Abstract
Biological rhythm is one or more biological events or functions that reoccur in time in a repeated order and with a repeated interval between occurrences and allow an organism to harmonize successfully with its environment. Aging leads to a functional deterioration of many physiological systems, including the biological clock – an internal time-keeping system – that generates ∼24-h rhythms in physiology and behavior. Latest data from experiments in model organisms, gene expression studies, and clinical trials imply that dysfunctions of the circadian clock contribute to aging and age-associated pathologies, thereby suggesting a functional link between the circadian clock and age-associated decline of brain functions and various biochemical and physiological processes. With the advancement in understanding the molecular aspects of aging and circadian system, several therapeutic strategies such as antioxidants, feeding regimen, and most advanced small molecule modulators have been extensively studied. In this chapter, we discuss the functional aspects of biological clock, its association with aging and give an insight into the existing therapeutic interventions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Biological Rhythms
A regular and predictable pattern of any biological phenomenon which reoccurs with certain periodicity is considered as biological rhythm [106]. A biological rhythm can be endogenous, where it is controlled by the internal biological clock or exogenous where it is controlled by synchronizing internal cycles with external stimuli. Such stimuli are mostly with respect to the position of Earth to the Sun and to the Moon as well as on the immediate effects of such variations, for example, day alternating with night, high tide alternating with low tide, etc. [33]. Biological rhythms are genetically regulated, temperature independent, and resistant to pharmacological and chemical disruption. Based on the set of cues, the organism entrains and generates rhythms (Fig. 20.1) with varied periodicity such as, circannual rhythms – occurring in cycles of approximately 1 year; circalunar rhythms – occurring in cycles of approximately one lunar cycle; circatidal rhythms – occurring in cycles of approximately one ocean tide; infradian rhythms – occurring in cycles of frequency more than a day (>24 hours (h)); ultradian rhythms – occurring in cycles of frequency less than a day (<24 h) and circadian rhythms – occurring in cycles of approximately 24 h. In anticipation of these day–night phases, living organisms have evolved cellular and physiological rhythms having a periodicity of approximately 24 h known as circadian rhythms [124].
Diagrammatic representation of mammalian circadian timing system. The biological localized in suprachiasmatic nucleus (SCN) gets entrained through photic input and then time information from SCN regulates physiological, biochemical, and transcriptional rhythms and hence synchronizing the entire body processes
Circadian Rhythms
Circadian rhythms regulate physiology and behavior with a period near 24 h. Circadian is derived from a Latin phrase meaning about (circa) a day (dia). There are several physiological, biochemical and behavioral aspects which follow circadian rhythms that include: sleep/wake cycle, body temperature, hormone secretion, blood pressure, digestive secretions, levels of alertness, etc. [69]. Exogenously they are set and entrained by zeitgebers, derived from German meaning time (Zeit) giver (Geber). The primary being Light–Dark cycles due to the rotation of earth around its axis though food, social cues, temperature, etc., can be considered as nonphotic zeitgebers. The circadian rhythms persist even in the absence of zeitgebers under constant conditions such as constant darkness or constant light. That means rhythms are endogenous. The persistence of rhythms in the absence of cues leads to free running situation, that is, rhythms continue to run, but with a slight deviation from 24 h. The rhythms are genetically determined [66] (Fig. 20.2). There are several other factors that act to the external stimuli and the result is the generation of the rhythms collectively known as the circadian timing system (CTS) [26]. These self-sustained, endogenous, and entrainable rhythms of sleep and wakefulness, foraging and feeding, body temperature, enzyme activity, hormonal release, energy metabolism, and several other molecular and behavioral parameters helped the organisms to effectively cope with ever-changing environment, thus improving their survival [56, 137, 150]. The importance of these oscillations on health and diseases has been rightly recognized with 2017 Nobel Prize in physiology and medicine jointly awarded to Jeffrey Hall, Michael Rosbash, and Michael Young “for their discoveries of molecular mechanisms controlling the circadian rhythm” [29].
Circadian Time-Keeping System (CTS)
The presence of temporally regulated rhythms in physiology and behavior hinted at the possible existence of a circadian clock which was confirmed by lesion experiments and was discovered to be located in the hypothalamic suprachiasmatic nucleus (SCN) in mammals [150]. SCN, the principal pacemaker, consisting of nearly 20,000 tightly packed neurons, acts as “master clock” by synchronizing the peripheral oscillators located in all other cells and tissues [1]. This hierarchical architecture of the CTS in mammals function robustly based on specialized input and output pathways.
Input Pathways to the SCN
Three major pathways convey information to the SCN resulting in entrainment of the master clock. Blue light activates photosensitive retinal ganglion cells in retina and will be communicated to SCN via retinohypothalamic tract (RHT) with the release of excitatory neurotransmitter glutamate and the neuropeptide pituitary adenylatecyclase-activating protein (PACAP). The release of these neurotransmitters leads to stimulation of signaling pathways involving Ca2+ and cAMP and leads to induction of clock genes [1, 22, 55].
RHT also indirectly communicates photic information to SCN via intergeniculate leaflets (IGL) geniculohypothalamic tract (GHT) pathway through gamma aminobutyric acid (GABA) and neuropeptide Y (NPY) [64]. However, the IGL also processes non-photic information such as arousal status received via pathway originating from the dorsal raphe nucleus (DRN), suggesting assimilation of photic and non-photic signals to entrain the SCN [30].
Another important input to the SCN comes directly from both the median raphe nucleus (MRN) and dorsal raphe nucleus (DRN) [96]. Here the serotonergic tract participates in entrainment of the circadian clock by non-photic cues such as physical activity and exercise [30].
Output Pathways from the SCN
Within hypothalamus, SCN axons project to the preoptic area, lateral septum, bed nucleus of the striaterminalis, the subparaventricular zone, and also to the arcuate nucleus and the dorsomedial hypothalamus. In thalamus, efferents from the SCN innervate the IGL and paraventricular nucleus (PVN). Glutamate, GABA, peptide neurotransmitters, AVP, VIP, prokineticin 2 (PK2), cardiolipin-like cytokine, and transforming growth factor α (TGFα) have been shown as SCN output signals [30, 54].
Melatonin, a multitasking molecule, also a messenger of darkness, is secreted from pineal gland and is considered to be an internal zeitgeber which communicates the time information from SCN to all other peripheral clocks through circulation. As the photic information reaches SCN via the RHT, the subsequent activation of signaling pathways lead to induction of clock genes which ultimately results in the regulation of biosynthesis and release of melatonin from pineal. The pathway governing melatonin synthesis is triggered during scotophase in the absence of light. In pineal, tryptophan is converted into serotonin (5-HT) by 5-hydroxytryptophan. Serotonin subsequently undergoes N-acetylation and methylation by arylalkylamine N-acetyltransferase (AANAT) and hydroxyindole-O-methyl-transferase (HIOMT) respectively, ultimately resulting in melatonin synthesis (reviewed in Jagota [55] and Reiter et al. [123]).
The neural circuitry from SCN to pineal gland involves a multisynaptic pathway that includes PVN, intermediolateral cell column (ILCC), superior cervical ganglia (SCG), and finally terminate on pinealocytes and release norepinephrine (NE) which acts on both α1- and β-adrenergic receptors potentiating cAMP production to activate protein kinase A (PKA) and stimulating adenylatecyclase (AC) respectively. PKA phosphorylates cAMP response element-binding protein (CREB) which in turn activates N-acetyl transferase (Nat) gene that leads to melatonin synthesis and secretion. However, the cAMP also suppresses Nat expression by inducible cAMP early repressor (ICER) which competes with P-CREB [55, 138].
Melatonin exerts its effects and influences cellular physiology majorly by membrane bound G-protein-coupled receptors such as MT1 and MT2. These receptors regulate cellular processes by inhibition of adenylatecyclase, followed by a decrease in cAMP levels and modulation of PKA activity [123, 160].
Molecular Mechanisms
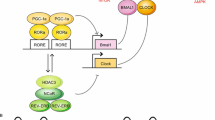
The molecular mechanism governing the mammalian CTS involves two main processes such as the posttranslational modifications (PTMs) of proteins (e.g., phosphorylation) and the autoregulatory transcriptional–translational feedback loop (TTFL) that consist of tightly interconnected positive and negative limbs [137].The positive limb composed of transcriptional activators BMAL1 and CLOCK (or NPAS2 in brain), hetero-dimerize and bind to the E-box elements present in the promoter of several clock-controlled genes (CCGs) including the clock genes Period (Per1 and Per2) and Cryptochrome (Cry1 and Cry2). PER-CRY heterodimers enter the nucleus to repress BMAL-CLOCK activity by deacetylating histones 3 and 4 by recruiting PSF/Sin3-HDAC complex [34].The auxiliary feedback loops consist of nuclear receptors REV-ERBs and RORs which are transcriptionally controlled by the BMAL1-CLOCK. RORs activate the transcription of Bmal1 while REV-ERBs inhibit the transcription of Bmal1, there by regulating their own activator [22, 137].
Moreover, the gene coding for adenine dinucleotide (NAD+) synthesis in the mammalian salvage pathway, nicotinamide phosphoribosyltransferase (Nampt), is a CCG.NAD+, a metabolic oscillator, modulates the transcriptional activity of clock through a histone deacetylase, SIRT1 [99]. This indicates that the cellular metabolism via NAD+ can feedback to the clock, suggesting an interplay between the elements of clock output and the clock itself [1].
Transcriptional regulation of circadian clock is also controlled by D-box elements [140]. The PAR-Zip transcription factors such as D-box-binding proteins (DBP) which are under the E-box-mediated transcriptional control bind to these elements, and hence, they indirectly regulate CCGs [72].
In addition to transcriptional regulation, PTMs regulate the subcellular localization and stability of PER–CRY complexes [45] allowing progressive and delayed (circa 24 h) maturation of PER/CRY as transcriptional repressors. CK1ϵ and mitogen-activated protein kinase play an important role in the activation or repression of BMAL1, while CK2α and GSK-3β help in cellular localization and proteasomal degradation respectively [75]. In case of PER, site-specific phosphorylation at a “priming site” (FASP site) delays phosphorylation at “sites” (PERs or βTrCP site) that would signal for nuclear entry and degradation by proteasomal pathways [141]. In addition, salt inducible kinase 3 (SIK3 kinase) modulates PER2 phosphorylation rhythms and abundance [47]. Similarly, microRNAs (miRNAs) and several RNA-binding protein complexes regulate RNA stabilization and degradation, circadian polyadenylation and splicing [112]. Overall, the molecular mechanisms underlying the regulation of circadian rhythms in a cell involves numerous complex processes.
Aging and Theories of Aging
Aging is an inevitable unidirectional process that eventually leads to the progressive decline of metabolism, physiology, and behavior and ultimately culminates in death. Aging is explained through a couple of theories. Programmed theory deems the timeline of different stages of life linked to change of metabolism, physiology, and behavior. First theory falls into three subcategories: (i) Programmed longevity – programmed switching of particular genes leading to senescence resulting in overt manifestations. (ii) Endocrine theory – evolutionarily conserved hormonal signaling such as insulin/IGF-1 signaling pathway regulates the process of aging. We have vividly discussed the endocrine regulation of aging elsewhere [59]. (iii) Immunological theory – preprogrammed deterioration of immune system.
Second, damage or error theory postulates that the accumulated damages or errors at several levels over the period of time would cause aging and is linked to metabolic disorders, epigenetic alterations, genomic instability, telomere attrition, loss of proteostasis, altered intercellular communications, cellular senescence, deregulated nutrient sensing, stem cell exhaustion and mitochondrial dysfunction, and DNA damage [82]. Of all the macromolecules that are being damaged as the age progresses, DNA is very important because it cannot be replaced like other macromolecules [38] and also slowing down of DNA repair mechanisms progresses aging process.
Age-Associated Circadian Dysfunction
Age has a marked effect on CTS, which impacts the temporal organization of circadian physiology and behavior (Fig. 20.3, Table 20.1). In humans, fragmented sleep and progressive advance in sleep phase has been recorded in elderly [35, 53]. Similarly, the amplitude of feeding rhythms, secretion of hormones, and body temperature are known to decline with age [149]. In aged animals, decline in locomotor activity rhythms and disrupted sleep–wake cycles suggests age-associated circadian alterations [9]. Reports from mice have demonstrated that the aged animals are more vulnerable to negative effects of photoperiodic phase shifts as the adaptability of circadian system is compromised with aging [7]. In rodents, age-related changes in circadian rhythms have been reported for body temperature, activity–wakefulness, locomotor activity patterns, drinking behavior, and serotonin rhythms [57, 87, 121, 148, 152]. In addition, core clock in aged mice showed diminished response to the external stimuli suggesting CTS deterioration [83].
Influence of Aging on Central and Peripheral Clocks
As the SCN communicates directly and indirectly to various peripheral clocks, circadian clock and aging may be interconnected by pathology at the level of the SCN and SCN output signals [91]. Though there appears no reduction in the total number of cells in aged SCN [50], the age-related loss of amplitude in SCN electrical activity [100] suggests alterations in cellular properties, neuronal circuitry, and clock genes [10]. At single cell level, the neurons of aged SCN showed diminished amplitude of potassium currents and resting membrane potential as a result of possible alterations in large conductance calcium-activated potassium channels (BK channels) [36]. Age-associated alterations in cellular communication in SCN has been evident with reports showing age-dependent loss of neuronal connectivity, marked by decline in synaptic spines and shortened dendrites, altered electrical activity, and altered signaling molecules [107]. Moreover, alterations in the expression of neuropeptide vasoactive-intestinal polypeptide (VIP) and arginine-vasopressin (AVP) reported upon aging would hamper the SCN output as they are essential for intracellular coupling within the SCN [93]. Disrupted GABAergic signaling in aged SCN indicates clock deterioration [107]. Weakened melatoninergic feedback to the SCN is suggested by reports showing diminished MT1 receptor expression in aged human SCN [145]. Age-linked circadian disruption is significantly contributed by desynchrony between SCN and oscillators in peripheral tissues. Phase-shifting studies in elderly involving exposure to different LD regimens showed decline in the ability to re-entrain in several parameters such as rhythms of activity, rest/sleep and body temperature [51]. Similarly, phase advance study using PER2::LUC mouse demonstrated that oscillators in esophagus, thymus gland and lungs in older mice took longer time period to get entrained to specific light–dark schedule, in comparison with younger mice [128].
Aging is also known to be resulting in declined total melatonin secretion. Studies in humans, primates, and hamsters indicate that the normal nighttime peak in elderly is reduced and phase advanced compared to younger adults [51]. In addition, diminished pineal melatonin synthesis and SCN expression of melatonin receptors have been reported in individuals with Alzheimer’s or Parkinson’s disease [142]. Severe alterations in daily rhythms and levels of serotonin metabolism in SCN of aged rats and a rat model of PD have been reported from our lab [57, 89, 120]. Similarly, cortisol, a hormone under clock control, which also synchronizes peripheral clocks was observed to show age-related reduction in amplitude and advance in phase [51]. In a recent study analyzing hepatic transcriptome, it was observed that 2626 genes (44.8%) were exclusively oscillatory in young mice whereas in old mice only 1626 genes (28.4%) were rhythmic [126]. Further, age-dependent decline in cyclic global protein acetylation was observed in peripheral clock liver [126]. Recently, from our laboratory, we have reported the age-associated day–night variations of proteins in SCN, substantianigra (SN), and pineal gland of rats [61]. In SCN, the number of proteins showing day–night variations were found to have decreased from 32 (in young adults) to 9 (in old age). Similarly, SN also showed a decrease from 59 to 9. However, in pineal, the number of proteins showing oscillations increased from 51 to 62 [61]. Our earlier studies investigating daily rhythms of lipid peroxidation and antioxidant enzyme activities in rats showed age-dependent variations in liver [87]. Further, reports from our laboratory have demonstrated differential alterations in daily rhythms and levels of NO and Socs1 expression in various peripheral clocks of aged rats [143, 144] suggesting desynchrony [61].
Influence of Aging on Clock Genes and Proteins
The canonical genes and proteins constituting the TTFL of the core clock machinery show drastic variations upon aging (Table 20.2). We have reported severe alterations in rhythms and levels of various clock genes in the SCN of mid- and old-aged rats [90]. Similarly, studies in aged mice SCN showed changes in Rev-erb a, Dec1, and Dbp expression [12]. Earlier studies in mice showed altered expression of the CLOCK and BMAL1 proteins in the SCN, hippocampus, amygdala, and several other brain regions by mid-age [153]. Additionally, altered expression of Bmal1 and Per2 transcripts in various non-SCN brain regions in aged hamsters has been reported [32]. Studies on Per1:luc rats showed slight age-related changes in Per1 expression in SCN, whereas robust changes in peripheral oscillators [155]. Though, reports on rhythmic PER2, PER3, and BMAL1 expression in cortex of elderly humans suggest persistence of clock function in old age [76], altered PER1, 2, 3 rhythms in leukocytes indicated desynchrony [49]. A detailed account on age-linked alterations in the core clock gene expression in the SCN is discussed elsewhere [10].
The mechanisms underlying the modulation of clock gene expression in aging might be involving the cross-talk between circadian and metabolic regulatory systems [116]. The NAD+-dependent protein deacetylase SIRT1 is known to play a significant role in the age-related deterioration of the circadian clock [20]. The role of SIRT1 in the modulation of circadian clock is well known [99] and studies in the SCN of aged mice have demonstrated decline in SIRT1 levels concomitantly with levels of BMAL1 and PER2 [20]. Further, Sirt1 knockout mice resulted in senescent-like phenotype and Sirt1 overexpression resulted in antiaging phenotype with respect to alterations in clock [20]. In addition, role of SIRTs in age-associated epigenetic changes in the clock has also gained considerable importance [104].
Therapeutic Interventions
Understanding the molecular components and their feedback mechanisms that are involved in aging and circadian dysfunction helps the researchers to identify the target molecules and to develop the therapeutic drugs to delay the progress of aging and age-associated disorders. Here we discuss few of the potential therapeutic strategies that are showing promising results in combating the aging process and restoring the circadian clock.
Effect of Antioxidants on Aging and CTS
-
(i)
Melatonin
The primary function of melatonin is to relay the circadian signals to the peripheral clocks and to synchronize them with the central clock [138]. It is also well established that melatonin relays seasonal temporal information [113]. Melatonin is shown to regulate several clock genes and considered as chronobiotic [59]. It is a multitasking molecule with several properties like anti-inflammatory and antioxidant, and can stimulate antioxidant enzymes like glutathione reductase, glutathione peroxidase, and catalase [87, 118]. Melatonin irregularities have been attributed to several circadian dysfunctions that are involved in cancers [122]. The secretary rhythm of melatonin has been linked with the immune changes and thyroid hormone in aging mice [114]. And also, the nocturnal secretion of melatonin is related to the cell-mediated immunity [84]. The peak expression of IL-1b, IL-2, IL-6, and TNF-α is observed shortly after the maximum melatonin serum levels [25].
In humans, melatonin metabolite 6-hydroxymelatonin sulfate showed similar pattern in both young and healthy centenary subjects; this is evident to claim that melatonin can be a proper aging marker [37]. Melatonin reduces the microglial activity in brain which is considered as anti-inflammaging property [46]. It also has shown to alleviate age-induced memory impairments and neuronal degeneration [130]. Cognitive deficits and neurodegeneration were shown to be alleviated with melatonin administration [103]. Melatonin has also shown the beneficiary effects on the sleep deprivation-induced memory deficits [4] and also improved sleep efficacy [135]. Further, melatonin supplementation has slowed down the progress of cognitive deficits and also ameliorated the sundowning syndrome in Alzheimer’s disease patients [18]. In correspondence to Alzheimer’s disease, melatonin has reduced β-amyloid, anomalous nitration of proteins, β-fibrillogenesis, tau phosphorylation, and also increased survival rate of AD transgenic mice [79, 80, 88]. We have recently demonstrated the restoratory effects of melatonin on age-induced alterations in NO daily rhythms and Socs1 expression in peripheral oscillators [143, 144]. We further demonstrated that melatonin administration could differentially restore the circadian phase, amplitude and the expression levels of clock genes such as Bmal1, Per1, Per2, Cry1, and Cry2 [89, 90] in aged and a rat model of PD. Additionally, we demonstrated age and PD-related changes in the number of oscillatory proteins which shows day–night variations, and the melatonin administration has resulted in differential restoration of these proteins in both aging and PD [61]. In concordance with it, several other researchers have shown the beneficial effects of melatonin in age-associated disorder like Parkinson’s disease [19].
The beneficiary effects of melatonin can be attributed to its amphiphilic nature that can easily cross the blood brain barrier [123]. The effect of melatonin on several clock-associated genes has been extensively discussed elsewhere [59].
-
(ii)
Resveratrol
Resveratrol (3,5,4′-trihydroxy-trans-stilbene), a polyphenol purified from natural sources is known to modulate CTS [102] and also rescues from various age-related impairments [41]. Studies in nocturnal primate gray lemur further revealed the influence of resveratrol on circadian clock. Resveratrol improved the synchronization of old animals to light–dark cycles and restored the rhythms of locomotor activity and body temperature [115]. Interestingly, few researchers have demonstrated its role in clock-mediated rescue from disorder of lipid metabolism in a rodent model [134]. Corroborative evidence on beneficial effects of resveratrol came from a report where it attenuated the insulin resistance in liver by modulating core clock elements as well as SIRT1 [163]. Considering the importance of clock in healthy aging [28], an earlier study emphasized SIRT1 and its activator resveratrol’s role in BMAL1-CLOCK-mediated transcription of clock genes, highlighting its influence on CTS [109].
-
(iii)
Curcumin
Curcumin (diferuloylmethane), a potent antioxidant, is a polyphenol derived from rhizomes of Curcuma longa and known for its multiple beneficiary effects [129]. It has been reported that curcumin can cross blood brain barrier [139]. Its role as antioxidative, anti-inflammatory, anticancerous, neuro-protective, and clock restoratory agent is widely explored in various animal models [17]. Several studies have also suggested curcumin as a potential SIRT1 activator [157], it could mediate antiaging effects. Reports showing Bmal1 and SIRT1 activation by curcumin suggest a modulatory role for curcumin in age and associated circadian disorders [43]. We have shown the influence of curcumin on circadian clock by studying serotonin (5-HT) and its metabolite 5-hydroxy indole acetic acid (5-HIAA) rhythms in the SCN and pineal of rats [58]. Interestingly, a recent study demonstrated a synergistic function of melatonin and curcumin in tumor suppression [131]. Overall, these reports suggest curcumin can be a potential drug candidate in reversing age-linked clock dysfunction.
-
(iv)
Withania somnifera
Withania somnifera (WS), known as Ashwagandha, is known to promote health, enhance longevity, and create a sense of well-being [85]. With various biologically active constituents, the leaf, root, and fruit extracts of the plant have potential regenerative properties and is used for the treatment of various disorders [146]. Several studies have explored the free radical scavenging activity, regulation of lipid peroxidation, glutathione-S-transferase activity, and anti-inflammatory property of WS [63, 108]. Similarly, clinical and preclinical experiments have revealed the potential of WS against cancer, insomnia, anxiety, stress, cognitive, and age-associated neurodegenerative disorders such as AD and PD [68, 85, 127]. Evidence for antiaging effects of WS has also come from studies reporting downregulation of senescence in human fibroblasts and lifespan extension in C. elegance [71]. Moreover, we have recently reported the differential restoratory effect of Ashwagandha leaf extract on age-induced alterations in SCN core clock transcript expression rhythms [60]. Our results showed an age-specific action of WS, as we observed restorations in the phase of Per1, Cry1, and Bmal1 in the SCN of mid-aged (12 m) rats and only Per1 in old-aged (24 m) rats [60].
Effect of Calorie Restriction (CR) on Aging and CTS
CR is demonstrated to be a potential strategy in lifespan extension and improved health in various organisms [14]. A recent report elucidating the role of CR in rescuing age-dependent circadian alterations by involving SIRT1 activation in peripheral clock liver [126] corroborated the previous knowledge on antiaging effects of CR [81]. CR, a strong metabolic cue and known to function as a zeitgeber for peripheral clocks has been shown to modulate peripheral gene expression [6, 111]. Studies investigating the SCN VIP expression, pineal melatonin, blood glucose, and locomotor activity rhythms suggested the influence of CR on circadian clock. In addition to it, CR could also synchronize the peripheral clocks and influence clock-controlled output systems, such as the food anticipatory activity (FAA) and body temperature. Further, studies exploring the influence of CR on photic responses suggested a role for CR in entrainment of circadian clock [94]. Transcriptome analysis in various peripheral clocks under CR demonstrated Per2 as the most upregulated gene in majority of the clocks [136]. Emerging studies have also linked CR with elevated expression of several core clock genes including Per1, Per2, Cry2, and Bmal1. Similarly, some of the key CCGs which code for transcription factors such as Dbp, Dec1, Dec2, Hlf, Tef, and E4bp4 were differentially affected in under CR [110]. These observations indicate that CR could directly synchronize central clock as well as peripheral clocks and rescue from the age-associated circadian ailments [126]. Interestingly, a recent study using Bmal1 knockout mice has highlighted the role of BMAL1 in CR-mediated longevity effects [110].
Effect of Small Molecules as Modulators in Aging and CTS
In the recent years, there is an upsurge in the studies related to usage of small molecules as drugs. Through chemical screening approaches more than 2,00,000 small molecules have been identified as circadian regulators that may act as modifiers of period length, phase delay, phase advance, phase attenuation, and amplitude (Table 20.3) [22]. Small molecules such as CKI inhibitors and synthetic ligands for the nuclear receptors CRY1, REV-ERBs, and RORs have been proposed as therapeutic alternatives for several CTS dysfunction [22]. Studies on small molecules would open a new avenue in modulating age-related circadian dysfunctions toward healthy and slowly progressive aging.
Conclusion
Aging is associated with disruption of the chronobiological cycle. CTS undergoes reduced sensitivity to external cues with aging in various physiological, biochemical, and molecular parameters. Numerous clinical studies have established a direct correlation between abnormal circadian clock functions and the severity of neurodegenerative and sleep disorders. Therapeutic interventions using various pharmacological agents such as antioxidants, CR, and small molecules may help to restore CTS dysfunction in elderly.
References
Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74:246–60.
Alessi C, Martin JL, Fiorentino L, Fung CH, Dzierzewski JM, Rodriguez Tapia JC, Song Y, Josephson K, Jouldjian S, Mitchell MN. Cognitive behavioral therapy for insomnia in older veterans using nonclinician sleep coaches: randomized controlled trial. J Am Geriatr Soc. 2016;64:1830–8.
Ali AA, Schwarz-Herzke B, Stahr A, Prozorovski T, Aktas O, von Gall C. Premature aging of the hippocampal neurogenic niche in adult Bmal1-deficient mice. Aging (Albany NY). 2015;7:435–49.
Alzoubi KH, Mayyas FA, Khabour OF, BaniSalama FM, Alhashimi FH, Mhaidat NM. Chronic melatonin treatment prevents memory impairment induced by chronic sleep deprivation. Mol Neurobiol. 2016;53:3439–47.
Antoch MP, Gorbacheva VY, Vykhovanets O, Toshkov IA, Kondratov RV, Kondratova AA, Lee C, Nikitin AY. Disruption of the circadian clock due to the clock mutation has discrete effects on aging and carcinogenesis. Cell Cycle. 2008;7:1197–204.
Asher G, Sassone-corsi P. Review time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161:84–92.
Azzi A, Dallmann R, Casserly A, Rehrauer H, Patrignani A, Maier B, Kramer A, Brown SA. Circadian behavior is light-reprogrammed by plastic DNA methylation. Nat Nurosci. 2014;17:377–82.
Baird AL, Coogan AN, Siddiqui A, Donev RM, Thome J. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry. 2012;17:988–95.
Banks G, Heise I, Starbuck B, Osborne T, Wisby L, Potter P, Jackson IJ, Foster RG, Peirson SN, Nolan PM. Genetic background influences age-related decline in visual and nonvisual retinal responses, circadian rhythms, and sleep. Neurobiol Aging. 2015;36:380–93.
Banks G, Nolan PM, Peirson SN. Reciprocal interactions between circadian clocks and aging. Mamm Genome. 2016;27:332–40.
Belden WJ, Dunlap JC. Aging well with a little wine and a good clock. Cell. 2013;153:1421–2.
Bonaconsa M, Malpeli G, Montaruli A, Carandente F, Grassi-Zucconi G, Bentivoglio M. Differential modulation of clock gene expression in the suprachiasmatic nucleus, liver and heart of aged mice. Exp Gerontol. 2014;55:70–9.
Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6:109–20.
Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, Di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P, Morgan TE, Dorff TB, Longo VD. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22:86–99.
Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, Colman RJ, Bradfield CA. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis. 2005;41:122–32.
Cagnin A, Fragiacomo F, Camporese G, Turco M, Bussè C, Ermani M, Montagnese S. Sleep-wake profile in dementia with Lewy bodies, Alzheimer’s disease, and normal aging. J Alzheimers Dis. 2017;55:1529–36.
Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT, Rizzarelli E. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33:2444–71.
Cardinali DP, Brusco LI, Liberczuk C, Furio AM. The use of melatonin in Alzheimer’s disease. Neuro Endocrinol Lett. 2002;1:20–3.
Carocci A, Sinicropi MS, Catalano A, Lauria G, Genchi G. Melatonin in Parkinson’s disease. In: Abdul QR, editor. A synopsis of Parkinson’s disease. Intech; 2014. pp 71–99.
Chang HC, Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell. 2013;153:1448–60.
Chen Z, Yoo SH, Park YS, Kim KH, Wei S, Buhr E, Ye ZY, Pan HL, Takahashi JS. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc Natl Acad Sci U S A. 2012;109:101–6.
Chen Z, Yoo S, Takahashi JS. Development and therapeutic potential of small-molecule modulators of circadian systems. Annu Rev Pharmacol Toxicol. 2018;58:231–52.
Chun SK, Jang J, Chung S, Yun H, Kim NJ, Jung JW, Son GH, Suh YG, Kim K. Identification and validation of Cryptochrome inhibitors that modulate the molecular circadian clock. ACS Chem Biol. 2014;9:703–10.
Cooke JR, Ancoli-Israel S. Normal and abnormal sleep in the elderly. Handb Clin Neurol. 2011;98:653–65.
Couto-Moraes R, Palermo-Neto J, Markus RP. The immune–pineal axis: stress as a modulator of pineal gland function. Ann N Y Acad Sci. 2009;1153:193–202.
Curtis J, Burkley E, Burkley M. The rhythm is gonna get you: the influence of circadian rhythm synchrony on self-control outcomes. Soc Personal Psychol Compass. 2014;8:609–25.
De Cata A, D’Agruma L, Tarquini R, Mazzoccoli G. Rheumatoid arthritis and the biological clock. Expert Rev Clin Immunol. 2014;10:687–95.
Declerck K, Berghe WV. Back to the future: epigenetic clock plasticity towards healthy aging. Mech Ageing Dev. 2018;174:18–29. https://doi.org/10.1016/j.mad.2018.01.002.
Dibner C, Schibler U. Body clocks: time for the nobel prize. Acta Physiol (Oxford). 2018;222(2):e13024. https://doi.org/10.1111/apha.13024.
Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49.
Dubrovsky YV, Samsa WE, Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY). 2010;2:936–44.
Duncan MJ, Prochot JR, Cook DH, Tyler Smith J, Franklin KM. Influence of aging on Bmal1 and Per2 expression in extra-SCN oscillators in hamster brain. Brain Res. 2013;1491:44–53.
Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–90.
Duong HA, Robles MS, Knutti D, Weitz CJ. A molecular mechanism for circadian clock negative feedback. Science. 2011;332:1436–9.
Espiritu J. Aging-related sleep changes. Clin Geriatr Med. 2008;24:1–14.
Farajnia S, Meijer JH, Michel S. Age-related changes in large-conductance calcium-activated potassium channels in mammalian circadian clock neurons. Neurobiol Aging. 2015;36:2176–83.
Ferrari E, Cravello L, Falvo F, Barili L, Solerte SB, Fioravanti M, Magri F. Neuroendocrine features in extreme longevity. Exp Gerontol. 2008;43:88–94.
Freitas AA, de Magalhães JP. A review and appraisal of the DNA damage theory of ageing. Mutat Res. 2011;728:12–22.
Gleason K, McCall WV. Current concepts in the diagnosis and treatment of sleep disorders in the elderly. Curr Psychiatry Rep. 2015;17:45.
Gloston G, Yoo S, Chen Z. Clock-enhancing small molecules and potential applications in chronic diseases and aging. Front Neurol. 2017;8:100.
Gocmez S, Gacar N, Utkan T, Gacar G, Scarpace PJ, Tumer N. Protective effects of resveratrol on aging-induced cognitive impairment in rats. Neurobiol Learn Mem. 2016;131:131–6.
Golombek DA, Ralph MR. KN-62, an inhibitor of Ca2+/calmodulin kinase II, attenuates circadian responses to light. Neuroreport. 1994;5:1638–40.
Grabowska W, Sikora E, Bielak-Zmijewska A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology. 2017;18:447–76.
Gruszka A, Hampshire A, Barker RA, Owen AM. Normal aging and Parkinson’s disease are associated with the functional decline of distinct frontal-striatal circuits. Cortex. 2017;93:178–92.
Gustafson CL, Partch CL. Emerging models for the molecular basis of mammalian circadian timing. Biochemistry. 2015;54:134–49.
Hardeland R. Deacceleration of brain aging by melatonin. In: Stephen CB, Campbell A, editors. Oxidative stress in applied basic research and clinical practice. New York: Springer; 2016. p. 345–76.
Hayasaka N, Hirano A, Miyoshi Y, Tokuda IT, Yoshitane H, Matsuda J, Fukada Y. Salt-inducible kinase 3 regulates the mammalian circadian clock by destabilizing PER2 protein. elife. 2017;6:e24779. https://doi.org/10.7554/eLife.24779.
Helleboid S, Haug C, Lamottke K, Zhou Y, Wei J, Daix S, Cambula L, Rigou G, Hum DW, Walczak R. The identification of naturally occurring neoruscogenin as a bioavailable, potent, and high-affinity agonist of the nuclear receptorRORα (NR1F1). J Biomol Screen. 2014;19:399–406.
Hida A, Kusanagi H, Satoh K, Kato T, Matsumoto Y, Echizenya M, Shimizu T, Higuchi S, Mishima K. Expression profiles of PERIOD1, 2, and 3 in peripheral blood mononuclear cells from older subjects. Life Sci. 2009;84:33–7.
Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5:33–51.
Hood S, Amir S. The aging clock: circadian rhythms and later life. J Clin Invest. 2017;127:437–46.
Hu Y, Spengler ML, Kuropatwinski KK, Comas-Soberats M, Jackson M, Chernov MV, Gleiberman AS, Fedtsova N, Rustum YM, Gudkov AV, Antoch MP. Selenium is a modulator of circadian clock that protects mice from the toxicity of a chemotherapeutic drug via upregulation of the core clock protein, BMAL1. Oncotarget. 2011;2:1279–90.
Jagota A. Aging and sleep disorders. Indian J Gerontol. 2005;19:415–24.
Jagota A. Suprachiasmatic nucleus: the center for circadian timing system in mammals. Proc Indian Natl Sci Acad. 2006;B71:275–88.
Jagota A. Age- induced alterations in biological clock: therapeutic effects of melatonin. In: Thakur M, Rattan S, editors. Brain aging and therapeutic interventions. Dordrecht: Springer; 2012. p. 111–29.
Jagota A, de la Iglesia HO, Schwartz WJ. Morning and evening circadian oscillations in the suprachiasmatic nucleus in vitro. Nat Neurosci. 2000;3:372–6.
Jagota A, Kalyani D. Effect of melatonin on age induced changes in daily serotonin rhythms in suprachiasmatic nucleus of male Wistar rat. Biogerontology. 2010;11:299–308.
Jagota A, Reddy MY. The effect of curcumin on ethanol induced changes in suprachiasmatic nucleus (SCN) and pineal. Cell Mol Neurobiol. 2007;27:997–1006.
Jagota A, Thummadi NB. Hormones in clock regulation during ageing. In: Rattan SIS, Sharma R, editors. Hormones and ageing and longevity. Healthy ageing and longevity, vol. 6. Cham: Springer; 2017. p. 243–65.
Jagota A, Kowshik K. Chapter 21: therapeutic effects of Ashwagandha in brain aging and clock disfunction (Invited chapter). In: Kaul S, Wadhwa R, editors. Science of Ashwagandha: preventive and therapeutic potentials. Cham: Springer; 2017. p. 437–56.
Jagota A, Mattam U. Daily chronomics of proteomic profile in aging and rotenone-induced Parkinson’s disease model in male Wistar rat and its modulation by melatonin. Biogerontology. 2017;18:615–30.
Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:479–504.
Khan MA, Subramaneyaan M, Arora VK, Banerjee BD, Ahmed RS. Effect of Withania somnifera (Ashwagandha) root extract on amelioration of oxidative stress and autoantibodies production in collagen-induced arthritic rats. J Complement Integr Med. 2015;12:117–25.
Kim HJ, Harrington ME. Neuropeptide Y-deficient mice show altered circadian response to simulated natural photoperiod. Brain Res. 2008;1246:96–100.
Kim MJ, Lee JH, Duffy JF. Circadian rhythm sleep disorders. J Clin Outcomes Manag. 2013;20:513–28.
Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic nucleus: the mind’s clock. New York: Oxford University Press; 1991.
Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov. 2014;13:197–216.
Konar A, Shah N, Singh R, Saxena N, Kaul SC, Wadhwa R, Thakur MK. Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS One. 2011;6:e27265.
Korenčič A, Bordyugov G, Lehmann R, Rozman D, Herzel H. Timing of circadian genes in mammalian tissues. Sci Rep. 2014;4:5782.
Krishnan N, Kretzschmar D, Rakshit K, Chow E, Giebultowicz JM. The circadian clock gene period extends healthspan in aging Drosophila melanogaster. Aging (Albany NY). 2009;1:937–48.
Kumar R, Gupta K, Saharia K, Pradhan D, Subramaniam JR. Withania somnifera root extract extends lifespan of Caenorhabditis elegans. Ann Neurosci. 2013;20:13–6.
Lavery DJ, Lopez-molina L, Margueron R, Fleury-Olela F, Conquet F, Schibler U, Bonfils C. Circadian expression of the steroid 15 alpha-hydroxylase (Cyp2a4) and coumarin 7-hydroxylase (Cyp2a5) genes in mouse liver is regulated by the PAR leucine zipper transcription factor DBP. Mol Cell Biol. 1999;19:6488–99.
Lavoie CJ, Zeidler MR, Martin JL. Sleep and aging. Sleep Sci Pract. 2018;2:3.
Lee CC. Tumor suppression by the mammalian period genes. Cancer Causes Control. 2006;17:525–30.
Lee Y, Kim K. Posttranslational and epigenetic regulation of the CLOCK/BMAL1 complex in the mammalian. Anim Cells Syst. 2012;16:1–10.
Lim ASP, Myers AJ, Yu L, Buchman AS, Duffy JF, De Jager PL, Bennett DA. Sex difference in daily rhythms of clock gene expression in the aged human cerebral cortex. J Biol Rhythm. 2013;28:117–29.
Lim ASP, Fleischman DA, Dawe RJ, Yu L, Arfanakis K, Buchman AS, Bennett DA. Regional neocortical gray matter structure and sleep fragmentation in older adults. Sleep. 2016;39:227–35.
Lindemer ER, Greve DN, Fischl BR, Augustinack JC, Salat DH. Regional staging of white matter signal abnormalities in aging and Alzheimer’s disease. Neuroimage Clin. 2017;14:156–65.
Ling ZQ, Tian Q, Wang L, Fu ZQ, Wang XC, Wang Q, Wang JZ. Constant illumination induces Alzheimer–like damages with endoplasmic reticulum involvement and the protection of melatonin. J Alzheimers Dis. 2009;16:287–300.
Lin L, Huang QX, Yang SS, Chu J, Wang JZ, Tian Q. Melatonin in Alzheimer’s disease. Int J Mol Sci. 2013;14:14575–93.
Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23:1048–59.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217.
Lupi D, Semo M, Foster RG. Impact of age and retinal degeneration on the light input to circadian brain structures. Neurobiol Aging. 2012;33:383–92.
Maestroni GJ, Sulli A, Pizzorni C, Villaggio B, Cutolo M. Melatonin in rheumatoid arthritis: synovial macrophages show melatonin receptors. Ann N Y Acad Sci. 2002;966:271–5.
Manchanda S, Mishra R, Singh R, Kaur T, Kaur G. Aqueous leaf extract of Withania somnifera as a potential neuroprotective agent in sleep-deprived rats: a mechanistic study. Mol Neurobiol. 2017;54:3050–61.
Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94:19–36.
Manikonda PK, Jagota A. Melatonin administration differentially affects age-induced alterations in daily rhythms of lipid peroxidation and antioxidant enzymes in male rat liver. Biogerontology. 2012;13:511–24.
Matsubara E, Bryant–Thomas T, Quinto JP, Henry TL, Poeggeler B, Herbert D, Cruz–Sanchez F, Chyan YJ, Smith MA, Perry G, Shoji M, Abe K, Leone A, Grundke–Ikbal I, Wilson GL, Ghiso J, Williams C, Refolo LM, Pappolla MA. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J Neurochem. 2003;85:1101–8.
Mattam U, Jagota A. Daily rhythms of serotonin metabolism and the expression of clock genes in suprachiasmatic nucleus of rotenone-induced Parkinson’s disease male Wistar rat model and effect of melatonin administration. Biogerontology. 2015;16:109–23.
Mattam U, Jagota A. Differential role of melatonin in restoration of age-induced alterations in daily rhythms of expression of various clock genes in suprachiasmatic nucleus of male Wistar rats. Biogerontology. 2014;15:257–68.
Mattis J, Sehgal A. Circadian rhythms, sleep, and disorders of aging. Trends Endocrinol Metab. 2016;27:192–203.
Maurya PK, Noto C, Rizzo LB, Rios AC, Nunes SO, Barbosa DS, Sethi S, Zeni M, Mansur RB, Maes M, Brietzke E. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2016;65:134–44.
Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605.
Mendoza J, Graff C, Dardente H, Pevet P, Challet E. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J Neurosci. 2005;25:1514–22.
Meng QJ, Maywood ES, Bechtold DA, Lu WQ, Li J, Gibbs JE, Dupré SM, Chesham JE, Rajamohan F, Knafels J, Sneed B, Zawadzke LE, Ohren JF, Walton KM, Wager TT, Hastings MH, Loudon AS. Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc Natl Acad Sci U S A. 2010;107:15240–5.
Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol. 1997;389:508–34.
Murri MB, Pariante C, Mondelli V, Masotti M, Atti AR, Mellacqua Z, Antonioli M, Ghio L, Menchetti M, Zanetidou S, Innamorati M, Amore M. HPA axis and aging in depression: systematic review and meta-analysis. Psychoneuroendocrinology. 2014;41:46–62.
Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, Herzog ED, Hogenesch JB, Wozniak DF, Dikranian K, Giasson BI, Weaver DR, Holtzman DM, Fitzgerald GA. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–400.
Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–7.
Nakamura TJ, Takasu NN, Nakamura W. The suprachiasmatic nucleus: age-related decline in biological rhythms. J Physiol Sci. 2016;66:367–74.
Nangle S, Xing W, Zheng N. Crystal structure of mammalian cryptochrome in complex with a small molecule competitor of its ubiquitin ligase. Cell Res. 2013;23:1417–9.
Oike H, Kobori M. Resveratrol regulates circadian clock genes in rat-1 fibroblast cells. Biosci Biotechnol Biochem. 2008;72:3038–40.
Olcese JM, Cao C, Mori T, Mamcarz MB, Maxwell A, Runfeldt MJ, Wang L, Zhang C, Lin X, Zhang G, Arendash GW. Protection against cognitive deficits and markers of neurodegeneration by long–term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res. 2009;47:82–96.
Orozco-Solis R, Sassone-Corsi P. Circadian clock: linking epigenetics to aging. Curr Opin Genet Dev. 2014;26:66–72.
Palesh O, Aldridge-Gerry A, Zeitzer JM, Koopman C, Neri E, Giese-Davis J, Jo B, Kraemer H, Nouriani B, Spiegel D. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37:837–42.
Palmer J. An introduction to biological rhythms. Saint Louis: Elsevier; 2012.
Palomba M, Nygård M, Florenzano F, Bertini G, Kristensson K, Bentivoglio M. Decline of the presynaptic network, including GABAergic terminals, in the aging suprachiasmatic nucleus of the mouse. J Biol Rhythm. 2008;23:220–31.
Panchawat S. In vitro free radical scavenging activity of leaves extracts of Withania somnifera. Recent Res Sci Technol. 2011;3:40–3.
Park I, Lee Y, Kim H, Kim K. Original article effect of resveratrol, a SIRT1 activator, on the interactions of the CLOCK/BMAL1 complex. Endocrinol Metab. 2014;29:379–87.
Patel SA, Chaudhari A, Gupta R, Velingkaar N, Kondratov RV. Circadian clocks govern calorie restriction – mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. FASEB J. 2017;30:1634–42.
Patel SA, Velingkaar N, Makwana K, Chaudhari A, Kondratov R. Calorie restriction regulates circadian clock gene expression through BMAL1 dependent and independent mechanisms. Sci Rep. 2016;6:25970.
Pegoraro M, Tauber E. The role of microRNAs (miRNA) in circadian rhythmicity. J Genet. 2008;87:505–11.
Pevet P, Challet E. Melatonin: both master clock output and internal time–giver in the circadian clocks network. J Physiol Paris. 2011;105:170–82.
Pierpaoli W, Yi C. The involvement of pineal gland and melatonin in immunity and aging I. Thymus–mediated, immunoreconstituting and antiviral activity of thyrotropin–releasing hormone. J Neuroimmunol. 1990;27:99–109.
Pifferi F, Dal-pan A, Languille S, Aujard F. Effects of resveratrol on daily rhythms of locomotor activity and body temperature in young and aged grey mouse lemurs. Oxidative Med Cell Longev. 2013;2013:187301.
Popa-Wagner A, Buga AM, Dumitrascu DI, Uzoni A, Thome J, Coogan AN. How does healthy aging impact on the circadian clock? J Neural Transm. 2017;124:89–97.
Rabadi MH, Mayanna SK, Vincent AS. Predictors of mortality in veterans with traumatic spinal cord injury. Spinal Cord. 2013;51:784–8.
Radogna F, Diederich M, Ghibelli L. Melatonin: a pleiotropic molecule regulating inflammation. Biochem Pharmacol. 2010;80:1844–52.
Rakshit K, Thomas AP, Matveyenko AV. Does disruption of circadian rhythms contribute to beta-cell failure in type 2 diabetes? Curr Diab Rep. 2014;14:474.
Reddy MY, Jagota A. Melatonin has differential effects on age-induced stoichiometric changes in daily chronomics of serotonin metabolism in SCN of male Wistar rats. Biogerontology. 2015;16:285–302.
Reddy VDK, Jagota A. Effect of restricted feeding on nocturnality and daily leptin rhythms in OVLT in aged male Wistar rats. Biogerontology. 2014;15:245–56.
Reiter RJ, Tan DX, Korkmaz A, Erren TC, Piekarski C, Tamura H, Manchester LC. Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit Rev Oncog. 2007;13:303–28.
Reiter RJ, Tan DX, Galano A. Melatonin: exceeding expectations. Physiology. 2014;29(5):325–33.
Reppert SM, Wever DR. Coordination of circadian timing system. Nature. 2002;418:935–41.
Robillard R, Naismith SL, Hickie IB. Recent advances in sleep-wake cycle and biological rhythms in bipolar disorder. Curr Psychiatry Rep. 2013;15:402.
Sato S, Solanas G, Peixoto FO, Bee L, Symeonidi A, Schmidt MS, Brenner C, Masri S, Benitah SA, Sassone-Corsi P. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell. 2017;170:664–70.
Sehgal N, Gupta A, Valli RK, Joshi SD, Mills JT, Hamel E, Khanna P, Jain SC, Thakur SS, Ravindranath V. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc Natl Acad Sci. 2012;109:3510–5.
Sellix MT, Evans JA, Leise TL, Castanon-Cervantes O, Hill DD, DeLisser P, Block GD, Menaker M, Davidson AJ. Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J Neurosci. 2012;32:16193–202.
Shen LR, Parnell LD, Ordovas JM, Lai CQ. Curcumin and aging. Biofactors. 2013;39:133–40.
Shin EJ, Chung YH, Le HLT, Jeong JH, Dang DK, Nam Y, Wie MB, Nah SY, Nabeshima YI, Nabeshima T, Kim HC. Melatonin attenuates memory impairment induced by Klotho gene deficiency via interactive signaling between MT2 receptor, ERK, and Nrf2–related antioxidant potential. Int J Neuropsychopharmacol. 2015;18(6):pii: pyu105. https://doi.org/10.1093/ijnp/pyu105.
Shrestha S, Zhu J, Wang Q, Du X, Liu F, Jiang J, Song J, Xing J, Sun D, Hou Q, Peng Y. Melatonin potentiates the antitumor effect of curcumin by inhibiting IKK β/NF- κ B/COX-2 signaling pathway. Int J Oncol. 2017;51:1249–60.
Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, Takahashi JS, Butler AA, Kamenecka TM, Burris TP. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–8.
Somanath PR, Podrez EA, Chen J, Ma Y, Marchant K, Antoch M, Byzova TV. Deficiency in core circadian protein Bmal1 is associated with a prothrombotic and vascular phenotype. J Cell Physiol. 2011;226:132–40.
Sun L, Wang Y, Song Y, Cheng XR, Xia S, Rahman MR, Shi Y, Le G. Resveratrol restores the circadian rhythmic disorder of lipid metabolism induced by high-fat diet in mice. Biochem Biophys Res Commun. 2015;458:86–91.
Sun X, Ran D, Zhao X, Huang Y, Long S, Liang F, Guo W, Nucifora FC Jr, Gu H, Lu X, Chen L, Zeng J, Ross CA, Pei Z. Melatonin attenuates hLRRK2–induced sleep disturbances and synaptic dysfunction in a Drosophila model of Parkinson’s disease. Mol Med Rep. 2016;13:3936–44.
Swindell WR. Comparative analysis of microarray data identifies common responses to caloric restriction among mouse tissues. Mech Ageing Dev. 2008;129:138–53.
Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18:164–79.
Tan DX, Xu B, Zhou X, Reiter RJ. Associated health consequences and rejuvenation of the pineal gland. Molecules. 2018;23(2):pii: E301. https://doi.org/10.3390/molecules23020301.
Tsai YM, Chien CF, Lin LC, Tsai TH. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int J Pharm. 2011;416:331–8.
Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37:187–92.
Vanselow K, Vanselow JT, Westermark PO, Reischl S, Maier B, Korte T, Herrmann A, Herzel H, Schlosser A, Kramer A. Differential effects of PER2 phosphorylation: molecular basis for the human familial advanced sleep phase syndrome (FASPS). Genes Dev. 2006;20:2660–72.
Videnovic A, Zee PC. Consequences of circadian disruption on neurologic health. Sleep Med Clin. 2015;10:469–80.
Vinod C, Jagota A. Daily NO rhythms in peripheral clocks in aging male Wistar rats: protective effects of exogenous melatonin. Biogerontology. 2016;17:859–71.
Vinod C, Jagota A. Daily Socs1 rhythms alter with aging differentially in peripheral clocks in male Wistar rats: therapeutic effects of melatonin. Biogerontology. 2017;18:333–45.
von Gall C, Weaver DR. Loss of responsiveness to melatonin in the aging mouse suprachiasmatic nucleus. Neurobiol Aging. 2008;29:464–70.
Wadhwa R, Konar A, Kaul SC. Nootropic potential of Ashwagandha leaves: beyond traditional root extracts. Neurochem Int. 2016;95:109–18.
Wang RH, Zhao T, Cui K, Hu G, Chen Q, Chen W, Wang XW, Soto-Gutierrez A, Zhao K, Deng CX. Negative reciprocal regulation between Sirt1 and Per2 modulates the circadian clock and aging. Sci Rep. 2016;6:28633.
Weinert D. Age-dependent changes of the circadian system. Chronobiol Int. 2000;17:261–83.
Weinert D. Circadian temperature variation and ageing. Ageing Res Rev. 2010;9:51–60.
Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–77.
Winkelman JW, Armstrong MJ, Allen RP, Chaudhuri KR, Ondo W, Trenkwalder C, Zee PC, Gronseth GS, Gloss D, Zesiewicz T. Practice guideline summary: treatment of restless legs syndrome in adults: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of Neurology. Neurology. 2016;87:2585–93.
Witting W, Mirmiran M, Bos NP, Swaab DF. The effect of old age on the free-running period of circadian rhythms in rat. Chronobiol Int. 1994;11:103–12.
Wyse CA, Coogan AN. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res. 2010;1337:21–31.
Yamaguchi Y, Suzuki T, Mizoro Y, Kori H, Okada K, Chen Y, Fustin JM, Yamazaki F, Mizuguchi N, Zhang J, Dong X, Tsujimoto G, Okuno Y, Doi M, Okamura H. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science. 2013;342:85–90.
Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A. 2002;99:10801–6.
Yan SS, Wang W. The effect of lens aging and cataract surgery on circadian rhythm. Int J Ophthalmol. 2016;9:1066–74.
Yang Y, Duan W, Lin Y, Yi W, Liang Z, Yan J, Wang N, Deng C, Zhang S, Li Y, Chen W, Yu S, Yi D, Jin Z. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic Biol Med. 2013;65:667–79.
Yang G, Chen L, Grant GR, Paschos G, Song WL, Musiek ES, Lee V, McLoughlin SC, Grosser T, Cotsarelis G, FitzGerald GA. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci Transl Med. 2016;8:324ra16.
Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–5.
Yonei Y, Hattori A, Tsutsui K, Okawa M, Ishizuka B. Effects of melatonin: basics studies and clinical applications. Anti-Aging Med. 2010;7:85–91.
Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY). 2011;3:479–93.
Zhou QP, Jung L, Richards KC. The management of sleep and circadian disturbance in patients with dementia. Curr Neurol Neurosci Rep. 2012;12:193–204.
Zhou B, Zhang Y, Zhang F, Xia Y, Liu J, Huang R, Wang Y, Hu Y, Wu J, Dai C, Wang H, Tu Y, Peng X, Wang Y, Zhai Q. CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology. 2014;59:2196–206.
Acknowledgments
AJ is thankful to Professor Pramod Rath for giving this opportunity and sincere patience during preparation of manuscript. The work is supported by DBT (102/IFD/SAN/5407/2011-2012), ICMR (Ref. No. 55/7/2012-/BMS), and UPE II Grants to AJ. KK is thankful to DST-INSPIRE for SRF and NBT is thankful to UGC for SRF.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jagota, A., Kukkemane, K., Thummadi, N.B. (2020). Biological Rhythms and Aging. In: Rath, P. (eds) Models, Molecules and Mechanisms in Biogerontology. Springer, Singapore. https://doi.org/10.1007/978-981-32-9005-1_20
Download citation
DOI: https://doi.org/10.1007/978-981-32-9005-1_20
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-32-9004-4
Online ISBN: 978-981-32-9005-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)