Abstract
The utilization of non-biodegradable disposable plastic has increased exponentially due to its lightweight and user-friendliness. Nonetheless, the non-biodegradable plastic waste produced by disposable plastics increases the environmental carbon footprint. The utilization of biodegradable plastics is one solution to mitigate the environmental pollution problem. Currently, only 1% of 300 million tons of plastic produced annually is biodegradable. Biodegradable plastics are obtained from both natural and synthetic resources. Due to their lower strength, natural-origin biodegradable plastics are only used in a few applications. For a large number of applications, the mechanical strength and other properties of synthetic-origin biodegradable plastics can be engineered. This chapter covers the biodegradable plastics of synthetic-origin including aliphatic polyesters, aromatic copolymers, vinyl polymers, and biodegradable polyurethanes. Biodegradation (both biotic and abiotic) in synthetic-origin biodegradable polymers has been discussed followed by the classification and degradation tendencies in synthetic-origin biodegradable plastics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Why the Planet Needs Biodegradable Plastics?
Modern lifestyle is highly dependent on the use of plastic materials due to their versatility and cost-effectiveness. A majority of such plastics are polyolefins derived from petroleum-based resources. Since these resources are finite in quantity, their sustained supply cannot be assured. Moreover, the end-of-life plastic is deposited into the environment as persistent pollutants posing a serious threat to our ecosystem.
Global plastic production was 367 million metric tons in the year 2020 and is expected to rise to over 445 million metric tons by the year 2025 [1]. Global plastics consumption is always higher than that produced in a year because a certain quantity is also recycled from the past years. Out of all the end-of-life plastic produced globally, 40% is dumped into landfills, 25% is combusted, 12% is subjected to mechanical recycling, 1% is utilized in monomer recovery, and 22% remains unattended [2].
Landfilling is a cheaper opportunity for managing plastic waste, but it may result in a bunch of undesirable effects. The undesirable effects include groundwater and air pollution, soil contamination, and disturbance in the natural environment. Landfilling broadly falls into two major categories, namely, (i) sanitary or managed landfills and (ii) non-sanitary or unmanaged dumps. In sanitary landfills, bottom liners and top covers are essentially required to minimize the penetration of leachates into the soil. Bottom liners and top covers also minimize the unpleasant odor and dust. In non-sanitary or unmanaged dumps, no bottom liners and top covers are required. In unmanaged dumps, the plastic waste is dumped in holes extending below the groundwater level. Pre-treatment of plastic waste is necessary for both managed and unmanaged landfilling. If the plastic waste is landfilled without pre-treatment or segregation, highly toxic leachates are produced. These leachates eventually reach humans through the food and water chain. Additionally, landfilling of untreated plastic waste results in longer (up to several hundred years) land-reclamation times [3].
Incineration focuses on the reduction of the quantity of plastic waste since it is a material destruction technique rather than a material recovery technique. Incineration results in the emission of a variety of hazardous and toxic organic pollutants. The organic pollutants include brominated and polychlorinated compounds, dioxins, furans, soot, and ash. Persistent exposure to the aforementioned pollutants is severely damaging to humans, animals, and vegetation. The brominated compounds enter humans and animals via the respiratory tract and cause carcinogenesis and mutagenesis. Likewise, the dioxins enter humans and animals via the water and food chain ultimately causing damage to reproductive and respiratory systems [4].
Recycling plastic waste can remarkably reduce environmental pollution and consumption of crude oil. Collection, sorting, and pre-treatment are essentially required for the effective recycling of plastic waste. Plastic waste recycling can be advantageous only after the implementation of an effective waste collection strategy. To avoid the generation of undesirable byproducts, plastic waste needs to be segregated before recycling [5].
All of the unattended end-user plastic waste is accumulated in the ecosystem for being non-biodegradable. A significant part of unattended plastic waste strews about on land and leaks into the ocean and rivers. An additional increase of 15% in plastic waste quantity each year brings further pollutes the ecosystem. With an estimated 8 million tons of plastic waste discarded annually into the oceans, there may be more plastic in the ocean than fish by 2050.
All the aforementioned shortcomings associated with conventional plastics require the world to pay more attention to the production and utilization of bioplastics. Bio-based plastics can assist the polymer industry to improve its sustainability and reduce crude oil dependency. Due to the environmental compatibility, bioplastics (both natural and synthetic) are the solution to environmental challenges [6].
2 Introduction to Biodegradability
Biodegradability or biodegradation is the breakdown of polymer molecules into salts, gases, and biomass. Biodegradation occurs under the influence of biotic and abiotic factors. The factors include algae, fungi, bacteria, moisture, air, sunlight, ultraviolet radiation, temperature, and soil. Some biodegradable polymers require a combination of these factors to degrade. A biodegradable polymer usually undergoes (i) erosion or fragmentation, (ii) depolymerization, and (iii) mineralization to completely degrade into non-toxic substances.
Biodegradable polymers originated in the early 1980s. Biodegradable polymers can cleave or erode themselves after a specific period under specific conditions. Biodegradability in polymers is attributed to the presence of hydrolyzable and oxidizable linkages in the backbone. While natural biodegradable polymers (NBPs) inherently possess this capability, synthetic biodegradable polymers (SBPs) are purposefully engineered for this capability. Both renewable and non-renewable resources can be utilized for the production of biodegradable polymers [7].
Biodegradation in polymers can occur aerobically or anaerobically. Aerobic biodegradation (in the presence of oxygen) produces carbon dioxide along with water, biomass, and residue. Anaerobic biodegradation yields methane gas instead of carbon dioxide, along with water, biomass, and residue. For a polymer to be completely biodegradable, aerobic and anaerobic biodegradation must be followed by mineralization. The process of mineralization refers to the complete transformation of produced biomass into gases, water, minerals, salts, and residue [8].
The extent or degree of biodegradability in polymers can be measured by various approaches. If the process of mineralization is complete, the amount of biomass produced can be used as a measure of biodegradability. For oxo-biodegradable polymers, the rate of oxygen uptake gives the measure of biodegradability. Aerobically biodegradable polymers yield carbon dioxide upon biodegradation and the quantity of carbon dioxide produced in a certain amount of time may be used as a measure of biodegradability. Similarly, the products of hydrolysis or other chemical reactions occurring during biodegradation may be used to assess the degree of biodegradability.
Fourier transform infrared spectroscopy (FTIR), ultraviolet spectroscopy (UV), or nuclear magnetic resonance spectroscopy (NMR) are commonly employed for measuring biodegradability. During biodegradation, the mechanical and physical properties of polymers are reduced and the quantitative value of the reduction in properties represents the extent of biodegradability. For measuring the reduction in mechanical properties, a universal test frame (UTM) or dynamic mechanical analyzer (DMA) is most frequently used. The reduction in physical properties is measured using an X-ray diffraction analyzer (XRD), Thermo gravimetric analyzer (TGA), gel permeation chromatography (GPC), and differential scanning calorimeter (DSC) [9].
Keeping the physical factors aside, biodegradability can broadly be classified into biotic and abiotic degradability. Biotic degradation is always predominant over abiotic degradation. Although some physical and abiotic factors are essentially involved in biotic biodegradation.
2.1 Biotic Degradation
Biodegradation that takes place in the presence of oxygen by the microorganism is termed biotic degradation. The microorganisms recognize the subject polymer molecule as feed and yield non-toxic products of biodegradation along with the energy. The liberated energy is then utilized by the microorganisms for their life functioning. The process of biodegradability is accomplished by the microorganisms in three stages. The first stage corresponds to the reduction in molecular weight while the second stage is attributed to the reduction in crystallinity of the polymer. The mechanical properties of the subject polymer are reduced in the third and final stage of biodegradation.
Biotic degradation can be further classified as physically, chemically, and enzymatically induced biotic degradation.
2.2 Abiotic Degradation
Abiotic degradation takes place in the absence of oxygen by abiotic factors like mechanical stresses, heat, sunlight, soil, and chemicals. Abiotic factors usually commence the process of biodegradation by dwindling the polymeric structure [10]. Abiotic degradation is categorized into the following sub-classes.
2.2.1 Mechanical Stress-Induced Abiotic Degradation
Abiotic degradation at the molecular level is initiated by tensile, compressive, and shear forces in polymers. The forces may arise from a variety of factors including but not limited to air and water-induced turbulences, aging, mechanical loading, and soil-burial pressures. The stress-induced abiotic degradation is only evident at the microscopic level. Only the macroscopic level stress-induced abiotic degradation is observable by the naked eye. The microscopic level stress-induced changes, when aided by other factors, usually result in defragmentation or rupture [11].
2.2.2 Temperature-Induced Abiotic Degradation
Thermally-induced abiotic degradation takes place at temperatures above the melting points in polymers. A solid-to-liquid phase transformation is always involved in this type of abiotic degradation. Since the environmental temperature in most parts of the world is below the melting temperature of thermoplastic polymers, biodegradation is not initiated at ambient conditions. Some polymeric composites, however, possess a melting point near the ambient temperature. The macromolecular structure and the crystallinity are altered when the polymers are heated near the glass transition temperature. The aspect is typically evident in semi-crystalline polymeric materials containing both crystalline and amorphous regions in their structure. Such materials when heated beyond their glass transition temperature (Tg) become more vulnerable to biotic or abiotic degradation. The addition of transition metal ions in polymers serves as pro-oxidants. Thermo-oxidative abiotic degradation is depicted by the polymers containing such pro-oxidants. For instance, the thermo-oxidative degradation in pro-oxidant-containing polyethylene (PE) films is initiated and controlled by varying the temperature [12].
2.2.3 Sunlight and UV Induced Abiotic Degradation
In some polymers, abiotic degradation is triggered by exposure to sunlight or ultraviolet (UV) radiation. When the polymers containing UV-sensitive functional groups are exposed to ultraviolet radiation, a Photolysis reaction is initiated. Likewise, the presence of photoinitiators in the polymer results in the initiation of an oxidation reaction. The incorporation of carbonyl groups in the polymer backbone usually imparts UV-sensitivity to the polymer. The copolymer of ethylene and carbon mono oxide depicts UV-sensitivity due to the presence of the carbonyl group in the backbone. Despite being photo-degradable, the carbonyl-containing polymers are more expensive than their non-carbonyl-containing competitors. A cost-effective method to inculcate photo biodegradability in polymers is the addition of non-toxic transition metal compounds. Pro-oxidant nature is also imparted to polymers by the addition of transition metal compounds. Polymers with pro-oxidant nature are unstable at processing conditions due to the initiation of thermally induced oxidation during processing. UV stabilizers and antioxidants are added to polymers to prevent undesirable thermally induced oxidation. The addition of antioxidants and UV stabilizers also provides an opportunity to engineer the lifespan of the polymer from a few weeks to several years. The lifespan of secondary food packaging films is controlled by this phenomenon [13].
2.2.4 Chemically Induced Abiotic Degradation
Chemically induced abiotic degradation is predominantly the result of oxidation or hydrolysis. The chemical pollutants, agrochemicals, molecular oxygen, and traces of ozone present in the atmosphere result in the oxidative degradation of polymers. The molecular oxygen present in the atmosphere attacks the covalent bonds in polymers and produces free radicals. Chain scissions and crosslinking are initiated by the generated free radicals [10]. Oxidative degradation in the polymer can be induced either catalytically or non-catalytically. In a polymer, it is possible to carry out abiotic oxidative degradation at ambient or elevated temperatures (both above and below the glass transition temperature Tg). The rate of oxidative degradation in polymers can be enhanced by coupling different modes of oxidation. For this purpose, chemical oxidation is usually coupled with ultrasonic-assisted oxidation, UV/sunlight photo-oxidation, thermal oxidation, or nuclear radiation-assisted oxidation. Some polymers exhibit an induction period upon exposure to oxygen during which oxidative degradation is absent. No structural change or oxygen absorption occurs during the induction period. The exposure of such polymers to oxygen during the induction period usually results in the formation of hydroperoxides responsible for rapid auto-oxidation at later stages. The oxidative degradation starts immediately in the polymers containing metallic salts due to the absence of an induction period. The oxidation of polymers is usually accomplished in three steps namely the initiation, the propagation, and the termination step.
The initiation step is characterized by the formation of free radicals either by physical (UV radiation, temperature, or ultrasonics) or chemical (peroxides, hydroperoxides, inhibitors, solvent, catalyst, and atomic state) factors. The propagation step is characterized by a variety of transformations including the formation of multiple functional groups and radicals. Firstly, the macro-sized radicals formed during the initiation step are further oxidized to peroxy radicals. The conversion of macro-sized free radicals to peroxy radicals is inversely related to the stability of free radicals. The peroxy radical formed further reacts with the polymer substrate to preferentially eliminate the secondary and tertiary hydrogen from the polymer. When the eliminated hydrogen atoms interact with the free radicals, hydroperoxides are formed. The formation of hydroperoxides is usually favored by low temperatures for being exothermic. Further decomposition of hydroperoxides then yields the alkoxy radicals and the hydroxyl groups. These alkoxy radicals give rise to polymer chain scission thereby directly contributing to fragmentation. The free radicals obtained during the initiation and propagation step combine to form inactive products thereby resulting in the termination of the reaction [14].
Hydrolytic degradation refers to the cleavage of water-sensitive functional groups in the polymer backbone for a partial or complete degradation of the polymer. Hydrolytic degradation is a combination of a physical and chemical phenomenon, with the physical phenomenon referring to the absorption of water by polymer molecules while the chemical phenomenon refers to the cleavage of hydrolyzable functional groups. Hydrolytic degradation is referred to as “bulk degradation” if the physical water absorption by the polymer is quicker than the chemical bond breaking. Most of the hydrolytically degradable polyesters, polyglycolic acids, and polylactic acids fall under this class. Contrastingly, if the polymer linearly unhooks its mass due to the hydrolytic degradation occurring at the polymer-aqueous interface only, it is referred to as “surface erosion degradation.” This phenomenon is mostly predominant in polyanhydrides [15]. Some biodegradable polymers may undesirably undergo hydrolytic degradation during their processing due to high humidity or temperature. The phenomenon can occur even at moderate processing temperatures leading to difficulties in their processing. This undesirable effect can be countered by incorporating modifying chemicals like carbodiimides and epoxides in the polymers [16].
3 Classification of Synthetic-Origin Polymers
The synthetic-origin polymers are categorized mainly into two types, i.e., biodegradable, and non-biodegradable as per their synthesis and processing method, chemical composition, economic importance, and end applications. In this chapter, synthetic-origin biodegradable polymers will be discussed in detail owing to their importance in recent environmental pollution issues and concerns. Classification of biodegradable and non-biodegradable common synthetic-origin polymers is given in Fig. 1.
3.1 Synthetic-Origin Biodegradable Polymers
Synthetic-origin polymers with hydrolyzable backbones are prone to biodegradation under specific conditions. The class of polymers having these types of chain backbones includes aliphatic polyesters, aromatic copolyesters, some vinyl polymers (e.g., PVA and PVAc), and polyurethanes (Fig. 1).
3.1.1 Aliphatic Polyesters
They are the most extensively studied and in-use class of synthetic-origin biodegradable polymers, from the polyesters family because of their versatility and diversity. In the process of their development, various monomers from large varieties and different polymerization routes can be in consideration. If we are discussing the polymerization routes, then for high molecular weight polymers like polyesters, ring-opening polymerization is chosen over polycondensation [17]. Six-membered lactones are used in ring-opening polymerization (ROP) for the preparation of most biodegradable polyesters. Being the only high molecular weight biodegradable polymers class, aliphatic polyesters are broadly explored, and their hydrolyzable ester bonds give biodegradability [18].
They can be categorized into two different types related to the bonding mechanism of their integrant monomers. Polyhydroxyalkanoates are the first type synthesized by the polymerization of hydroxy acids (HO-R-COOH). Some of the examples are poly (glycolic acid) (PGA) and poly(lactic acid) (PLA). Polymers of the second type are poly (alkene dicarboxylate)s and prepared by the polycondensation reaction of dicarboxylic acids and diols. Examples are poly(ethylene succinate) (PES) and poly(butylene succinate) (PBS) [19].
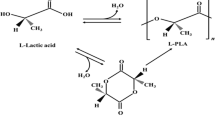
3.1.2 Polylactic Acid (PLA)
Polylactic acid is a linear aliphatic, thermoplastic polyester synthesized normally using cyclic dilactone by ring-opening polymerization capable of renewability along with high biocompatibility, UV stability, and strong rigidity [20]. Various structural parameters of PLA like composition (stereo-regularity content) and molecular weight (Mw) may decide thermal properties. It is a semi-crystalline or near amorphous polymer having a glass transition temperature (Tg) of 55 °C and melting temperature (Tm) ranging from 180 to 190 °C. Figure 2 shows the chemical structure of Polylactic acid (PLA) [21].
Fragility and low resistance to temperature are some apparent defects that PLA suffers usually. For addressing some other disadvantages are intrinsic brittleness, water sensitivity, and low impact strength, the use of suitable fibers and fillers in the processing of PLA is an efficient and convenient way of enhancing the overall properties [22].
In recent years consumption of PLA has increased because of advanced manufacturing technologies results enhanced production rates due to which a gradual decrease in price for PLA was observed [23]. It can be used for the production of suitable biomaterials for several different applications by using its excellent mechanical properties and remarkable barrier capability.
3.1.3 Polyglycolic Acid (PGA)
PGA is a simple linear aliphatic polyester, usually synthesized by the ring-opening polymerization of glycolide and cyclic lactone and has a chemical structure (Fig. 3) identical to polylactic acid (PLA) but without having a methyl side group along the main chain, that brings a high degree of crystallinity around (45–55%) due to the tight packing of polymer chains, results in high melting temperature (thermal stability) about (Tm = 220–230 °C), extraordinarily high gas barrier properties (more than 3 times higher to EVOH), along with high stiffness (7 GPa) and mechanical strength (115 MPa) [24].
On the other hand, PGA normally shows a similar rapid biodegradation trend along with a 100% composability ratio as that of cellulose. Till now, PGA has been mostly used in its copolymer form, like poly(lactic-co-glycolic acid) (PLGA). Its distinctive properties have often been neglected and so far, not been fully investigated yet. The reason behind this is intrinsic properties associated with it such as rapid degradation profile, brittle behavior, high hydrophilicity, and insolubility issue in many organic solvents that have obstructed its applications on practical grounds [25].
Besides all these limitations PGA finds applications in many active fields today such as in drug delivery [26] and tissue engineering [27] because of its human’s friendly nature, and functional use like in sensors, coating, packaging, etc.
3.1.4 Polycaprolactone (PCL)
PCL is usually produced by the ring-opening polymerization of comparatively low-cost cyclic monomer known as ε-caprolactone and in the existence of a catalyst called tin octoate (Tin(II) 2-ethyl hexanoate) [28]. The chemical structure of PCL can be seen in Fig. 4.
PCL is an extremely tough aliphatic linear polyester and semi-crystalline in nature having adequate biocompatibility and biodegradability. It can be soluble in a wide range of inorganic and organic solvents. Being a temperature-sensitive biodegradable polyester, PCL has a low glass transition temperature (Tg) of about −60 °C and melting temperature (Tc) around 60–65 °C. Because of its low Tg, PCL is used as a soft block or as a compatibilizer in different polyurethane formulations [29].
At room temperature, it acts as a semi-rigid material and has lower values of tensile strength (i.e., 23 MPa) and relatively higher elongation to break values (more than 700%), if we are talking about the modulus of PCL it usually lies in between high-density polyethylene (HDPE) and low-density polyethylene (LDPE).
Crystal dominations remain intact during the degradation process of PCL because it usually takes place in amorphous constituent domains. It can easily be degradable under the action of microorganisms like enzymes and fungi. To improve the degradation pace, several copolymers of PCL can be made with lactide or glycolide [30].
3.1.5 Polyethylene Succinate (PES)
PES is usually synthesized either by ring-opening polymerization (ROP) of succinic anhydride with ethylene oxide or polycondensation reaction of succinic acid and ethylene glycol [31]. The chemical structure of polyethylene succinate (PES) can be seen in Fig. 5.
It has a melting temperature (Tm) in the range of 103–106 °C, with many good mechanical properties, specifically elongation. For film making and utility, it can be considered in application because of its high-level gas barrier properties along with excellent biodegradability.
3.1.6 Polybutylene Succinate (PBS) and Its Copolymer
A white crystalline aliphatic thermoplastic polyester is generally synthesized by the polycondensation reaction of succinic acid with 1,4-butanediol. PBS has glass low transition temperature (Tg) of about −45 to −10 °C and melting temperature (Tc) around 90–120 °C. PBS is chemosynthetic polyester in nature and its melting, as well as crystallization behavior, is well reported in the literature [32]. The chemical structure of Polybutylene succinate (PBS) can be visible in Fig. 6.
Mechanical properties like tensile strength are 32 MPa and elongation at break is about 330%. These are comparable with polyethylene and polypropylene which are widely used polymers. Other interesting properties such as chemical and thermal resistance, melt processability, and most importantly biodegradability [33].
Because of its excellent processability, it can be used in the polymer processing field easily in the temperature range of 160–200 °C. Applications in all related products including containers, mulch film, cutlery, bags, packaging film, and flushable hygiene products. Due to the high crystallization rate and crystallinity, it has a comparatively low biodegradation rate. To overcome this issue several approaches have been adopted in this regard, such as the formation of composites, copolymerization, or physical blending [34].
Polybutylene succinate adipate (PBSA):
Low biodegradation rates of polybutylene succinate (PBS) urged scientists to work on copolymerization. PBSA is a copolymer of PBS synthesized by the addition reaction of aliphatic dicarboxylic acids with glycols. Figure 7 shows the chemical structure of polybutylene succinate adipate (PBSA). The presence of succinic acid (prepared by the fermentation of sugar obtained from sugarcane) confirms its biomaterial class [35].
It can be used in different applications like injection molded cutlery, sheet extrusions, blow-molded containers, foam cushions, monofilaments, multifilaments, and films. For composting purposes, PBSA film is suitable especially for kitchen waste because it has properties closer to linear low-density polyethylene (LLDPE) and has a relatively high biodegradability rate [36].
3.2 Aromatic/Aliphatic Co-polyesters
Using aliphatic monomers of different sizes, a large number of polyesters or copolyesters have been synthesized in recent years. However, their mechanical properties are lower in range than those of conventional non-biodegradable polymers. They typically show sensitivity toward microbial or enzymatic attack and hydrolytic degradation. To overcome this issue, a combination of aliphatic–aromatic copolyesters was introduced. Aromatic and aliphatic monomers are used in a mixture and are usually based on terephthalic acid [37].
3.2.1 Polybutylene Adipate Terephthalate (PBAT)
PBAT is one of the most regularly studied and used copolyesters. A mixture of terephthalic acid and adipic acid along with 1,4-butanediol is used in the synthesis of PBAT and done by a polycondensation reaction. The chemical structure of polybutylene adipate terephthalate (PBAT) is visible in Fig. 8.
The concentration of terephthalic acid can play a vital role in the overall properties of the final polymer. For instance, a concentration higher than 35% mol will give better mechanical and thermal properties. But higher concentrations above 55% mol results in a rapid decrease in the biodegradation rate [38].
Like polyethylene, PBAT has lots of appealing properties such as puncture toughness and modest high impact. Low stiffness and normal tensile strength along with relatively high elongation at break (30–40%) are some main characteristics of PBAT reported. Moreover, because of the characteristics of fully biodegradable material (compostable), it can be managed and processed on typical blown film equipment usually employed for polyethylene. PBAT can work as a toughening agent in the processing of poly(lactic acid) PLA [39].
3.2.2 Polyethylene Adipate (PEA)
It is from the class of aliphatic copolyesters. Mostly synthesized using adipic acid and ethylene glycol as base units by choosing polycondensation reaction. The chemical structure of polyethylene adipate (PEA) is presented in Fig. 9.
Because of its biodegradable nature, through various mechanisms and as compared to other common polymers PEA is relatively inexpensive. The reason behind biodegradability is its lower molecular weight compared to many other polymers [40].
3.3 Vinyl Polymers
3.3.1 Polyvinyl Alcohol (PVA)
It is the most quickly biodegradable polymer from the vinyl polymers family and is known as “Bioplastic.” Wastewater-activated sludge easily degrades it. In contrast to many vinyl polymers, polymerization of PVA has not been done usually from its subsequent monomer. Wholly, acetaldehyde and tautomeric forms of vinyl alcohol and monomer exist. Chemical structural depiction of polyvinyl alcohol (PVA) available in Fig. 10.
PVA has a molecular weight ranging between 26,300 and 30,000 with a melting point of around 180–190 °C precisely and values 86.5–89% degree of hydrolysis [41]. The film-forming, emulsifying, and adhesion capabilities of PVA are exceptional. It also has oil, grease, and solvent resistance. In addition to its high tensile and flexibility, this material also possesses excellent oxygen and odor barrier characteristics. However, these attributes are influenced by humidity, in other words, more water is absorbed when the humidity is higher. Its tensile strength is reduced with the addition of water, while elongation and tear strength are enhanced [42].
As far as synthetic water-soluble polymers in concern, PVA is the most widely available and largely produced polymer on the planet. PVA’s biodegradability in the environment is one of the most notable features of its properties set. The two-step biodegradation reaction involving the oxidation of the hydroxyl group and subsequent hydrolysis is the most widely accepted mechanism. Because of its excellent biodegradability and mechanical characteristics, PVA has received a great deal of research attention. PVA has become a popular material for disposable and biodegradable plastic alternatives because of these characteristics. For example, it could be used as a flocculant, metal-ion remover, and excipient in controlled release systems because of its water-soluble, biodegradable, and reactive properties [43].
3.3.2 Polyvinyl Acetate (PVAc)
A synthetic biodegradable polymer available in the form of a rubber-like compound is polyvinyl acetate (PVAc). It belongs to the family of polyvinyl esters and thermoplastic polymers in nature. For the synthesis of PVAc, vinyl acetate monomer undergoes free radical vinyl polymerization [44]. The chemical structure of polyvinyl acetate (PVAc) is shown in Fig. 11.
According to reports, PVAc biodegrades more slowly. In soil-burial testing, ethylene/vinyl acetate copolymers allow a relatively slow biodegradation process. Increasing the acetate content led to greater weight reduction for 120 days. There are several ways to manage PVAc’s hydrolysis and subsequent oxidation, which should provide degradation materials with a wide variety of characteristics, degradability, and the ability to decompose in an environment-friendly manner [45].
It is widely used as glue-type material, generally referred to as carpenter’s glue. In the paint industry, paper coating, and some other industrial coatings, and for nonwovens, sanitary napkins, and filter paper are used as a binder [46].
3.4 Biodegradable Polyurethanes
A diisocyanate, chain extender, and polyol combine to form polyurethanes. Hard and soft segments are present alternatively in a segmented polymer formed by the reaction of the two substances. Polyols like polyester polyols and polyether polyols are the building blocks for soft segments. With the help of the chain extender, a hard segment is synthesized from diisocyanate. Polyurethane biodegradation is influenced by the chemical composition of the polymer segments [47].
It is possible to control the degradation by selecting the right soft segment. Polyether-based polyurethanes are resistant to decomposition by microorganisms. Polyurethanes are quickly biodegradable if the polyol used is polyester. There are three types of biodegradable polyesters: PCL, PLA, and PGA, which are employed. When esters are placed in soft segments, it is considered that their breakdown rate is controlled. Hydrolysis is difficult for the urethane boundaries in the hard portion [48].
To satisfy the ever-changing needs of current technologies including coatings, adhesives, fiber, foams, and thermoplastic elastomers, the unique material polyurethane having a broad range of chemical and physical properties has been thoroughly adapted and tailored.
4 Conclusions
The ability to easily biodegrade towards the end of their service life is one of the most valuable advantages of biodegradable polymers over other types of polymers. But this property is limited and subjected to such conditions that facilitate their biological degradation. Biodegradable polymers have gained more attention during the last decades because of their prospective applications in the fields which are addressing environmental protection and sustainability. Currently, only a few synthetic-origin biopolymers have market acceptance because of a lack of price competitiveness with common synthetic polymers. To improve the different properties of biodegradable polymers, a lot of methods have been studied, developed, and applied accordingly. A promising future for the development of biopolymer materials is ahead.
References
Tiseo, I.: Production forecast of thermoplastics worldwide from 2025 to 2050 (2022)
Degnan, T.: Waste-plastic processing provides global challenges and opportunities. MRS Bull. 44(6), 436–437 (2019). https://doi.org/10.1557/mrs.2019.133
Daria, M.: Landfill impacts on the environment—review. 1–16 (2019)
Verma, R., Vinoda, K.S., Papireddy, M., Gowda, A.N.S.: Toxic pollutants from plastic waste—a review. Procedia Environ. Sci. 35, 701–708 (2016). https://doi.org/10.1016/j.proenv.2016.07.069
Thiounn, T., Smith, R.C.: Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 58(10), 1347–1364 (2020). https://doi.org/10.1002/pol.20190261
Cho, R.: The truth about bioplastics
Pillai, C.K.S.: Recent advances in biodegradable polymeric materials. Mater. Sci. Technol. (United Kingdom) 30(5), 558–566 (2014). https://doi.org/10.1179/1743284713Y.0000000472
Leja, K., Lewandowicz, G.: Polymer biodegradation and biodegradable polymers—a review. Polish J. Environ. Stud. 19(2), 255–266 (2010)
Arutchelvi, J., Sudhakar, M., Arkatkar, A., Doble, M., Bhaduri, S., Uppara, P.V.: Biodegradation of polyethylene and polypropylene. Indian J. Biotechnol. 7(1), 9–22 (2008)
Lucas, N., Bienaime, C., Belloy, C., Queneudec, M., Silvestre, F., Nava-Saucedo, J.E.: Polymer biodegradation: mechanisms and estimation techniques—a review. Chemosphere 73(4), 429–442 (2008). https://doi.org/10.1016/j.chemosphere.2008.06.064
Briassoulis, H.: The institutional complexity of environmental policy and planning problems: the example of Mediterranean desertification. J. Environ. Plan. Manag. 47(1), 115–135 (2004). https://doi.org/10.1080/0964056042000189835
Jakubowicz, I.: Evaluation of degradability of biodegradable polyethylene (PE). Polym. Degrad. Stab. 80(1), 39–43 (2003). https://doi.org/10.1016/S0141-3910(02)00380-4
Scott, G.: Photo-biodegradable plastics : their role in the protection of the environment. 29, 135–154
Rabek, J.F.: Chapter 4. Oxidative degradation of polymers. Compr. Chem. Kinect. 14(C), 425–538 (1975). https://doi.org/10.1016/S0069-8040(08)70336-4
Hofmann, D., Entrialgo-Castaño, M., Kratz, K., Lendlein, A.: Knowledge-based approach towards hydrolytic degradation of polymer-based biomaterials. Adv. Mater. 21(32–33), 3237–3245 (2009). https://doi.org/10.1002/adma.200802213
Dreier, J., Brütting, C., Ruckdäschel, H., Altstädt, V., Bonten, C.: Investigation of the thermal and hydrolytic degradation of polylactide during autoclave foaming. Polymers (Basel). 13(16) (2021). https://doi.org/10.3390/polym13162624
Vroman, I., Tighzert, L.: Biodegradable polymers. Materials (Basel) 2(2), 307–344 (2009). https://doi.org/10.3390/ma2020307
Albertsson, A.C., Varma, I.K.: Aliphatic polyesters: synthesis, properties and applications. Adv. Polym. Sci. 157, 1–40 (2002). https://doi.org/10.1007/3-540-45734-8_1
Douka, A., Vouyiouka, S., Papaspyridi, L.M., Papaspyrides, C.D.: A review on enzymatic polymerization to produce polycondensation polymers: the case of aliphatic polyesters, polyamides, and polyesteramides. Prog. Polym. Sci. 79, 1–25 (2018). https://doi.org/10.1016/j.progpolymsci.2017.10.001
Avérous, L.: Polylactic acid: synthesis, properties and applications. Monomers Polym. Compos. Renew. Resour. 433–450 (2008). https://doi.org/10.1016/B978-0-08-045316-3.00021-1
Kaseem, M.: Properties and medical applications of polylactic acid related papers melt rheology of poly(Lactic Acid)/low density polyet ethylene polymer blends Mosab Kaseem preparation and characterization of binary and ternary blends with poly(Lactic Acid), pol [Online]. www.expresspolymlett.com
Siakeng, R., Jawaid, M., Ariffin, H., Sapuan, S.M., Asim, M., Saba, N.: Natural fiber reinforced polylactic acid composites: a review. Polym. Compos. 40(2), 446–463 (2019). https://doi.org/10.1002/pc.24747
Nagarajan, V., Mohanty, A.K., Misra, M.: Perspective on polylactic acid (PLA) based sustainable materials for durable applications: focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 4(6), 2899–2916 (2016). https://doi.org/10.1021/acssuschemeng.6b00321
Lu, D.R., Xiao, C.M., Xu, S.J.: Starch-based completely biodegradable polymer materials. Express Polym. Lett. 3(6), 366–375 (2009). https://doi.org/10.3144/expresspolymlett.2009.46
Samantaray, P.K., et al.: Poly(glycolic acid) (PGA): a versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 22(13), 4055–4081 (2020). https://doi.org/10.1039/D0GC01394C
Li, Y., Liao, C., Tjong, S.C.: “Synthetic biodegradable aliphatic polyester nanocomposites reinforced with nanohydroxyapatite and/or graphene oxide for bone tissue engineering applications. Nanomaterials 9(4) (2019). https://doi.org/10.3390/nano9040590
Gorth, D., Webster, T.J.: Matrices for tissue engineering and regenerative medicine. Biomater. Artif. Organs. 270–286. Elsevier (2011)
Labet, M., Thielemans, W.: Synthesis of polycaprolactone: a review. Chem. Soc. Rev. 38(12), 3484–3504 (2009). https://doi.org/10.1039/b820162p
Guarino, V., Gentile, G., Sorrentino, L., Ambrosio, L.: Polycaprolactone: synthesis, properties, and applications (2017)
Christen, M.O., Vercesi, F.: Polycaprolactone: how a well-known and futuristic polymer has become an innovative collagen-stimulator in esthetics. Clin. Cosmet. Investig. Dermatol. 13, 31–48 (2020). https://doi.org/10.2147/CCID.S229054
Niaounakis, M.: Definitions of terms and types of biopolymers (2015)
Aliotta, L., Seggiani, M., Lazzeri, A., Gigante, V., Cinelli, P.: A brief review of poly(Butylene Succinate) (PBS) and its main copolymers: synthesis, blends, composites, biodegradability, and applications. Polymers (Basel) 14(4), 844 (2022). https://doi.org/10.3390/polym14040844
Rafiqah, S.A., et al.: A review on properties and application of bio-based poly(Butylene succinate). Polymers (Basel) 13(9), 1–28 (2021). https://doi.org/10.3390/polym13091436
Stȩpień, K., et al.: Biocopolyesters of poly(butylene succinate) containing long-chain biobased glycol synthesized with heterogeneous titanium dioxide catalyst. ACS Sustain. Chem. Eng. 7(12), 10623–10632 (2019). https://doi.org/10.1021/acssuschemeng.9b01191
Bondeson, D., Syre, P., Niska, K.O.: All cellulose nanocomposites produced by extrusion. J. Biobased Mater. Bioenergy 1(3), 367–371 (2008). https://doi.org/10.1166/jbmb.2007.011
Ren, M., et al.: Crystallization kinetics and morphology of poly(butylene succinate-co-adipate). J. Polym. Sci. Part B Polym. Phys. 43(22), 3231–3241 (2005). https://doi.org/10.1002/polb.20539
Nagata, M., Machida, T., Sakai, W., Tsutsumi, N.: Synthesis, characterization, and enzymatic degradation studies on novel network aliphatic polyesters. Macromolecules 31(19), 6450–6454 (1998). https://doi.org/10.1021/ma980644f
Jian, J., Xiangbin, Z., Xianbo, H.: An overview on synthesis, properties, and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 3(1), 19–26 (2020). https://doi.org/10.1016/j.aiepr.2020.01.001
Burford, T., Rieg, W., Madbouly, S.: Biodegradable poly(butylene adipate-co-terephthalate) (PBAT). Phys. Sci. Rev. (2021). https://doi.org/10.1515/-2020-0078
Monvisade, P., Loungvanidprapa, P.: Synthesis of poly(ethylene adipate) and poly(ethylene adipate-co-terephthalate) via ring-opening polymerization. Eur. Polym. J. 43(8), 3408–3414 (2007). https://doi.org/10.1016/j.eurpolymj.2007.05.009
Havstad, M.R.: Biodegradable plastics. Plastic Waste and Recycling, pp. 97–129. Elsevier (2020)
Aslam, M., Kalyar, M.A., Raza, Z.A.: Polyvinyl alcohol: a review of research status and use of polyvinyl alcohol-based nanocomposites. Polym. Eng. Sci. 58(12), 2119–2132 (2018). https://doi.org/10.1002/pen.24855
Gaaz, T., Sulong, A., Akhtar, M., Kadhum, A., Mohamad, A., Al-Amiery, A.: Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 20(12), 22833–22847 (2015). https://doi.org/10.3390/molecules201219884
Emblem, A.: Plastics properties for packaging materials. Packaging Technology, pp. 287–309. Elsevier (2012)
Amann, M., Minge, O.: Biodegradability of poly(vinyl acetate) and related polymers, pp. 137–172
Kaboorani, A., Riedl, B.: Mechanical performance of polyvinyl acetate (PVA)-based biocomposites. Biocomposites, pp. 347–364. Elsevier (2015)
Kim, G.B., Guo, J., Hu, J., Shan, D., Yang, J.: Novel applications of urethane/urea chemistry in the field of biomaterials. Advances in Polyurethane Biomaterials, pp. 115–147. Elsevier (2016)
Tatai, L., et al.: Thermoplastic biodegradable polyurethanes: the effect of chain extender structure on properties and in-vitro degradation. Biomaterials 28(36), 5407–5417 (2007). https://doi.org/10.1016/j.biomaterials.2007.08.035
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mehmood, M., Ahmad, A., Khan, M.T. (2023). Synthetic-Origin Biodegradable Polymers. In: Shaker, K., Hafeez, A. (eds) Advanced Functional Polymers. Engineering Materials. Springer, Singapore. https://doi.org/10.1007/978-981-99-0787-8_7
Download citation
DOI: https://doi.org/10.1007/978-981-99-0787-8_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-0786-1
Online ISBN: 978-981-99-0787-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)