Abstract
High-risk human papillomavirus (HPV) is associated with the carcinogenesis of not only cervical cancer but anal, penile, vulvar, vaginal, and oropharyngeal cancers. Although molecular biological mechanisms of high-risk HPV (HR-HPV)-associated carcinogenesis is well studied, it remains unclarified why cervical cancer is the most common among these HPV-associated cancers. Two major causes are that the cervix is a susceptible site to viral infection because of its immune deficiency to protect allogenic sperm in reproductive function and that the squamocolumnar junction (SCJ) where cervical neoplastic diseases develop is composed of tissue stem cells with self-renewal and pluripotency. This specific environment of the cervix allows HPVs to be persistently infected into the cervical epithelial cells, followed by the immortalization of the HPV-infected cells. We here focused on the carcinogenesis specific to the cervix as novel therapeutic strategies for cervical cancer, targeting cancer stem cells and mucosal immunotherapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cervical cancer
- Human papillomavirus
- Cancer stem cell
- Squamocolumnar junction
- Mucosal immunity
- HPV therapeutic vaccine
11.1 Epidemiology of HPV-Associated Cancers and Cancer Prevention
Cervical cancer is the second most common cancer in women worldwide. Over 95% of cervical cancer is caused by high-risk human papillomavirus (HR-HPV) infection. High-risk HPVs are also reported to be the cause of about 90% of anal cancer, 40% of vaginal cancer, 60% of pharyngeal cancer, 40% of vulvar cancer, and 50% of penile cancer [1].
In Japan, the age-adjusted mortality rate of various cancers, including the five major cancers, has been decreasing in the last two decades, but that of only cervical cancer is increasing since about 10,000 women per year develop and 2500 women die from cervical cancer. The incidence of cervical cancer in Japan is increasing although the HPV vaccine is implemented into the national immunization program. HPV vaccine can protect against sexual transmission of HPV16 and 18, the most oncogenic types and prevention of high-risk HPV infection is the most fundamental cancer prevention. HPV vaccine is implemented in 2007 worldwide and recently HPV vaccine has been reported to have a great population impact on the prevention of HPV-associated cancers [2]. In the subjects of clinical trials of the 4-valent HPV vaccine, the precursor or precancer lesions of cervical cancer are not found during 14 years from 2007 [3]. World Health Organization (WHO) has declared at the board in 2019 that it will eliminate cervical cancer in the world by 2060 [4]. However, it will be 40 years later that cervical cancer will be eliminated even if the HPV vaccine is implemented worldwide. Low-income countries have not yet implemented the HPV vaccine due to the limitation of financial issues.
Furthermore, as for Japan, the Ministry of Health, Labor and Welfare has suspended proactive vaccination of HPV vaccine because of the reporting of various adverse events after vaccination. Japanese citizens hesitant to vaccinate the HPV vaccine due to this government policy. Although the safety and efficacy of the HPV vaccine was confirmed by epidemiological studies in Japan, the government has not changed its position on HPV vaccination [5].
Taken together, the development of a new therapeutic agent for precursor lesions of cervical cancer, cervical intraepithelial neoplasia (CIN), is a medical need worldwide even after the implementation of HPV vaccination.
11.2 Mechanisms and Feature of HPV-Associated Carcinogenesis
Genital HPVs infect various mucosal sites, including the cervix, penis, vagina, vulva, anus, and oropharynx, by sexual transmission and are widespread worldwide regardless of gender. Among genital HPVs, about 13 genotypes are high-risk (oncogenic) HPVs that can transform the infected cells to malignant cells [6]. Therefore, HPV-associated cancers can develop anywhere on the infected sites; cervical, penile, vaginal, vulvar, anal, and oropharyngeal cancers.
Numerous molecular biological studies have demonstrated the mechanisms by which HPV infection occurs carcinogenic change in the infected epithelium [6]. When HPV E6 and E7 oncogenes of HR-HPV are expressed strongly and ubiquitously in the infected cell, the function of p53 and Rb anti-oncoproteins is suppressed, hTERT is inactivated, and the cell cycle is accelerated. These actions suppress apoptosis and promote the immortalization of the infected cells. The epithelium consisting of the immortalized cells is histologically diagnosed as high-grade intraepithelial neoplasia, a precancer lesion, with disorder of polarity and proliferation of undifferentiated cells. The immortalized cells can acquire malignant features by chromosomal instability and finally invasive cancers develop. It is a feature of HPV-associated carcinogenesis that molecular biological change correlates to pathological change of the epithelium.
Among HR-HPVs, HPV types 16 and 18 are the viruses with the highest risk of cervical cancer. The odds ratio of developing cervical cancer is 434 times higher in women infected with HPV16 and 248 times higher in women with HPV18, compared with HPV-negative women [7]. Seventy to ninety percent of HPV-associated cancers are caused by HPV16 or 18. Notably, 90% of cervical cancer patients of 20–40 years old are caused by HPV16 or 18 [8], meaning that HPV16 and 18 infected cells are rapidly immortalized and easy to transform into malignant cells. We have estimated the fate of CIN using the Markov model, an epidemiological predictive simulation model, and a large scale of retrospective cohort of CIN patients and we found that HPV16-positive CIN patients most frequently had a progression to the invasive cancer when compared with other HR-HPVs [9].
11.3 Specificity of HPV-Associated Carcinogenesis to the Cervix
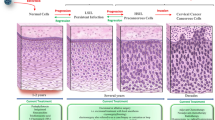
Cervical cancer develops most frequently and most quickly after infection among HPV-associated cancers although the epithelium of cervix, vulvar, vagina, penis, anus, and oropharynx are similarly exposed to HR-HPVs. This suggests that the cervix has unique characteristics in HPV-associated carcinogenesis, which allow persistent infection with HPV and result in the infected cells becoming more susceptible to tumorigenesis. CIN and cervical cancer arise from transformation zone (TZ) including the squamocolumnar junction (SCJ) of the cervix as shown in Fig. 11.1. SCJ is the unique site consisting of tissue stem cells, called “reserve cell,” which retain embryonic features such as self-renewal and pluripotency. Herfs and Crum et al. examined immunologically the features of the embryonic cervix of fetus at 16, 18, and 20 gestational weeks and showed that the stem cell markers p63, keratin 5, and keratin 7 were strongly expressed throughout the TZ. Like the fetal cervix, it was shown that these markers remained in the cervix of adult females, consistent with SCJ [10]. This indicates that SCJ retains the properties of embryonic stem cells even in adulthood, and has the ability to differentiate into “squamous” and “columnar” epithelium (pluripotency) and self-renewal (Fig. 11.1b). Interestingly, the most susceptible and favorable site to HPVs is the TZ and SCJ among the cervix. The proliferative infection of HPVs is dependent on squamous differentiation [11]. The tissue stem cells in the SCJ can spontaneously differentiate into squamous epithelium, which is called squamous metaplasia, and infection of HPVs to the stem cells seems to promote the differentiation [10]. In contrast, the squamous differentiation provides DNA replication of the HPV genome [11], and thereby SCJ is a really suitable site for the proliferation of HPVs.
(a) Squamocolumnar junction (SCJ) and cervical intraepithelial neoplasia (CIN); a picture shows a representative CIN lesion (white epithelium) that arises from the SCJ. The SCJ is the border region between the squamous and columnar epithelium on the mucosa of the cervix. CIN is likely to arise in the SCJ. (b) Tissue stem cells (called reserve cells) and HPV infection in the SCJ; Cell populations, called reserve cells, in the SCJ maintain the stem cell features expressed from embryonic stages [5]. Reserve cells have the pluripotency to differentiate into squamous (ectocervix) and columnar (endocervix) epithelium, and they are also capable of self-renewal. This is where HPVs prefer to infect, as they can use the stem cell features to maintain their own persistent infection of HPVs
Since the tissue stem cells locating at the SCJ possess the self-renewal potential, HR-HPVs that once infected into the cells can persistently retain viral genome there and HPV viral genes are expressed when SCJ move toward differentiation to squamous metaplasia for proliferation. These mechanisms lead to persistent infection of HPVs at the cervix alternating latent and proliferative infections, by which HPVs can evade immunological clearance.
The above-mentioned HPV-associated carcinogenesis begins when the oncogenes are accidentally integrated into the host genome in the proliferative infection and over-expressed in a disorderly manner [6]. Interestingly, Hu et al. demonstrate by whole-genome sequencing that the overexpression of oncogenes occur without integration in some cases [12]. It is confirmed that persistent proliferative infection, which is persistently positive for HR-HPV DNA, is the most critical risk factor for the development of cervical cancer and SCJ is a unique site to favor such infection and transformation toward cancer.
11.4 A Possible Therapeutic Strategy Targeting Cancer Stem Cells of Cervical Cancer
Heterogeneity is an important theory for understanding cancer behavior and biology of all cancers and is demonstrated in various gynecologic cancers at the cell line level [13, 14]. On the other hand, cancer stem cells (CSC) are focused on various cancers. Numerous studies on CSC have demonstrated that CSC possess specific characteristics distinct from cancer cells: stem cell-like features such as self-renewal and pluripotency, malignant features such as metastasis/recurrence, therapy-resistance, anti-apoptosis, and specific metabolism [14]. CSC is thought to be a unique population among the “heterogeneity” of cancer.
In CSC research of cervical cancer, cultured cells positive for some stem cell markers (ALDH1 and CD44 variant 6, etc.) or sphere-forming cells (spheroid) derived from cervical cancer cell lines are often used as a substitute for CSC or cancer stem-like cells. We have demonstrated sphere-forming cells of cervical cancer cell lines are positive for ALDH1 [15]. On the other hand, we focused on the development of cervical cancer from SCJ as mentioned above (Fig. 11.2). To study on CSC of cervical cancer, we have first generated a novel cervical tissue stem cells locating at SCJ, called “reserve cells,” from induced pluripotency stem (iPS) cells [16, 17]. The iPS cell-derived reserve cells we have generated (called induced reserve cells: iRCs) have pluripotency to differentiate into squamous and columnar epithelium and express the cervical stem cell markers in SCJ described above. Furthermore, by transducing HPV16 or 18 oncogenes, E6 and E7, into the iRC cells, the iRCs were immortalized by these oncogenes (called 16E6/E7-iRC and 18E6/E7-iRC). Since the stem cells of the SCJ mimicked by the iRCs are likely to transform into CSC of cervical cancer by HPV oncogene expression, E6/E7-iRCs we generated may show the original features of CSC derived from the SCJ (Fig. 11.2). Then, by using HPV16/18 E6/E7-iRCs, we plan to address the candidates for the development of therapeutic agents targeting the original CSC of cervical cancer. It is well known that HPV18 causes cervical cancer with the highest risk ratio and is most likely to cause adenocarcinoma among HR-HPVs [7]. These clinical features of HPV18-associated cancer might be explained by the characteristics of HPV18 different from those of other HR-HPVs including HPV16. We now try to examine the difference between HPV16- and 18-carcinogenesis using HPV16 and 18 E6/E7-iRCs and focusing on stem cell-like features. The E6/E7-iRCs provides new insights to explore therapeutic strategy targeting CSC of cervical cancer.
Hypothesis of cancer stem cells (CSCs) of cervical cancer derived from HR-HPV-infected reserve cells; when HR-HPV-infected reserve cells become immortalized and transform to malignant cells, they become cancer cells with stem cell features. Unlike the process of CSC formation from cancer cells, the direct formation of CSCs by HR-HPVs may be related to the rapidity of cervical cancer carcinogenesis
We have approached comprehensive gene expression of each E6/E7-iRCs using RNA sequencing and some interesting genes that overexpress in 18E6/E7-iRCs but 16E6/E7-iRCs are not found. Furthermore, the TCGA database shows some genes of interest are expressed higher in HPV18-associated cervical cancer tissue compared with HPV16-associated one (unpublished data). Interestingly, the gene is barely expressed in human keratinocytes immortalized by HPV18 E6/E7, suggesting the gene expression is enhanced by HPV18 E6/E7 only in the stem cell-like cells (iRCs). The suppression of the gene expression by the siRNA method downregulated cell proliferation of E6/E7-iRCs. The gene might be a target gene against CSCs of cervical cancer.
11.5 Therapeutic for Precancer Lesion of Cervical Cancer Is an Unmet Need
Preventing the infection of HR-HPVs is the most fundamental cancer prevention, and HPV vaccines for the prevention of infection are having a major impact. Although it is expected that HPV vaccines will be able to eradicate cervical cancer in the future, at present, HPV vaccination rates are limited in many countries and regions (due to cost issues), and it will take some time before the global elimination of cervical cancer and to eradicate cervical cancer worldwide. The development of therapeutics to treat precursor lesions (CIN) is still necessary even now that the HPV vaccine has been implemented worldwide.
Surgical resection is the only treatment that can be given for early cervical cancer and its precancerous lesions (CIN2-3), which peak in the 20s and 30s. At present, there are no pharmacologic treatments. Hysterectomy terminates fertility, and cervical and conization worsen obstetrical outcomes in subsequent pregnancies. In other words, the risk of preterm birth is approximately three times higher at the time of pregnancy after conization, and the rate of cesarean delivery and low birth weight are also increased approximately threefold [18]. Since the age at which CIN2-3 develops in a woman coincides with the age at which she becomes pregnant and gives birth, the worsening of obstetrical outcomes through cervical incompetence due to partial resection of the cervix is a major issue for the reproductive health of young women. Therefore, the development of a therapeutic agent for CIN as a nonsurgical treatment for CIN2-3 is an unmet medical need.
11.6 Immunotherapy Targeting HPV Molecules Is a Promising Therapeutic Strategy
The ubiquitous and overexpression of HR-HPV oncoproteins, E6 and E7, in cervical epithelial cells leads from CIN2-3 to cervical carcinoma. E6 and E7 are essential viral proteins for the progression of CIN to cervical cancer and maintenance of HPV-associated cancer [6]. On the other hand, E7 is known to be highly immunogenic in humans while E6 is less likely to induce immune responses in humans. Therefore, HR-HPV E7 is not only a viral protein but a “tumor antigen” of HPV-associated cancer, suggesting that E7 is the most definitive tumor antigen and the target molecule of immunotherapy in the case of HPV-associated cancer, including cervical cancer.
Previous prospective cohort studies on the natural history of CIN have demonstrated that CIN regresses spontaneously by host immune responses. Matsumoto et al. reveal that approximately 70% of CIN1 and 50–60% of CIN2 patients will spontaneously regress within 2 years of follow-up [19]. Another study shows about 20% of CIN3 regresses within 2 years of follow-up with no intervention [20]. Since CIN1 expresses HPV E2 protein whereas CIN2-3 expresses HPV E7 and E2 and E7 are immunogenic for humans, these antigens are recognized by host immune cells and TH1 immune responses occur followed by immunological clearance. This process of spontaneous regression could be used to develop a novel noninvasive therapeutic strategy for CIN, referred to as cancer immunotherapy or HPV therapeutic vaccine. Immunization with CIN patients by various vaccine carriers expressing HPV molecules (E7, E6, and E2) can elicit HPV-specific TH1 cellular immunity to eliminate CIN or cervical cancer.
A number of clinical trials (Phase I–III trials) of various HPV therapeutic vaccines have been conducted for treatment of CIN2-3 since the 1990s, the majority of which have used HPV E7 as the target molecule (Table 11.1) [21]. Immunologists and gynecologists have considered that the immunotherapy targeting HPV E7 is a promising therapeutic agent to treat CIN based on its natural history. In earlier trials, vaccine antigens were administered either intramuscularly or subcutaneously to induce E7-specific cell-mediated immunity (E7-CMI) in the peripheral blood of immunized patients. However, their immune responses do not always correlate with clinical efficacy, and none have been applied clinically at this time. The U.S. phase III trial (VGX-3100, Invio) and our phase I/II trial (IGMKK16E7) are ongoing (bold in Table 11.1). Trimble et al. have reported that the plasmid DNA vaccine, VGX-3100, is intramuscularly administered to 167 patients with HPV16-positive CIN2-3, in a randomized placebo-controlled trial [22]. The regression to normal was observed in 48% of the VGX-3100 group and 30% of the placebo group with a significantly higher regression rate in the VGX-3100 group (p = 0.034). However, CIN2 patients are enrolled in 30% of the VGX-3100 and 26% of the placebo group in this trial. Since CIN2 is likely to regress spontaneously compared with CIN3, the difference in patients background might influence the result. Although there was a significant difference in clinical efficacy, adverse events at the inoculation site occurred in 98% of cases due to intramuscular injection. The VGX-3100 is currently in a phase III trial in the USA.
11.7 Development of a Novel Therapeutic Agent Targeting HPV E7 Using Mucosal Immunity
Noting that CIN is an intraepithelial lesion locating at the cervical mucosa, we hypothesized that mucosal immunity to HPV E7 must be induced in order to immunologically eliminate the mucosal lesion. In the mucosal immune system, the Peyer’s patches (GALT: gut-associated lymphoid tissues) or mesenteric lymph nodes are known to be the inductive sites for the genital mucosa including the cervical mucosa (Fig. 11.3). Gut-derived mucosal lymphocytes are recruited and activated at GALT and mesenteric lymph nodes home to the genital mucosa via the peripheral blood. The mucosal lymphocytes have a unique surface antigen called integrin β7 which binds to its natural ligands (MadCAM) expressed at the endothelial cells of the mucosal vessels, and infiltrate into the mucosa. The integrin β7 also binds to E-cadherin expressed at the cervical epithelium and mucosal lymphocytes can accumulate the epithelium. We have previously revealed that approximately 20–40% of CD3+ T cells in the cervical epithelium of CIN patients were integrin β7+ T cells that are gut-derived and furthermore, we found that CIN was more likely to regress when the content of integrin β7+ T cells was high [23]. Thus, we considered that memory helper and killer T cells educated in the gut mucosa can infiltrate into CIN2-3 lesions, and TH1 immune cells activated by HPV E7 antigen will recognize CIN2-3 cells and induce TH1 immune responses to eliminate the lesion.
Mechanism of HPV E7-targeting mucosal immunotherapy to treat CIN2-3; Oral administration of E7-exposed lactobacillus-based therapeutic vaccine elicits E7-specific mucosal Th1 cells at the mucosal inductive site (GALT: Peyer’s patches etc.). E7-specific TH1 cells home through the peripheral blood to the mucosal effector site (cervical mucosa) and infiltrate the mucosal epithelium and recognize and activate E7-overexpressed CIN2-3. This leads to an E7-specific TH1 immune response and immunological clearance of CIN2-3
We have developed a new HPV16 E7-targeting therapeutic vaccine using Lactobacillus casei (L. casei) that is known to have an adjuvant effect on TH1 immune responses. Our concept of the therapeutic vaccine is that oral administration with the agent provides induction of mucosal TH1 immune response to HPV16 E7 at the GALT and the mucosal T cells infiltrate into the CIN2-3 lesions followed by immunological clearance by E7-specific TH1 immune responses including natural killer activity (Fig. 11.3). L. casei is safe because it is already used as a lactic acid-based beverage and the immunization route is oral administration of capsule tablets, which is a completely different route of administration than the other HPV therapeutic vaccines mentioned above.
The first lactobacillus-based vaccine generated was GLBL101c which expressed the HPV16 E7 gene on the cell surface [24]. But the number of E7 molecule expressed on the cell surface was not optimized. Then, we generated a second-generation lactobacillus-based vaccine on which the maximum amount of HPV16E7 molecules is expressed, called IGMKK16E7. Oral immunization of mice with these agents have demonstrated that the ability of IGMKK16E7 to induce the number of IFNγ-producing cells in response to E7 was approximately four times greater than that of GLBL101c [25].
After these preclinical studies, we conducted an exploratory Phase I/IIa clinical study with the approval of the Research Ethics Review (IRB) Committee. Patients were treated with GLBL101c once a day for 5 days a week for 1, 2, 4, and 8 weeks in CIN3 patients with HPV16 positive. In all 17 patients, there were no grade 2 or higher adverse events and none of the grade 1 adverse events were causally related to GLBL101c. The regression rate of CIN3 to CIN1/normal was 38.4% in the first 12 months of treatment, which was clearly higher than the rate of spontaneous regression (about 10% per year). In addition, the group that regressed to CIN2 or less clearly had a higher induction of mucosal E7-specific IFNγ-producing cells into cervical intraepithelial lymphocytes than the non-regressed group [26]. Next, we conducted a randomized, placebo-controlled double-blind Phase IIb clinical trial of GLBL101c to treat HPV16-positive CIN2. Compared to the placebo group, there was no difference in adverse events and safety was confirmed although the trial did not show the significant clinical efficacy on regression of CIN2 (in preparation for submission).
Since the first generation GLBL101c used in these two exploratory clinical studies was considered to have limited pharmacological efficacy, we developed a next-generation agent, IGMKK16E7, mentioned above [25]. Then, in June 2019, a phase I/II physician-initiated clinical trial of IGMKK16E7 began in HPV16-positive CIN2-3 in four groups; placebo, low dose, medium dose, and high dose (1:1:1:1). This was a multicenter study conducted at our hospital and other university hospitals, with a target enrollment of 164 patients (124 with CIN3 and 40 with CIN2). The primary endpoint was pathological remission (CR = normal, PR = CIN1, SD = CIN2-3, PD = invasive cancer), and the protocol was designed to assess efficacy at 16 weeks from the start of treatment [27]. We plan to conduct a Phase III trial after proving the efficacy of IGMKK16E7.
References
Cutts FT, Franceschi S, Goldie S, Castellsague X, de Sanjose S, Garnett G, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ. 2007;85:719–26., https://www.who.int/bulletin/volumes/85/9/06-038414/en/. https://doi.org/10.2471/blt.06.038414.
Lei J, Ploner A, Elfstrom KM, Wang J, Roth A, Fang F, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340–8. https://doi.org/10.1056/NEJMoa1917338.
Kjaer S, MariNygard S, Sundstrom K, Dillner J, Tryggvadottir L, Munk C, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;100401:1–11. https://doi.org/10.1016/j.eclinm.2020.100401.
World Health Organization (WHO). Health topic, Cervical cancer, eliminating cervical cancer, January 2019. https://www.who.int/health-topics/cervical-cancer#tab=tab_1
Iwata S, Okada K, Kawana K, On behalf of the Expert Council on Promotion of Vaccination. Consensus statement from 17 relevant Japanese academic societies on the promotion of the human papillomavirus vaccine. Vaccine. 2007;35:2291–2. https://doi.org/10.1016/j.vaccine.2017.03.015.
zur Hausen H. Papillomavirus and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. https://doi.org/10.1038/nrc798.
Bosch FX, de Sanjose S, Human papillomavirus and cervical cancer—burden and assessment of causality. J Natl Cancer Inst Monogr, 2003; 31: 3-13. doi: https://doi.org/10.1093/oxfordjournals.jncimonographs.a003479.
Matsumoto K, Yaegashi N, Iwata T, Yamamoto K, Aoki Y, Okadome M, et al. Reduction in HPV16/18 prevalence among young women with high-grade cervical lesions following the Japanese HPV vaccination program. Cancer Sci. 2019;110:3811–20. https://doi.org/10.1111/cas.14212.
Taguchi A, Hara K, Tomio J, Kawana K, Tanaka T, Baba S, et al. Multistate Markov model to predict the prognosis of high-risk human papillomavirus-related cervical lesions. Cancers. 2020;12(2):270. https://doi.org/10.3390/cancers12020270.
Herfs M, Vargas SO, Yamamoto Y, Howitt BE, Nucci MR, Hornick JL, et al. A novel blueprint for ‘top down’ differentiation defines the cervical squamocolumnar junction during development, reproductive life, and neoplasia. J Pathol. 2013;229:460–8. https://doi.org/10.1002/path.4110.
Kukimoto I, Mori S, Sato H, Takeuchi T, Kanda T. Transcription factor human Skn-1a enhances replication of human papillomavirus DNA through the direct binding to two sites near the viral replication origin. FEBS J. 2008;275:3123–35. https://doi.org/10.1111/j.1742-4658.2008.06468.x.
Hu Z, Zhu D, Wang W, Li W, Jia W, Zeng X, et al. Genome-wide profiling of HPV integration in cervical cancer identifies clustered genomic hot spots and a potential microhomology-mediated integration mechanism. Nat Genet. 2015;47:158–63. https://doi.org/10.1038/ng.3178.
Curley MD, Therrien VA, Cummings CL, Sergent PA, Koulouris CR, Friel AM, et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27:2875–83. https://doi.org/10.1002/stem.236.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;1(414):105–1011. https://doi.org/10.1038/35102167.
Fujimoto A, Kawana K, Taguchi A, Adachi K, Sato M, Nakamura H, et al. Inhibition of endoplasmic reticulum (ER) stress sensors sensitizes cancer stem-like cells to ER stress-mediated apoptosis. Oncotarget. 2016;7:51854–64. https://doi.org/10.18632/oncotarget.10126.
Sato M, Kawana K, Adachi K, Fujimoto A, Yoshida M, Nakamura H, et al. Targeting glutamine metabolism and focal adhesion kinase additively inhibits the mammalian target of the rapamycin pathway in spheroid cancer stem-like properties of ovarian clear cell carcinoma in vitro. Int J Oncol. 2017;50:1431–8. https://doi.org/10.3892/ijo.2017.3891.
Sato M, Kawana K, Adachi K, Fujimoto A, Yoshida M, Nakamura H, et al. Regeneration of cervical reserve cell-like cells from human induced pluripotent stem cells (iPSCs): A new approach to finding targets for cervical cancer stem cell treatment. Oncotarget. 2017;8:40935–45. https://doi.org/10.18632/oncotarget.16783.
Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–98. https://doi.org/10.1016/S0140-6736(06)68181-6.
Matsumoto K, Oki A, Furuta R, Maeda H, Yasugi T, Takatsuka N, et al. Predicting the progression of cervical precursor lesions by human papillomavirus genotyping: a prospective cohort study. Int J Cancer. 2011;128:2898–910. https://doi.org/10.1002/ijc.25630.
Holowaty P, Miller AB, Rohan T. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst. 1999;91:252–8. https://doi.org/10.1093/jnci/91.3.252.
Kawana K, Yasugi T, Taketani Y. Human papillomavirus vaccines: current issues and future: Review. Indian J Med Res. 2009;130:341–7.
Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386:2078–88. https://doi.org/10.1016/S0140-6736(15)00239-1.
Kojima S, Kawana K, Fujii T, Yokoyama T, Miura S, Tomio K, et al. Characterization of gut-derived intraepithelial lymphocyte (IEL) residing in human papillomavirus (HPV)-infected intraepithelial neoplastic lesions. Am J Reprod Immunol. 2011;66:435–43. https://doi.org/10.1111/j.1600-0897.2011.01041.x.
Adachi K, Kawana K, Yokoyama T, Fujii T, Tomio A, Miura S, et al. Oral immunization with Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocyte against HPV16 E7. Vaccine. 2010;28:2810–7. https://doi.org/10.1016/j.vaccine.2010.02.005.
Komatsu A, Igimi S, Kawana K. Optimization of human papillomavirus (HPV) type 16 E7-expressing lactobacillus-based vaccine for induction of mucosal E7-specific IFNγ-producing cells. Vaccine. 2018;36:3423–6. https://doi.org/10.1016/j.vaccine.2018.05.009.
Kawana K, Adachi K, Kojima S, Taguchi A, Tomio K, Yamashita A, et al. Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients. Vaccine. 2014;32:6233–9. https://doi.org/10.1016/j.vaccine.2014.09.020.
Ikeda Y, Uemura Y, Asai-Sato M, Nakao T, Nakajima T, Kawana K, et al. Safety and efficacy of mucosal immunotherapy using human papillomavirus (HPV) type 16 E7-expressing Lactobacillus-based vaccine for the treatment of high-grade squamous intraepithelial lesion (HSIL): the study protocol of a randomized placebo-controlled clinical trial (MILACLE study). Jpn J Clin Oncol. 2019;49:877–80. https://doi.org/10.1093/jjco/hyz095.
Acknowledgment
We are very thankful to Mrs. Naoko Tomita for her helpful support and effort to our studies including clinical trials.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kawana, K. et al. (2021). Novel Approach for Therapeutics of Cervical Cancer Based on HPV-Associated Carcinogenesis at the Cervix. In: Isonishi, S., Kikuchi, Y. (eds) Molecular Diagnosis and Targeting for Gynecologic Malignancy. Current Human Cell Research and Applications. Springer, Singapore. https://doi.org/10.1007/978-981-33-6013-6_11
Download citation
DOI: https://doi.org/10.1007/978-981-33-6013-6_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6012-9
Online ISBN: 978-981-33-6013-6
eBook Packages: MedicineMedicine (R0)