Abstract
For decades, pharmaceutical drug product development has been carried out using the traditional hit and trial approaches, which are considered to be less efficient and reliable for attaining desired quality and batch-to-batch consistency. Moreover, such issues create product recalls and rejects, often shortage of medicines. In order to avoid such issues, the federal agencies in the twenty-first century adopted a pharmaceutical quality assessment system (PQAS) for the holistic development of the drug products. Quality by Design (QbD) and design of experiments (DoE) are considered as two pivotal elements of the PQAS for maintaining product quality consistency. DoE analyzes the cause-and-effect relationship among the factors and responses, thus considered as an ultimate resource saving tool for amalgamating the science and risk-based development into the practice. The present chapter provides an overview account on the evolution, principles, and various applications of DoE for pharmaceutical product manufacturing with high robustness and performance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
Design of experiments (DoE) or also referred to as the experimental designs are the systematic tools used for decades by the technology-driven industries for rationally optimizing the product and process performance and robustness during manufacturing. As any scientific experiment tends to involve a series of factors which encounter high variability in the end results, thus use of systematic tools has now become a routine practice [1]. DoE particularly emphasizes on rational planning and execution on the basis of a planned set of experiments and utilizes the mathematical and statistical principles to suggest an optimal solution. Experimental designs are very helpful in establishing the relationship between the input and output variables and furnishes the end results in the form of output and reduces the variability [2]. In another way, the experimental designs are very efficient for their predictive ability to detect and plug the error and to reduce the experimental variability to attain batch-to-batch consistency. However, the experimental designs are unable to reduce the impact due to the uncontrollable or noise factors [3, 4].
1.2 Early History and Evolution of Experimental Designs
The concept of experimental design was first envisioned by Sir Ronald A. Fisher in 1920s with his work pioneering to deal with agricultural models implementing the statistical methods. After Fisher, Rothamsted also put forward stepping stones for the application of systematic mathematical models in the agricultural experiments [3]. Later, George E.P. Box and K.B. Wilson in 1951 worked on the response surface mapping (RSM) technique for implementation in the manufacturing sector to improve the product and process robustness. Additionally, Kiefer and Wolfowitz in 1959 worked on multifactorial optimization techniques using factorial designs, while Genichi Taguchi in 1987 proposed the concept of Taguchi experimental designs for robustness testing and optimization [2].
1.3 Experimental Design Principles
Every experimental design relies on three key principles such as replication, randomization, and blocking (or error control), which should be carefully adhered for meaningful execution of the experimental conditions and rational prediction of the optimum response variables [1]. The details regarding the aforesaid principles of experimental designs are provided below.
1.3.1 Replication
It is ideal to use a good number of replicates on any point of an experimental design for reducing the experimental error, if any. There is no fixed rule for selection of the number of replicates, but it is better to use at least three or more replicates for any design.
1.3.2 Randomization
It is ideal to randomize all the experimental runs and perform them in a random manner. This helps in avoiding the experimental bias which may be encountered owing to performing the experiments in a serial manner. Use of randomization helps in reducing the effect of extraneous factors that may present during experimental practice.
1.3.3 Blocking or Error Control
Blocking is used to reduce or eliminate the variability transmitted from nuisance factors, which may influence the experimental response. Block represents a set of relatively homogeneous experimental conditions and helps in classifying the experiments performed in identical set of conditions.
1.4 General Considerations in Performing DoE
The application of DoE into actual practice involves a multistep process, thus requires defining the experimental objectives, identification of the critical factors and responses, mathematical modeling and statistical validity analysis, and selection of the optimum solutions [5,6,7]. Figure 1.1 illustrates the typical five-step approach used for implementation of DoE in an experimental setup.
1.4.1 Defining the Problem
Before execution of an experimental design, the study objectives are defined to prepare a list of specific problems that are to be addressed by the experiment. It is also important to keep all the objectives of the experiment in mind before execution of an experimental design.
1.4.2 Establishment of Cause-and-Effect Relationship
The cause-and-effect relationship is established by drawing rational relationship between the input and output variables based on the prior knowledge, experience, and expertise on the similar type of experiments. The cause-and-effect diagram is drawn, which is also known as fishbone diagram by considering the various causes of variability such as men, materials, machine, methods, milieu, and management.
1.4.3 Selection of Critical Factors by Screening
When a system or process is new, it is usually important to identify the vital factors which have the most influence on the response(s) of interest. Often there are a lot of factors involved in any pharmaceutical product or process, and the experimenters do not know much about the system. Hence, it is better to perform screening in the beginning of the experiment in order to eliminate any unwanted factors. Usually, prioritization exercise is carried out on the basis of prior knowledge and experience. Also, screening experimental designs are used for identifying suitable factors. Figure 1.2 pictorially depicts a list of commonly used screening experimental designs.
1.4.4 Selection of the Critical Response Variable(s)
The selection of critical response variables is carried out on the basis of product-specific and patient-centric attributes. While selecting the response variable(s), the experimenter must consider useful information related to the impact of input factors on the safety and efficacy of the product. It is ideal to access the likely impact of the factors on the response and measures to mitigate the same.
1.4.5 Experimental Design-Based Factor Optimization
Use of response surface mapping (RSM) designs is very helpful for optimizing the factor effect on the related responses. Figure 1.3 pictorially depicts a list of commonly used screening experimental designs. With the help of a RSM design, a set of experiments are performed containing rational combination of the factors. The obtained data is subjected to analysis by fitting with various mathematical models such as linear, quadratic, cubic, and quartic models. A suitable model was chosen based on the validity of the model for statistical parameters like correlation coefficient, predicted error, and lack of fit analysis.
1.4.6 Selection of Optimum Solution and Model Validation
The selection of optimum solution is carried out on the basis of varied tools like graphical optimization (by brute-force method, overlay plots) and numerical optimization (by desirability and objective function) and artificial neural networks (ANN) (Fig. 1.4). The optimum solution is usually demarcated in the design space which is the narrower region within the knowledge space, as shown in Fig. 1.5.
1.5 Experimental Design Selection and Types
The approach of experimental design selection depends on the nature of experiment, number of factors, and flexibility of conducting the number of experiments. Design selection also depends on the selection of empirical model to describe statistical cause-and-effect relationship. Experimental designs are basically classified into two types such as the screening designs and the response surface designs [1, 8, 9]. The details regarding various experimental designs have been discussed below.
1.5.1 Screening Designs
Screening designs are an efficient way to identify the significant main effects of the factors. The term “screening” refers to an experimental plan that is intended to find the few significant factors from a list of many potential ones. Alternatively, a screening design is primarily used to identify significant main effects only, while the interaction effects are biased due to confounding nature of the factors. Fractional factorial design, Taguchi design, and Plackett–Burman design are primarily used for the purpose of factor screening.
1.5.1.1 Fractional Factorial Design
FFDs are recommended when the number of factors in an experiment ranges between 3 and 7. Such designs require two levels (−1 and +1) for preparing a matrix with combination of the factors. FFDs are expressed by Xk−p, where “X” indicates the number of levels and “k” indicates the number of factors and p describes the fractionation used.
1.5.1.2 Taguchi Design
Such design relies on the use of orthogonal arrays which provide a set of well balanced (minimum) experiments and serve as objective functions for optimization. Taguchi design starts with minimum of three factors and produces four experimental runs at two levels without requisite of any center point runs.
1.5.1.3 Plackett–Burman Design
Plackett–Burman design (PBD) is used for screening study for a minimum of 11 factors and produces 12 experimental runs. The number of runs (n) produced by the design is produced in the multiple of 4, 8, 12, 24. Hence, PBD is useful in screening factors in an experiment with large number of factors.
1.5.2 Response Surface Designs
Response surface designs or optimization design are used for optimizing the critical factors identified by factor screening. Such design employs nonlinear models like quadratic, cubic, and quartic models. Select instances of the response surface designs include full factorial design, central composite design, Box–Behnken design, optimal design, and mixture designs, which have been discussed below.
1.5.2.1 Full Factorial Design
These designs are represented by Xk, where X indicates number of factors and k indicates number of levels. A full factorial design may also be called a fully crossed design. A full factorial design generates experimental runs based on the factorial points and generates a linear polynomial model. Moreover, center points can also be placed in a full factorial design to increasing its power for better predictability. Such design is also capable of identifying the main effect as well as interaction effects.
1.5.2.2 Central Composite Design
Central composite design (CCD) is an effective statistical design which provides information exclusively on the effect of experiment variables. It is widely used for response surface optimization of the experiments, which employs second-order (quadratic) model for the response variable without using a complete three-level factorial experiment. It is employed when factorial designs detect the presence of curvature in the data, thus requires augmentation from an erstwhile linear design to the quadratic response surface design.
1.5.2.3 Box–Behnken Design
Box–Behnken design (BBD) is an independent quadratic design in that it does not contain an embedded factorial or fractional factorial design. In this design the treatment combinations are at the midpoints of edges of the process space and at the center. These designs are rotatable (or near rotatable) and require three levels of each factor. The designs have limited capability for orthogonal blocking compared to the central composite designs.
1.5.2.4 Optimal Designs
The optimality of a design depends on the statistical model and is assessed with respect to a statistical criterion, which is related to the variance-matrix of the estimator. Specifying an appropriate model and specifying a suitable criterion function both require understanding of statistical theory and practical knowledge with designing experiments. Further, optimal designs are of different types such as D-optimal, I-optimal, and A-optimal. These designs utilize three levels for each of the selected factors and are most commonly used for factor optimization study.
1.5.2.5 Mixture Designs
In a mixture experiment, the independent factors are proportions of different components of a blend. In another way, the fact that the proportions of the different factors must sum to 100% complicates the design as well as the analysis of mixture experiments. Mixture designs can be of different types such as simplex lattice designs, simplex centroid designs, and optimal designs. Among these variants of mixture designs, optimal designs are most commonly used for optimization of factors. Further, optimal designs are of different types such as D-optimal, I-optimal, and A-optimal. These designs utilize three levels for each of the selected factors and are most commonly used for factor optimization study.
1.6 Applications of DoE in Drug Product Development
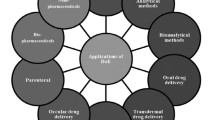
For the development of pharmaceutical drug products, the use of systematic approaches for product development has now become a routine practice. Federal regulatory authorities are bringing new regulations for efficient, effective, and cost-effective product development techniques for ultimately improving the cost of drug products to make them affordable without compromising their quality, safety, and efficacy [10, 11]. DoE, a multivariate approach to product development, has invariably proved its worth for producing robust drug products. Hence, applicability of DoE is possible at all stages of product development, which also helps in ultimately saving time, efforts, and resources. A variety of pharmaceutical products containing multitude of excipients for diverse functional applications for oral and non-oral drug delivery have been developed.
1.6.1 Oral Drug Delivery Systems
A variety of oral drug delivery systems have been developed using the systematic DoE approach. Such delivery systems include tablets, capsules, powders, granules, pellets, microspheres, etc., while liquid dosage forms include dry syrups, emulsions, suspensions, etc. Select instances of the literature reports available on DoE optimization of oral drug delivery systems have been described in Table 1.1.
1.6.2 Non-oral Drug Delivery Systems
A variety of non-oral drug delivery systems have been developed using the systematic DoE approach, which include dosage forms such as parenteral, topical, transdermal, ocular, otic, and inhalational preparations. Select instances of the literature reports on DoE optimization of non-oral drug delivery systems have been provided in Table 1.2.
1.7 Conclusion and Future Perspectives
In a holistic development practice, product and process understanding are the twin keystones of DoE approach. The more the formulator knows about the system, the better he can define it, and the higher precision he can monitor it with. As with any other coherent scientific methodology, DoE also requires holistic envisioning of the formulation development characteristics. Notwithstanding the enormous utility of this DoE-based philosophy in developing optimal solutions, it leads research mindsets to evolve the “out-of-box” strategies too. Besides, the current regulations laid by ICH through Q8 guidance highlight the pharmaceutical development using Quality by Design (QbD) principles. QbD particularly emphasizes on the application of DoE tools for systematic product development practice. Hence, the regulatory importance of DoE has augmented manifold in attaining efficient pharmaceutical development.
References
Singh B, Raza K, Beg S (2013) Developing “optimized” drug products employing “designed” experiments. Chem Ind Dig 23:70–76
Singh B, Saini S, Lohan S, Beg B (2017) Systematic development of nanocarriers employing quality by design paradigms. In: Mishra, Kesherwani PK, Amin MIM (eds) Nanotechnology-based approaches for targeting and delivery of drugs and genes. Academic Press, New York, pp 110–148
Beg S, Swain S, Rahman M et al (2019) Application of design of experiments (DoE) in pharmaceutical product and process optimization. In: Beg S, Hasnain MS (eds) Pharmaceutical quality by design. Academic Press, New York, pp 43–64
Beg S, Rahman M, Panda SS (2017) Pharmaceutical QbD: omnipresence in the product development lifecycle. Eur Pharm Rev 22:58–64
Beg S, Hasnain MS (2019) Pharmaceutical quality by design: principles and applications. Academic Press, New York
Beg S, Rahman M, Robaian MA et al (2020) Pharmaceutical drug product development and process optimization: effective use of quality by design. CRC Press, New York
Singh B, Beg S (2013) Quality by design in product development life cycle. Chron Pharmabiz 22:72–79
Beg S, Hasnain MS, Rahman M et al (2019) Chapter 1 - introduction to quality by design (QbD): fundamentals, principles, and applications. In: Beg S, Hasnain MS (eds) Pharmaceutical quality by design. Academic Press, New York, pp 1–17
Beg S, Rahman M, Swain S (2020) Quality by design applications in pharmaceutical product development. Pharma Focus Asia, pp. 1–5
Beg S, Al Robaian M, Rahman M, Imam SS, Alruwaili N, Panda SK (2020) Pharmaceutical drug product development and process optimization: effective use of quality by design. CRC Press, Boca Raton, FL
Taleuzzaman M, Chuhan S, Gilani SJ, Imam SS, Beg S (2020) Systematic product and process development tools in life cycle management. In: Pharmaceutical drug product and process optimization. CRC Press, Boca Raton, FL, pp 33–52
Bonthagarala B, Dasari V, Kotra V, Swain S, Beg S (2019) Quality-by-design based development and characterization of pioglitazone loaded liquisolid compact tablets with improved biopharmaceutical attributes. J Drug Deliv Sci Technol 51:345–355
Bansal S, Beg S, Garg B, Asthana A, Asthana GS, Singh B (2016) QbD-oriented development and characterization of effervescent floating-bioadhesive tablets of cefuroxime axetil. AAPS PharmSciTech 17(5):1086–1099
Beg S, Swain S, Gahoi S, Kohli K (2012) Design, development and evaluation of chronomodulated drug delivery systems of amoxicillin trihydrate with enhanced antimicrobial activity. Curr Drug Deliv 10(2):174–187
Singh B, Garg B, Bhatowa R, Kapil R, Saini S, Beg S (2017) Systematic development of a gastroretentive fixed dose combination of lamivudine and zidovudine for increased patient compliance. J Drug Deliv Sci Technol 37:204–215
Sethi S, Mangla B, Kamboj S et al (2018) A QbD approach for the fabrication of immediate and prolong buoyant cinnarizine tablet using polyacrylamide-g-corn fibre gum. Int J Biol Macromol 117:350–361
Iurian S, Tomuta I, Bogdan C et al (2016) Defining the design space for freeze-dried orodispersible tablets with meloxicam. Drug Dev Ind Pharm 42:1977–1989
Du J, Zhou Y, Wang L et al (2018) Deacetyl mycoepoxydience nanocrystals dispersible tablets formulation and in vitro study. J Nanosci Nanotechnol 18:3850–3855
Sultana S, Bhavna, Iqbal Z et al (2009) Lacidipine encapsulated gastroretentive microspheres prepared by chemical denaturation for Pylorospasm. J Microencapsul 26:385–393
Swain S, Behera UA, Beg S, Sruti J, Patro CN, Dinda SC, Rao MEB (2012) Design and characterization of enteric-coated controlled release mucoadhesive microcapsules of Rabeprazole sodium. Drug Dev Ind Pharm 39(3):468–480
Bansal S, Beg S, Asthana A, Garg B, Asthana GS, Kapil R, Singh B (2016) QbD-enabled systematic development of gastroretentive multiple-unit microballoons of itopride hydrochloride. Drug Deliv 23(2):437–451
Zhang AY, Fan TY (2009) Optimization of riboflavin sodium phosphate loading to calcium alginate floating microspheres by response surface methodology. Beijing Da XueXue Bao 41:682–686
Yadav KS, Sawant KK (2010) Formulation optimization of etoposide loaded PLGA nanoparticles by double factorial design and their evaluation. Curr Drug Deliv 7:51–64
Dong X, Mattingly CA, Tseng M et al (2009) Development of new lipid-based paclitaxel nanoparticles using sequential simplex optimization. Eur J Pharm Biopharm 72:9–17
Beg S, Katare OP, Singh B (2017) Formulation by design approach for development of ultrafine self-nanoemulsifying systems of rosuvastatin calcium containing long-chain lipophiles for hyperlipidemia management. Colloids Surf B Biointerfaces 159:869–879
Bandyopadhyay S, Beg S, Katare OP et al (2015) QbD-oriented development of self-nanoemulsifying drug delivery systems (SNEDDS) of valsartan with improved biopharmaceutical performance. Curr Drug Deliv 12:544–563
Beg S, Sandhu PS, Batra RS et al (2015) QbD-based systematic development of novel optimized solid self-nanoemulsifying drug delivery systems (SNEDDS) of lovastatin with enhanced biopharmaceutical performance. Drug Deliv 22:765–784
Beg S, Sharma G, Thanki K, Jain S, Katare OP, Singh B (2015) Positively charged self-nanoemulsifying oily formulations of olmesartan medoxomil: systematic development, in vitro, ex vivo and in vivo evaluation. Int J Pharm 493(1–2):466–482
Swain S, Beg S, Sahu PK, Jena BR, Babu SM (2019) Formulation development, statistical optimization and characterization of the self-microemulsifying drug delivery system (SMEDDS) of irbesartan. Nanosci Nanotechnol Asia 9(2):210–228
Beg S, Kaur R, Khurana RK, Rana V, Sharma T, Singh B (2019) QbD-based development of cationic self-nanoemulsifying drug delivery systems of paclitaxel with improved biopharmaceutical attributes. AAPS PharmSciTech 20(3):118
Beg S, Jena SS, Patra CN, Rizwan M, Swain S, Sruti J, Rao ME, Singh B (2012) Development of solid self-nanoemulsifying granules (SSNEGs) of ondansetron hydrochloride with enhanced bioavailability potential. Colloids Surf B: Biointerfaces 101:414–423
Garg B, Beg S, Kaur R, Kumar R, Katare OP, Singh B (2018) Long-chain triglycerides-based self-nanoemulsifying oily formulations (SNEOFs) of darunavir with improved lymphatic targeting potential. J Drug Target 26(3):252–266
Khurana RK, Beg S, Burrow AJ, Vashishta RK, Katare OP, Kaur S, Kesherwani P, Singh KK, Singh B (2017) Enhancing biopharmaceutical performance of an anticancer drug by long chain PUFA based self-nanoemulsifying lipidic nanomicellar systems. Eur J Pharm Biopharm 121:42–60
Sandhu PS, Kumar R, Beg S, Jain S, Kushwah V, Katare OP, Singh B (2017) Natural lipids enriched self-nano-emulsifying systems for effective co-delivery of tamoxifen and naringenin: systematic approach for improved breast cancer therapeutics. Nanomedicine 13(5):1703–1713
Sharma G, Beg S, Thanki K, Katare OP, Jain S, Kohli K, Singh B (2015) Systematic development of novel cationic self-nanoemulsifying drug delivery systems of candesartan cilexetil with enhanced biopharmaceutical performance. RSC Adv 5(87):71500–71513
Beg S, Sandhu PS, Batra RS, Singh B (2015) QbD-based systematic development of novel optimized solid self-nanoemulsifying drug delivery systems (SNEDDS) of lovastatin with enhanced biopharmaceutical performance. Drug Deliv 22(6):765–784
Beg S, Choudhry H, Zamzami MA, Alharbi KS, Rahman M, Singh B (2018) Nanocolloidal lipidic carriers of olmesartan medoxomil surface-tailored with Concanavalin-A for lectin receptor targeting. Nanomedicine 13(24):3107–3128
Beg S, Saini S, Bandopadhyay S, Katare OP, Singh B (2018) QbD-driven development and evaluation of nanostructured lipid carriers (NLCs) of olmesartan medoxomil employing multivariate statistical techniques. Drug Dev Ind Pharm 44(3):407–420
Rahman M, Beg S, Alharbi KS, Alruwaili NK, Alotaibi NH, Alzarea AI, Almalki WH, Alenezi SK, Altowayan WM, Alshammari MS, MAfzal S, Saleem VK (2020) Implications of solid lipid nanoparticles of ganoderic acid for the treatment and management of hepatocellular carcinoma. J Pharm Innov. https://doi.org/10.1007/s12247-020-09450-4
Beg S, Jain S, Kushwah V, Bhatti GK, Sandhu PS, Katare OP, Singh B (2017) Novel surface-engineered solid lipid nanoparticles of rosuvastatin calcium for low-density lipoprotein-receptor targeting: a quality by design-driven perspective. Nanomedicine 12(4):333–356
Garg B, Beg S, Kumar R, Katare OP, Singh B (2019) Nanostructured lipidic carriers of lopinavir for effective management of HIV-associated neurocognitive disorder. J Drug Deliv Sci Technol 53:101220
Poonia N, JK Narang V, Lather S, Beg T, Sharma B, Singh DP (2019) Resveratrol loaded functionalized nanostructured lipid carriers for breast cancer targeting: systematic development, characterization and pharmacokinetic evaluation. Colloids Surf B: Biointerfaces 181:756–766
Poonia N, Lather V, Narang JK, Beg S, Pandita D (2020) Resveratrol-loaded folate targeted lipoprotein-mimetic nanoparticles with improved cytotoxicity, antioxidant activity and pharmacokinetic profile. Mater Sci Eng C 114:111016
Qadir A, Aqil M, Ali A, Warsi MH, Mujeeb M, Ahmad FJ, Ahmad S, Beg S (2020) Nanostructured lipidic carriers for dual drug delivery in the management of psoriasis: systematic optimization, dermatokinetic and preclinical evaluation. J Drug Deliv Sci Technol 57:101775
Beg S, Dhiman S, Sharma T, Jain A, RK Sharma A, Jain BS (2020) Stimuli responsive in situ gelling systems loaded with PLGA nanoparticles of moxifloxacin hydrochloride for effective treatment of periodontitis. AAPS PharmSciTech 21(3):76
Raza K, Singh B, Singal P et al (2013) Systematically optimized biocompatible isotretinoin-loaded solid lipid nanoparticles (SLNs) for topical treatment of acne. Colloids Surf B Biointerfaces 105:67–74
Jana S, Ali SA, Nayak AK et al (2014) Development of topical gel containing aceclofenac-crospovidone solid dispersion by “quality by design (QbD)” approach. Chem Eng Res Des 92:2095–2105
Garg BJ, Garg NK, Beg S, Singh B, Katare OP (2016) Nanosized ethosomes-based hydrogel formulations of methoxsalen for enhanced topical delivery against vitiligo: formulation optimization, in vitro evaluation and preclinical assessment. J Drug Target 24(3):233–246
Negi P, Singh B, Sharma G, Beg S, Raza K, Katare OP (2016) Phospholipid microemulsion-based hydrogel for enhanced topical delivery of lidocaine and prilocaine: QbD-based development and evaluation. Drug Deliv 23(3):941–957
Akhlaq M, Arshad MS, Mudassir AM et al (2016) Formulation and evaluation of anti-rheumatic dexibuprofen transdermal patches: a quality-by-design approach. J Drug Target 24:603–612
Kaur A, Bhoop BS, Chhibber S et al (2017) Supramolecular nano-engineered lipidic carriers based on diflunisal-phospholipid complex for transdermal delivery: QbD based optimization, characterization and preclinical investigations for management of rheumatoid arthritis. Int J Pharm 533:206–224
Ahmed OAA, Kurakula M, Banjar ZM et al (2015) Quality by design coupled with near infrared in formulation of transdermal glimepiride liposomal films. Int J Pharm Sci 104:2062–2075
De T, Miller T, Yim Z et al (2018) Abstract 3718: an injectable nanoparticle formulation of valrubicin. Cancer Chem 78(13):3718. https://doi.org/10.1158/1538-7445.AM2018-3718
Cun D, Jensen DK, Maltesen MJ (2011) High loading efficiency and sustained release of siRNA encapsulated in PLGA nanoparticles: quality by design optimization and characterization. Eur J Pharm Biopharm 77:26–35
Khurana LK, Singh R, Singh H et al (2018) Systematic development and optimization of an in-situ gelling system for moxifloxacin ocular nanosuspension using high-pressure homogenization with an improved encapsulation efficiency. Curr Pharm Des 24:1434–1445
Lakhani P, Patil A, Taskar P (2018) Curcumin-loaded nanostructured lipid carriers for ocular drug delivery: design optimization and characterization. J Drug Deliv Sci Technol 47:159–166
Bartos C, Pallagi E, Szabó-Révész P et al (2018) Formulation of levodopa containing dry powder for nasal delivery applying the quality-by-design approach. Eur J Pharm Sci 123:475–483
Shah V, Sharma M, Pandya R et al (2017) Quality by design approach for an in situ gelling microemulsion of Lorazepam via intranasal route. Mater Sci Eng C Mater Biol Appl 75:1231–1241
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Beg, S., Swain, S. (2021). Introduction to the Application of Experimental Designs in Pharmaceutical Product Development. In: Beg, S. (eds) Design of Experiments for Pharmaceutical Product Development. Springer, Singapore. https://doi.org/10.1007/978-981-33-4351-1_1
Download citation
DOI: https://doi.org/10.1007/978-981-33-4351-1_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-4350-4
Online ISBN: 978-981-33-4351-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)