Abstract
Phasmids exhibit twig mimesis as defense mechanism against predators. Most detailed information on the neural basis of this particular behavior is existing for the femur-tibia joint of the stick insect leg. The neural network controlling the activities of the extensor tibiae and the flexor tibiae muscles of this leg joint generates the motor output for catalepsy. Catalepsy, an element of twig mimesis is characterized through extremely slow return movements in response to external perturbations. This property of the neural network governing the femur-tibia joint sets phasmids apart from other orthopteran insect species, e.g. locusts, which do not generate twig mimesis. Cybernetic and comparative analyses have shown that catalepsy is produced by an increased sensitivity of the belonging joint control network to movement velocity. This is achieved by the particular processing of sensory feedback signals about movements of the tibia provided by the femoral chordotonal organ, the main transducer of the femur-tibia joint. This chapter summarizes the present knowledge concerning the neural basis of catalepsy and twig mimesis in the stick insect.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Posture and movement of animals’ appendages are controlled by premotor networks controlling the activity of motor neurons innervating the muscles of the appendage. These networks controlled by the brain reside in the central nervous system of an animal close to the appendage (Orlovsky et al. 1999; Hooper and Büschges 2017): for example, legs of mammals are governed by premotor networks residing in the lumbar spinal chord; legs of insects are governed by neural networks residing in the respective segments, i.e. ganglia, of the thoracic nerve chord. In the resting animal these networks generate reflexes in the muscles of the appendages, i.e. the legs, to guarantee the desired posture of the animal. Here we describe the present knowledge about a neural network controlling posture and movement of a leg joint in phasmids, in which these networks have been specialized in order to allow the generation of twig mimesis, i.e. the defense mechanism towards predators.
9.2 Thanatosis and Catalepsy in the Stick Insect
In the bright light stick insects, Carausius morosus can assume several different postures: at one extreme, the femur-tibia joints are bent to different angles and the femurs stand out from the body (Fig. 9.1a), and at the other extreme, a stick posture is assumed by the animal with all femur-tibia joints being fully extended and the front legs stretched forward and middle and hind legs lying flat against the body pointing rearward (Fig. 9.1b). All intermediate postures between these two extremes can be observed while the overall passively imposed body postures are kept (e.g. Fig. 9.1a, b, c). The stick posture in Carausius is called thanatosis based on the plausible assumption that thanatosis emphasizes the twig-like body shape of a stick insect in which no movement occurs and the animal appears to be a twig for an observer.
When a free leg of a resting stick insect without tarsal contact is forced into a new position, it will return towards the original position extremely slowly, being as slow as the hour hand of a clock. Therefore, it appears to the casual observer as if the leg would remain in the new position. This behavior is called catalepsy, because of its resemblance to the cataleptic (catatonic) state in humans and other mammals. Catalepsy suppresses fast movements in response to passive deflections that could be detected by a predator. It is therefore plausible to allocate catalepsy as part of twig mimesis in stick insects.
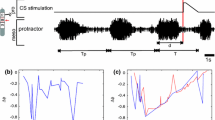
In the 70s, Ulrich Bässler and coworkers have addressed the question, whether thanatosis and catalepsy can be regarded as closely related behaviors or separate behavioral states. Depth of thanatosis was measured by quantification of the extensor forces generated by means of a force transducer in the almost fully extended position of the femur-tibia joint, i.e. at 170 deg. Depth of catalepsy was monitored by measuring the time of return movements of the tibia to the 90 deg-angle in response to a passive flexion from 170 deg to 50 deg and holding it there for 30 s before releasing it (Bässler 1972a, b; e.g. Fig. 9.2). Measuring both values, i.e. extensor force and return speed directly one after the other and plotting them against each other revealed a weakly negative correlation. This meant that the deeper the thanatosis the less pronounced is catalepsy (Bässler 1972b), indicating that thanatosis and catalepsy may not be considered as expressions of one behavior, as both of them may not fall into the same behavioral context. This conclusion was further corroborated by lesioning the proprioceptive sense organ of the femur-tibia joint, the femoral chordotonal organ. While this did not affect thanatosis, cataleptic behavior was no longer detectable (Bässler 1972b; Bässler and Foth 1982).
Catalepsy and tibial return movements. Return movements of the tibia to the 180 deg starting position after being passively bent to 50 deg for 30 s. Nine randomly selected examples of intact legs (modified after Bässler 1972b)
9.3 Neural Basis of Catalepsy in the Stick Insect
9.3.1 Anatomy of Sense Organs and Muscles Controlling the Femur-Tibia Joint
Due to its anatomical advantages the femur-tibia joint of the stick insect was chosen to study the neural basis of catalepsy (summary in Bässler 1983): the fact that the pivot of the femur-tibia joint is oriented exactly rectangular to the plane formed by the femur and the tibia was an important aspect. It allows measuring the femur-tibia angle in a plane with high resolution and without optical distortion.
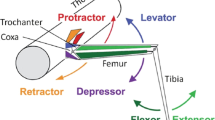
The femur-tibia joint is controlled by two antagonistic muscles, the flexor tibiae and the extensor tibiae. As common for many orthopteran insects these leg muscles are innervated by excitatory and inhibitory motoneurons and at least one efferent modulatory neuron: The extensor tibiae is innervated by only three motoneurons, i.e. one slow motoneuron (SETi), one fast motoneuron (FETi), and the common inhibitor I (Bässler and Storrer 1980) and in addition by modulatory dorsal unpaired median efferent neurons, called DUM-neurons (Mentel et al. 2008; for review see Bräunig and Pflüger 2001). The flexor tibiae is innervated by some 15 excitatory, two inhibitory motoneurons, the common inhibitor II and III (Storrer et al. 1986; Debrodt and Bässler 1990; Goldammer et al. 2012) and as well modulatory DUM-neurons (see above). Among the excitatory motoneurons innervating the flexor muscle are slow, semi-fast, and fast motoneurons, which show sequential recruitment from slow to fast upon activation of the flexor tibiae muscle with increasing contraction forces generated (Gabriel et al. 2003). Because of its simpler innervation the extensor tibiae lent itself for studying the neural basis of catalepsy in the femur-tibia joint (Fig. 9.3).
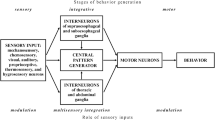
Schematic presentation of the identified information flow of sensory signals about tibial movement in the neural network governing the activity of the femur-tibia joint in the stick insect. The scheme shows the principle connectivity for the distributed processing of flexion signals in the neural network between sensory afferents and the two excitatory extensor motor neurons (slow extensor tibiae (SETi) and fast extensor tibiae (FETi)) and the fibers of the extensor muscle for three pathways: (1) direct monosynaptic pathways between fCO afferents and the extensor motor neurons, (2) polysynaptic pathways providing synaptic drive to nonspiking interneurons exciting extensor motor neurons (eNSI), and (3) polysynaptic pathways providing synaptic drive to nonspiking interneurons inhibiting extensor motor neurons (iNSI). For references and details see text
Movement and position of the tibia are measured by three sense organs, the femoral chordotonal organ (fCO) situated close to the proximal end of the femur and connected to the tibia via a long apodeme, and two single sensory cells, the RDPL and the RDAL located close to the tibia (Coillot and Boistel 1968; Bässler 1977; summary in Bässler 1983). The latter two play no obvious role in the control of tibial muscles. However, the fCO has been shown to serve as sensor in the control of the tibia (summary: Bässler 1983). It is two partite with a ventral part consisting of ca. 80 sensory neurons and a dorsal part encompassing ca. 420 sensory neurons (Füller and Ernst 1973). While the sensory neurons of the dorsal part monitor vibration of the tibia (Field and Pflüger 1989; Sauer and Stein 1999), the sensory neurons of the ventral part monitor position, velocity, and acceleration of tibia movements, either purely or in combinations (Hofmann et al. 1985; Hofmann and Koch 1985; Büschges 1994). fCO sensory neurons show considerable adaptation, e.g. sensory units responding to velocity and position of tibial movements (Sauer et al. 1996). This is at least partially due to sensitivity of fCO sensory neurons depending on the history of discharge controlled by a calcium-dependent adaptation mechanism (DiCaprio et al. 2002). Load on the femur and the tibia is measured by cuticular sensors, the so-called campaniform sensillae situated on the proximal femur and tibia (e.g. Hofmann and Bässler 1982, 1986; Zill et al. 2013, 2017). These load sensors are activated upon resisted forces generated, for example, by activity of the tibial muscles or gravity. They can, therefore, be neglected for the generation of unrestrained return movements of the tibia as generated in the generation of catalepsy.
9.3.2 Catalepsy in the Femur-Tibia Control Loop and Its Neural Basis
Catalepsy in the femur-tibia joint was quantitatively described by studying return movements of the tibia in experimental animals, which express thanatosis, i.e. showing a fully extended tibia (Fig. 9.3). This holds both for female Carausius morosus and Cuniculina impigra (Bässler and Foth 1982).
By selectively stimulating the fCO in an otherwise semi-intact, restrained experimental animal and recording the activity of tibial motoneurons and muscles it was found that the underlying neural mechanism for the generation of catalepsy arises from the fact that velocity and movement signals from the fCO strongly affect tibial motoneuron activity, however with differing times and gain of influence. Presently, the following picture applies: when the tibia is flexed by passive displacement and return of the tibia is allowed after some time, then the initial fast return movement is generated by a still effective resistance influence elicited in extensor motoneurons and muscles by fCO signals signaling flexion of the tibia. This influence has a rather short time constant of action and consequently decays relatively fast. At the same time the generated extension movement elicits activation of the antagonistic flexor tibiae, serving in addition in slowing down the return movement. The slow part of the return movement of the tibia results from the balance between the persistent positional feedback about flexion of the tibia activating the extensor motoneurons and muscle and the counteracting force generated by the flexor muscle upon the ongoing extension movement, elicited by fCO-signals about joint extension.
This action of the neural control system arises from the following properties: (1) a high gain with which signals about movements, i.e. position and velocity, of the tibia from the fCO are processed in the premotor network of the femur-tibia joint, (2) the relative dominance of velocity sensitivity over position sensitivity of the neural control system, and (3) the long time constants of decay for the processing of low velocity signals as compared to high velocity signals. Together, these serve in giving the neural control system of the tibial motoneurons the properties of a low pass filter, a characteristic the system needs to generate the very slow movements generated during catalepsy (for summary see Bässler 1983, 1993). Individual properties were traced to the level of their origin in the neural components involved, e.g. sensory neurons, interneurons, and motoneurons (Fig. 9.3; e.g. Driesang and Büschges 1993; Büschges and Wolf 1995; Sauer et al. 1996): by means of intracellular recordings. Bässler and coworkers studied the network architecture underlying the processing of sensory information from the fCO. Interestingly, they did not find evidence for the existence of labeled lines with respect to a given motor output generated, e.g. a resistance reflex in response to passive deflections of the tibia. In contrast they found a principle of information processing and network architecture processing sensory signals in a distributed fashion by means of parallel direct and polysynaptic neural pathways between sensory neurons in the fCO and the tibial motoneurons (e.g. Büschges 1990; Sauer et al. 1996). These parallel pathways generate the motor output based on their antagonistic nature with individual pathways not only supporting, but also opposing the motor activity generated. Thus, the motor activity generated is always the balance between the action of individual pathways. Due to its similarity to the generation of political decisions in a democratic society Bässler coined the term “parliamentary principle” for it (Bässler 1993). All in all Bässler and coworkers identified five levels of antagonistic interaction in the control of the femur-tibia joint: (1) fCO afferents of a given sensitivity influence each other by means of presynaptic inhibition (Fig. 9.3; Sauer et al. 1997), (2) premotor interneurons receive in parallel excitatory and inhibitory synaptic inputs from the same class of fCO signals, e.g. from velocity sensitive sensory neurons (Fig. 9.3; Sauer et al. 1995, 1996), (3) the membrane potential of tibial motoneurons is affected by antagonistic synaptic inputs from direct and polysynaptic pathways from the fCO (Fig. 9.3; Büschges 1990; Sauer et al. 1996), (4) the membrane potential of the tibial muscle fibers is affected by the parallel action of excitatory and inhibitory motoneurons (Bässler and Stein 1996), (5) the movement of the tibia results from the ongoing balance between the antagonistic actions of the respective muscles, i.e. the flexor and the extensor tibiae (for summary see: Bässler 1993; Büschges 1995). This means that all the different outputs use a common neuronal network. Only the internal parameters need to be changed.
Comparative studies on other orthopteran insects expressing catalepsy (proscopiids: Prosarthria; Wolf et al. 2001) and lacking catalepsy (locusts; Ebner and Bässler 1978; Büschges and Wolf 1995) have corroborated the notion that these are indeed the crucial factors for an insects capability of generating catalepsy. The different outputs apparently use the same neuronal networks (see above). It may be thus plausible that this is also the case for thanatosis, in other words, that thanatosis is the output of a network with parliamentary, i.e. distributed, structure. If this is true, there would be no specialized neural circuits for thanatosis but it rather would depend on special internal (and external) conditions of a particular behavioral context.
9.4 General Summary
In summary, it presently appears, that even though the ecological relevance renders similarities thanatosis and catalepsy are different behaviors. By means of a highly specific processing of proprioceptive signals catalepsy is aiding the generation of slow movements necessary for twig mimesis thereby allowing for camouflage. Thanatosis, on the other hand, appears to be an active component of behavioral camouflage.
References
Bässler U (1972a) Der “Kniesehnenreflex” bei Carausius morosus: Übergangsfunktion und Frequenzgang. Kybernetik 11:32–49
Bässler U (1972b) Der Regelkreis des Kniesehnenreflexes bei der Stabheuschrecke Carausius morosus: Reaktionen auf passive Bewegungen der Tibia. Kybernetik 12:8–20
Bässler U (1977) Verhaltensphysiologie bei Stabheuschrecken. BIUZ 7(2):48–54
Bässler U (1983) Neural basis of elementary behavior in stick insects. Springer, Cham, p 169
Bässler U (1993) The femur-tibia control system of stick insects--a model system for the study of the neural basis of joint control. Brain Res Rev 18:207–226
Bässler U, Foth E (1982) The neural basis of catalepsy in the stick insect cuniculina impigra. Biol Cybern 45:101–105
Bässler U, Stein W (1996) Contributions of structure and innervation pattern of the stick insect extensor tibiae muscle to the filter characteristics of the muscle-joint system. J Exp Biol 199:2185–2198
Bässler U, Storrer J (1980) The neural basis of the femur-tibia-control-system in the stick insect Carausius morosus. Biol Cybern 38:107–114
Bräunig P, Pflüger H-J (2001) The unpaired median neurons of insects. Adv Insect Physiol 28:185–266
Büschges A (1990) Nonspiking pathways in a joint control loop of the stick insect Carausius morosus. J Exp Biol 151:133–160
Büschges A (1994) The physiology of sensory cells in the ventral scoloparium of the stick insect femoral chordotonal organ. J Exp Biol 189:285–292
Büschges A (1995) Plasticity of sensori-motor networks that control posture and movement of insect leg joints. Verh Dtsch Zool Ges 88(2):139–152
Büschges A, Wolf H (1995) Nonspiking local interneurons in insect leg motor control. I. Common layout and species-specific response properties of femur-tibia joint control pathways in stick insect and locust. J Neurophysiol 73:1843–1860
Coillot JP, Boistel J (1968) Localisation et description des récepteurs à l'étirement au niveau de l'articulation tibio-fémorale de la patte sauteuse du criquet Schistocerca gregaria. J Insect Physiol 14:1661–1667
Debrodt B, Bässler U (1990) Responses of flexor motor neurons to stimulation of the femoral chordotonal organ of the phasmid Extatosoma tiaratum. Biol Cybernetics 94:101–119
DiCaprio R, Wolf H, Büschges A (2002) Activity-dependent sensitivity of proprioceptive sensory neurons in the stick insect femoral Chordotonal organ. J Neurobiol 88:2387–2398
Driesang RB, Büschges A (1993) The neural basis of catalepsy in the stick insect. J Comp Physiol A 173:445–454
Ebner I, Bässler U (1978) Zur Regelung der Stellung des Femur-Tibia-Gelenkes in Mesothorax der Wanderheuschrecke Schistocerca gregaria. Biol Cybern 29:83–96
Field L, Pflüger H-J (1989) The femoral chordotonal organ: a bifunctional orthopteran (Locusta migratoria) sense organ? Comp Biochem Physiol A 93:729–743
Füller H, Ernst A (1973) Die Ultrastruktur der femoralen Chordotonalorgane von Carausius morosus Br. Zool Jb Anat 91:74–601
Gabriel JP, Scharstein H, Schmidt J, Büschges A (2003) Control of flexor motoneuron activity during single leg walking of the stick insect on an electronically controlled treadwheel. J Neurobiol 56:237–251
Goldammer J, Büschges A, Schmidt J (2012) Motoneurons, DUM cells, and sensory neurons in an insect thoracic ganglion: a tracing study in the stick insect Carausius morosus. J Comp Neurol 520:230–257
Hoffmann T, Koch UT, Bässler U (1985) Physiology of the femoral chordotonal organ in the stick insect, Cuniculina impigra. J Exp Biol 114:207–223
Hofmann T, Bässler U (1982) Anatomy and physiology of trochanteral campaniform sensilla in the stick insect, Cuniculina impigra. Physiol Entomol 7:413–426
Hofmann T, Bässler U (1986) Response characteristics of single trochanteral campaniform sensilla in the stick insect, Cuniculina impigra. Physiol Entomol 11:17–21
Hofmann T, Koch UT (1985) Acceleration receptors in the femoral chordotonal organ of the stick insect, Cuniculina impigra. J Exp Biol 114:225–237
Hooper SL, Büschges A (2017) In: Hooper SL, Büschges A (eds) Neurobiology of motor control - fundamental concepts and new directions. Wiley, Hoboken, NJ
Mentel T, Cangiano L, Grillner S, Büschges A (2008) Neuronal substrates for state- dependent changes in coordination between motoneuron pools during fictive locomotion in the lamprey spinal cord. J Neurosci 28:868–879
Orlovsky GN, Deliagina TG, Grillner S (1999) Neuronal control of locomotion: from Mollusc to man. Oxford University Press, New York, p 322
Sauer AE, Büschges A, Stein W (1997) Role of presynaptic inputs to proprioceptive afferents in tuning sensorimotor pathways of an insect joint control network. J Neurobiol 32(4):359–376
Sauer AE, Driesang RB, Büschges A, Bässler U (1995) Information processing in an invertebrate joint control loop. J Comp Physiol A 177:145–158
Sauer AE, Driesang RB, Büschges A, Bässler U (1996) Distributed processing on the basis of parallel and antagonistic pathways simulation of the femur-tibia control system in the stick insect. J Comput Neurosci 3:179–198
Sauer AE, Stein W (1999) Sensorimotor pathways processing vibratory signals from the femoral chordotonal organ of the stick insect. J Comp Physiol A 185:21–31
Storrer J, Bässler U, Mayer S (1986) Motoneurone im Meso- und Metathorakalganglion der Stabheuschrecke Carausius morosus. Zool Jahrb Abt Zool Physiol Tiere 90:359–374
Wolf H, Bässler U, Spiess R, Kittmann R (2001) The femur-tibia control system in a proscopiid (Caelifera, Orthoptera): a test for assumptions on the functional basis and evolution of twig mimesis in stick insects. J Exp Biol 204:3815–3828
Zill SN, Chaudhry S, Büschges A, Schmitz J (2013) Directional specificity and encoding of muscle forces and loads by stick insect tibial campaniform sensilla, in particular by receptors with round cuticular caps. Arthropod Struct Dev 42:455–467
Zill SN, Neff D, Chaudhry S, Exter A, Schmitz J, Büschges A (2017) Effects of force detecting sense organs on muscle synergies are correlated with their response properties. Arthropod Struct Dev 46:564–578
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Büschges, A., Pflüger, HJ., Bässler, U. (2021). Catalepsy and Twig Mimesis in Insects and Its Neural Control. In: Sakai, M. (eds) Death-Feigning in Insects. Entomology Monographs. Springer, Singapore. https://doi.org/10.1007/978-981-33-6598-8_9
Download citation
DOI: https://doi.org/10.1007/978-981-33-6598-8_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6597-1
Online ISBN: 978-981-33-6598-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)