Abstract
The abdomen is unique to have two venous systems—the systemic and portal system. The systemic venous network drains directly into the inferior vena cava, whereas the portal system delivers the blood to the liver via the hepatic portal vein. The portal venous blood gets filtered through the hepatic sinusoids to enter the hepatic veins and finally the inferior vena cava. The mesenteric and splenic veins are its main tributaries but smaller veins from the stomach, pancreas, and gallbladder also contribute to this system. The portal venous system is subject to various congenital and acquired disorders, most importantly portal venous obstruction/thrombosis and portal hypertension. Proper understanding of the anatomy of the portal venous system is imperative for the diagnosis, management, and effective treatment planning of these disorders. Variant anatomy and congenital anomalies of the portal venous system are particularly important to identify in the context of consideration of liver transplantation or hepatic resections and interventional procedures like transjugular intrahepatic portosystemic shunt, portal vein embolization, etc. In this chapter, we will review the embryology and anatomy of the portal venous system, discuss its complex tributaries, and also succinctly learn about relevant anatomical and topographical variants in light of their significance prior to surgical or interventional treatments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Portal system
- Portal vein
- Splenic vein
- Superior mesenteric vein
- Anatomic variation
- Portal hypertension
- Portosystemic shunts
1.1 Introduction
The portal venous system consists of all veins draining the abdominal part of the gastrointestinal tract (from the lower esophagus to the upper rectum), spleen, pancreas, and gallbladder to the liver. Unlike other solid viscera, the liver receives about 75% of its blood through the portal vein (PV), whereas the remaining 25–30% comes from the hepatic artery. The blood flows through the hepatic sinusoids and enters the systemic circulation via the hepatic veins into the inferior vena cava (IVC).
The portal venous system may be affected by a wide spectrum of congenital variants and anomalies and acquired abnormalities. It is imperative to understand the conventional anatomy and identify variant anatomy to aid diagnosis and guide appropriate management of portal vein thrombosis or portal hypertension. This chapter reviews the normal anatomy and congenital variations involving the portal venous system and their clinical significance.
1.2 Embryology of the Portal Vein System
Formation of the portal venous system takes place between the fourth and twelfth weeks of gestation. The system is formed from the paired vitelline and umbilical veins (Fig. 1.1). Initially, the right and left vitelline veins enter the liver, branch into the hepatic sinusoids, coalesce, and then drain into the sinus venosus—the primitive heart [1, 2]. These two vitelline veins form three pre-hepatic anastomoses around the developing duodenum: the cranial-ventral, dorsal, and caudal-ventral. The right and left umbilical veins initially lie outside of the liver and also drain into the sinus venosus (Fig. 1.1a). As gestation advances, there is involution of parts of the vitelline and umbilical veins (Fig. 1.1b). The caudal aspect of the right vitelline vein, cranial part of the left vitelline vein, and the caudal-ventral anastomosis involute leaving the dorsal anastomosis and cranial-ventral anastomosis to become the main portal vein (MPV) and the left portal vein (LPV). The right umbilical vein and cranial aspect of the left umbilical vein involute leaving the caudal aspect of the left umbilical vein. The remaining left umbilical vein bifurcates forming a communication with the LPV and the IVC, the latter known as the ductus venosus (Fig. 1.1c). The ductus venosus transports blood from the placenta to the fetus and the communication between the left umbilical vein and LPV transports blood from the placenta to the liver. The ductus venosus and the left umbilical vein involute postpartum, becoming ligamentum venosum and ligamentum teres [1,2,3].
Illustration of the development of the portal venous system, reproduced from [1]. (a) tomoses join the vitelline veins around the duodenum to supply the liver. (b) Fragmentation and involution of parts of the vitelline and umbilical veins. (c) Formation of the portal venous system and ductus venosus
1.3 Gross Anatomy
The PV is a thin-walled, valve-less vascular structure that measures approximately 6–8 cm in length in adults with a maximum diameter of 13 mm [1, 2]. It drains the abdominal part of the alimentary tract as well as the spleen, pancreas, and gallbladder, and is formed by the confluence of the superior mesenteric and splenic veins behind the neck of the pancreas at the level of L1–L2 vertebrae (Fig. 1.2) [4, 5].
The PV contributes 40 mL/min or 72% of the total oxygen supply to the liver. Normal portal blood flow in human beings is about 1000–1200 mL/min. The normal portal pressure is about 7 mmHg.
The MPV ascends within the hepatico-duodenal ligament at an angle of 40°–90° with respect to the spine and enters the liver at the porta hepatis. The portal trunk divides in the liver hilum into two branches: LPV and right portal vein (RPV) [1,2,3,4,5,6].
The RPV subsequently divides into an anterior branch and a posterior branch. The right anterior portal vein branch (RAPV) supplies Couinaud segments V and VIII while the right posterior portal vein branch (RPPV) supplies segment VI and VII. The LPV supplies hepatic segments II, III, and IV and also supplies the caudate lobe (segment I) (Fig. 1.3).
These hepatic segmental branches divide further, forming smaller venous branches and finally portal venules which empty into the hepatic sinusoids. Hepatic sinusoids which are lined by endothelial cells and surrounded by hepatocytes process the blood and deliver it to the central veins. The blood then flows through the hepatic veins into the IVC [1,2,3,4,5,6,7].
1.4 Tributaries
1.4.1 Portal Vein
In addition to the splenic vein (SV) and superior mesenteric vein (SMV) which constitute the PV, the other tributaries of the MPV are the left gastric, right gastric, and superior pancreaticoduodenal veins (Fig. 1.4). The cystic vein drains into the RPV while the umbilical vein drains into the LPV [5,6,7].
The left gastric vein (LGV) or coronary vein is one of the most important tributaries of the MPV which is responsible for the formation of esophageal and gastric fundal varices in portal hypertension [8,9,10,11]. The LGV starts from small branches of the lower esophagus and anterior and posterior gastric walls [4,5,6]. It passes along the lesser curvature and typically drains into the MPV (30%) or at the splenoportal junction (33%) [5]. In about 37% of cases, the LGV may instead drain into the SV [4, 5].
The RGV travels close to the gastric pylorus in the lesser curvature and separately enters into the MPV behind the duodenal cap. It drains the lesser curve of the stomach [4,5,6]. It also receives the prepyloric vein which drains the duodenal bulb.
The pancreaticoduodenal veins drain the second and third parts of the duodenum as well as the pancreatic head and neck. They are four in number and form a venous arcade around the duodenum. The posterior superior pancreaticoduodenal vein drains into MPV, whereas the anterior superior pancreaticoduodenal vein joins the SMV. The anterior and posterior inferior pancreaticoduodenal veins also empty into the SMV [4, 5].
The cystic vein drains the gallbladder and typically opens into the RPV while the paraumbilical vein, which drains the anterior abdominal wall, runs within the ligamentum teres to join the LPV [4, 5, 10].
1.4.2 Superior Mesenteric Vein
The SMV drains the major portion of the small bowel and the colon up to the splenic flexure. It ascends along the root of the mesentery to join the SV posterior to the pancreatic neck [1, 4,5,6].
The SMV receives tributaries corresponding to the superior mesenteric artery (SMA) branches, namely the jejunal vein (draining the jejunum), ileal vein (draining the ileum), ileocolic vein (draining the ileum and cecum), right colic vein (draining the ascending colon), and middle colic vein (draining the proximal two-thirds of the transverse colon) (Fig. 1.5) [4, 5].
In addition, it receives the right gastroepiploic vein (draining the greater curve of the stomach), and the pancreaticoduodenal veins (draining the head of the pancreas and second and third parts of the duodenum). These include the anterior superior pancreaticoduodenal vein and the inferior pancreaticoduodenal veins (anterior and posterior) [4,5,6].
1.4.3 Splenic Vein
Multiple small venous tributaries at the splenic hilum constitute the SV. The SV receives the short gastric veins (draining the gastric fundus), left gastroepiploic vein (draining the greater curve of the stomach), pancreatic veins (draining the neck, body, and tail of the pancreas), and typically the inferior mesenteric vein (IMV) (Fig. 1.4) [4,5,6].
1.4.4 Inferior Mesenteric Vein
The IMV drains into the SV in 38% of cases. In the remaining, it drains into the splenoportal confluence (32.7%), SMV (29.3%), or rarely the first jejunal vein (Fig. 1.6) [5].
IMV drains the left-sided colon starting from the splenic flexure to the mid rectum and receives blood from the left colic vein (drains the splenic flexure and descending colon), sigmoid veins (drains the sigmoid colon), and the superior rectal vein (drains the upper and mid rectum) (Fig. 1.5) [4,5,6].
In addition to the superior rectal vein, the rectal (or hemorrhoidal) venous plexus is constituted by middle and inferior rectal veins which in contrast to the superior rectal vein drain into systemic circulation (iliac veins) (Fig. 1.7) [4, 5, 8, 9].
The rectal (or hemorrhoidal) venous plexus. The upper and mid third rectum drains via the superior rectal vein (SRV) into the inferior mesenteric vein (IMV). The lower third rectum drains via the middle rectal vein into the internal iliac vein (systemic circulation). The anorectal junction and anal canal drain via the internal pudendal vein into the inferior rectal vein—a tributary of the internal iliac vein
1.5 Normal Portosystemic Anastomoses
1.6 Portosystemic Collateral Pathways
Resistance to normal portal venous blood flow either secondary to venous thrombosis/occlusion or due to liver parenchymal disease results in the formation of portosystemic collateral circulation. Portosystemic collateral pathways are a result of recanalization of embryonic portosystemic channels and/or reversal of flow in preexisting normal veins of the adult portal venous systems [6,7,8,9,10,11].
Portosystemic collateral pathways (or shunts) allow shunting of blood from high-pressure portal system to low pressure systemic vascular beds. Large volumes of blood passing through these anastomoses over a sustained period of time result in abnormal dilatation of the end-organ veins around the anastomoses—known as varices. Varices can be located outside the gut wall (para-esophageal, para-gastric, para-rectal), adjacent to the muscular layer (peri-esophageal, peri-gastric, peri-rectal) or subepithelial/submucosal in location (esophageal, gastric, rectal varices) [6].
Traditionally, the portosystemic varices have been broadly classified into two types: gastro-esophageal varices and ectopic varices [6, 8, 10, 11]. Ectopic varices encompass all varices other than those in the stomach or esophagus, e.g., duodenum, jejunum, ileum, colon, rectum, omentum, gallbladder, bile duct, bladder, uterus, vagina, diaphragm, and peristomal [6, 8, 10].
In addition to the formation of varices, spontaneous portosystemic shunts may also develop between the portal and systemic venous circulation so as to allow larger amounts of flow across them (e.g., gastrorenal or gastrocaval shunts) [8].
A wide spectrum of portosystemic collateral pathways (i.e., varices and shunts) can be encountered in patients with portal hypertension [6, 8,9,10,11]. Based upon their prevalence, these can be classified into common and uncommon pathways (Tables 1.2 and 1.3) [8].
1.7 Anatomical Variants of Portal Vein
Typical PV anatomy (i.e., the MPV trunk bifurcating into RPV and LPV at the liver hilum and the RPV subsequently dividing RAPV and RPPV) is encountered in 65–80% using multi-detector CT, as has been described by published case series [3, 12, 15,16,17]. Any deviation from this conventional anatomy is considered an anatomical variant. Due to the increasing number of liver transplants, hepatic resections, and interventional procedures, a thorough understanding of portal vasculature and potential variations is of paramount importance [13].
There are four main types of PV branching variants described in the literature [14]:
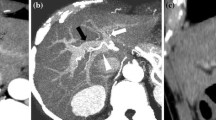
Type 1: This variant is also known as “trifurcation” pattern and has a reported occurrence of 9–11% [14]. In this variant, the MPV divides into three branches: LPV, RAPV, and RPPV (Fig. 1.8).
Trifurcation variation, reproduced from [13]. (a) Myrian three-dimensional (3D) volume-rendered (VR) image. (b) CT coronal-oblique maximum intensity projection (MIP)
Type 2: RPPV originates as the first branch of portal vein (PV); this has a prevalence of 9.7–23% (Fig. 1.9).
RPPV arising from the MPV variation, reproduced from [13]. (a) Myrian 3D VR image. (b) CT coronal-oblique MIP
Type 3: RAPV directly originates from the LPV (Fig. 1.10).
RAPV arising from the LPV variation, reproduced from [13]. (a) Myrian 3D VR image. (b) CT coronal-oblique MIP
Type 4: This variant is the least common (<2%) and comprises of absent portal vein bifurcation (Fig. 1.11). The MPV trunk continues as a single intrahepatic arch and traverses from the right to the left liver lobe [14, 18].
Absence of the portal vein bifurcation variation, reproduced from [13]. (a) Myrian 3D VR image. (b) CT coronal-oblique MIP
1.7.1 Clinical Significance
Knowledge and comprehensive understanding of these variants is extremely important for surgeons, physicians, and radiologists while planning surgeries and interventional procedures (e.g., transjugular intrahepatic portosystemic shunt [TIPS]) to avoid untoward complications.
1.7.2 Liver Transplantation
With improved diagnostic and surgical technology, PV variations can be managed rather safely; however, some variants can make surgery difficult and remain contraindications to living donor right lobectomy [19, 20]. Conventional PV branching, in which the RAPV and RPPV originate from the RPV, is the most suitable for living donor liver transplant. This is due to the fact that it facilitates only one surgical anastomosis between the recipient’s MPV and donor’s RPV. The risk of intraoperative complications increases in Type 1 (trifurcation) variant as clamping becomes difficult. In Type 2 and Type 3 variation, more than one portal vein anastomosis is required, predisposing to portal vein thrombosis [21]. If the variant RPV branches are close to each other, the recipient’s PV can be bifurcated facilitating an easy reconstruction. However, when the RAPV branches from the LPV more distally, an interposed vein graft is required for reconstruction, thereby increasing the complexity of transplant manifold [20]. In Type 2 variant where the RPPV originates from the MPV, there is a high risk of unintentional ischemia/infarction of hepatic segments V and VIII when the left lobe is harvested for liver transplantation or a left trisegmentectomy (segments II, III, and IV) is performed [22].
1.7.3 Transjugular Intrahepatic Portosystemic Shunt
The relative prevalence of PV anatomic variants mandates attentive consideration to PV anatomy prior to undertaking interventions such as TIPS. Indications for TIPS include recurrent (uncontrolled) variceal bleeding and refractory ascites in patients in whom medical treatment has failed. The procedure is often used as a bridging treatment for those awaiting liver transplantation [23,24,25]. It involves the percutaneous creation of an intrahepatic parenchymal shunt between a large hepatic vein and a major PV branch by inserting an expandable stent. TIPS is usually created between the RPV and the right hepatic vein, sometimes the middle hepatic vein [26, 27]. In the presence of variant PV anatomy, the direction of puncture and techniques may have to be tailored to the size, location, and direction of PV. It can also impact transhepatic access and procedural success rates, thereby reiterating the importance of cross-imaging and planning prior to TIPS [23, 28].
1.7.4 Portal Vein Embolization (PVE)
Preoperative PVE is an important tool to be considered before major hepatectomy. PVE is a minimally invasive interventional procedure which involves percutaneous selective cannulation and embolization of a peripheral branch of the PV that supplies the liver segments that are to be removed [18]. This procedure aims to reduce postoperative morbidity and mortality by producing ischemia/infarction of the embolized segment and reactive hypertrophy/hyperplasia of the remaining segments. This helps in achieving a sufficient non-tumoral future liver remnant (FLR) volume, thus preventing the occurrence of postoperative liver failure [19, 20, 29].
Diagnostic portal venography provides a road map to liver segments that require embolization and any variant anatomy that might complicate the procedure. This is to ensure that non-targeted segments are not inadvertently embolized, which might compromise the FLR. This also prevents incomplete embolization of the hepatic segments that are to be resected, which would reduce the stimulus for growth of the FLR [20].
1.7.5 Liver Resection
Embolization of both RPV and LPV branches is required in major uncommon hepatectomy procedures like extended right hepatectomy and extended left hepatectomy. PVE prior to extended right hepatectomy (which includes segment IV) is of particular significance as embolization of the segment IV branch results in better regeneration of segments I, II, and III. Preprocedural road mapping is vital to prevent reflux of the embolizing material into branches of FLR tissue [13, 18, 30].
1.8 Congenital Anomalies
As discussed, embryologically the PV is formed at 4–10 weeks via involution of the peri-intestinal vitelline venous loop. Aberrant involution and/or anastomoses of the vitelline ducts can lead to a variety of topographical variants or congenital anomalies of the portal venous system. While some are totally asymptomatic, others can have serious consequences as discussed below.
1.8.1 Congenital Agenesis of the PV
Atypical involution may cause a prebiliary, preduodenal, or duplicated PV, and excessive involution may result in agenesis of the PV. Agenesis of PV is a rare malformation characterized by the absence of the PV and anomalous drainage of SMV and SV into the systemic circulation [31,32,33,34].
In 1793, John Abernathy described the first case of congenital PV absence with a portosystemic shunt between the mesenteric vein and IVC on an autopsy of a 10-month old child [35]. Subsequently, the term “Abernethy malformations” has been used to describe and classify different varieties of extrahepatic congenital portosystemic shunts that are associated with an atretic PV. They can be classified as follows (Fig. 1.12):
-
Abernethy type 1 (end-to-side) portosystemic shunt consists of either a completely atretic PV with the SV and SMV draining separately in the IVC (type 1a) or incomplete PV atresia with only a short common trunk which terminates into the IVC (type 1b) (Fig. 1.13).
-
An Abernethy type 2 portosystemic shunt consists of a hypoplastic PV with partial shunting of blood into the IVC via a side-to-side shunt.
Liver morphology is generally preserved but altered hemodynamics secondary to portosystemic shunting may lead to the development of hepatic encephalopathy, hepatopulmonary syndrome, metabolic dysfunction, and cirrhosis. These patients are prone to develop focal nodular hyperplasia like liver nodules, hepatic adenomas, and HCC [31,32,33,34,35,36].
Associated visceral abnormalities include congenital cyanotic/acyanotic heart disease, duplicated SVC/IVC, hepatobiliary abnormalities like biliary atresia, congenital hepatic fibrosis, choledochal cyst, urological abnormalities like multicystic dysplastic kidney, cross-fused ectopia, hypospadias, and skeletal abnormalities like radial hypoplasia, vertebral anomalies, etc. [34].
1.8.2 Congenital Intrahepatic Portosystemic Shunt
Intrahepatic portosystemic shunts are abnormal intrahepatic communications >1 mm in caliber between branches of the PV and the hepatic veins or IVC [34, 37]. They are the result of persistent embryonic communication between the portal and vitelline veins during the fourth week of embryonic life [34].
Intrahepatic portosystemic shunts can be subdivided into the following:
-
Type 1: single uniform-sized channel connecting the RPV to the IVC.
-
Type 2: has one or more communications between peripheral branches of the PV and the hepatic veins, localized to one hepatic segment.
-
Type 3: an aneurysmal shunt between the peripheral PV and the hepatic veins (Fig. 1.14).
-
Type 4: PV branches and the hepatic veins communicating through multiple channels distributed diffusely in both lobes.
The first two varieties are the most common [34].
1.8.3 Congenital Arterio-Portal Shunt
Intrahepatic arterio-portal shunts are rare and represent abnormal communication between hepatic arterial system and portal venous system. The vast majority of these shunts are acquired and associated with cirrhosis and tumors or occur secondary to penetrating trauma or iatrogenic injury (e.g., post-liver biopsy). Congenital arterio-portal shunts are extremely rare and seen in patients with hereditary hemorrhagic syndromes, total anomalous pulmonary venous return (TAPVR), etc. [36,37,38]. Depending on the size of the shunt, they may be completely asymptomatic or may manifest with hepatomegaly, portal hypertension, or heart failure [39, 40].
1.8.4 Portal Vein Aneurysm
Portal vein aneurysm (PVA) is increasingly being diagnosed incidentally as a consequence of increased imaging. It is a rare clinical entity described as a focal dilatation of the MPV or splenoportal confluence.
Although there is no strict size criteria, MPV diameter of more than 20 mm is considered aneurysmal (Fig. 1.15). Intrahepatic portal venous caliber more than 9 mm or any disproportionately dilated segment in comparison to adjacent segments may also be considered as aneurysms [41, 42].
PVA can be congenital or acquired. Congenital PVA may result from absent/incomplete involution of the vitelline vein in utero, which later dilates due to increased portal venous pressure. Systemic disorders like collagen vascular disorders or neurofibromatosis can also cause congenital aneurysms due to weakness in the portal vein wall. Cirrhosis, portal hypertension, pancreatitis, and invasive malignancy have been attributed to cause acquired aneurysms. Complications include thrombosis, secondary portal hypertension, compression of adjacent structures, or rarely rupture [43].
1.9 Topographical Variants
1.9.1 Preduodenal Portal Vein
Preduodenal portal vein (PDPV) is a rare congenital anomaly due to embryonic maldevelopment of the portal venous system. As the name suggests, this results in the portal vein lying anterior to the duodenum (Fig. 1.16). Although PDPV can occur as an isolated defect, it is typically associated with other congenital anomalies, including heterotaxia or polysplenia syndrome, situs inversus, cardiac defects, malrotation, biliary or duodenal atresia, and annular pancreas [44].
Clinically, 50% of patients with this anomaly may present with symptomatic duodenal obstruction, caused by itself or coexisting anomalies such as malrotation, duodenal web, and annular pancreas. Most symptomatic cases occur in the pediatric age group [45]. In the remaining 50% of asymptomatic patients who are predominantly adults, PDPV is generally an incidental finding either at surgery or picked up on imaging.
1.9.2 Circumportal Pancreas
Circumportal pancreas (CP) is actually a rare congenital fusion anomaly of the pancreas where the pancreatic tissue at the region of the uncinate process anomalously encases the PV and/or the SMV [46]. CP is a clinically important anatomical variant as it has been associated with a higher rate of postoperative pancreatic fistula (POPF) following pancreatectomy. This is attributed to the anomalous course of the pancreatic duct which results in two cut surfaces (dorsal and ventral to the PV) following resection of the pancreatic tissue at the level of the PV–SMV junction [47, 48].
1.10 Conclusion
The complex network of veins constituting the portal venous system may be affected by a wide spectrum of anatomical variability, congenital anomalies, and acquired abnormalities. A thorough understanding of conventional and commonly seen variant anatomy of the portal vein system is essential to aid diagnosis and guide appropriate management of portal vein thrombosis and portal hypertension.
References
Carneiro C, Brito J, Bilreiro C, et al. All about portal vein: a pictorial display to anatomy, variants and physiopathology. Insights Imaging. 2019;10:38.
Collardeau-Frachon S, Scoazec JY. Vascular development and differentiation during human liver organogenesis. Anat Rec (Hoboken). 2008;291(6):614–27. https://doi.org/10.1002/ar.20679.
Marks C. Developmental basis of the portal venous system. Am J Surg. 1969;117(5):671–81.
Sharma M, Babu CS, Garg S, Rai P. Portal venous system and its tributaries: a radial endosonographic assessment. Endosc Ultrasound. 2012;1(2):96–107. https://doi.org/10.7178/eus.02.008.
Rameshbabu CS, Wani ZA, Rai P, Abdulqader A, Garg S, Sharma M. Standard imaging techniques for assessment of portal venous system and its tributaries by linear endoscopic ultrasound: a pictorial essay. Endosc Ultrasound. 2013;2(1):16–34. https://doi.org/10.7178/eus.04.005.
Sharma M, Rameshbabu CS. Collateral pathways in portal hypertension. J Clin Exp Hepatol. 2012;2(4):338–52. https://doi.org/10.1016/j.jceh.2012.08.001.
Rajesh S, Mukund A, Arora A. Imaging diagnosis of splanchnic venous thrombosis. Gastroenterol Res Pract. 2015;2015:101029. https://doi.org/10.1155/2015/101029.
Philips CA, Arora A, Shetty R, Kasana V. A comprehensive review of portosystemic collaterals in cirrhosis: historical aspects, anatomy, and classifications. Int J Hepatol. 2016;2016:6170243. https://doi.org/10.1155/2016/6170243.
Bandali MF, et al. Portal hypertension: imaging of portosystemic collateral pathways and associated image-guided therapy. World J Gastroenterol. 2017;23(10):1735–46. https://doi.org/10.3748/wjg.v23.i10.1735.
Arora A, Rajesh S, Meenakshi YS, Sureka B, Bansal K, Sarin SK. Spectrum of hepatofugal collateral pathways in portal hypertension: an illustrated radiological review. Insights Imaging. 2015;6(5):559–72. https://doi.org/10.1007/s13244-015-0419-8.
Abby Philips C, Sahney A. Oesophageal and gastric varices: historical aspects, classification and grading: everything in one place. Gastroenterol Rep (Oxf). 2016;4(3):186–95. https://doi.org/10.1093/gastro/gow018.
Smith CS, Sheehy N, McEniff N, Keogan MT. Magnetic resonance portal venography: use of fast acquisition true FISP imaging in the detection of portal vein thrombosis. Clin Radiol. 2007;62(12):1180–8.
Sureka B, Patidar Y, Bansal K, Rajesh S, Agrawal N, Arora A. Portal vein variations in 1000 patients: surgical and radiological importance. Br J Radiol. 2015;88:20150326.
Guerra A, De Gaetano AM, Infante A, et al. Imaging assessment of portal venous system: pictorial essay of normal anatomy, anatomic variants and congenital anomalies. Eur Rev Med Pharmacol Sci. 2017;21(20):4477–86.
Koc Z, Ulusan S, Oguzkurt L, Tokmak N. Venous variants and anomalies on routine abdominal multi-detector row CT. Eur J Radiol. 2007;61(2):267–78. https://doi.org/10.1016/j.ejrad.2006.09.008.
Koç Z, Oğuzkurt L, Ulusan S. Portal vein variations: clinical implications and frequencies in routine abdominal multidetector CT. Diagn Interv Radiol. 2007;13(2):75–80.
Akgul E, Inal M, Soyupak S, Binokay F, Aksungur E, Oguz M. Portal venous variations. Prevalence with contrast-enhanced helical CT. Acta Radiol. 2002;43(3):315–9. https://doi.org/10.1080/j.1600-0455.2002.430314.x.
Schmidt S, Demartines N, Soler L, Schnyder P, Denys A. Portal vein normal anatomy and variants: implication for liver surgery and portal vein embolization. Semin Interv Radiol. 2008;25(2):86–91. https://doi.org/10.1055/s-2008-1076688.
Nakamura TN, Tanaka K, Kiuchi T, et al. Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation. 2002;73:1896–903.
Iqbal S, Iqbal R, Iqbal F. Surgical implications of portal vein variations and liver segmentations: a recent update. J Clin Diagn Res. 2017;11(2):AE01–5. https://doi.org/10.7860/JCDR/2017/25028.9453.
Kamel IR, Kruskal JB, Pomfret EA, Keogan MT, Warmbrand G, Raptopoulos V. Impact of multidetector CT on donor selection and surgical planning before living adult right lobe liver transplantation. AJR. 2001;176:193–200.
Covey AM, Brody LA, Getrajdman GI, Sofocleous CT, Brown KT. Incidence, patterns, and clinical relevance of variant portal vein anatomy. Am J Roentgenol. 2004;183:1055–64. https://doi.org/10.2214/ajr.183.4.1831055.
Gunasekaran SS, Gaba RC. Anatomic variations of the right portal vein: prevalence, imaging features, and implications for successful transjugular intrahepatic portosystemic shunt creation. J Clin Imaging Sci. 2017;7:14.
Saad N, Darcy M, Saad W. Portal anatomic variants relevant to transjugular intrahepatic portosystemic shunt. Tech Vasc Interv Radiol. 2008;11(4):203–7. https://doi.org/10.1053/j.tvir.2009.04.008.
Farsad K, Kaufman JA. Novel image guidance techniques for portal vein targeting during transjugular intrahepatic portosystemic shunt creation. Tech Vasc Interv Radiol. 2016;19(1):10–20. https://doi.org/10.1053/j.tvir.2016.01.002.
Zhuang ZW, Teng GJ, Jeffery RF, Gemery JM, Janne d’Othee B, Bettmann MA. Long-term results and quality of life in patients treated with transjugular intrahepatic portosystemic shunts. AJR Am J Roentgenol. 2002;179(6):1597–603.
Vignali C, Bargellini I, Grosso M, et al. TIPS with expanded polytetrafluoroethylene-covered stent: results of an Italian multicenter study. AJR Am J Roentgenol. 2005;185(2):472–80.
Kanterman RY, Darcy MD, Middleton WD, Sterling KM, Teefey SA, Pilgram TK. Doppler sonography findings associated with transjugular intrahepatic portosystemic shunt malfunction. AJR Am J Roentgenol. 1997;168(2):467–72.
Camelo R, Luz JH, Gomes FV, Coimbra E, Costa NV, Bilhim T. Portal vein embolization with PVA and coils before major hepatectomy: single-center retrospective analysis in sixty-four patients. J Oncol. 2019;2019:4634309. https://doi.org/10.1155/2019/4634309.
Anwar AS, Srikala J, Papalkar AS, Parveez MQ, Sharma A. Study of anatomical variations of hepatic vasculature using multidetector computed tomography angiography. Surg Radiol Anat. 2020;42:1449–57. https://doi.org/10.1007/s00276-020-02532-5.
Franchi-Abella S, Gonzales E, Ackermann O, et al. Congenital portosystemic shunts: diagnosis and treatment. Abdom Radiol (NY). 2018;43(8):2023–36. https://doi.org/10.1007/s00261-018-1619-8.
DiPaola F, Trout AT, Walther AE, et al. Congenital portosystemic shunts in children: associations, complications, and outcomes. Dig Dis Sci. 2020;65(4):1239–51. https://doi.org/10.1007/s10620-019-05834-w.
Bernard O, Franchi-Abella S, Branchereau S, Pariente D, Gauthier F, Jacquemin E. Congenital portosystemic shunts in children: recognition, evaluation, and management. Semin Liver Dis. 2012;32(4):273–87. https://doi.org/10.1055/s-0032-1329896.
Stringer MD. The clinical anatomy of congenital portosystemic venous shunts. Clin Anat. 2008;21(2):147–57.
Howard ER, Davenport M. Congenital extrahepatic portocaval shunts—the Abernethy malformation. J Pediatr Surg. 1997;32(3):494–7. https://doi.org/10.1016/s0022-3468(97)90614-x.
Eduardo Alonso-Gamarra, Manuel Parrón, Ana Pérez, Consuelo Prieto, Loreto Hierro, Manuel López-Santamaría Clinical and radiologic manifestations of congenital extrahepatic portosystemic shunts: a comprehensive review. Radiographics 2011: 31: 707
Bhargava P, Vaidya S, Kolokythas O, Katz DS, Dighe M. Hepatic vascular shunts: embryology and imaging appearances. Br J Radiol. 2011;84:1142–52.
Lupescu I, Masala N, Capsa R, Câmpeanu N, Georgescu SA. CT and MRI of acquired portal venous system anomalies. J Gastrointestin Liver Dis. 2006;15(4):393–8.
Zhang DY, Weng SQ, Dong L, Shen XZ, Qu XD. Portal hypertension induced by congenital hepatic arterioportal fistula: report of four clinical cases and review of the literature. World J Gastroenterol. 2015;21(7):2229–35. https://doi.org/10.3748/wjg.v21.i7.2229.
Norton SP, Jacobson K, Moroz SP, et al. The congenital intrahepatic arterioportal fistula syndrome: elucidation and proposed classification. J Pediatr Gastroenterol Nutr. 2006;43(2):248–55. https://doi.org/10.1097/01.mpg.0000221890.13630.ad.
Gallego C, Velasco M, Pilar Marcuello P, Tejedor D, De Campo L, Friera A. Congenital and acquired anomalies of the portal venous system. Radiographics. 2002;22:141–59.
Lopez-Machado E, Mallorquin-Jimenez F, Medina-Benitez A, Ruiz-Carazo E, Cubero-Garcia M. Aneurysms of the portal venous system: ultrasonography and CT findings. Eur J Radiol. 1998;26:210–4.
Laurenzia A, Ettorrea GM, Lionettib R, Meniconia LR, Colasantia M, Vennareccia G. Portal vein aneurysm: what to know. Dig Liver Dis. 2015;47(11):918–23.
Rusu S, Zaghal A, Choudhry MS. Surgical decision making in preduodenal portal vein: report of two cases in neonates. Eur J Pediatr Surg Rep. 2018;6(1):e40–2. https://doi.org/10.1055/s-0038-1661409.
Kim SH, Cho YH, Kim HY. Preduodenal portal vein: a 3-case series demonstrating varied presentations in infants. J Korean Surg Soc. 2013;85(4):195–7. https://doi.org/10.4174/jkss.2013.85.4.195.
Arora A, Velayutham P, Rajesh S, Patidar Y, Mukund A, Bharathy KG. Circumportal pancreas: a clinicoradiological and embryological review. Surg Radiol Anat. 2014;36(4):311–9. https://doi.org/10.1007/s00276-013-1189-y.
Kabir T, Xuan ZTZ, Chung AYF. Circumportal pancreas: a report of two cases. Ann Hepatobil Pancreat Surg. 2019;23(3):300–4. https://doi.org/10.14701/ahbps.2019.23.3.300.
Ohtsuka T, Mori Y, Ishigami K, Fujimoto T, Miyasaka Y, Nakata K, et al. Clinical significance of circumportal pancreas, a rare congenital anomaly, in pancreatectomy. Am J Surg. 2017;214:267–72.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Das, R., Chambers, J., Arora, A. (2021). Anatomy of Portal Vein System. In: Qi, X., Xie, W. (eds) Portal Vein Thrombosis. Springer, Singapore. https://doi.org/10.1007/978-981-33-6538-4_1
Download citation
DOI: https://doi.org/10.1007/978-981-33-6538-4_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6537-7
Online ISBN: 978-981-33-6538-4
eBook Packages: MedicineMedicine (R0)