepp HC, Li J-L, McAllister MJ, Sai H, Herrera-Alonso M, Adamson DH, Prud’homme RK, Car R, Saville DA, Aksay IA (2006) Functionalized single graphene sheets derived from splitting graphite oxide. J Phys Chem B 110(17):8535–8539
Shao Y, Wang J, Engelhard M, Wang C, Lin Y (2010) Facile and controllable electrochemical reduction of graphene oxide and its applications. J Mater Chem 20(4):743–748
Shin H-J, Kim KK, Benayad A, Yoon S-M, Park HK, Jung I-S, Jin MH, Jeong H-K, Kim JM, Choi J-Y (2009) efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv Func Mater 19(12):1987–1992
Si Y, Samulski ET (2008) Synthesis of water soluble graphene. Nano Lett 8(6):1679–1682
Singh K, Srivastava G, Talat M, Srivastava ON, Kayastha AM (2015) α-amylase immobilization onto functionalized graphene nanosheets as scaffolds: its characterization, kinetics and potential applications in starch based industries. Biochem Biophys Rep 3:18–25
Singh PK, Singh U, Bhattacharya B, Rhee HW (2014) Electrochemical synthesis of graphene oxide and its application as counter electrode in dye sensitized solar cell. J Renew Sustain Energy 6(1). https://doi.org/10.1063/1.4863834
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Graphene-based composite materials. Nature 442(7100):282–286
Staudenmaier L (1898) Verfahren Zur Darstellung Der Graphitsäure. Ber Dtsch Chem Ges 31:1481–1487
Su C, Loh KP (2013) Carbocatalysts: graphene oxide and its derivatives. Acc Chem Res 46(10):2275–2285
Sun X, Liu Z, Welsher K, Robinson JT, Goodwin A, Zaric S, Dai H (2008) Nano-graphene oxide for cellular imaging and drug delivery. Nano Res 1(3):203–212
Szabó T, Berkesi O, Forgó P, Josepovits K, Sanakis Y, Petridis D, Dékány I (2006) Evolution of surface functional groups in a series of progressively oxidized graphite oxides. Chem Mater 18(11):2740–2749
Tang L, Li X, Ji R, Teng KS, Tai G, Ye J, Wei C, Lau SP (2012) Bottom-up synthesis of large-scale graphene oxide nanosheets. J Mater Chem 22(12):5676–5683. https://doi.org/10.1039/c2jm15944a
Torrisi F, Hasan T, Wu W, Sun Z, Lombardo A, Kulmala TS, Hsieh G-W et al (2012) Inkjet-printed graphene electronics 4:2992–3006
Tung VC, Allen MJ, Yang Y, Kaner RB (2009) High-Throughput solution processing of large-scale graphene. Nat Nanotechnol 4(1):25
Wang X, Zhi L, Tsao N, Tomović Ž, Li J, Müllen K (2008) Transparent carbon films as electrodes in organic solar cells. Angewandte Chemie—Int Edition 47(16):2990–2992. https://doi.org/10.1002/anie.200704909
Wang Y, Wan Y, Zhang D (2010) Reduced graphene sheets modified glassy carbon electrode for electrocatalytic oxidation of hydrazine in alkaline media. ElectrochemCommun 12(2):187–190
Warner JH, Schäffel F, Bachmatiuk A, Rümmeli MH (2013) Properties of graphene. In Graphene. https://doi.org/10.1016/B978-0-12-394593-8.00003-5
Yang X, Wang Y, Huang X, Ma Y, Huang Y, Yang R, Duan H, Chen Y (2011) Multi-functionalized graphene oxide based anticancer drug-carrier with dual-targeting function and PH-sensitivity. J Mater Chem 21(10):3448–3454
Yu W, Sisi L, Haiyan Y, Jie L (2020) Progress in the functional modification of graphene/graphene oxide: a review. RSC Adv 10(26):15328–15345
Zanin H, Margraf-Ferreira A, Da Silva NS, Marciano FR, Corat EJ, Lobo AO (2014) Graphene and carbon nanotube composite enabling a new prospective treatment for trichomoniasis disease. Mater Sci Eng, C 41:65–69
Zhang L, Xia J, Zhao Q, Liu L, Zhang Z (2010) Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 6(4):537–544
Zhang X, Xie H, Liu Z, Tan C, Luo Z, Li H, Lin J et al (2015) Black phosphorus quantum dots. Angewandte Chemie—Int Edition 54(12):3653–3657. https://doi.org/10.1002/anie.201409400
Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 22(35):3906–3924
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Khalilullah, A., Anwer, R., Uddin, I. (2023). Synthesis of Graphene Oxide and Its Metal Composites. In: Uddin, I., Ahmad, I. (eds) Synthesis and Applications of Nanomaterials and Nanocomposites. Composites Science and Technology . Springer, Singapore. https://doi.org/10.1007/978-981-99-1350-3_3
Download citation
DOI: https://doi.org/10.1007/978-981-99-1350-3_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-1349-7
Online ISBN: 978-981-99-1350-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)