Abstract
Summarized the current development situation of low permeability oil reservoirs at home and abroad, and sorted out the development process of three stages: indoor research, pilot testing, and field application of CO2 flooding at home and abroad. The micro oil displacement mechanism in the CO2 oil displacement process was systematically elaborated, and some widely used calculation methods for the minimum miscibility pressure (MMP) of CO2 and crude oil were summarized, providing a theoretical basis for significantly improving oil recovery. On the basis of tracking and researching the on-site application and effectiveness of CO2 flooding technology for improving oil recovery in 33 low permeability reservoirs both domestically and internationally, 5 rock properties and 2 fluid properties of reservoirs using CO2 flooding method were summarized, and the impact of 2 displacement modes and 5 injection methods on CO2 flooding efficiency was analyzed. The research results indicate that the displacement method of the miscible flooding and the injection method of periodic gas injection have better CO2 oil displacement effect. Provide on-site application reference and experience support for the efficient application of CO2 flooding technology to improve oil recovery in low permeability reservoirs in China.
Copyright 2023, IFEDC Organizing Committee.
This paper was prepared for presentation at the 2023 International Field Exploration and Development Conference in Wuhan, China, 20–22 September 2023.
This paper was selected for presentation by the IFEDC Committee following review of information contained in an abstract submitted by the author(s). Contents of the paper, as presented, have not been reviewed by the IFEDC Technical Team and are subject to correction by the author(s). The material does not necessarily reflect any position of the IFEDC Technical Committee its members. Papers presented at the Conference are subject to publication review by Professional Team of IFEDC Technical Committee. Electronic reproduction, distribution, or storage of any part of this paper for commercial purposes without the written consent of IFEDC Organizing Committee is prohibited. Permission to reproduce in print is restricted to an abstract of not more than 300 words; illustrations may not be copied. The abstract must contain conspicuous acknowledgment of IFEDC. Contact email: paper@ifedc.org.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
As exploration and development of onshore oil fields continue, techniques for enhancing oil recovery in low-permeability reservoirs have become a focus of attention [1]. Both domestically and internationally, low-permeability oil reserves are abundant, with recoverable reserves reaching 483 × 108 t [2]. In China, low-permeability oil and gas resources account for 49% of the total oil and gas resources, making it a strategic area for oil resource replacement [3, 4]. However, due to poor pore-permeability conditions and strong reservoir heterogeneity, the development of low-permeability oil reservoirs is challenging, with the current overall recovery rate only around 18% [1, 5].
During the tertiary oil recovery process, the use of carbon dioxide to enhance oil recovery (CO2-EOR) has been applied in oil fields for over 40 years and has become a relatively mature tertiary oil recovery development method [6]. CO2-EOR not only promotes an increase in oil field production but also utilizes and sequesters CO2 resources, reducing greenhouse gas emissions. In September 2020, China clearly stated its goal of striving to reach peak CO2 emissions by 2030 and achieving carbon neutrality by 2060. In recent years, China’s CO2 emissions have been increasing year by year, with CO2 emissions reaching 119 × 108 t in 2021, accounting for 33% of the world’s total CO2 emissions. The use of CO2 flooding in low-permeability reservoirs can achieve the dual purpose of enhancing oil recovery and geological sequestration, achieving both environmental and economic benefits [6, 7].
The United States first applied water containing carbonate to the K&S project in northeastern Oklahoma in 1958, marking the beginning of the field application of CO2-EOR. In 1964, the first pure CO2 miscible flooding project was implemented in the Mead Strawn oil field in Texas. Nine years later, in 1972, the first CO2-EOR commercial project in the world was put into production in the SACROC block of the Kelly-Snyder oil field in Texas. This project established the CO2 water-gas alternating drive displacement method, which initially increased the average production rate of a single well by more than three times. Its success marked the beginning of the maturity of CO2 flooding technology. From 1980 to 1992, in response to the oil crisis, the United States introduced a series of policies to stimulate the enthusiasm of oil companies and private capital, promoting the rapid development of CO2-EOR technology. During this period, the number of CO2-EOR projects increased to 54, with annual EOR production increasing to 783 × 104 t. By 2014, the United States had a total of 137 CO2-EOR projects, with an annual EOR production of 1371 × 104 t [8, 9].
China began researching CO2-EOR in the late 1960s, initially conducting indoor research and pilot trials in small test areas in the Daqing oil field, but research into injection was largely suspended due to CO2 source limitations [10]. After 2000, the discovery of CO2-containing natural gas reservoirs in the Songliao Basin enabled CO2-EOR research in Jilin and Daqing oil fields. The Yushulin oil field in Daqing conducted oil displacement experiments using CO2, and as of 2022, the cumulative increased oil amount reached 11 × 104 t. In 2005, the CO2 huff-n-puff enhanced oil recovery (EOR) field test was initiated in Caoshe oilfield, and the huff-n-puff was achieved in 2008. By the end of 2020, the cumulative oil production increment was 13.52 × 104 t. In 2017, the CO2 flooding EOR field test was conducted in the Huang 3 area of Changqing oilfield. By the end of 2018, the effective good ratio corresponding to the well network reached 56.5% in just one year, and the cumulative oil production increment was 748.1 t, indicating a good EOR effect. In September 2020, with the proposal of the “dual carbon” target, CO2-EOR ushered in a rapid development opportunity. By the end of 2021, CO2-EOR experimental areas of different types, such as Daqing low permeability, Jilin ultra-low permeability, and Changqing super-low permeability, had been established [11]. CO2-EOR experiments are blooming everywhere, and major oil companies and oilfields are exploring this technology [12].
2 CO2 EOR Mechanism
The critical temperature of CO2 is 31.1 °C, and the corresponding critical pressure is 7.495 MPa. CO2 fluid above the critical temperature and critical pressure are in a supercritical state [13]. When CO2 reaches a supercritical state, its density is similar to that of liquid, and its viscosity is similar to that of gas, but its diffusion coefficient is nearly 100 times that of liquid [14]. In addition, CO2 is more soluble in water than hydrocarbon gases, and its solubility in crude oil is greater than that in water, indicating strong solubilization [13]. The physical and chemical properties of CO2 determine its displacement mechanism in the EOR process, which mainly includes changing the rock properties of the reservoir, altering the fluid properties of the reservoir, dissolving gas drive, and huff-n-puff effect [15].
2.1 Changing Rock Properties of Reservoir
Physical Properties: The chemical reaction CO2 + H2O → H2CO3 indicates that CO2 injected into the reservoir reacts with formation water to generate carbonic acid, and the hydrogen ions produced by the ionization of carbonic acid decrease the pH value of the formation fluid, making it acidic. The acidic fluid reacts with the rock and its cementation materials, thereby dissolving rock minerals, and increasing porosity and permeability [15,16,17]. However, this process is also accompanied by the precipitation of new minerals and the transport of debris particles. When the actual well spacing is large, the mineral components dissolved near the injection well may precipitate near the production well with the decrease of pressure, thereby reducing the permeability of the reservoir [15].
Wettability: After CO2 is injected into the reservoir and dissolved in water, it corrodes upon contact with the rock surface, enhancing the hydrophilicity of the rock. On the other hand, CO2 dissolved in crude oil produces asphaltene precipitation, and polar substances such as asphaltenes adsorb on the surface of rock particles, causing the wettability of the rock to shift toward oil-wet [15].
2.2 Altering Fluid Properties of Reservoir
Expansion: After CO2 is injected into the reservoir and dissolved in crude oil, the volume of the oil expands, and its internal kinetic energy increases, making it separate from the confinement of the formation water and rock surface to become movable oil. At the same time, the elastic energy of the formation increases, enhancing the efficiency of oil displacement [15,16,17,18,19].
Reduction of viscosity: CO2 dissolved in crude oil disperses in a supercritical state, reducing the interaction forces between hydrocarbon molecules and lowering the viscosity of the crude oil, thereby significantly enhancing its flowability. The higher the original viscosity of the crude oil, the higher the percentage of viscosity reduction under the action of carbon dioxide [15,16,17,18,19].
Molecular diffusion: CO2 gas enters the formation water and crude oil through slow dissolution and diffusion of molecules, thereby increasing the volume of the crude oil, reducing its viscosity, and improving the oil displacement efficiency. At the same time, after CO2 is dissolved into the crude oil, sufficient single-phase diffusion can slow down the gas channeling of CO2 and increase the spreading coefficient. Therefore, during CO2 flooding, it is necessary to appropriately reduce the injection rate to ensure that the gas molecules fully diffuse in the oil phase, thereby obtaining the best oil recovery efficiency [15, 17].
Improvement of water-oil mobility ratio: After CO2 is dissolved in the formation water and crude oil, they are carbonated separately. After the formation water is carbonated, its viscosity increases by more than 20%, and its mobility decreases; after the crude oil is carbonated, its viscosity decreases, and its mobility increases. The mobility of water and oil tends to be closer, improving the water-oil mobility ratio, expanding the sweep area, and improving the oil displacement efficiency [15].
Reduction of oil-water interfacial tension: During CO2 flooding, CO2 can mix with light hydrocarbons in crude oil, reducing the interfacial tension between the oil and water phases, thereby reducing the resistance during CO2 displacement and ultimately increasing the oil recovery rate. The greater the CO2 concentration, the more significant the reduction in interfacial tension [15,16,17,18,19].
Extraction and vaporization: Supercritical CO2, due to its strong solubility and high fluidity, is often used as an extractant. Supercritical CO2 can effectively extract and vaporize light hydrocarbons in crude oil, especially residual oil that has been expanded but not yet released from the constraints of the formation water, and undergoes interphase mass transfer with CO2 gas. As the extraction and vaporization deepen, CO2 approaches or reaches a state of miscibility with the crude oil, and the interfacial tension drops significantly, thereby significantly improving the displacement efficiency [15,16,17,18,19].
2.3 Gas Dissolution Drive
During the oil displacement process, CO2 is dissolved in the crude oil. When the reservoir pressure drops, the dissolved CO2 separates from the oil and forms bubbles. As the pressure drops further, the CO2 gas expands, producing elastic expansion energy that promotes the flow of crude oil into the wellbore. This is the gas dissolution drive effect [15,16,17,18,19].
2.4 Miscible Effect
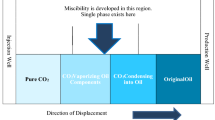
During CO2 flooding, when the pressure reaches the miscibility pressure, the CO2 and crude oil undergo multiple contacts, resulting in the evaporation or extraction of light hydrocarbons from the crude oil. This causes the injected gas at the front of the gas flood to mix with the crude oil, forming a miscible zone, as shown in Fig. 1. The advancement of the miscible zone can effectively displace oil, and the oil recovery can reach more than 90% in some cases [15]. CO2 flooding can be classified into two types based on whether CO2 and crude oil reach miscibility: CO2 immiscible flooding and CO2 miscible flooding [18].
-
(1)
CO2 non-miscible displacement
In the CO2 non-miscible displacement process, the pressure of the formation is lower than the minimum miscible pressure between CO2 and crude oil, resulting in a clear interface between the two and making it difficult for them to mix. In this mode of oil recovery, only a portion of the CO2 dissolves in the crude oil, causing the volume of the crude oil to expand and the viscosity to decrease, thereby achieving the purpose of oil displacement. However, since only a portion of the CO2 interacts with the crude oil, the displacement efficiency is low and issues such as rapid breakthrough of CO2 gas can easily occur. Therefore, this method has limited application in the field and its maturity level is much lower than that of the miscible displacement mode. However, after years of research and exploration, effective monitoring techniques for CO2 injection, methods for removing blockages in the injection formation and production profile, and effective selection of suitable reservoirs for CO2 non-miscible displacement have been developed. Therefore, in recent years, CO2 non-miscible displacement technology has been revived [8].
-
(2)
CO2 miscible displacement
When the formation pressure is greater than the minimum miscible pressure between CO2 and crude oil, but less than the formation’s fracturing pressure, CO2 and crude oil mix to achieve CO2 miscible displacement. After CO2 and crude oil are mixed, they dissolve in each other without a clear interface, eliminating interfacial tension and greatly improving displacement efficiency. Since CO2 miscible displacement has a better effect on increasing the recovery rate than non-miscible and near-miscible displacement, most oilfield experiments and developments at home and abroad use CO2 miscible displacement mode. For oilfields where the original formation conditions cannot achieve miscibility, the minimum miscible pressure can be reduced or the formation pressure can be rapidly increased to improve the degree of miscibility. For example, “CO2 flooding” technology can be used to lower the minimum miscible pressure by enhancing CO2’s ability to dissolve in crude oil and extract light hydrocarbons from crude oil while injecting large amounts of liquid CO2 into the local well group through rapid pressure buildup to improve the degree of miscibility [7].
3 Calculation Methods for Minimum Miscibility Pressure (MMP)
One of the key issues in achieving CO2 miscible flooding technology is determining the minimum miscibility pressure (MMP) for CO2 flooding. Many experts at home and abroad have researched this, and currently, the methods for determining MMP mainly include experimental methods, empirical formula methods, equation of state methods, and numerical simulation methods.
3.1 Experimental Method
-
(1)
Capillary tube experiment
Among the traditional laboratory methods for determining MMP, the capillary tube experiment is currently the most accurate and reproducible method. Due to its direct and reliable method, it has become a standard method for determining MMP at home and abroad.
The experimental process of the capillary tube experiment is shown in Fig. 2. The capillary tube is a long metal tube filled with sand, glass beads, or other particles inside. The experiment requires saturating the capillary tube with crude oil at the desired temperature and pressure and using a backpressure valve to control the experimental pressure. After the system reaches equilibrium, CO2 displacement experiment begins. When using this method to determine MMP, CO2 injection is usually performed at different pressure conditions, and the oil recovery rate when injecting 1.2 times the pore volume of CO2 is recorded. The oil recovery rate-pressure curve is established, and the inflection point of the curve when the recovery rate is about 90% is defined as the MMP [20,21,22].
Although the capillary tube experiment is currently considered the most reliable laboratory method for determining MMP, the standard for the length, diameter, and internal filling of the experimental device has not been determined yet. Due to the inability to simulate factors such as viscosity fingering and gravity override and the long experimental time required, the capillary tube experiment has certain limitations.
-
(2)
Bubble Rising Apparatus Method
The experimental apparatus of the bubble-rising method is shown in Fig. 3. The core device of the experiment is the glass tube mounted vertically on the high-pressure observation gauge in the temperature-controlled bath. In the experiment, the CO2-oil miscible state can be directly observed through the glass tube. At the beginning of the experiment, the glass tube is filled with crude oil and CO2 gas is slowly injected into the glass tube at different pressure conditions. The changes during the CO2 bubble rising process are observed and photographed. In the glass tube, the gas-liquid mass transfer occurs at the interface between the CO2 bubble and crude oil. At low pressure conditions, due to the larger interfacial tension, the bubble shape is larger. As the pressure increases, the interfacial tension decreases, and the bubble becomes smaller. When the interfacial tension is extremely low and the bubble shape is extremely small, it can be considered that the CO2 and crude oil have reached miscibility [20, 23].
Although the bubble-rising method is very fast, with the experiment taking about 1 h to complete, the results obtained do not have a quantitative standard and are greatly influenced by human factors, leading to large errors. The reliability of the experimental results needs to be verified.
-
(3)
Interfacial Tension Disappearance Method
The interfacial tension disappearance method determines the MMP of CO2 and reservoir crude oil by directly measuring the interfacial tension between them and using extrapolation [20, 22,23,24]. Generally, the methods for measuring interfacial tension between gas and liquid include the ring detachment method, pendant drop method, sessile drop method, capillary rise method, and drop volume method. The pendant drop method is usually used in high-pressure systems [23].
The pendant drop method determines the interfacial tension between the two phases by measuring the shape parameters of the liquid droplet suspended at the top of a capillary probe. The principle can be expressed as:
where γ is the interfacial tension between gas and liquid, N/m; De is the diameter of the widest part of the droplet, m; g is the acceleration due to gravity, m/s2; Δρ is the density difference between the gas and liquid phases, kg/m3; and H is the corrected shape factor [24].
The interfacial tension at different pressures can be measured using the pendant drop method. However, since the interfacial tension measuring device cannot measure the pressure at which the interfacial tension becomes zero at the point of phase mixing, the MMP can be calculated by combining the experimental results with the extrapolation method. Compared with the capillary tube method, this method can quickly and simply determine the MMP. However, it has limitations because it cannot account for the impact of porous media on the measurement results [20, 23].
3.2 Empirical Formula Method
Due to its simple and rapid calculation, the empirical formula method has become increasingly favored by researchers. Currently, there are more than 20 published empirical formulas for determining MMP. Below, some of them are selected for introduction.
-
(1)
Yelling and Metcalfe formula [25]
In 1980, Yelling and Metcalfe extrapolated the CO2 vapor pressure curve to the MMP and predicted CO2 flooding MMP based on reservoir temperature. At the same time, the Petroleum Institute for Studies in Recovery of Oil (PRI) also proposed a similar empirical formula.
Yelling and Metcalfe formula:
Petroleum Institute for Studies in Recovery of Oil (PRI) formula:
-
(2)
Glaso formula [26]
Glaso added the consideration of the effect of intermediate components in crude oil on the minimum miscibility pressure of CO2 based on the prediction chart made by Benham and others. Two relationship formulas were given based on the content of intermediate components (mol %) in crude oil as a limit:
① When the mole percentage of intermediate components in crude oil is less than 18%:
② When the mole percentage of intermediate components in crude oil is greater than 18%:
where Mc7+ is the molecular weight of C7+ in the dewaxing oil, and fRF is the mole content of C2-C6 in the reservoir fluid.
-
(3)
Alston formula [27]
Alston and others proposed a method to predict the MMP of a gas-containing oil system that reaches miscible displacement through multiple contacts with pure or impure CO2 gas.
① Pure CO2 displacement:
where TR is the reservoir temperature, °F, MC5+ is the molar mass above C5+, g/mol, Xvol is the mole number of volatile components (such as N2 and C1) in crude oil, and Xint is the mole number of intermediate hydrocarbon components (C2-4, CO2, and H2S) in crude oil.
② Impure CO2 displacement:
where Wi is the weight fraction of component I, Tcm is the average critical temperature of the injected gas, °F, Ti is the critical temperature (Rankine temperature R) of component I, °R, and Xvol and Xint are the mole numbers of the volatile components and intermediate hydrocarbon components in crude oil, respectively.
Detailed composition analysis of the CO2-rich phase and oil-rich phase formed by the mixture of CO2 and crude oil shows that the distribution of molecular sizes in crude oil has a much greater impact on MMP than the changes in hydrocarbon structure. Silva used this to establish a formula for MMP as a function of the molecular weight distribution of crude oil.
First, based on the analysis of the gas phase, normalized weight fractions WIC2+ of each fraction of C2–C31 hydrocarbons (excluding non-hydrocarbons such as C1, N2, CO2, and H2S) are given:
where Wi is the weight fraction of component I, and I = 31 corresponds to the weight fraction of all components above C31.
The distribution coefficients Ki of component I between the CO2-rich phase and oil-rich phase are obtained when CO2 and crude oil reach phase equilibrium:
where Ci is the number of carbon atoms in component I, and C31+ is replaced by the average number of carbon atoms C33. If the weight fraction of the component is given in segments, the corresponding Ki can be calculated according to the average number of carbon atoms in the component.
Weight composition parameter F:
CO2 density ρρmm at MMP:
When F < 1.1467, \(\rho \rho_{mm} = - 0.524F + 1.189\);
When F ≥ 1.1467, \(\rho \rho_{mm} = 0.42\).
The pressure corresponding to this density of CO2 at MMP can be calculated based on the state equation or by looking up the CO2 temperature-pressure-density table.
3.3 State Equation Method [30]
The PR state equation was proposed by Peng et al., and has high accuracy in calculating the critical point of mixtures. Its form is as follows:
For the i component in the mixture: \(a_{i} = \Omega_{a} \times \frac{{R^{2} T_{ci}^{2} }}{{p_{ci} }}\), Ωa = 0.45724; \(b_{i} = \Omega_{b} \times \frac{{RT_{ci} }}{{p_{ci} }}\), Ωb = 0.07780.
Peng et al. used Soave’s method to determine the parameter α:
where \(m_{i} = 0.37464 + 1.5422\omega_{i} - 0.26992\omega_{i}^{2}\).
Substituting the state equation of a real gas \(pv = z_{m} nRT\) into Eq. (14), the compressibility factor cubic equation can be obtained:
where \(A_{m} = \frac{{a_{m} (T)p}}{{(RT)^{2} }}\), \(B_{m} = \frac{{b_{m} p}}{RT}\).
For mixtures, the following mixing rules are generally used:
From Eqs. (16) and (17), the fugacity coefficient of the i component can be derived as \(\Phi_{i}\):
where \(H_{i} = \frac{{A_{m} }}{{2\sqrt 2 B_{m} }}(2\frac{{\psi_{j} }}{{a_{m} }} - \frac{{b_{i} }}{{b_{m} }})\ln [\frac{{z_{m} + (1 + \sqrt 2 )B_{m} }}{{z_{m} + (1 - \sqrt 2 )B_{m} }}]\), \(\psi_{j} = \sum\limits_{j = 1}^{n} {x_{j} (a_{i} \alpha_{i} a_{j} \alpha_{j} )^{0.5} (1 - k_{ij} )}\).
3.4 Numerical Simulation Method
To determine MMP using the numerical simulation method, it is necessary to establish a numerical simulation model based on a good fit of the PVT properties of the reservoir fluids. The actual data from the reservoir is used to establish a numerical simulation model of the slim tube experiment, with injection and production wells set at the two ends of the model. The inflow and outflow of CO2 and crude oil at different pressures are simulated, and MMP is determined by plotting the relationship between recovery rate and displacement pressure [31,32,33].
The numerical simulation method is a relatively mature method for determining MMP and has a wide range of applications. Currently, reservoir simulation software such as Eclipse and CMG can be used for this purpose. However, the accuracy of the calculation is greatly affected by the precision of the experiment and the selection of data.
4 Field Application and Effect
This paper investigated a total of 33 low-permeability oilfields that have implemented CO2 EOR technology, extracting their geological characteristics and relevant parameters of field application effectiveness, as shown in Appendix and Fig. 4, and summarized them as follows.
4.1 Lithology
Different sedimentary environments of oil reservoirs lead to different lithologies. The internal mineral composition and structure of reservoirs with different lithologies are also different, resulting in varying properties of the oil reservoirs and influencing the choice of development methods.
The surveyed blocks mainly contain Sandstone, carbonate, and siliceous rock reservoirs. Among them, there are 19 Sandstone reservoirs, accounting for 57.6%, including 16 mixed-phase reservoirs and 3 non-mixed-phase reservoirs; 14 carbonate reservoirs, accounting for 42.4%, are all mixed-phase reservoirs; and 1 siliceous rock reservoir, accounting for 3%, is a mixed-phase reservoir. The average daily oil production of Sandstone, carbonate, and siliceous rock reservoirs are 6.376t, 7.02t, and 3.02t, respectively. The CO2 displacement effect is better in carbonate reservoirs.
4.2 Reservoir Depth and Temperature
The depth of the reservoir affects the temperature and pressure of the reservoir to a certain extent, thereby affecting the CO2 displacement effect. When the depth of the reservoir is high, the temperature of the reservoir also increases, which can easily lead to excessively high mixed-phase pressure and difficulty in forming a mixed-phase; when the depth of the reservoir is low, the pressure of the reservoir is also low, and it is also difficult to form a mixed-phase. Nevertheless, because the mixed-phase displacement effect is better than non-mixed-phase displacement, some reservoirs will take corresponding measures to increase the formation pressure before starting CO2 displacement to achieve mixed-phase displacement. The surveyed blocks are mostly medium-deep reservoirs in terms of depth and low- to medium-high temperature reservoirs according to temperature classification.
The lowest depth of the reservoir is 394.4 m, the highest is 3200 m, and the average depth is 1960.6 m. Among them, there are 4 shallow reservoirs (≤1220 m), accounting for 12.12%, all of which are mixed-phase reservoirs; 19 medium-deep reservoirs (1220–2440 m), accounting for 57.58%, including 16 mixed-phase reservoirs and 3 non-mixed-phase reservoirs; and 9 deep reservoirs (>2440 m), accounting for 27.27%, are all mixed-phase reservoirs. The average daily oil production of single wells in medium-deep and deep reservoirs is 5.1 t and 14.575 t, respectively, and the CO2 displacement effect is better in deep reservoirs.
The lowest temperature of the reservoir is 22.8 °C, the highest is 126 °C, and the average temperature is 71.6 °C. Among them, there are 13 low-temperature reservoirs (≤60 °C), accounting for 39.39%, including 12 mixed-phase reservoirs and 1 non-mixed-phase reservoir; 12 medium-high temperature reservoirs (60–120 °C), accounting for 36.36%, including 10 mixed-phase reservoirs and 1 non-mixed-phase reservoir; and 1 high-temperature reservoir (>120 °C), accounting for 3.03%, is a mixed-phase reservoir. The average daily oil production of single wells in low-temperature and medium-high temperature reservoirs is 4.44 t and 7.88 t, respectively, and the CO2 displacement effect is better in medium-high temperature reservoirs.
4.3 Porosity and Permeability
Porosity and permeability of reservoirs are important parameters for evaluating the storage capacity and permeability characteristics of reservoirs [34], and are also two important factors that affect the selection of development methods. Low porosity and permeability of reservoirs can lead to poor oil flowability, and conventional displacement agents may have difficulty achieving ideal results. However, supercritical CO2, due to its strong permeability, can easily enter the tiny pores of reservoirs and displace oil more effectively. The surveyed blocks are mostly characterized by ultra-low porosity and permeability reservoirs.
The minimum porosity of the reservoir is 6%, the maximum is 23.4%, and the average porosity is 13.3%. Among them, there are 5 ultra-low porosity reservoirs (≤10%), accounting for 15.15%; 20 low-porosity reservoirs (10–15%), accounting for 60.61%; and 8 medium-porosity reservoirs (15–25%), accounting for 24.24%. The daily production of a single well for ultra-low porosity, low porosity, and medium porosity reservoirs is 10.88 t, 4.89 t, and 3.57 t, respectively. The CO2 displacement effect in ultra-low porosity reservoirs is better.
The minimum permeability of the reservoir is 0.27 mD, the maximum is 46 mD, and the average permeability is 11.16 mD. There are 2 ultra-low permeability reservoirs (0.1–1 mD), accounting for 6.06%; 19 special low-permeability reservoirs (1–10 mD), accounting for 57.58%; and 12 low-permeability reservoirs, accounting for 36.36%. The daily production of a single well for ultra-low permeability and low permeability reservoirs is 3.26t and 10.96t, respectively. The CO2 displacement effect in low-permeability reservoirs is better.
4.4 Crude Oil Density and Viscosity
Most of the low-permeability reservoirs for CO2 displacement both domestically and abroad are light crude oil reservoirs, with a relative density ranging from 0.725 to 0.881, and an average relative density of 0.828. Among them, there are 19 light crude oil reservoirs (≤0.852), accounting for 57.58%; and 13 medium crude oil reservoirs (0.852–0.930), accounting for 39.39%. The daily production of a single well for light crude oil and medium crude oil reservoirs is 8.51 t and 5.39 t, respectively. Light crude oil reservoirs have a better CO2 displacement effect.
Classified by crude oil viscosity, most of them are low viscosity oil reservoirs, with crude oil viscosity ranging from 0.2 mPa·s to 12.8 mPa·s, and an average crude oil viscosity of 2.1 mPa·s. Among them, there are 30 low viscosity oil reservoirs (≤10 mPa·s), accounting for 90.91%, and 1 medium viscosity oil reservoir (10–100 mPa·s), accounting for 3.03%.
4.5 Displacement Mode
There are mainly two types of CO2 displacement modes that increase the recovery rate, namely, CO2 miscible flooding and non-miscible flooding (see Sect. 2.4 for more details on miscible effect). For low-permeability reservoirs with CO2 flooding in China and abroad, the miscible flooding mode is mainly used, with 30 reservoirs accounting for 90.91% and 3 non-miscible flooding reservoirs accounting for 9.09%.
CO2 miscible flooding reservoirs are covered by Sandstone, carbonate rock, and siliceous rock. The depth of the reservoir is about 394.4–3200 m, with an average depth of 1966.3 m. The reservoir temperature is about 22.8–126 °C, with an average temperature of 70.4 °C. The porosity is about 6–23.4%, with an average porosity of 13.4%. The permeability is about 0.27–46 mD, with an average permeability of 12.1 mD. The relative density of crude oil is 0.725–0.881, with an average relative density of 0.828. The viscosity of crude oil is 0.2–12.8 mPa·s, with an average viscosity of 2.05 mPa·s.
The non-miscible CO2 flooding reservoirs are all Sandstone reservoirs, with a concentration of depths in the middle and deep layers, about 1617–2100 m, with an average depth of 1905.7 m. The reservoirs are low-porosity and ultra-low-permeability reservoirs, with porosity of 10–12.8% and permeability of 1.16–1.9 mD. The distribution of reservoir temperature, crude oil relative density, and viscosity is similar to that of the miscible CO2 flooding reservoirs. The temperature is 44–108 °C, the crude oil is light, with a relative density of about 0.79–0.86, and the crude oil is low viscosity, with a viscosity of 1.91–3.6 mPa·s.
The single-well daily oil production of CO2 miscible flooding reservoirs and non-miscible flooding reservoirs are 7.42 t and 1.025 t, respectively, with the miscible flooding effect being much better than that of non-miscible flooding.
4.6 Injection Method
The injection methods that improve the recovery rate of CO2 flooding mainly include continuous gas injection, water-gas alternating injection, periodic gas injection, etc.
Continuous gas injection refers to the direct injection of continuous CO2 gas into the formation. This method has a fast effect and high oil recovery efficiency, but the consumption of CO2 is large, and early gas channeling is prone to occur, with a small affected area and rapid gas production increase [17].
Water-gas alternating (WAG) injection is a method of driving oil by alternately injecting CO2 and water into the reservoir with smaller segment plug sizes. Although injection of water may cause water blockage and CO2 to bypass crude oil [17], this method can effectively improve the problem of early gas channeling during continuous gas injection. Injecting CO2 into the reservoir reduces the viscosity of crude oil and expands its volume, which is beneficial to expanding the affected area and improving oil recovery efficiency [18].
Periodic injection involves injecting a cycle of CO2 into a formation, followed by shutting the well for a period of time before injecting the next cycle. This method has the advantages of continuous injection, alternating between water and gas, and CO2 sequestration. During the shut-in period, CO2 comes into contact with the crude oil, reducing its viscosity and increasing its volume, thereby improving oil recovery through the mechanism of CO2 EOR. However, this method requires a long time due to the need to shut-in the well [35].
In addition, some oil fields have developed their own targeted CO2 EOR models based on their specific conditions. For example, the Hua 26 block in the Subei Basin uses an asynchronous injection and production method, while the Weyburn oil field in Canada uses a method of injecting water and gas separately but simultaneously [36, 37].
Of the surveyed blocks, 9 blocks, accounting for 27.27%, used continuous injection and all were using miscible flooding; 14 blocks, accounting for 42.42%, used alternating injection of water and gas, with one block being a non-miscible reservoir; 4 blocks, accounting for 12.12%, started with continuous injection and then switched to alternating injection or used both injection methods simultaneously, with one block being a non-miscible reservoir; 3 blocks, accounting for 9.09%, used periodic injection, with one block being a non-miscible reservoir; and there were 1 block each for asynchronous injection and separate injection of water and gas, both accounting for 3.03%, and both using miscible flooding. Among them, the periodic injection method had a daily oil production rate of 13.64 t per well, far better than other injection methods.
5 Conclusion and Outlook
-
(1)
CO2-EOR technology achieves its oil recovery purpose mainly through the changes in reservoir rock and fluid properties, gas dissolution drive and miscibility effects. The minimum miscibility pressure (MMP) of CO2 and crude oil can be obtained through experimental methods, empirical formulae, equation of state, and numerical simulation methods. Among them, the experimental method yields more accurate results, the empirical formula and equation of state methods are more convenient, and the numerical simulation method has a wide range of applications, but the results are greatly affected by the original data.
-
(2)
The application of CO2-EOR technology in low-permeability reservoirs is mainly concentrated in the following types of reservoirs: classified by reservoir depth, mainly in middle and deep reservoirs; classified by reservoir temperature, mainly in low-temperature and medium-to-high-temperature reservoirs; classified by pore structure, mainly in ultra-low porosity and ultra-low permeability reservoirs; classified by crude oil properties, mainly in light crude oil reservoirs and low-viscosity crude oil reservoirs. The displacement mode tends to be miscible displacement, and the CO2 injection methods are mainly water alternating gas injection and continuous injection.
-
(3)
In the oil fields where CO2 is currently used to improve oil recovery, carbonate reservoirs have better displacement effects than other rock types; deep, medium-to-high temperature, ultra-low porosity, low-permeability, and light crude oil reservoirs have better displacement effects than other reservoir properties; in terms of displacement and injection methods, miscible displacement and cyclic injection have better effects than other methods.
-
(4)
CO2-EOR can not only significantly increase the recovery rate of low-permeability reservoirs, but also achieve effective CO2 sequestration, which is of great significance to ensure national energy security and achieve the carbon peaking and carbon neutrality goals, and has broad application prospects. However, attention should also be paid to balancing the increase in recovery rate and geological sequestration, so as to achieve a more secure and economically benign promotion.
References
Tang, W., Zhang, G., Xu, P.: Analyze key areas and orientations of technological innovation for oil and gas exploration and development in 14h five-year plan period. Pet. Sci. Technol. Forum 41(5), 7–15 (2022)
U.S Energy Information Administration: Technically recoverable shale oil and shale gas resources: an assessment of 137 shale formations in 41 countries outside the United States. U.S Energy Information Administration, 10 June 2013
Wang, C., Hong, L., Gao, R., et al.: Status-quo and challenges of enhanced oil recovery in low permeability reservoirs. Unconventional Oil Gas 5(3), 102–108 (2018)
Wenrui, H.: The present and future of low permeability oil and gas in China. Strateg. Study CAE 11(8), 29–37 (2009)
Wang, Z., Cao, G., Bai, Y., et al.: Development status and prospect of EOR technology in low- permeability reservoirs. Special Oil Gas Reservoirs, 1–17 (2023)
Ding, S., Xi, Y., Liu, G., et al.: Adaptability of different injection methods of CO2 flooding in low permeability reservoirs to enhanced oil recovery and geological storage. Petrol. Geol. Recovery Effic. 30, 1–8 (2023)
Wang, X., Yang, H., Wang W., et al.: Technology and practice of CO2 flooding and storage in low-permeability tight reservoirs. Petrol. Geol. Recovery Effic., 1–9 (2023)
Qin, J., Han, H., Liu, X.: Application and enlightenment of carbon dioxide flooding in the United States of America. Pet. Explor. Dev. 42(2), 209–216 (2015)
Koottungal, L.: 2014 worldwide EOR survey. Oil Gas J. 112(4), 79–91 (2014)
Yuan, S., Wang, Q., Li, J., et al.: Technology progress and prospects of enhanced oil recovery by gas injection. Acta Petrolei Sinica 41(12), 1623–1632 (2020)
Song, X., Wang, F., Ma, D., et al.: Progress and prospect of carbon dioxide capture, utilization and storage in CNPC oilfields. Pet. Explor. Dev. 50(1), 206–218 (2023)
Li, S., Tang, Y., Hou, C.: Present situation and development trend of CO2 injection enhanced oil recovery technology. Petrol. Reservoir Eval. Dev. 9(03) (2019)
Gao, H., He, Y., Zhou, X.: Research progress on CO2 EOR technology. Special Oil Gas Reservoirs 16(1), 6–12, 106 (2009)
Jia, W., Song, S., Li, C., et al.: Progress of oily sludge extraction by supercritical CO2. Chem. Ind. Eng. Progress 41(12) (2022)
Wang, X.: CO2 Flooding Technology in Low Rermeability Sandstone Reservoir. Petroleum Industry Press, Beijing (2017)
Gu, L., Li, Z., Ou, J.: The existing state of enhanced oil recovery by utilizing carbon dioxide. China Min. Mag. 10, 66–69 (2007)
Zhu, C, Cheng, L.: Reserch on CO2 flooding in low permeability reservoir. Drill. Prod. Technol. 30(6), 55–57, 60, 144–145 (2007)
Jia, K., Ji, D., Gao, J., et al.: The existing state of enhanced oil recovery by CO2 flooding in low permeability reservoirs. Unconventional Oil Gas 6(1), 107–114, 61 (2019)
Cheng, S., Liu, S., Zhu, S.: The mechanism and popularization application of single well CO2 huff and puff for increasing oil production. Oil-Gas Field Surface Eng. 10, 16–17 (2003)
Dai, B., Wang, L., Zhuang, J.: Experiment of minimum miscible pressure of CO2 flooding in ultra-low permeability reservoir. Lithologic Reservoirs 32(2), 129–133 (2020)
Fang, Z.: Calculation of Minimum Miscibility Pressure for CO2-Hydrocarbon System. China University of Petroleum, Beijing (2019)
Liang, M., Yuan, H., Yang, Y., et al.: Research progress on miscible gas displacement and determination of minimum miscibility pressure. J. Southwest Petrol. Univ. (Sci. Technol. Edn.) 39(5), 101–112 (2017)
Wang, Q.: Review on determine methods of minimum miscibility phase pressure of CO2 miscible flooding. Chem. Ind. Eng. Progress 30(S1), 805–808 (2011)
Huang, C., Tang, R., Yu, H., et al.: Determination of the minimum miscibility pressure of CO2 and crude oil system by hanging drop method. Lithologic Reservoirs 27(1), 127–130 (2015)
Yellig, W.F., Metcalfe, R.S.: Determination and prediction of CO2 minimum miscibility pressures (includes associated paper 8876). J. Petrol. Technol. 32(01), 160–168 (1980)
Glaso, O.: Generalized minimum miscibility pressure correlation. SPE J. (12), 927934 (1985)
Alston, R.B., Kokolis, G.P., James, C.F.: CO2 minimum miscibility pressure: a correlation for impure CO2 streams and live oil systems. SPE J. 25(2), 268–274 (1985)
Silva, M.K., Orr, F.M., Jr.: Effect of oil composition on minimum miscibility pressure - part 1: solubility of hydrocarbons in dense carbon dioxide. SPE Reservoir Eng. 2 (1987)
Yang, X.: Study on the Minimum Miscibility Pressure of Gas Injection in Oil Reservoirs. Southwest Petroleum University, Chengdu (2003)
Ye, A., Guo, P., Wang, S., et al.: Determination of minimum miscibility pressure for CO2 flooding by using PR equation of state. Lithologic Reservoirs 24(6), 125–128 (2012)
Gao, Z.: The Production Technology by Injecting Gas of Oil Field. Petroleum Industry Press, Beijing (1994)
Hao, Y., Chen, Y., Yu, H.: Determination and prediction of minimum miscibility pressure in CO2 flooding. Petrol. Geol. Recovery Effic. (6), 64–66, 87 (2005)
Zhang, G., Li, Z., Liu, J., et al.: Study on determine methods of minimum miscibility pressure of hydrocarbon injection miscible flooding. Drill. Prod. Technol. 144(03), 99–102+157 (2008)
Lu, P.: Research on Porosity and Permeability Model of Tight Sandstone Reservoirs Based on Machine Learning-Taking the Eight Member of the Shihezi Formation of the Upper Paleozoic in the Middle Part of the Western Margin of the Ordos Basin as an Example. Northwest University, Xi’an (2022)
He, Y., Zhou, X., Li, M., et al.: Injection mode of CO2 displacement in extra-low permeability reservoirs. J. Oil Gas Technol. 32(06), 131–134+531 (2010)
Li, Y., Huang, W., Jin, Y., et al.: Different reservoir types of CO2 flooding in Sinopec EOR technology development and application under “dual carbon” vision. Petrol. Reservoir Eval. Dev. 11(6), 793–804, 790 (2021)
Shen, P., Liao, W.: Geological Storage of Carbon Dioxide and Technology for Improving Oil Recovery. Petroleum Industry Press, Beijing (2009)
Klins, M.A.: Carbon Dioxide Flooding Mechanism and Engineering Design. Shaojin, C. (Trans.). Petroleum Industry Press, Beijing (1989)
Holm, L.W., O’Brien, L.J.: Carbon dioxide test at the Mead-Strawn field. J. Pet. Technol. 23, 431–442 (1971)
Manrique, E.J., Muci, V.E., Gurfinkel, M.E.: EOR field experiences in carbonate reservoirs in the United States. SPE Res. Eval. Eng. 10, 667–686 (2007)
Graham, B.D., Bowen, J.F.: Design and implementation of a Levelland unit CO2 Tertiary pilot. SPE 8831, Tulsa (1980)
Brannan, G., Whitington, H.M.: Enriched Gas MiscibleFlooding: A Case History of the Levelland Unit Secondary Miscible Project, SPE 5826, IOR. Tulsa, OK (1976)
Liu, Y., Liu, J., et al.: Polymer and Carbon Dioxide Oil Displacement Technology. China Petrochemical Press, Beijing (2001)
Youngren, G.K., Charlson, G.S.: History match analysis of the little creek CO2 pilot test. J. Pet. Technol. 32, 2042–2052 (1980)
Merchant, D.H., Thakur, S.C.: Reservoir management in tertiary CO2 floods. Paper presented at the SPE Annual Technical Conference and Exhibition, Houston, Texas, October 1993
Stein, M.H., Frey, D.D., Walker, R.D., Pariani, G.J.: Slaughter estate unit CO2 flood: comparison between pilot and field-scale performance. J. Pet. Technol. 44, 1026–1032 (1992)
Watts, R.J., Conner, W.D., Wasson, J.A., Yost, A.B.: CO2 injection for tertiary oil recovery, Granny’s creek field, Clay county, West Virginia. Paper presented at the SPE Enhanced Oil Recovery Symposium, Tulsa, Oklahoma, April 1982
Watts, R.J., Gehr, J.B., Wasson, J.A., Evans, D.M., Locke, C.D.: A single CO2 injection well minitest in a low-permeability carbonate reservoir. J. Pet. Technol. 34, 1781–1788 (1982)
Brummert, A.C., Watts, R.J., Boone, D.A., Wasson, J.A.: Rock creek oil field CO2, pilot tests, Roane County, West Virginia. J. Pet. Technol. 40, 339–347 (1988)
Desch, J.B., Larsen, W.K., Lindsay, R.F., Nettle, R.L.: Enhanced oil recovery by CO2 miscible displacement in tie little knife field, Billings County, North Dakota. In: SPE 10696, 3rd EOR, Tulsa (1982)
Fox, M.J., Simlote, V.N., Beaty, W.G.: Evaluation of CO2 flood performance, Springer ‘A’ Sand, NE Purdy Unit, Garvin County, Oklahoma. Paper SPE 12665 presented at the 1984 SPE/DOE Enhanced Oil Recovery Symposium, Tulsa, Oklahoma, 15–16 April (1984)
Fox, M.J., Simlote, V.N., Stark, K.L., et al.: Review of CO2 flood, Springer “A” sand, Northeast Purdy Unit, Garvin County, Oklahoma. SPE Reserv. Eng. 3(4), 1161–1167 (1988)
Tanner, C.S., Baxley, P.T., Crump, J.G., et al.: Production performance of the Wasson Denver unit CO2 flood. In: Society of Petroleum Engineers. Society of Petroleum Engineers (1992)
Pittaway, K.R., Albright, J.C., Hoover, J.W., et al.: The Maljamar CO2 pilot: review and results. J. Petrol. Technol. 39(10), 1256–1260 (1987)
Merritt, M.B., Groce, J.F.: A case history of the Hanford San Andres Miscible CO2 project. J. Petrol. Technol. 44(08), 924–929 (1992)
Brownlee, M.H., Sugg, L.A.: East vacuum Grayburg-SanAndres Unit CO2 injection project: development and results to date. In: SPE 16721, 62nd ATCE, Dallas (1987)
Poole, E.S.: Evaluation and implementation of CO2 injection at the Dollarhide Devonian Unit (1988)
Burbank, D.E.: Early CO2 flood experience at the South Wasson Clearfork unit. In: SPE/DOE Enhanced Oil Recovery Symposium (1992)
Fullbright, G.D., Hild, G.P., Korf, T.A., et al.: Evolution of conformance improvement efforts in a major CO2 WAG injection project. In: SPE/DOE Improved Oil Recovery Symposium. Society of Petroleum Engineers (1996)
Beliveau, D., Payne, D.A., Mundry, M.: Waterflood and CO2 flood of the fractured Midale field (includes associated paper 22947). J. Petrol. Technol. 45(09), 881–887 (1993)
Beliveau, D.A.: Midale CO flood pilot. J. Can. Petrol. Technol. 26(06) (1987)
Ji, B., He, Y.: Practice and understanding about CO2 flooding in low permeability oil reservoirs by Sinopec. Petrol. Reservoir Eval. Dev. 11(6), 805–811, 844, 790 (2021)
Zhang, F., Wang, Z.: The field experiment and results analysis of CO2 miscible displacement in Caoshe oilfield of the north Jiangsu Basin. Pet. Geol. Exp. 32(3), 296–300 (2010)
Zhang, Y.: CO2-EOR and Geological Storage Study of Jiangsu Caoshe Oil-Field. China University of Petroleum (East China), Qingdao (2010)
Song, Y.: Evaluation of Oil Displacement Effect by CO2 Flooding of FUYANG Reservoirs in DAQING Oilfield. China University of Petroleum, Beijing (2016)
Zhang, Y., Yang, T., Yang, Z., et al.: Evaluation of oil displacement by CO2 at Fuyang extra-low permeability layer in Yushulin oilfield. Sci. Technol. Rev. 33(5), 52–56 (2015)
Yuan, S., Ma, D., Li, J., et al.: Progress and prospects of carbon dioxide capture, EOR-utilization and storage industrialization. Pet. Explor. Dev. 49(4), 828–834 (2022)
Hu, Y., Hao, M., Chen, G., et al.: Technologies and practice of CO2 flooding and sequestration in China. Pet. Explor. Dev. 46(4), 716–727 (2019)
Zhang, X., Kou, G., Wang, Z., et al.: Technical discussion on development effects of CO2 flooding in Jilin extra-low permeability reservoir. Drill. Prod. Technol. 42(2), 64–67, 4–5 (2019)
Guofeng, W.: Carbon dioxide capture, enhanced-oil recovery and storage technology and engineering practice in Jilin Oilfield, NE China. Pet. Explor. Dev. 50(1), 219–226 (2023)
Cao, X., Lyu, G., Wang, J., et al.: Technology and application of CO2 flooding in ultra-low permeability beach-bar sand reservoir. Petrol. Reservoir Eval. Dev. 9(3), 41–46 (2019)
Li, C.: Application of CO2 miscible flooding technology in the development of ultra-low permeability reservoirs in Block Gao 89-1. J. Oil Gas Technol. 33(06), 328–329 (2011)
Zhang, Z., Lyu, G., Wang, J.: CCUS and its application in Shengli Oilfield. Petrol. Reservoir Eval. Dev. 11(06), 812–822+790 (2021)
Wang, J., Gao, Y., Zong, C., et al.: Response characteristics of CO2-immiscible-flooding wag in the ultra-low permeability oil reservoirs. Petrol. Geol. Oilfield Dev. Daqing 35(02), 116–120 (2016)
Liao, H.: Numerical simulation of oil displacement with carbon dioxide in DB34 well block, Yaoyingtai Oilfield. J. Xi’an Shiyou Univ. (Nat. Sci. Edn.) 25(05), 50–53+109 (2010)
Dong, C., Gao, Z., Li, X.: Pilot study on CO2 displacement in Yaoyingtai Oilfield. Liaoning Chem. Ind. 47(06), 578–579+582 (2018)
Kang, Y., Bai, Y., Jiang, S., et al.: Technical features and engineering practice of Yanchang full-chain carbon capture, utilization and storage project. Appl. Chem. Ind. 49(7), 1768–1771, 1775 (2020)
Wang, Y., Li, W., Jia, Z., et al.: The development practice and effect analysis for CO2 injection in Chang 6 reservoir of Qiaojiawa Oilfield. Unconventional Oil Gas 3(03), 75–79 (2016)
Yang, H., Jiang, S., Wang, H., et al.: Research of adaptability of gas channeling controlling methods in CO2 flooding of fractured ultra- low permeability reservoir. J. Xi’an Shiyou Univ. (Nat. Sci. Edn.) 32(02), 105–109+115 (2017)
Yang, Y.: Research and application of CO2 flooding technology in extra-low permeability reservoirs of Shengli oilfield. Petrol. Geol. Recovery Effic. 27(1), 11–19 (2020)
Qi, C., Li, R., Zhu, S., et al.: Pilot test on CO2 flooding of Chang 4+51 oil reservoir in Yougou region of the Ordos Basin. Oil Drill. Prod. Technol. 41(2), 249–253 (2019)
Ren, B.: Analysis of CO2 displacement mechanism in the third member of Fujiang formation in Zhangjiaduo oilfield. Petrochem. Ind. Technol. 27(04), 97–98 (2020)
Li, M., Li, Y., Yang, J., et al.: Research and on-site testing of CO2 flooding technology in Huang 3 district. Petrochem. Ind. Appl. 39(5), 71–75 (2020)
Li, K., Lo, P., Wei, M., et al.: A pilot project of CO2 enhanced oil recovery and storage in Chang 8 extra-low permeability reservoir in Huang 3 district of Changqing oilfield. J. Eng. Geol. 29(5), 1488–1496 (2021)
Tang, Y., Liao, S., Lei, X., et al.: Study on improving the sweep efficiency of CO2 flooding in low permeability fractured reservoirs in Huang-3 block. Petrol. Reservoir Eval. Dev. 9(03), 9–13 (2019)
Wang, S., Ma, G., Lang, Q., et al.: Study on formation mechanism and prevention technology of gas channeling by CO2 flooding in low permeability fractured reservoirs. Energy Chem. Ind. 43(03), 29–34 (2022)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix
Appendix
See Table 1.
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Zhou, W., Niu, My., Luo, Wj., Liu, Xt. (2024). Research Progress on Enhanced Oil Recovery by CO2 Flooding in Low Permeability Reservoirs. In: Lin, J. (eds) Proceedings of the International Field Exploration and Development Conference 2023. IFEDC 2023. Springer Series in Geomechanics and Geoengineering. Springer, Singapore. https://doi.org/10.1007/978-981-97-0268-8_28
Download citation
DOI: https://doi.org/10.1007/978-981-97-0268-8_28
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-97-0267-1
Online ISBN: 978-981-97-0268-8
eBook Packages: EngineeringEngineering (R0)