Abstract
Groundwater contamination assessment using geochemical methods and management were carried out in the shallow aquifer, Ramganga Sub-basin (RSB), India. Groundwater quality in the shallow aquifer is an important parameter to manage groundwater recharge and abstraction for various uses in the RSB. In order to identify the pollution sources and geochemical processes in detail, groundwater samples were collected from shallow (n = 37) and few deep wells and analysed for major and minor ions and trace metals. Results reveal that the water chemistry in the RSB is controlled by the carbonate and silicates weathering and ion exchange reactions. Ion activity ratios and stability field diagrams, obtained from PHREEQC geochemical modeling, show that silicate weathering products (kaolinite and gibbsite) regulate water chemistry in the study area. Results of the present study and previous literatures imply that the groundwater quality in the shallow aquifer is degraded by the vertical leakage of wastewater derived from the anthropogenic sources (irrigation return flow, animal waste accumulation, domestic sewage water) and nitrification process. Hence, management program including periodic groundwater quality monitoring and awareness program among the local populace will help to preserve the groundwater resources in the shallow aquifer of the RSB.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
18.1 Introduction
Groundwater quality monitoring is an important task in sustainable groundwater management especially in the shallow aquifer. Recent days, shallow aquifers are under stress due to high abstraction for irrigation and domestic need as well as quality degradation. Water quality degradation in the shallow aquifers are widely reported worldwide especially South Asian countries due to porous and permeable soil, shallow water table and vadose zone thickness (Nolan et al. 2002; Davraz et al. 2009; Jiang et al. 2009; Rajmohan and Prathapar 2013, 2014). Groundwater quality in the shallow aquifers is governed by various factors and processes that can be broadly classified into natural and man-made activities. Climate variability, rock water interaction, adsorption/desorption, dissolution/precipitation, etc. are natural processes that affect water quality (Matthess 1982; Rajmohan and Elango 2004; Das et al. 2016; Patel 2019a, b). Dumping sites, landfills, industrial and domestic wastewater, irrigation return flow, excess fertilizers and pesticide usage are some of the man-made activities degrade water quality through vertical infiltration. In the Ganges basin and sub basins, groundwater quality deterioration by arsenic, selenium and iron is extensively reported (Saha et al. 2009; CGWB 2014; Rajmohan and Prathapar 2013, 2014; Shah 2014). Besides, nitrate, chloride, phosphate and heavy metals contaminations in groundwater are also documented in this region (Raju et al. 2009; Khan et al. 2015; Rajmohan and Amarasinghe 2016).
In the Ganges basin, Ramganga Sub-basin is a high runoff and water yield basin (Surinaidu et al. 2016; Amarasinghe et al. 2016). In this basin, groundwater is a soul source to balance the domestic and irrigation water requirements due to surface water scarcity (CPCB 2013; Amarasinghe et al. 2016; Khan et al. 2016). Thus, this study was performed to understand the overall groundwater quality status in this region using published literature as well as to identify the geochemical processes and pollution sources through groundwater sampling and analysis. Besides, this study provided the recommendations to manage the shallow aquifer efficiently.
18.2 Study Area
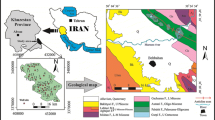
Ramganga Sub-basin (RSB) existed in the Central Ganga Alluvial Plain (78° 14′ to 80° 8′ and 27° 7′ to 30° 6′) in India (Fig. 18.1). In this region, groundwater requirement is increased tremendously due to the agricultural development, rapid urbanization and industrialization as well as inadequate surface water resources (CGWB 2009). River Ramganga is one of the important tributaries in the Ganga River and formed from lower Himalayas (about 3110 m above mean sea level) and flows in the states of Uttarakhand and Uttar Pradesh (UP). Length of the Ramganga river is 596 km and it covers 30,839 km2 (Fig. 18.1) (Rajmohan and Amarasinghe 2016). RSB experiences subtropical monsoon climate and the temperature varies from 40.5 to 8.6 °C during May and January, respectively. The annual average rainfall is 923 mm and 90% of the rainfall occurs from July to September. RSB is sloped from north to south and almost plain. This study region is classified into Lower piedmont plain of Tarai, Older alluvial plain/upland, Younger alluvial plain/low land and Meander flood plain based on geomorphology. In this region, soil is classified into Tarai soils (locally known as “Mar”), Khadar or low-land soils (silty loamy sand or sandy) and Upland or Bangar soils (existed at upland tract of older alluvial plain).
Hydrogeologically, the RSB is covered by the older alluvium (Clay with Kankar and sand), younger alluvium (Fine sand, silty clay with gravel) and Tarai formations (Clay-sandy, sand, gravel and clay). The main water bearing formations are alluvial sediments which formed by the alternate beds of clay and granular material. Based on groundwater exploration work, CGWB (2014) identified the multi-layer aquifer (up to 750 m) in this basin. During premonsoon, the groundwater level ranges from 2.45 to 14.88 mbgl whereas during postmonsoon, it varies from 1.95 to 14.65 mbgl (CGWB 2009).
18.3 Materials and Methods
The groundwater sampling was carried out randomly in the Bareilly, Rampur and Shajahanpur districts in the study region (Fig. 18.1). Survey was carried out to select the wells for water sampling. Groundwater samples were stored in the pre-cleaned two HDPE bottles (1000 and 250 ml) from shallow (n = 37) and deep (n = 7) wells. Water samples were obtained after removing the stagnate water in the borewell casing. In the field, pH and EC were determined by portable meters and water samples were stored in the icebox (4 °C) until analysis. Collected samples were filtered using 0.45 µm Millipore filter. For metal analysis, filtered samples (250 ml) were acidified (pH < 2) with ultra-pure HNO3.
Well depth details were obtained from well owners. Major and minor ions and trace metals were analysed using standard methods (APHA 2012). Trace metals namely As, Cr, Cu, Fe, Mn, Ni and Zn were analysed using Atomic Absorption Spectrophotometer (AAS4141, ECIL). All the analyses were performed at Water Technology Center, Indian Agricultural Research Institute (IARI), New Delhi, India. Analysis accuracy was tested using the ion balance error, which is within ±5%.
SPSS (v 16.0) was used for Pearson correlation analysis. Saturation indices of mineral phases, aqueous species, ion activities and ionic strength were calculated using PHREEQC software (Parkhurst and Appelo 1999). The detailed procedure is given in Parkhurst and Appelo (1999). Ion activities were used to identify the thermodynamic stability of the reaction phases in the groundwater. Further, published literatures was also used to discuss the pollution status of shallow aquifer in the RSB.
18.4 General Groundwater Quality in the RSB
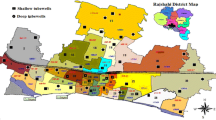
In the RSB, groundwater quality is influenced by the various elements especially salinity, fluoride, arsenic, nitrate and trace metals. Rajmohan and Amarasinghe (2016) carried out a detailed study in all 15 districts in the RSB using secondary data and published literatures. They reported high salinity (Bareilly > Hardoi > Bijnor > Budaun > Shahjahanpur; TDS > 500 mg/l), chloride (Bareilly > J.P. Nagar > Pilibhit > Budaun > Shahjahanpur; Cl− > 250), sulphate (Bareilly > Budaun; SO42− > 200), nitrate (Pilibhit > Shahjahanpur > Hardoi; NO3− > 45), arsenic (Pilibhit; As > 0.05 mg/l) iron (Bareilly > Pilibhit > Bijnor > Hardoi > Shahjahanpur; Fe > 0.3 mg/l) and fluoride (Pilibhit > Shahjahanpur; F > 1.5 mg/l) content in groundwater in the RSB districts. Figure 18.2 indicates the groundwater contamination status in the RSB districts. Groundwater contamination by the Iron and nitrate are widely identified in these districts. Among these districts, groundwater in the Pilibhit is contaminated by the arsenic, nitrate, salinity, iron, fluoride and total hardness. Based on groundwater contamination status (Fig. 18.2), RSB districts can be classified in the order as Pillibhit > Hardoi = Shahjahanpur = J.P. Nagar = Bijnor > Budaun = Bareilly = Moradabad > US Nagar = Chamoli > Almora = Farrukhabad > Nainital = Garhwal = Rampur. In the RSB, groundwater in the upstream districts has low TDS and chloride compared to downstream districts (Rajmohan and Amarasinghe 2016).
Groundwater pollution status in the RSB districts (i.e. N—Nitrate contamination (NO3 > 45 mg/l)) (after Rajmohan and Amarasinghe 2016)
Besides, other studies are also performed in the RSB to assess the groundwater contamination status. In the RSB, high salinity is reported in the Bareilly, Chamoil and U.S. Nagar districts (CGWB 2009). High salinity is also reported in the Ganges basin states (i.e. Bihar, Haryana, Rajasthan and Uttar Pradesh) due to irrigation return flow and excess groundwater usage (Chakraborti et al. 2011). Groundwater with high TDS (>500 mg/l) is reported in the Moradabad city.
Like salinity, nitrate contamination in the groundwater is extensively reported in the RSB. CGWB (2009) noted nitrate contamination (NO3 > 45 mg/l) in Moradabad and Bareilly districts due to improper waste disposal and fertilizer uses for irrigation. Raju et al. (2009) and Khan et al. (2015) also reported nitrate contamination in the lower Kali watershed and Varuna River basin. Uttar Pradesh. Shallow aquifer is highly affected by nitrate compared to deep aquifer and it is documented in the Varanasi city (Nandimandalam 2012).
Apart from nitrate and salinity, iron, arsenic and fluoride contaminations are also reported in the RSB. Iron contamination in groundwater is encountered in most of these districts. Iron contamination is reported in the Moradabad city (Kumar and Sinha 2008), Bareilly district (Rastogi and Sinha 2008; Singh et al. 2009) and Hasanpur in JP district (Sinha and Saxena 2006). Groundwater in the Bareilly and Moradabad districts is polluted by the arsenic as well (CGWB 2009). According to Agarwal (2014), high arsenic in groundwater is identified in the 19 villages (Bareilly district) during Jal Nigam survey. In the RSB, iron and arsenic are mostly derived from geogenic sources (Rajmohan and prathapar 2013, 2014). Several processes are responsible of metal releases to groundwater such as pH, natural weathering, adsorption/desorption, oxidation/reduction, organic matter, soil texture, etc. (Rajmohan et al. 2014). Reducing environment in the anoxic condition will enhance reduction of oxides/hydroxides/sulphides/carbonates, which results metals enrichment in the groundwater (Kumar et al. 2017; Das and Kumar 2015; Das et al 2015). In the RSB, Sinha and Saxena (2006) and Singh et al. (2009) documented that iron, manganese and zinc have strong interrelationship as well as positive correlation. Likewise, arsenic also released to groundwater from young alluvial deposits in the RSB (Pandey et al. 2009; MDWS 2011; Rajmohan and Prathapar 2014).
Fluoride contamination is documented in the study region as well as Ganges basin. In the Ganges basin, high fluoride in groundwater is identified in the states of Bihar, Chhattisgarh, Delhi, Haryana Jharkhand, Madhya Pradesh, Rajasthan, Uttar Pradesh and West Bengal (CWC/NRSC 2014; Rajmohan and Prathapar 2014). In the RSB, high fluoride is noted in the Shahzad Nagar block, Rampur district (Kumar and Yadav 2011), Bareilly district (Singh et al. 2009) and Hasanpur, J.P. Nagar district (Sinha and Saxena 2006). Besides, Rastogi and Sinha (2008) recorded high manganese concentration in the groundwater in the Moradabad city. Sinha and Saxena (2006) mentioned that shallow hand pumps have high concentration of major ions and low dissolved oxygen compared to deeper hand pumps in the Hasanpur (J.P. Nagar district). Kamal et al. (2014) suggested that anthropogenic sources are responsible for high nitrate, sulphate and phosphate in groundwater in the J.P. Nagar district.
18.5 Groundwater Quality in the Shallow Wells
Previous studies implied that groundwater in the shallow unconfined aquifer is polluted by the various elements. Hence, in order to assess the sources of contamination and detailed geochemical processes, groundwater samples were obtained from 37 shallow and 7 deep wells in the RSB and analysed for major and minor ions and metals. Results suggest that groundwater quality is potable in the study region (TDS < 1000 mg/l; Freeze and Cherry 1979). In the shallow wells (n = 37), the depth ranges from 6 to 26 m (Table 18.1). The average EC, TDS and pH are 896 µS/cm, 573 mg/l and 7.4, respectively and the groundwater is alkaline in nature (Table 18.1). CaMgHCO3 (n = 17) > CaMgCl (n = 15) > NaHCO3 (n = 4) > NaCl (n = 1) are dominant water types in the shallow wells, which justify that the water quality is influenced by the minerals dissolution, recharge, mixing and ion exchange reactions.
Figure 18.3 illustrates that groundwater in the shallow wells are more mineralized (Deep wells are plotted in the Figs. 18.3, 18.4, 18.5 and 18.6) just for comparison only). This variation is significant in the major ions, nitrate, phosphate and metals. Figure 18.3 justify that the water quality in the shallow wells is not governed by the natural processes alone. But, anthropogenic sources are also affected the water quality. Metal distributions (i.e. Mn, Cu and Cr) indicate that these elements are partially/totally derived from the non-lithological sources (Fig. 18.3). In addition, high standard deviations (Table 18.1) also justify that these metals are derived from multiple sources. In the case of Mn, it is widely used in various products such as varnish, batteries, cleaning, fireworks, bleaching, fungicides, fertilizers, livestock food supplements, etc. (ATSDR 2000; HSDB 2001; WHO 2011). Likewise, Cu compounds are used in the pipes, valves electrical wiring, building materials, cooking utensils, algicides, fungicides, insecticides, fertilizers, wood preservatives and animal feeds (Landner and Lindestrom 1999; ATSDR 2002; WHO 2004). Dye, wood preservatives, paint pigments, metal coatings, paints pigments, paper, cement and rubber contain Cr compounds. These elements are entered into the aquifer through irrigation return flow and sewage water.
Pearson’s correlation analysis was performed to understand the possible sources, processes and elements association in the aquifer. pH, Ca, PO4, H4SiO4, Fe, Mn, Zn, Cu and Cr do not show correlation with other variables; hence, excluded in Table 18.2.
Table 18.2 indicates that bicarbonate shows significant positive correlation with EC, TDS and major ions. Major ions show strong positive correlation with each other. Fluoride and nitrate have positive correlation but not correlating with other variables. Chloride shows positive correlation with major ions. Variables correlating with chloride suggest that the water quality is highly affected by surface contamination sources.
-
Processes regulating water quality
In the RSB, groundwater quality is regulated by the mineral weathering (carbonates and silicates), ion exchange reactions and surface sources. Ionic ratios are informative tool to assess geochemical processes in the aquifer. In this study, Na/Cl ratio ranges from 0.41 to 2.11 with a mean value of 0.85. Higher ratios (Na/Cl >1) shows silicate weathering/cation exchange whereas lower ratio (<1) expresses reverse ion exchange reactions. Likewise, Na/Cl = 1 shows halite dissolution or surface input. As halite is highly undersatured in this region (SI <−6), chloride is derived from anthropogenic sources. To explain the impact of mineral weathering process on groundwater quality, Na normalized molar ratios of Ca/Na, Mg/Na and HCO3/Na are employed (Gaillardet et al. 1999).
Figure 18.4 depicts that most of the samples plotted between silicate and carbonate weathering zones, which suggests that the water quality is predominantly controlled by the carbonate dissolution. In fact, weathering rate of carbonates is faster (12 times) than silicates (Meybeck 1987). In addition, mHCO3/mNa + K ratio is higher than one in most of the samples. Molar Ca/Mg ratio is generally used to distinguish the impact of dolomite (mCa/Mg = 1), calcite (2 > mCa/Mg > 1) and silicate (mCa/Mg > 2) weathering processes on water quality (Mayo and Loucks 1995; Rajmohan and Elango 2004). In this study, this ratio is generally less than two, which justify that carbonate dissolution is a major process that regulates water quality in the RSB.
In order to explain the mineral water interaction, silicate weathering products and carbonate minerals dissolution in details, aqueous geochemical modelling (PHREEQC code) is employed. Saturation indices (SI) and ion activities were calculated using PHREEQC. Dissolution and precipitation of particular mineral phases are identified using SI values. Figure 18.5 illustrates the saturation state of SiO2, carbonate, silicates, hydroxides and fluorite phases. The dissolved CO2 in the shallow wells ranges from −2.5 to −1.3 with an average value of −1.9, which is higher than atmospheric CO2 (−3.5). This observation indicates that system is opened to atmosphere and dissolved CO2 is likely derived from organic matter degradation/root respiration (Appelo and Postma 2005) or vertical leakage of wastewater (Sinha and Saxena 2006). In the study region, carbonate phases (calcite, aragonite and dolomite) show saturation and over saturation in the groundwater in most of the samples. Hence, dissolution and precipitation of carbonate minerals largely governed the groundwater quality in the study region. In contrast, sulphate phases (anhydrite, gypsum, melanterite; SI: −10.3 to −1.6) fluorite (SI < −1.9) and halite (SI < −5.9) are undersaturated. SI of silicates indicate that phases such as sepiolite (−7 to −2.7), chrysotile (−7.9 to −1.7), amorphous silica (−1.8 to −1.3) and chalcedony (−0.5 to −1.0) are undersaturated while quartz (−0.5 to −0.1), willemite (−3.6 to 1.7) and talc (−5.8 to 0.7) are saturated or near saturation in the groundwater.
In the case of iron, groundwater shows saturation/oversaturation with hydroxides (Fe(OH)3(amorphous), Goethite), oxides (Hematite) and carbonates [Siderite (FeCO3)]. In contrast, manganese oxides (Hausmannite, Pyrolusite) and hydroxides (Manganite, Pyrochroite) show undersaturation but manganese carbonate [rhodochrosite (MnCO3)] expresses saturation/oversaturation in the groundwater. Biswas et al. (2012) reported similar observation in the eastern part of the Ganges basin. Like manganese, smithsonite (ZnCO3) also indicates near saturation in these wells. Further, near saturation is also observed in the willemite (Zn2SiO4). The saturation indices of mineral phases evident that the water quality is governed by weathering of carbonate and silicate minerals along with oxides and hydroxide phases (i.e. Fe)
Ion activities, calculated by the geochemical modeling, are employed to draw mineral stability diagrams to describe the equilibrium status of silicate weathering products formed from incongruent dissolution of silicates with groundwater (Stumm and Morgan 1996). In this aquifer, clay-water interaction is very common because RSB is formed by the alluvial deposits. Groundwater samples are plotted in the stability diagram of the partial system Na2O–Al2O3–H2O–SiO2 and CaO–Al2O3–H2O–SiO2 to explain the reactions governing the system (Nesbitt and Young 1984; Rogers 1989; Rajmohan and Elango 2004). Groundwater samples clustered predominantly in the Kaolinite field and few of them in the Kaolinite-Gibbsite interface (Fig. 18.6). Hence, the water chemistry is influenced by the kaolinite clay formations and explained below.
\( \begin{aligned} & 2{\text{NaAlSi}}_{3} {\text{O}}_{8} + \, 11{\text{H}}_{2} {\text{O}} + 2{\text{CO}}_{2} \leftrightarrow {\text{Al}}_{2} {\text{Si}}_{2} {\text{O}}_{5} \left( {\text{OH}} \right)_{4} + 2{\text{Na}} + 2{\text{HCO}}_{3} + 4{\text{H}}_{4} {\text{SiO}}_{4} \\ & {\text{Albite }}\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad {\text{Kaolinite}} \\ \end{aligned} \)
\( \begin{aligned} & 6{\text{CaAl}}_{2} {\text{SiO}}_{10} \left( {\text{OH}} \right)_{2} + 2{\text{H}}^{ + } + 23{\text{H}}_{2} {\text{O}} \leftrightarrow 7{\text{Al}}_{2} {\text{Si}}_{2} {\text{O}}_{5} \left( {\text{OH}} \right)_{4} + {\text{Ca}}^{2 + } + 8{\text{H}}_{4} {\text{SiO}}_{4} \left( {\text{a}} \right) + 8{\text{H}}_{4} {\text{SiO}}_{4} \\ & {\text{Ca - smectite}}\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad {\text{Kaolinite}} \\ \end{aligned} \)
\( \begin{aligned} & {\text{Al}}_{2} {\text{Si}}_{2} {\text{O}}_{5} \left( {\text{OH}} \right)_{4} + 5{\text{H}}_{2} {\text{O}} \leftrightarrow 2{\text{Si}}\left( {\text{OH}} \right)_{4} + 2{\text{Al}}\left( {\text{OH}} \right)_{3} \\ & {\text{Kaolinite}}\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad {\text{Gibbsite}} \\ \end{aligned} \)
Based on sediments analysis using XRD, Shah (2014) identified the occurrence of silicate minerals (feldspar, kaolinite, quartz, montmorillonite, muscovite and goethite) in the Varanasi, Ganges Basin.
As mentioned earlier, ion exchange reactions are also affected the water quality in the RSB. The ion exchange reactions are classified into cation exchange [Na(K)-Clay + Ca2+(Mg2+)aq = 2Na+(K+)aq + Ca(Mg)-Clay] and reverse ion exchange (RIE) [Na+(K+)aq + Ca(Mg)-Clay = Na(K)-Clay + Ca2+(Mg2+)aq]. Ion exchange reactions can be explained by Na/Cl ratio and chloro-alkaline indices (CA1 and CA2; Schoeller 1977). In this study, Na/Cl ratio is less than one in 26 samples (70%) and highlight the role of RIE processes. CA1 {CAI1 = [Cl − (Na + K)]/Cl} and CA2 {CAI2 = [Cl − (Na + K)]/[Cl + HCO3 + SO4 + NO3]} are calculated in this study. In this calculation, positive and negative values denote RIE and cation exchange, respectively. Results suggest that 20 wells (54%) show positive indices (Fig. 18.4). Hence, RIE and cation exchange are contributed well; however, former is predominant in this aquifer. Besides, Cl corrected Na + K is plotted against HCO3 and SO4 corrected Ca and Mg to validate the role of ion exchange reactions in this aquifer (Fisher and Mulican 1997). Figure 18.4 depicts negative slope (−1.15) with strong correlation (r2 = 0.95) and prove that ion exchange reactions governed the water chemistry in the shallow wells along with mineral dissolutions.
-
Impact of land use pattern on shallow aquifer
Groundwater quality in the RSB is affected by the surface contamination sources. High dissolved CO2 value in the shallow wells (average −1.9 > atmospheric CO2) suggests that the groundwater is contaminated by the vertical leakage of wastewater from various contamination sources from surface (drainage/sewage lines, domestic wastewater and other sources) (Sinha and Saxena 2006; Mukherjee et al. 2007). Sinha and Saxena (2006) also reported high free CO2 and low dissolved oxygen in the shallow aquifer. Similar observations are also stated by the Raju et al. (2009), Nandimandalam (2012) and Khan et al. (2015).
In this study, dissolved silica is not varying with depth and justify that the depth wise variation is not due to silicate weathering alone. Figure 18.3 displays that shallow wells have high Cl, NO3, SO4, HCO3 and K. Further, undersaturation is observed in the sulphate (gypsum and anhydride) and chloride (halite) minerals in the groundwater. Hence, shallow wells are more vulnerable to surface pollution sources. Excessive fertilizers (NPK, muriate of potash, gypsum) application, irrigation return flow and domestic sewage water are claimed the enrichment of the above parameters in the shallow well water. In the Muzaffarnagar district (Uttar Pradesh), high chloride and sulphate are noticed in the groundwater due to wastewater derived from chemical fertilizers and sugar factories (Tyagi et al. (2009). During the fieldwork, it is found that domestic sewage water is directly discharged into the ground/ditches near to hand pumps/houses and storage of animal waste by the local populace. Other studies also reported that high nitrate in the groundwater is derived from these sources (Somasundaram et al. 1993; Chakraborti et al. 2011; Raju et al. 2009; Tyagi et al. 2009; Nandimandalam 2012; Khan et al. 2015). Nitrate in the groundwater is governed by the nitrification and denitrification processes. Nitrification (oxidation of ammonium and organic matter degradation) results acidic proton, which is neutralized by the carbonates and silicates in the vadose zone. Denitrification observes the acidic proton and increases pH in the water. In the study region, nitrification is predominant process in the shallow aquifer. Vertical infiltration of wastewater from land surface and nitrification processes induce mineral weathering and ion exchange reactions in the vadose zone, which resulted the enrichment of major ions and nitrate in this aquifer. Water types (CaMgHCO3 (n = 17) > CaMgCl (n = 15)) and correlation between Cl− and SO42− with major ions justify this argument. Likewise, high fluoride in these wells are likely derived from surface (evaporated water from surface, clays in ceramic industries and phosphatic fertilizers) (Datta et al. 1996; Sinha and Saxena 2006; Misra and Mishra 2007; Kundu and Mandal 2009) because F− shows significant positive correlation with Cl−.
-
Groundwater management
Groundwater development and management is an immediate task to preserve the shallow aquifer in the RSB. This study recommends the following options to preserve groundwater resources.
-
Periodic groundwater quality monitoring is a right option to protect and manage the aquifer.
-
Care should be taken during the hand pumps/tube wells installation to avoid the vertical leakage from wastewater accumulated in the ground. Wastewater accumulation is noticed during fieldwork.
-
Local populace should be trained for disposal and reuse of animal waste (i.e. cow dung) to protect the aquifer. Huge heap of animal waste storage is observed during fieldwork.
-
Managed Aquifer Recharge (MAR) and rainwater harvesting methods should be implemented in the village and household level to improve the water quality and aquifer storage.
-
Unused wells and surface structures (i.e. ponds, pits) can be used as a groundwater recharge options.
-
Village level awareness program will be carried out about water borne diseases, water demand, water quality and waste management to protect the shallow aquifer.
-
Smart agricultural practices (high production with less water and agrochemicals) will be introduced in the local populace to save water and protect water quality.
18.6 Conclusions
Groundwater quality in the shallow unconfined aquifer is deteriorated by the natural and man-made activities. Results of this study suggest that the groundwater quality is governed by the carbonate and silicate weathering reactions in the RSB. Ion activity ratios and stability diagrams suggest that kaolinite, formed from silicate weathering reactions, is governed the water chemistry in this region. In addition, ion exchange reactions also contributed well in groundwater quality variation. Saturation indices reveal that carbonate minerals are saturated/over saturated whereas fluorite, halite and sulphate minerals are under saturated in this aquifer. Besides, carbonates, oxides and hydroxides phases have substantial contribution in trace metals occurrence in the groundwater. In addition to natural processes, high concentrations of Cl−, SO42−, NO3−, Cu, Mn, Fe and Cr and dissolved CO2 justify that vertical infiltration of wastewater (derived from domestic sewage water, irrigation return flow, animal waste accumulation) leads to mineral weathering, ion exchange reactions and nitrification in the vadose zone, which increases pollutants load in groundwater. The present study concluded that well planned groundwater management program is required to secure the shallow aquifer in the RSB.
References
Agarwal P (2014) Arsenic in ground water causing cancer? Times of India, 26 June 2014. http://timesofindia.indiatimes.com/city/bareilly/Arsenic-in-ground-water-causing-cancer/articleshow/37264298.cms. Accessed on Aug 2015
Amarasinghe UA, Muthuwatta L, Smakhtin V, Surinaidu L, Natarajan R, Chinnasamy P, Kakumanu KR, Prathapar SA, Jain SK, Ghosh NC, Singh S, Sharma A, Jain SK, Kumar S, Goel MK (2016) Reviving the Ganges water machine: potential and challenges to meet increasing water demand in the Ganges River Basin. International Water Management Institute (IWMI), Colombo, Sri Lanka, 42 p (IWMI Research Report 167). https://doi.org/10.5337/2016.212
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Water Works Association, USA
Appelo CAJ, Postma D (2005) Geochemistry. Groundwater and pollution. Balkema, Rotterdam
ATSDR (2000) Toxicological profile for manganese. Atlanta, GA, United States Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry
ATSDR (2002) Toxicological profile for copper (draft for public comment). Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry
Biswas A, Nath B, Bhattacharya P, Halder D, Kundu AK, Mandal U, Mukherjee A, Chatterjee D, Mörth CM, Jacks G (2012) Hydrogeochemical contrast between brown and grey sand aquifers in shallow depth of Bengal Basin: consequences for sustainable drinking water supply. Sci Total Environ 431:402–412
CGWB (2009) District groundwater profiles. Central Groundwater Board, Ministry of water resources, Government of India. http://cgwb.gov.in/District_Profile/UP_districtprofile.html. Accessed Mar 2017
CGWB (2014) State profile. Central Groundwater Board, Ministry of water resources, Government of India. http://cgwb.gov.in/gw_profiles/st_up.html. Accessed Mar 2017
Chakraborti D, Das B, Murrill MT (2011) Examining India’s groundwater quality management. Environ Sci Technol 45:27–33
CPCB (2013) Pollution assessment: River Ganga. Central Pollution Control Board, Ministry of Environment and Forests, Govt. of India. http://www.indiaenvironmentportal.org.in/files/file/pollution%20assessment%20-%20Ganga_report.pdf. Accessed Mar 2017
CWC/NRSC (2014) Ganga Basin. Ministry of Water resources, Government of India
Das N, Deka JP, Shim J, Patel AK, Kumar A, Sarma KP, Kumar M (2016) Effect of river proximity on the arsenic and fluoride distribution in the aquifers of the Brahmaputra floodplains, Assam, northeast India. Groundw Sustain Dev 2:130–142
Das A, Kumar M (2015) Arsenic enrichment in the groundwater of Diphu, Northeast India: coupled application of major ion chemistry, speciation modeling, and multivariate statistical techniques. CLEAN–Soil, Air, Water 43(11):1501–1513
Das N, Patel AK, Deka G, Das A, Sarma KP, Kumar M (2015) Geochemical controls and future perspective of arsenic mobilization for sustainable groundwater management: A study from Northeast India. Groundw Sustain Dev 1(1–2):92–104
Datta PS, Deb DL, Tyagi SK (1996) Stable isotope (18O) investigations on the processes controlling fluoride contamination of groundwater. J Contam Hydrol 24:85–96
Davraz A, Karaguzel R, Soyaslan I, Sener E, Seyman F, Sener S (2009) Hydrogeology of karst aquifer systems in SW Turkey and an assessment of water quality and contamination problems. Environ Geol 58:973–988
Fisher RS, Mulican WFIII (1997) Hydrochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the Northern Chihuahuan desert, Trans-Pecos, Rexas, USA. Hydrogeol J 10(4):455–474
Freeze RA, Cherry JA (1979) Groundw. Prentice Hall, Englewood Cliffs, p 604
Gaillardet J, Dupre B, Louvat P, Allegre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30
HSDB (2001) Manganese compounds. Bethesda, MD, National Library of Medicine, Hazardous Substances Data Bank. http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB. Accessed Mar 2017
Jiang YJ, Wu YX, Groves C, Yuan DX, Kambesis P (2009) Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan, China. J Contam Hydrol 109:49–61
Kamal V, Mukherjee S, Srivastava D, Hazarika N, Singh N (2014) Geoenvironmental study of alluvial aquifer in Upper Gangetic plain, a case study of J.P. Nagar, Uttar Pradesh, India. IOSR J Environ Sci Toxicol Food Technol (IOSR-JESTFT) 8(5):56–67
Khan A, Umar R, Khan HH (2015) Hydrochemical characterization of groundwater in lower Kali watershed, Western Uttar Pradesh. J Geol Soc India 86:195–210
Khan MYA, Gani KM, Chakrapani GJ (2016) Assessment of surface water quality and its spatial variation. A case study of Ramganga River, Ganga Basin, India. Arab J Geosci 9:28. https://doi.org/10.1007/s12517-015-2134-7
Kumar N, Sinha DK (2008) Assessment of underground aquatic environment at Moradabad (Uttar Pradesh), India. Pollut Res 27(3):425–430
Kumar R, Yadav SS (2011) Correlation analysis of groundwater quality in and around Shahzad Nagar block of Rampur district, Uttar Pradesh, India. Int J Chem Sci 9(1):440–447
Kumar M, Patel AK, Das A, Kumar P, Goswami R, Deka P, Das N (2017) Hydrogeochemical controls on mobilization of arsenic and associated health risk in Nagaon district of the central Brahmaputra Plain, India. Environ Geochem Health 39(1):161–178
Kundu MC, Mandal B (2009) Assessment of potential hazards of fluoride contamination in drinking groundwater of an intensively cultivated district in West Bengal, India. Environ Monit Assess 152:97–103
Landner L, Lindestrom L (1999) Copper in society and in the environment. Vasteras, Swedish Environmental Research Group (MFG) (SCDA S-721 88)
Matthess G (1982) The properties of groundwater. Wiley, New York, p 498
Mayo AL, Loucks MD (1995) Solute and isotopic geochemistry and groundwater flow in the Central Wasatch Range, Utah. J Hydrol 172:31–59
MDWS (2011) Report of the Central Team on Arsenic mitigation in rural drinking water sources in Ballia district, Uttar Pradesh State. Ministry of Drinking Water and Sanitation, Government of India, New Delhi. http://www.indiaenvironmentportal.org.in/content/343568/report-of-the-central-team-on-arsenic-mitigation-in-rural-drinking-water-sources-in-ballia-district-uttar-pradesh-state/. Accessed Mar 2017
Meybeck M (1987) Global chemical weathering from surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Misra AK, Mishra A (2007) Study of quaternary aquifers in Ganga Plain, India: focus on groundwater salinity, fluoride and fluorosis. J Hazard Mater 144:438–448
Mukherjee A, Fryar AE, Rowe HD (2007) Regional-scale stable isotopic signatures of recharge and deep groundwater in the arsenic affected areas of West Bengal, India. J Hydrol 334:151–161
Nandimandalam JR (2012) Evaluation of hydrogeochemical processes in the Pleistocene aquifers of middle Ganga Plain, Uttar Pradesh, India. Environ Earth Sci 65:1291–1308
Nesbitt HW, Young GM (1984) Prediction of some weathering trends of plutonic and volcanic rocks based on thermodynamic and kinetic considerations. Geochim Cosmochim Acta 48:1523–1534
Nolan BT, Hitt KJ, Ruddy BC (2002) Probability of nitrate contamination of recently recharged groundwaters in the conterminous United States. Environ Sci Technol 36:2138–2145
Pandey DS, Singh KK, Tripathi PK, Rai P, Singh PK (2009) Arsenic contamination in groundwater: an alarming problem and its remedial measures in Ballia district (U.P.). Bhu-Jal News 24(2–3):114–118
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (Version 2)—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. United States Geological Survey, Water Resources Investigations Report 99-4259, Washington, DC, p 326
Patel AK, Das N, Goswami R, Kumar, M (2019a) Arsenic mobility and potential co-leaching of fluoride from the sediments of three tributaries of the Upper Brahmaputra floodplain, Lakhimpur, Assam, India. J Geochem Explor 203:45–58
Patel AK, Das N, Kumar M (2019b). Multilayer arsenic mobilization and multimetal co-enrichment in the alluvium (Brahmaputra) plains of India: A tale of redox domination along the depth. Chemosphere 224:140–150
Rajmohan N, Amarasinghe UA (2016) Groundwater quality issues and management in Ramganga Sub-Basin. Environ Earth Sci 75:1030. https://doi.org/10.1007/s12665-016-5833-9
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ Geol 46(1):47–61
Rajmohan N, Prathapar SA (2013) Hydrogeology of the Eastern Ganges Basin: an overview. International Water Management Institute (IWMI), Colombo, 42 p (IWMI Working Paper 157). https://doi.org/10.5337/2013.216
Rajmohan N, Prathapar SA (2014) Extent of arsenic contamination and its impact on the food chain and human health in the eastern Ganges Basin: a review. International Water Management Institute (IWMI), Colombo, 47 p (IWMI Working Paper 161). https://doi.org/10.5337/2014.224
Rajmohan N, Prathapar SA, Jayaprakash M, Nagarajan R (2014) Vertical distribution of heavy metals in soil profile in a seasonally waterlogging agriculture field in Eastern Ganges Basin. Environ Monit Assess 186(9):5411–5427
Raju NJ, Ram P, Dey S (2009) Groundwater quality in the lower Varuna River Basin, Varanasi District, Uttar Pradesh. J Geol Soc India 73:178–192
Rastogi GK, Sinha DK (2008) Metal toxicity in underground drinking water at Moradabad, Uttar Pradesh, India. Int J Chem Sci 6(2):1074–1080
Rogers RJ (1989) Geochemical comparison of groundwater in areas of New England, New York, and Pennsylvania. Groundwater 27(5):690–712
Saha D, Dwivedi SN, Sahu S (2009) Arsenic in groundwater in parts of middle Ganga plain in Bihar—an appraisal. Bhu-Jal News 24(2–3):82–94
Schoeller H (1977) Geochemistry of groundwater. In: Groundwater studies—an international guide for research and practice, vol 15. UNESCO, Paris, pp 1–18
Shah BA (2014) Arsenic in groundwater, quaternary sediments, and suspended river sediments from the middle Gangetic Plain, India: distribution, field relations, and geomorphological setting. Arab J Geosci 7(9):3525–3536
Singh SK, Singh CK, Kumar KS, Gupta R, Mukherjee S (2009) Spatial-temporal monitoring of groundwater using multivariate statistical techniques in Bareilly district of Uttar Pradesh, India. J Hydrol Hydromech 57(1):45–54
Sinha DK, Saxena R (2006) Statistical assessment of underground drinking water contamination and effect of Monsoon at Hasanpur, J.P. Nagar (Uttar Pradesh), India. J Environ Sci Eng 48(3):157–164
Somasundaram MV, Ravindran G, Tellam JH (1993) Ground-water pollution of the Madras Urban aquifer, India. Ground Water 31:4–11
Stumm W, Morgan JJ (1996) Aquatic chemistry. Wiley-Interscience, New York
Surinaidu L, Muthuwattab L, Amarasinghe UA, Jain SK, Ghosh NC, Kumar S, Singh S (2016) Reviving the Ganges Water machine: accelerating surface water and groundwater interactions in the Ramganga sub-basin. J Hydrol 540:207–219
Tyagi SK, Datta PS, Pruthi NK (2009) Hydrochemical appraisal of groundwater and its suitability in the intensive agricultural area of Muzaffarnagar district, Uttar Pradesh, India. Environ Geol 56:901–912
WHO (2004) Copper in drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality. WHO/SDE/WSH/03.04/88, p 23. http://www.who.int/water_sanitation_health/dwq/chemicals/copper.pdf. Accessed Mar 2017
WHO (2011) Manganese in drinking-water. Background document for development of WHO guidelines for drinking-water quality. WHO/SDE/WSH/03.04/104/Rev/1, p 21. http://www.who.int/water_sanitation_health/dwq/chemicals/manganese.pdf. Accessed Feb 2017
Acknowledgements
The author gratefully acknowledge the financial support provided by the CGIAR Research Program Water, Land, and Ecosystems (WLE). This work is a part of Gangetic Aquifer Management for Ecosystem Services (GAMES) project funded by WLE. Author would like to thank Dr. Upali A. Amarasinghe, International Water Management Institute (IWMI), Sri Lanka for his constant support and encouragement. Author would also like to thank Dr. Neelam Patel, Water Technology Center, IARI, New Delhi, India for her help in the water analysis.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rajmohan, N. (2020). Groundwater Contamination Issues in the Shallow Aquifer, Ramganga Sub-basin, India. In: Kumar, M., Snow, D., Honda, R. (eds) Emerging Issues in the Water Environment during Anthropocene. Springer Transactions in Civil and Environmental Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-32-9771-5_18
Download citation
DOI: https://doi.org/10.1007/978-981-32-9771-5_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-32-9770-8
Online ISBN: 978-981-32-9771-5
eBook Packages: EngineeringEngineering (R0)