Abstract
Wet chemical processing of textiles requires a large quantity of water as a processing medium, which is finally discharged as an effluent contaminated with residual dyes, pigments, and other hazardous chemicals. However, plasma and UV photons can be effectively used for nanoscale surface engineering of various textile substrates while avoiding the usage of water as a processing medium. Plasma- and UV-induced surface activation, oxidation, etching, increase in surface area/roughness, and polymerization of textile substrates have also been utilized for improvement in water and oil absorbency, dyeing, printing, antistatic, and anti felting properties. Specialty fabrics, such as with one hydrophilic side and other side hydrophobic could also be produced by UV treatment. On the other hand, fragmentation of a precursor molecule in the plasma zone leads to in situ plasma reaction resulting in the development of pinhole-free hydrophobic textiles. In plasma and UV treatment, as only the surface of the sample is modified, they require a minimum amount of chemicals and energy. In addition, the cost of the final product can also be reduced due to the shorter processing time, exclusion of multistep operations, and partial reduction in effluent treatment. In the plasma- and UV-treated samples, the dyeing time, temperature, and dye bath auxiliaries can be reduced to achieve similar or better depth of shade compared to the untreated sample without compromising the fastness properties.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The chemical processing of textiles is important for their improvement in aesthetic and functional value. However, in wet chemical processing of textiles, the industry causes significant water and air pollution during the padding, drying, curing, and post washing operations. Approximately 100 L of water are used to process 1 kg of cotton textile. The cost of the final product also increases due to the multiple numbers of drying operations and effluent treatment. Currently, due to stringent environmental regulations, the textile industry is slowly moving towards implementation of environment-friendly processing technology with a minimal usage of water or without water at all along with usage of eco-friendly chemicals and auxiliaries. This approach will help to develop a sustainable wet chemical processing value chain. In the last two decades, several technological advancements have been demonstrated in textile chemical processing, such as spray and foam finishing, digital printing, and low material-to-liquid processing to reduce the consumption of water and generation of effluents [2, 39]. In addition, present consumers are more conscious of health and hygienic textiles. As a result of this, the demand for cellulosic (cotton) apparel textile processed and finished with natural products such as natural dyes for coloration, enzyme for biopolishing, and neem and aloevera extract for antimicrobial finishing is gaining attention. As far as water conservation is concerned, low-temperature plasma, an emerging technology, can be used for water-free textile processing and finishing by modifying the surface at the nanometer level. Plasma is a partially ionized gas composed of many types of species, such as positive and negative ions, electrons, neutrals, excited molecules, photons, and UV light. It has the potential to be commercialized in textile processing for the development of valued-added home, apparel, and technical textiles at a lower cost, while addressing the problems associated with environmental pollution. Unlike bulk modification of textiles by conventional processes, surface modification of the textile using plasma can be utilized to develop products with minimal usage of chemicals and energy. The surface modification technique is obligated to restrict the modification in the first few atomic layers of the fiber surface, while keeping the bulk properties unaltered. Plasma can exist over a wide range of temperature and pressure. The lightning bolt and the solar corona are examples of plasma present in nature, and as their temperature is quite high (>1,500 °C) they cannot be used for processing of polymeric material. In contrast, cold plasma, also known as low-temperature plasma or nonthermal plasma with bulk temperature of 20–250 °C, can be used for surface modification of polymeric/textile substrates. Plasma is an energetic chemical environment, where the generation of plasma species opens up diverse reactions resulting in various end applications. The surface modification of textiles using plasma can be carried out using non polymerizing gases (small molecule), such as oxygen (O2), nitrogen (N2), air, argon (Ar), helium (He), or fluorine (F) for surface activation, cleaning, oxidation, changes in surface energy, increases in surface roughness/area, etching, coating/deposition, and the creation of nanostructures. These help in improving textile properties in terms of water absorbency, wetting, wicking, oil absorbency, rate of dyeing, adhesion, and antifelting of wool. On the other hand, plasma reaction with a precursor molecule (big molecule) containing vinyl, hydroxyl, carbonyl, carboxyl, acrylate, or fluorocarbon backbone leads to the development of functional textiles, such as hydrophobic, UV protective, antimicrobial, and flame retardant.
Similar to the plasma treatment of textiles, UV excimer lamps have also been explored for similar surface modification. The UV treatment of textiles is also an emerging technology, where surface modification does not involve any in situ polymerization with a liquid or gaseous precursor. However, generation of free radicals by UV treatment followed by graft polymerization of a suitable monomer is possible, and has been carried out for tailor-made surface engineering. It is quite a simple, cost-effective, and dry process, hence it has been explored for surface modification of wool, silk, polyester, and nylon substrate improvement in wetting, wicking, dyeing, antifelting, hydrophilic, and specialty finishing. The UV excimer lamp with 172 nm photons is most commonly used for surface modification of heat-sensitive textile substrates to alter physicochemical properties. Mostly, the textile samples are UV treated in the presence of air, oxygen (O2), and nitrogen (N2) gases for surface etching, activation, oxidation, and radical generation.
The present chapter discusses in detail various types of plasma and UV used in wet chemical processing and finishing of textiles with their merits and demerits. Use of these two emerging technologies for the improvement of hydrophilic, oleophilic, dyeing, antistatic, and antifelting of cotton, wool, silk, nylon, and polyester fabrics has been reported in detail. In addition, the use of plasma technology for hydrophobic finishing of textiles using various polymerizable precursors has also been discussed. The advantages of plasma and UV treatments in wet chemical processing of textiles in terms of environmental friendliness and cost-effectiveness in terms of time, temperature, and pollution load have been summarized.
2 Surface Modification of Textiles
Surface modification techniques of polymeric and textile substrates can be classified broadly into two methods, physical and chemical, as discussed below.
2.1 Physical Method
In the physical methods, surface modification of polymer/textile substrates is carried out by either chemically altering the surface of the material or depositing an extraneous layer on the top of the existing material. For the nonreactive precursor, treatment in the presence of high-energy species, for example, radicals, ions, and molecules in excited states helps to generate radicals that on successive reaction produce polar groups. On the other hand, in the second approach, the material surface is superficially coated in the presence of high-energy atoms or clusters of atoms. Different physical techniques of surface modification are discussed below.
-
(a)
Flame Treatment
Flame treatment is an old technology and widely used in the printing industry for improving surface hydrophilicity of polyolefin film to enhance printability. Active species such as radicals, ions, and molecules in excited states are formed by high temperature. Distance between the flame and the polymer, number of flames, flame gas composition, temperature, and speed of the machine are adjusted depending upon the material to be processed and functionality required.
-
(b)
Metallization
Metallization is the process of coating the polymeric and textile substrate by metal species, deposited by evaporation induced by the Joule effect or electron-beam excitation. The most widespread application is the aluminium coating of plastic films for packaging purposes.
-
(c)
Sputtering
The sputtering process involves the creation of ions, accelerating them on a target, and forming atoms or clusters, which are then deposited on the substrates. It is used for producing inorganic coating, when evaporation is not possible. A typical example is ultrathin silver or gold coating of textile/polymeric substrates for scanning electron microscope (SEM) analysis.
-
(d)
UV Eximer and Laser Treatment
UV lamps and lasers in the 172–400 nm wavelength are widely used for the treatment of polymer/textile substrates to improve hydrophilic functionality, dye uptake, photon-activated cross-linking of paper coating, and fragmentation of polymer coating.
2.2 Chemical Method
The surface chemical composition of the polymeric/textile substrates can be changed either by direct reaction with a given solution (wet treatment) or by graft polymerization using a suitable monomer. Some of the important chemical methods of surface modification are briefly described below.
2.2.1 Wet Treatment
Wet treatment is the first surface modification technique used in order to improve the surface properties of polymer. For example, hot chromic acid is used to oxidize polyolefin.
2.2.2 Etching
Etching of the polymeric/textile surface is useful for improving adhesion strength. For example, due to the low surface energy of the fluorocarbon, polymers have poor interfacial adhesion strength to any matrix material. The treatment of such polymers using strong reducing agents such as sodium in liquid ammonia improves their interfacial properties due to the etching effect.
2.2.3 Hydrolysis
Among the different hydrolysis techniques, treatment of polyester (PET) by hot sodium hydroxide is the oldest and probably the most exploited method used thus far. This method is used to improve the surface hydrophilicity of the polyester sample.
2.2.4 Grafting
Grafting is achieved by formation of a covalent bond with a molecule on the top of the substrate surface. This is done by first creating radicals on the sample surface by UV/plasma/γ irradiation, followed by grafting polymerization of a vinyl-based monomer.
2.2.5 Plasma Treatment
Plasma, an ionized gas, can be used for surface modification of polymeric/textile substrates by generating polar groups with surface activation, improving surface roughness by plasma etching, and functional properties by plasma polymerization. Therefore, based on the physicochemical changes brought out, the plasma-induced surface modification may be considered either a physical or a chemical method.
3 Generation and Classification of Plasma and UV
Plasma is a partially ionized gas normally generated by electrical breakdown of a gaseous molecule in the presence of high-frequency alternating current (AC) and most commonly used for surface modification of fibrous material. An alternating current with a high-frequency power supply helps in dissociation of various gaseous molecules into a collection of ions, electrons, neutral particles, and other excited species. Plasma is often considered as the fourth state of matter. It was first identified by Sir William Crookes in 1879, and named “plasma” by Irving Langmuir in 1928. Different types of plasmas are discussed below.
3.1 Hot and Cold Plasma
Plasma can be classified into hot/thermal plasma and cold/low-temperature/nonthermal plasma based on the temperature of the plasma zone. Hot plasma occurs when the temperatures of the electrons, atomic and molecular species, are extremely high and remain near to the thermal equilibrium state. In that condition, molecules remain almost fully ionized (100 %). The sun and other stars in various galaxies of the universe, fusion reactors, and plasma torches are examples of hot plasma. In hot plasma, the temperature of the plasma zone is around 106–108 K with an electron density of ≥1020 m−3. On the other hand, in cold plasma, the electrons remain at a significantly higher temperature, and the ions and neutral molecules remain near to ambient temperature (Te ≫Tion ≈ Tgas = 25–250 °C, Telectrons ≈ 727 °C). In cold plasma, the electron density is significantly lower (ne ≈ 1010 m−3) and only a small fraction of gas molecules (~1 %) is ionized [42]. Cold plasma is suitable only for surface modification of heat-sensitive polymeric and textile substrates.
3.2 Low and Atmospheric Pressure Plasma
3.2.1 Low Pressure Plasma
It is easy to ionize a gaseous molecule by electrical breakdown under a low-pressure condition, and it has been extensively studied for material processing. The advantages of low-pressure plasma processing are: (i) presence of a high concentration of reactive species, (ii) uniform glow plasma, (iii) temperature of plasma below 250 °C, and (iv) lower breakdown voltages. However, some of the limitations of low-pressure plasma processing are: (i) longer processing time, (ii) limited sample size to the size of the reactor, and (iii) mostly batch processing.
3.2.2 Atmospheric Pressure Plasma
Unlike low-pressure plasma, it is quite challenging to generate plasma at atmospheric pressure due to the presence of high voltage in a narrow electrode gap and the difficulty in ionizing gaseous molecules and generation of uniform cold plasma. However, if the stable cold plasma can be generated at atmospheric pressure, it can overcome the limitations of low-pressure technology. It can also be easily integrated into existing textile processes for continuous treatment of textiles. The three major types of atmospheric pressure cold plasma commonly used in textile processing are briefly discussed below.
-
(a)
Dielectric Barrier Discharge
The dielectric barrier discharge provides a strong thermodynamic non equilibrium plasma at atmospheric pressure with a moderate gas temperature. It is produced by an arrangement consisting of two parallel flat electrodes. Among them, at least one electrode is covered with a dielectric plate. An AC voltage in the range of 1–100 kV with a RF frequency of 50 Hz–100 kHz is applied to ignite the DBD plasma. The textile sample is kept between the two electrodes having a gap of <1 mm to several mm, and gas or a mixture of gases is then injected for uniform treatment of the textile. The DBD plasma is used in plasma-assisted chemical vapor deposition, surface etching, surface cleaning, surface activation, and plasma polymerization.
-
(b)
Corona Discharge
Corona discharge is the characteristic of an asymmetric electrode pair either powered by a continuous or pulsed by an AC/DC electrical supply. In a highly nonuniform electric field such as a point, plane gap, or wire cylindrical gap, when the high electric field near the point electrode exceeds the breakdown strength of a gas, plasma is formed. The plasma discharge gap is kept at about 0.5–2 mm. The corona discharge is used in electrostatic precipitators in dust collection and activation of polymer/textile substrates to improve the hydrophilic property.
-
(c)
Plasma Jet
The atmospheric pressure plasma jet (AAPJ) consists of two concentric electrodes through which a flow of a mixture of gases is supplied. The inner electrode is coupled with 13.56 MHz or kHz RF power with a discharge voltage in the range of 250 V to 10 kV, and the outer electrode is grounded. The discharge is ignited by a RF power between the outer and the inner electrodes, thus producing a high velocity of highly reactive species for the downstream processing of textiles.
3.3 UV Treatment
Surface modification such as etching, ablation, deposition, and evaporation by laser treatment can be carried out in various ways depending on the purpose of such modification. A large number of lasers are capable of operating at different wavelengths, and different modes are available for the surface modification of various substrates. The operating principle is based on the radiative decomposition of excimer states created by a silent discharge in a high-pressure gas column. A typical lamp consists of two concentric quartz tubes, outer and inner metallic electrodes with a discharge gap of a few millimeters, and an external high-voltage generator. The discharge gap is filled with either a rare gas or a rare gas-halogen mixture. The photons are emitted through the quartz wall, which is transparent to the generated radiation. An alternative high voltage of 7–10 kV and frequency of 50 Hz to several MHz is adequate to run the arc discharge between the electrodes. The charge buildup on the dielectric surface immediately decreases the field in the discharge gap and extinguishes the arc [24]. Excimer lamps are used for surface modification of polymers and textile substrates for improving in antimicrobial, antisoiling, antifelting, antistatic, wettability, adhesion strength, and cross-linking properties.
3.3.1 Excimer Laser
Lasers, which use noble gas for laser generation, are generally referred to as excimer lasers. They operate in the ultraviolet to near UV region, that is, from 193 to 351 nm. For instance, CO2 and Nd: YAG lasers are the most powerful, however, they are not suitable for modification of polymeric substrates because of their longer wavelength and lower photon energies, insufficient to break the molecular bonds of polymers. Excimer lasers, however, are capable of operating in the UV region and are strongly absorbed by the polymers for photochemical reaction. It is difficult to expose large areas of polymers in an efficient way due to the requirement of a large and quite expensive facility [64].
3.3.2 Excimer Lamps
The term excimer stands for “excited dimmer.” Excimers, which exist only in the excited state and under normal condition, do not possess a stable ground state. The excimer molecules are not very stable and once formed, within a few nanoseconds give up their excitation energy in the form of UV photons. UV lamps work on the dielectric barrier discharge principle and emit intense monochromatic light in the UV region of the spectrum. Organic materials, which have nonbonding electron pairs, strongly absorb light with a wavelength shorter than 200 nm. The penetration depth of a photon is approximately 10 nm. The bond dissociation power of the photon is different for the various excimer systems. For example, if xenon gas is used, it emits 172 nm light, which corresponds to photon energy of 7.2 eV that is capable of breaking polymeric bonds, such as C–H, –C=O, and –OH.
4 Environment-Friendly Textile Processing Using Plasma and UV
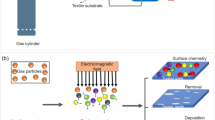
It is well known that during the wet chemical processing of textiles, industries consume a large quantity of water and generate a similar amount of effluent. The cost of final products also increases due to the multiple numbers of drying operations. Recently, due to the increase in environmental awareness and effluent norms, textile industries are slowly moving towards the implementation of environment-friendly low-water-based technologies, such as digital printing, spray, foam finishing, supercritical fluids, and solvent processing for the development of sustainable textiles. The industry is also using eco-friendly chemicals and agents such as natural dyes, enzymes, and plant extracts for textile processing and finishing, while avoiding usage of hazardous to nonhazardous chemicals to develop green/eco-textiles. In this context, cold plasma and UV treatment seem to be promising environmentally friendly technologies for the wet chemical processing of textiles. Plasma treatment of textiles modifies only the surface of the material without altering the bulk properties to increase the uptake of dyes and chemicals or to impart a unique functionality or both. Different value-added functionalities, such as water, stain and oil repellent, hydrophilic, antimicrobial, flame-retardant, UV-protective, antistatic properties, and improvements in dyeing, printing, biocompatibility, and adhesion can be accomplished by modifying the fiber surface at the nanometer level as shown in Fig. 1. The main advantages of plasma processing of textiles are (i) liquid-free dry single-step operation, (ii) requirement of a minimal amount of chemicals, (iii) cost-effectiveness in terms of time and temperature, (iv) imparted functionality independent of substrate chemistry, and (v) environmental friendliness. Low-pressure plasma has been extensively studied for such applications, but the process technology has not been commercialized in textiles due to its inherent technoeconomical limitation. On the other hand, atmospheric-pressure cold plasma can overcome the limitations of low-pressure plasma technology and is being explored for similar applications. Hence, it is becoming popular both in research as well as commercial applications in textile and allied sectors. Atmospheric pressure plasma is an emerging technology and several challenges associated with plasma generation for in situ plasma reactions with textile substrates are still not fully addressed for their inline integration with existing textile processes.
Surface modification with a desired functionality could be achieved by selecting the appropriate plasma process parameters along with a suitable precursor molecule. Fragmentation of a precursor followed by plasma reaction with textile substrates is the best way of surface engineering to develop value-added technical, apparel, smart, and home textiles. Similar to plasma processing of textiles, UV treatment can also be used for increasing surface area, hydrophilic property, antifelting, antistatic, dyeing, printing, and adhesion strength. In both processes, there is no requirement of any prior treatment, such as swelling the fibers in organic, aqueous, or alkaline solvents. The changes in properties induced by plasma and UV treatment are therefore restricted to the surface only. From the physical point of view, roughening of the fiber surface is responsible for changes in coefficient of friction, top cohesion, spinnability, yarn strength, and increase in antifelting of wool. From the chemical point of view, fiber surface oxidation and reaction with suitable precursor molecules are the main factors responsible for improving the various functional properties of textiles. The comparison of textile processing by conventional method, plasma treatment, and UV treatment is summarized in Table 1.
In comparison to UV treatment, plasma technology has an additional advantage of surface modification by in situ plasma coating, deposition, and polymerization. Plasma chemistry is a complex process that involves a large number of elementary homogeneous and heterogeneous reactions. The homogeneous reaction occurs between species in the gaseous phases and the heterogeneous reaction between the plasma species and solid surface. In UV treatment, UV excimers exist only in the excited dimer state and give up excitation energy in the form of UV photons (monochromatic light). The highly energized UV photon of shorter wavelength (<200 nm) could modify the fiber surface by approximately 10 nm. The energy of the photon depends upon the gases used to generate, for example, xenon gas (172 nm) has a photon energy of 7.2 eV and mercury (185.9 and 253.7 nm) also has similar energy capable of physical changes, such as etching, roughening, and increasing surface area and chemical changes, including breaking the chemical bond (C–H, –C=O, –OH) and generation of polar groups (C=O, –COOH, and NH2) depending upon the UV irradiation time and atmosphere used [4]. Different types of interaction of plasma and UV with substrates are discussed below.
-
(i)
Ion formation: Reactions due to the ion formation that would directly lead to a new chemical product, such as formation of NH3 and NO2.
-
(ii)
Recombination: When the rate of producing surface radicals is high and air is excluded, a tough cross-linked shell is formed that offers protection against solvent attack.
-
(iii)
Oxidation: In treatment by oxygen containing plasma/UV, surface excitation leads to the formation of polar groups, such as ketone, hydroxyl, ether, peroxide, and carboxylic acid that make the surface wettable.
-
(iv)
Peroxide formation: When a textile is exposed to argon (Ar) plasma followed by exposure to air, a high proportion of reactive sites is converted to peroxide form. Because peroxide is known to act as an initiator for vinyl polymerization, it is used for graft polymerization.
-
(v)
Radical formation: Carbon-free radicals are formed when the energetic ions/photons from the plasma/UV break the organic bonds of the polymeric substrate.
-
(vi)
Polymerization: Ionization of an organic monomer in the vapor phase in the plasma zone leads to a rapid in situ polymerization resulting in formation of thin pinhole-free films.
-
(vii)
Surface cleaning: A cleaning process in which argon (Ar), helium (He), and oxygen (O2) gases are used to ablate organic contaminants such as oil from the substrate surface.
5 Improvement on Liquid Absorbency
5.1 Improvement in Water Absorbency
5.1.1 Plasma Treatment of Textile
The effect of atmospheric pressure glow (APG) cold plasma in the improvement of the hydrophilic property in terms of water absorbency time was measured in nylon and polyester woven fabrics [53–55]. The samples were helium (He) plasma treated in an indigenously developed plasma reactor in a continuous manner. Water absorbency was measured according to the AATCC test 39-1971 method by putting a 37 µl distilled water droplet on the fabric. It was observed that a water droplet took 540 s to spread over an area of 3.79 cm2 in the untreated nylon sample, whereas it took only 1.1 s in the 60 s He-plasma–treated sample. In the polyester (PET) sample, the water absorbency time was found to reduce from 700 to 6.7 s in the untreated and 60 s He-plasma–treated samples, respectively. Absorption and spreading of water was very slow in the untreated sample because of the absence of polar groups in the polymer backbone. However, after plasma treatment, the surface energy of the samples increased significantly, probably due to the generation of hydrophilic groups. Surface energy of the as-procured nylon fabric was below 43.4 milliJ/m2 and after 60 s of plasma treatment, it was found to increase to 63 milliJ/m2 in the He, argon (Ar), or air–plasma treated samples. In the polyester sample, the surface energy was found to increase from 36 milliJ/m2 in the untreated sample to the maximum measurable value of 71 milliJ/m2 in the plasma-treated sample. The normalized peak intensity of –NH2 groups in the nylon sample increased significantly from 1.261 for the untreated sample to 1.409 and 1.428 for the 60 s air and He plasma-treated samples, respectively. In the air–plasma treated sample, there was a small increase in 1,633 cm−1 (–CONH–) peak as well. The small increase in the value of the normalized peak intensity for the –C=O group in the polyester sample could have happened due to the formation of more –COOH groups. The chemical changes in the plasma-treated nylon samples were found to be more profound than the plasma-treated polyester samples. The changes measured in ATR-FTIR analysis were marginal due to their low sensitivity to nanoscale analysis. Scanning electron microscope (SEM) and atomic force microscope (AFM) images of the plasma-treated samples showed significant improvement in surface area and surface capillaries, which might have helped in faster wetting and wicking of the samples. Therefore, it was concluded that the faster water absorbency in the plasma-treated samples was due to the cumulative action of nanosized channel’s (capillary action) formation and increase in surface energy due to the formation of polar groups. Samanta in [52] studied plasma treatment of polyamide 6 (nylon 6) woven textiles in a continuous manner for 10, 15, 30, and 60 s in helium (He) and a helium–oxygen (He–O2) mixture. The samples were exposed to air after the plasma treatment to allow the formation of various polar groups. In the untreated nylon sample, a water droplet of 37 µL did not get absorbed by the fabric even after 60 min (maximum measured time). After plasma treatment of 10 s, there was no improvement in the water absorbency time (Table 2). However, after 15 s of plasma treatment, there was an improvement in hydrophilic functionality. As a result of the same, the water absorbency time was found to reduce from >60 min in the untreated sample to 9.1 min in the He–O2 and 35.3 min in the He plasma-treated samples.
With increasing plasma treatment time to 30 s, the water absorbency time further reduced to 0.08 and 3.5 min for the He–O2 and He plasma-treated samples, respectively. The 60 s plasma-treated sample showed an excellent hydrophilic property with water absorbency time of 0.05 min (3.3 s) and 0.08 min (4.9 s) in the He–O2 and He plasma-treated samples, respectively, as shown in Fig. 2.
Conversion of hydrophobic nylon to hydrophilic upon He–O2 plasma treatment [52]
In the both 30 s He and He–O2 plasma-treated samples the water contact angle was found to be ~0°, whereas in the untreated sample it was as high as 130°. It was interesting to note that the He–O2 plasma treatment was more effective in improving the hydrophilic property compared to the He plasma treatment. This may be due to the formation of more oxygen-containing hydrophilic groups in the presence of oxygen in He–O2 plasma. Furthermore, it was observed that the imparted hydrophilic functionality in nylon fabric was durable to heat treatment at 70, 90, and 110 °C for a prolonged time and it can also be stored for more than 6 months. Similarly, it has been observed that the surface energy of the melt-blown nylon could be increased to 70 dynes/cm within 5 s of the glow plasma treatment. The imparted hydrophilic functionality was durable up to 6 months. Thereafter, surface energy reduced from 70 to 64 dynes/cm after 1 year of ageing in atmospheric condition [67].
The surface chemistry of both the untreated and plasma-treated samples were analyzed using X-ray photoelectron spectroscopy (XPS) to reveal the change in atomic percentage in different samples. In the untreated sample, the surface oxygen percentage was 18.6 % and it increased significantly to 21.8 % in the 60 s He plasma-treated and 29 % in the He–O2 plasma-treated samples. The increase in surface oxygen might have helped in formation of more oxygen-containing polar groups, such as ketone, hydroxyl, ether, peroxide, and carboxylic acid. In the plasma-treated samples, the surface nitrogen percentage was a little lower compared to the untreated sample. Surface molecules in both the untreated and plasma-treated samples were also analyzed using a secondary ion mass spectrometer (Model: MiniSIMS, Millbrook Company, UK). The untreated sample showed the presence of major fragments of nylon at different masses, such as 13 amu for CH−, 16 amu for O−, and 17 amu for OH−. In addition, other mass peaks were C− at 12 amu, CH2 − or N− at 14 amu, C–C− at 24 amu, and CH−C− at 25 amu, and CH−CH− at 26 amu. The presence of these masses in the untreated sample is an indication that during the SIMS analysis major bonds of nylon, such as \( - {\text{CH}}_{ 2} - {\text{CH}}_{ 2} , - {\text{CO}} - {\text{NH}} - \) and so on were broken down into smaller molecules and atoms, such as \( {\text{C}}^{ - } ,{\text{ CH}}^{ - } ,{\text{ CH}}_{ 2}^{ - } ,{\text{ N}}^{ - }, {\text{O}}^{ - } ,{\text{ HO}}^{ - } ,{\text{ C}} - {\text{C}}^{ - } ,^{{}} {\text{and C}} - {\text{CH}}^{ - } \). In the plasma-treated samples similar mass patterns were also observed. In the untreated sample, the peak intensity for OH− at 17 amu was smaller than the CH− peak at 13 amu. However, in the plasma-treated samples, the HO− peak intensity was more than that found with CH− at 13 amu. It was seen that in the untreated sample the O−/CH− ratio was 2.4 and it increased to 2.7 and 3.0 in the He and He–O2 plasma-treated samples, respectively. The O−/N− ratio in the untreated sample was 9.2 and it increased to 14.0 and 16.9 in the He and He–O2 plasma-treated samples, respectively. The result indicates that both plasma treatments followed by exposure of samples in air might have helped in formation of polar groups. The SEM of the untreated nylon sample showed smooth surface morphology. However, after treatment in He plasma very regular particulate type surface features were observed and these special features became even more dense when the sample was subjected to He–O2 plasma treatment. Such changes on the fiber surface might have resulted from plasma-enhanced surface etching. The changes in physical and chemical properties jointly have contributed to the improvement of the water absorbency of textiles.
Similarly the effect of He, He–O2 and He–maleic anhydride (He–MA) treatment on polyester fabric was used to study the effectiveness of different plasma for hydrophilic surface modification [45]. Different plasma parameters such as discharge voltage, frequency, and time of treatment were varied to study their individual effect on the hydrophilic property. During the plasma treatment, frequencies were kept at 19, 20, and 22 kHz and time of treatment was varied in the range of 0.5–5 min. It was seen that a water drop took 2,100 s to be fully absorbed by the hydrophobic polyester fabric. However, when the sample was He plasma-treated for 0.5 min, water absorbency time reduced to 478 s (V = 2.36, F = 19) and further to 145 s in the 5 min plasma-treated sample. With increasing He plasma treatment time, the water absorbency time also reduced. As discussed above for nylon samples, the He–O2 (He 1,500 ml/min, O2 350 ml/min) plasma-treated samples showed better absorbency time compared to the He plasma samples for the polyester samples also. In those samples, the water absorbency time was found to reduce from 252 s for the 0.5 min plasma-treated sample to 85 s for the 5 min plasma-treated sample. It was also seen that plasma treatment at lower frequency could impart better hydrophilic functionality. On the other hand, when the polyester fabric was first padded with maleic anhydride solution followed by plasma treatment in He at 19 kHz and 2.36 kV for 5 min, the sample showed minimum water absorbency time of 99 s. The imparted hydrophilic functionality was durable to storage time and number of washing cycles. Yet in an another study, the atmospheric pressure plasma with He–Ar or acetone–argon on wool and polyester (PET) fabrics were carried out and it was found that wettability increases with increasing plasma treatment time. Plasma treatment by He–Ar was found to have more effectiveness than the acetone–argon plasma [72]. Low-pressure plasma treatments using oxygen containing gaseous mixtures (Ar–O2 and He–O2) on polyester fabric, improved wettability significantly due to the formation of polar groups on the surface. The imparted hydrophilicity also depends on the fabric structure, such as a better result in a loose fabric structure compared to tightly woven fabric [20]. As indicated above, the atmospheric pressure air plasma treatment (300–1,000 W) of PET woven fabric improves surface energy and reduces the water contact angle and water wicking time. The weight of water absorbed by the capillary increased from 12 to 200 mg. The water contact angle on the plasma-treated PET sample decreased from 80 to 40° due to the chemical change of the fiber surface [14]. The wettability of polyester can be improved with a simultaneous decrease in soiling property by corona treatment in air. During plasma treatment, fabrics accumulate a negative charge and soils are also negatively charged and hence, they repel each other keeping the fabric free from soil. Plasma etching also increases the hydrophilic characteristic that also decreases soiling [75]. Atmospheric-pressure plasma treatment of polypropylene nonwovens with nitrogen and dry air resulted in surface activation and permanent hydrophilic functionalization of lightweight nonwovens. Similarly, with acetylene mixed with ammonia plasma (C2H2/NH3) treatment of textile improves wettability slightly [18].
The objectives of increasing hydrophilic features and reducing chemical waste from existing pretreatment processes for cotton fabrics were achieved by corona discharge in an air atmosphere [17]. It effectively increased the hydrophilic property without affecting the integrity of the fiber or yarn. The treatment causes chemical and physical changes in the waxy cuticle layer of the cotton without damaging the cellulose backbone. Pectinase enzyme treatment subsequent to air or Ar plasma treatment was applied in linen fabric preparation. Alkaline treatment was most effective in removing the hydrophobic outer layer of raw linen to give a wetting time of 10 ± 1 s. Argon–plasma-treated linen showed less wetting time (80 s) than the air–plasma treated one (100 s) indicating formation of polar species on the raw linen fabric. Air or argon–plasma pretreatment followed by Beisol PRO enzyme application can reduce the wetting times significantly to 20 and 16 s, respectively. Exposing the linen fabrics to either plasma or enzyme treatment causes a significant increase in the wetting rate and with a combination of argon–plasma treatment with Beisol PRO enzyme was the most effective next to alkaline scouring. Alkaline scoured, argon plasma treated, argon plasma followed by Beisol PRO enzyme treatment, or Sera-Zyme C-PE treatment was characterized by the highest total surface free energy values of 26.08, 26.40, 25.83, and 25.82 mJ/m2, respectively, compared to 21.89 mJ/m2 for untreated linen. Polyester/cotton (P/C) blended woven textile was treated with DBD plasma to improve hydrophilic properties using He–O2 gases [25]. It was found that plasma process parameters played a critical role in deciding the efficiency of the treatment, and at optimum conditions, the hydrophilicity in terms of vertical wicking was found to be higher than the untreated sample. Polyester has a hydrophobic surface as it is made up of ether oxygen (C–O–C) linkages, where the hydrophilic ester oxygen (C=O) linkages are facing towards the core of the fiber. When the sample was plasma treated, the ester oxygen (C=O) coming closer to the surface as a result of etching or formation of new C=O bonds, helped the sample to be more hydrophilic [35].
Atmospheric pressure plasma has also been used for surface modification of protein fiber, such as wool to improve wettability [44]. After the plasma treatment, the water absorption time decreased from >3,600 s in the untreated sample to <1 s in the helium–plasma treated sample. The change in wettability in protein fabric is likely because of the removal of a covalently bound fatty acid layer from the surface. This exposes the underlying hydrophilic protein material along with the generation of additional polar groups. As a result of this, there was an increase in the intensity of HO− and NH− groups in SIMS analysis. In the untreated sample, the intensity of the CH− peak was very high compared to HO− and NH2 − peaks probably due to the presence of a fatty acid layer on the wool cuticle. In the plasma-treated sample, the intensity of the CH− peak reduced significantly and intensities of HO− and NH2 − peaks increased profoundly. There was no detectable ageing effect of improved hydrophilic property after 9 weeks of storage and 30 wash cycles. Wool was also made highly hydrophilic by a different plasma treatment, as a result of the wetting time being reduced from 900 s in the untreated sample to less than 1 s in the plasma-treated sample [31]. The possible reason for this is the removal of the hydrophobic epicuticle of wool fiber and formation of cysteic acid from cystine. The improvement in the hydrophilic property and/or surface area/roughness was utilized for improvement in dyeing and antifelting of wool. The wettability in terms of water absorption time in the 5 min N2 plasma-treated fabric decreased from 180 to 1 s in the untreated sample. Similar results were also observed when the sample was plasma treated in O2 plasma. Upon plasma treatment, various physicochemical changes were observed in wool, such as (i) additional formation of –C=O, –OH, –COOH groups, (ii) slight improvement in crystallinity, and (iii) increase in surface area. It has been observed that on 5 min of O2 plasma treatment, the C percentage decreased by 12 % (XPS analysis). On the other hand, the N and O percentage increased by 1 and 48 %, respectively, due to formation of more –C=O, –OH, and –COOH groups on the surface. Similar to N2 plasma treatment, the reduction in sulphur (S) percentage was also noted in the O2 plasma-treated sample. In the O2 and N2 plasma-treated samples, the C/N decreased and the O/C ratio increased [30]. Plasma treatment was found to improve the wettability of degummed silk fabric. Similar to plasma treatment of wool as discussed above, 5 min O2 plasma-treated Tussar silk fabric showed a wicking height of 100 mm in ~5 min, whereas in the control silk the maximum height of 100 mm was observed after a prolonged time. In the Eri and Muga silk, the plasma treatment nearly doubled the wicking rate compared to the untreated silk. ATR-FTIR spectra showed an increase in β-sheet structure after 15 min of O2 plasma treatment and disappearance of the silk amide-II random coil band. However, the amide-II random coil band was observed if the characterization could be carried out within few hours of plasma treatment. It might be due to the formation of the β-sheet from amide-II because of surface ageing [76]. Unlike the hydrogen (H2) plasma treatment, the O/C and N/C atomic ratios improved slightly after O2 and N2 plasma treatment. The effect of NH3 plasma on silk has also been studied and was found to improve the N atomic percentage considerably.
5.1.2 UV Treatment of Textiles
Similar to plasma treatment of textiles, UV treatment has also been explored for surface modification of natural as well as synthetic textiles to improve hydrophilic functionality. The wool fabric surface was modified using a Xenon Excimer UV lamp, that has almost monochromatic light in VUV region (λ = 172 nm) with irradiation power of 50 mW/cm2 [4]. It can be seen from Fig. 3 that the untreated scoured wool fabric has a high wetting time of 280 s due to the presence of many hydrophobic molecules on the surface. However, similar to plasma treatment, after UV irradiation in an air atmosphere, the sample became highly hydrophilic. As result of this, wetting time reduced to 22.2 and 1.5 s in the 1 and 5 min treated samples, respectively.
Effect of UV excimer irradiation time on wetting of wool fabrics [4]
It can be seen from Fig. 3 that with increasing irradiation time, the wetting time initially decreases exponentially and later on linearly. The 15 min treated sample showed the lowest wetting time of 0.5 s. Similar trends were also observed when the samples were treated in the presence of O2 and N2 atmosphere. The wetting time in the nitrogen (N2) -treated sample was a little higher compared to air and O2 treated samples. This is because the presence of oxygen helped in formation of the polar group that assisted in wetting. It can be seen that 1–5 min of UV irradiation is sufficient to impart a high degree of hydrophilic functionality in wool fabrics. The improvement of the hydrophilic characteristic of wool fabric upon UV exposure is attributed to (i) removal/modification of the fatty lipid layer on the fiber surface, (ii) cleavage of disulphide protein linkages (–S–S–) to produce sulphonic acid groups (–SO3H) due to the presence of oxygen, and (iii) formation of oxygen-rich polar groups on pertinacious/lipid carbon sites. Wool fiber is characterized by the hydrophilic core protected by the fatty waxy hydrophobic thin film (0.9 nm) on the outer epicuticular layer. This layer does not get removed even after an alkaline scouring and to the contrary, acts as a barrier for penetration of water and dye molecules. UV excimer treatment reduces the wetting time by a factor of 10, even after 1 min of exposure, irrespective of the nature of atmosphere used.
Similar to wetting time, vertical wicking in the 1, 5, 10, and 15 min UV irradiated samples in air, O2 and N2 atmosphere was evaluated with a rectangular piece of fabric (100 mm × 20 mm). As wetting and wicking are the two interrelated phenomena, a liquid does not wick well until and unless it is truly wettable. It can be seen from Fig. 4 that the untreated sample has a very low rate of water wicking. However, upon 15 min of UV irradiation, the wicking rate increased significantly. It was found that irradiation beyond 15 min does not show further improvement in wicking. The irradiation in a nitrogen atmosphere showed the best result compared to the air- and oxygen-treated samples. It may be due to the formation of more striations in the N2 atmosphere. The observed wicking behavior can be explained with reference to the capillary action of water, which is defined as the upward movement of water against the gravitational force within the spaces of a porous material. The irradiation of wool by UV damages the fatty layer and develops hydrophilic groups on the surface. This phenomenon contributed positively to the adhesive forces between the fiber and water molecules, leading to an increase in wickability [62]. In addition, the effective striations present in the UV-treated wool fabric might have increased resulting in increasing the wickability.
Vertical wicking in the untreated and 15 min irradiated wool fabrics [3]
The UV-treated wool fabric took only 10 s to travel a height of 2 cm irrespective of atmosphere used in comparison to 2 min required in the untreated sample [6]. The high-energy UV photon etched the wool fabric surface, broke its waxy smooth scales, and created a capillary as observed under the SEM. Oxygen incorporation in the treated fabric surface by the ozone mechanism was proposed by Basak in [3]. Surface oxidation of disulphide protein linkages (–S–S–) of wool produced sulphonic acid (–SO3H), removed the surface fatty waxy layer, and formed extra polar groups, such as C=O, –COOH, and –NH2 [6, 15]. All these factors together helped in better wetting and wicking. Periyasamy et al. in [46] reported that 172 nm UV excimer treatment of silk fabrics could improve the wetting and wicking properties. It might be due to the increased surface roughness and formation of nano pores in the treated fabric. The wettability of polyester fabric was improved using a UV excimer lamp, emitting mostly monochromatic light of 172 nm with an irradiation power of 50 mW/cm2. The samples were irradiated for 1, 3, 5, and 10 min keeping 5 mm distance from the lamp. The high-energy photon (7.2 eV) is capable of breaking even a C=C bond, leading to the generation of free radicals. The polyester samples previously equilibrated with the surrounding atmospheric gases (air) contain a quantity of dissolved oxygen, particularly on the superficial layer. The diffused or ambient oxygen quickly reacts with these free radicals producing oxidized molecules, such as ketone, carboxylic acid, alcohol, and peroxide species. In the UV-treated hydrophilic polyester fabric, the wetting time was significantly reduced from 6.7 (untreated) to 3.1 s and wicking time from 100 (untreated) to 65 s. The effect intensifies with an increase in irradiation time. The improvement in wetting and wicking is attributed to the creation of oxygen-rich species and submicron-level surface roughness. There was no significant change in the crystallinity and tensile strength as UV treatment only renders change in the surface of the fibers.
5.2 Improvement in Oil Absorbency
The atmospheric pressure glow plasma was generated in the presence of helium (He), argon (Ar), oxygen (O2), and air gases to improve oil absorbency in the various textile substrates [54]. A drop of 37 µl of mustard oil was placed on the fabric, and the time was measured to spread over an area of 3.79 cm2. For cotton, three oil drops were placed simultaneously, as one drop of oil was found insufficient to spread over the specified area. In all the plasma-treated samples, there was a significant improvement in oil absorbency. In the nylon sample case, the oil spreading time decreased from 152 to 52 s in the untreated to 60 s He plasma-treated samples. Similar results were also found when the samples were plasma treated in the presence of Ar, O2, and Air gases. Air plasma-treated samples took slightly more time (75 s) compared to the helium plasma-treated sample (52 s). Plasma treatment helped to reduce the oil spreading time approximately by half to one third compared to the untreated sample. Similar to nylon, the oil spreading time in polyester woven fabrics was found to decrease from 28.6 min in the untreated sample to 2.8 min in the 60 s He plasma-treated sample. It was interesting to note that in the plasma-treated sample, the rate of oil absorption and spreading was more, although the sample turned from hydrophobic to hydrophilic. Furthermore, even on the highly hydrophilic cotton textile, oil (hydrophobic liquid) spreading time decreased from 59.5 s in the untreated sample to 30.4 s in the 60 s He plasma-treated sample. Similar results were also observed in air, Ar, and O2 plasma-treated samples. The SEM pictures at a magnification of 35 KX revealed the surface features of <100 nm due to the formation of hills and valleys. The AFM micrograph of the untreated samples over an area of 4 µm × 4 µm (Z-axis 500 nm/div) appeared to have a smooth surface. After the plasma treatment, vertical channel-like features with dimensions of <200 nm were easily visible in the nylon sample due to the bombardment of high-energy plasma species. In the plasma-treated polyester sample, horizontal channel-like features of ~100 nm in height and separated from each other by about 350 nm in the horizontal direction were easily visible (Z-axis 200 nm/div). In both the plasma-treated samples, nanosized horizontal and vertical channels were uniformly distributed over the entire surface area. The formation of such channels upon plasma treatment increased the effective capillary radius resulting in better fluid spreading. In the untreated cotton textile, better oil absorption was due to the presence of a textured and convoluted structure, and on plasma treatment these features might have enhanced, resulting in further improvement in oil absorbency. Cotton has more surface energy than the oil, hence oil could spread easily. Upon plasma treatment, the surface energy might have enhanced further. Also formation of pores, microcracks, and an increase in surface roughness in the plasma-treated cotton might have also helped in better fluid transport. Improvement in oil absorbency of textile substrates would have possible applications in cleaning of surfaces contaminated with oil in the metal industry, household cleansers, and cosmetics.
The UV eximer has also been used similarly to the plasma treatment of textiles. It was found that oil spreading time was much lower in the untreated wool fabric than the water spread time [3]. This is because for spreading of any liquid on a solid surface, the surface energy of the solid surface must be equal or more than the surface energy of the liquid. Due to the presence of a hydrophobic fatty layer on the wool fiber surface, it has low surface energy, similar to surface energy of oil. As a result, oil could spread easily in the untreated sample. However, water has a surface energy of 71 dynes/cm, which is much more than the surface energy of wool, and as a result of this, water did not get absorbed/spread by the untreated sample. After UV irradiation, surface energy of the wool fabrics increased due to the generation of polar groups and increased in surface roughness resulting in faster spreading of oil and water also (as discussed above). The oil spreading time decreased from 2.7 s in the untreated sample to 0.5 s in the 15 min UV irradiated samples. With increasing irradiation time, the oil spreading time was found to decrease further. Air and O2 irradiated samples showed similar results, however, the N2-treated sample showed a slightly better result.
6 Improvement in Dyeing
6.1 Textile Coloration Using Plasma
Plasma treatment has been utilized for surface modification of cotton, ramie, wool, silk, and like fibers for improvement in their coloration. The improvement in the hydrophilic property (chemical changes) and increase in surface area/roughness (physical changes) were utilized in faster dye exhaustion and/or better K/S value, in addition to reduction in dyeing time and temperature. The DBD plasma treatment of cotton, wool, and polypropylene was found to change the hydrophobic character into hydrophilic. Specific surface area was found to increase significantly from 0.1 to 0.35 m2/g in the cotton fabrics, resulting in increase in dye uptake [19]. Air and dichlorodifluoromethane (DCFM) plasma treatment on cotton fabrics led to improvement in dyeability with reactive and natural dyes. However, there was a small decrease in dyeability with direct dye [7]. With the use of dichloromethane with RF plasma (10 Pa) for 10–45 s on cotton and polyester fabrics, the dyeability with reactive dyes could be enhanced without affecting other properties [22]. Low-pressure plasma polymerization was carried out for 5–30 min on cotton fabric in amine ethylenediamine or triethylenetetramine (TETA) [43]. The treated fabrics were dyed with a reactive Remazol Black B dye. The maximum improvement in color value was 33.9 % compared to the untreated sample. This improvement in dyeing was due to the creation of chemical groups that were suitable for dye exhaustion and reaction. In the plasma-modified dyed samples, the rubbing fastness was very good for higher shade depths. Plasma treatment of cotton followed by TETA application could also be dyed with acid dyes with better color yield due to the formation of new amine groups as confirmed by FTIR analysis. Sun and Stylios in [66] reported an increase in reactive dyeing rate in the plasma-treated samples compared to the untreated cotton sample, which was visible after 40 min of dyeing time. The exhaustion of dye was possibly due to the formation of holes during O2 plasma treatment that provided a new pathway of dye penetration. Dyeing of plasma pretreated cotton woven fabrics showed deeper and brighter shades [41]. Plasma treatment in the presence of air or oxygen increased both the rate of dyeing and direct dye uptake in the absence of electrolyte in the dye bath (Chloramine Fast Red K) [65]. The oxygen plasma treatment was found to be more effective for textile coloration than the air plasma treatment. The increase in dye uptake in the cotton fabric was due to the cumulative effect of (i) the oxidative attack of cotton fiber that modifies the surface properties, (ii) change in fabric surface area per unit volume caused by surface erosion, (iii) the etching effect of fiber by plasma and removal of impurities such as wax, or any remaining size material, (iv) chemical changes in cotton fiber leading to the formation of carbonyl and carboxyl groups, and (v) the possibility of the formation of free radicals on the fiber surface [13]. When the nylon 6 fabrics were treated with cold plasma using nonpolymerizing gases, such as oxygen, argon, and tetrafluoromethane, improvement in dyeability with basic dyes was noticed due to etching of fiber and introduction of polar groups. When different monomer coatings were applied to polyester, polyamide, and polypropylene (PP) fabrics, it improved the affinity to other classes of dyes [68]. Low-temperature plasma treatment of grey, mercerized cotton and polyester/cotton blended fabrics before dyeing has been reported to be effective for improvement in dyeing. Silicon tetrachloride hydrophilic plasma coating on PET fabric was found to increase in surface roughness resulting in improvement of PET dyeing [34]. Plasma polymerization of acrylic-like coatings on polyester and polyamide fabrics was found to enhance wettability and dyeability with basic dyes and also improved soil-resistance properties [10]. The color depth of the dyed fabric (polyester/cotton blend) increased with the acrylic-like film thickness. The surface modification of PP fabrics by acrylonitrile cold plasma to deposit poly-acrylonitrile–like layers was reported to improve in water absorption and dyeing properties [59]. This was due to the presence of nitrogen- and carbon-based unsaturated linkages and the formation of secondary generation =C=O groups. Dyeing of polyamide fabrics was carried out after surface modification with DBD plasma (power –0.5–2.5 kW/min/m) [63], and it was found that the change from hydrophobic to hydrophilic was the key point for adsorption of aqueous dye solutions to achieve excellent dye uptake, a high rate of dyeing, good uniformity, and good fastness for darker shades using less concentration of dyestuffs at a lower time and temperature.
Protein fibers such as wool and silk were plasma treated in the presence of various gases and their effects on the rate of dye exhaustion, final dye uptake, K/S, and fixation have been reported by several research groups. Wool fiber was plasma treated using various nonpolymerizing gases to improve the hydrophilic properties in terms of water absorbency and surface roughness as discussed in Sect. 5.1. The improvement in water absorbency time might have enhanced the uptake of an anionic dye by 5 % at equilibrium compared to the control sample, when the dyeing was carried out at 70 °C. The improvement in dye uptake in the plasma-treated sample was mainly due to (i) partial destruction of the outer epicuticle layer of wool fiber, (ii) reduction of covalently bond fatty acid (18 methyl esacanoic acid) layer on the upper epicuticle, (iii) cystine (S–S) linkage reduced to cysteic acid (–SO3H), and (iv) formation of an additional NH2 group. XPS analysis showed that after 5 min N2 plasma treatment, the carbon (C) percentage decreased by 8.5 % due to partial etching and removal of the long carbon hydrophobic fatty acid chain from the fiber surface. As expected, the N and O percentages in the treated samples increased by 16 and 39 %, respectively. It might be noted that the increase in N2 percentage promotes the formation of additional NH2 groups. The surface oxygen percentage increased due to surface oxidation and the same was confirmed by the reduction in sulphur (S) atomic percentage from 2.58 to 2.23 with a shift in sulphur atomic peak from 163 to 168 eV binding energy in the untreated to the plasma-treated samples, respectively [77]. Electrospin resonance (ESR) results showed the increase in the area under the curve by 18.2 times with G factor 2.007 in the plasma-treated sample due to the formation of more nitrogen-centered free radicals [38]. Similar results were also observed when the wool fabric was plasma treated in the presence of O2 plasma. Two more physicochemical changes were observed, such as (i) additional formation of –C=O, –OH, and –COOH groups and (ii) a slight improvement in crystallinity. Plasma treatment enhanced the surface etching leading to an increase in surface area from 0.1 to 0.35 m2/gm in the untreated to treated samples, respectively [19]. Sun and Stylios in [66] reported that plasma treatment could increase in scouring efficiency and dyeing rate by nearly 50 %. Improved dye uptake of the wool fabric as well as the depth of color irrespective of plasma gases used were reported by Ratnapandian et al. in [50]. In a similar study, wool fabric was treated for 5 min in O2 plasma and dyed with acid, chrome, and reactive dyes at 50 °C with pH of 4.5. It was observed that in the case of acid, chrome, and reactive dyes, after 1 h of dyeing, the dye uptake increased by 72.6, 6.5, and 39.4 %, respectively. The result showed that the plasma treatment helped in faster dye exhaustion. However, after 2 h of dyeing at equilibrium, the total dye uptake increased by only 2 % compared to the untreated fabric for the acid- and the chrome-dyed fabric. On the other hand, 25 % more dye uptake was found for the reactive dyed sample at equilibrium. It might be possibly due to the formation of oxygen containing functional groups that could react with dye molecules. However, plasma etching and new dye site generation was not sufficient for the improvement of acid and chrome dyes at equilibrium [27]. According to Rombaldoni et al. [51], if O2 plasma could be used for 5 min at 30 W for surface modification of wool followed by dyeing using a combination of 1:2 metal complex dyes at 98, 85, and 80 °C at a neutral pH, the final bath exhaustion of the plasma-treated sample could be comparable with the control sample dyed at 85 °C. The effect of atmospheric pressure on He plasma treatment for 6 min on wool fiber was studied using dichlorotriazine-based reactive dye (C.I. Reactive Red 2) at 35 °C by the pad batch method [44]. Plasma treatment resulted in significant improvement of color yield. Both the dye pick-up and the fixation of dye on wool fabric increased almost twice based on K/S values after plasma treatment of the samples. The liquid expression was found to increase by approximately 40–50 % in the plasma-treated sample resulting in high concentration of dye being applied on the fiber. Even after the removal of unfixed dye, the K/S values were found to increase by almost 100 % (1.5–2.5 for the untreated sample and 3.7–4.6 for the plasma-treated samples). The percentage of fixation was in the range of 80 ± 5 % for both the untreated and plasma-treated wool. The SIMS analysis showed an increase in the intensity of HO− and NH− groups, whereas the intensity of the CH− peak decreased after the plasma treatment. This is an indication of formation of more numbers of –NH2 groups resulting in better dye exhaustion and fixation.
Similar to wool, in Eri and Muga silk, the plasma treatment nearly doubled the wicking rate compared to the untreated silk [49]. After 30 min of low-pressure plasma treatment in the presence of O2, N2, and H2 atmosphere, the coefficient of friction increased to 0.7–0.8 from 0.27 for the untreated sample. It was observed that after 10 and 30 min of O2 plasma treatments, there was 13 and 22 % loss of crystallinity. Unlike hydrogen (H2) plasma treatment, the O/C and N/C atomic ratios improved slightly in the O2 and N2 plasma treated samples. The effect of NH3 plasma treatment on silk fabric has also been studied and found to improve the N atomic percentage considerably. However, improvement in reactive dye uptake was not so promising. Plasma treatment improved the dyeability and color strength of the silk fabric irrespective of gases used for treatment. Degummed silk fabrics were plasma treated in O2, N2, and H2 atmospheres for 5 min and dyed with Ramazol reactive dye at 50 °C for 90 min. The K/S value of the treated fabrics improved significantly compared to the control fabric. The 5 min plasma-treated dyed fabric at 6 % shade exhibited the equal color strength of the 10 % dyed control sample. This might be because the plasma treatment helped in the formation of more active sites required for dyeing [21].
6.2 Textile Coloration Using UV
Wool is one of the important natural protein fibers and it has 1 % mass nonprotein material and 25–75 % impurities, such as grease, perspiration suint, dirt, and vegetable matter. Clean wool contains 82 % keratinous proteins with 18 different types of amino acids. Amino acids, such as glycine, alanine, phenyl alanine, valine, leucine, and isoleucine, are hydrophobic in nature, whereas serine, threonine, and tyrosine are hydrophilic in nature. The wool surface also has a hydrophobic characteristic due to the presence of a large number of disulphide cystine crosslinks in the exocuticle. Fatty acids are covalently bound to the protein by means of ester or thioester bonds. These chains are oriented away from the fiber to produce a polyethylene-like layer on the fiber surface, thus making the epicuticle hydrophobic. For this reason, wool exhibits a hydrophobic (water-repellent) characteristic even after the removal of wool grease by a scouring process or solvent extraction. Together, these act as a barrier against penetration of dye molecules and other chemicals in the fiber structure. Low-temperature plasma (LTP) has been explored for removal of this hydrophobic layer and improvement in dyeing properties as discussed above. UV pretreatment has also been used for similar surface modification and improvement in dyeing as discussed below.
6.2.1 Dyeing with Acid Dye
Effect of Dyeing Time
Wool fabrics were dyed with acid dye (Navimill yellow 56 N) with 2 % shade keeping the dye bath at 60 °C, material-to-liquor ratio of 1:40, pH at 5. and acetic acid at 0.5 g/l [3]. The dye exhaustion was measured by sampling the dye bath concentration after 20, 30, 60, 90, and 120 min of dyeing. Absorbance of the dye solution was measured in UV-Vis spectrophotometer at 425 nm (λ max). Figure 5 shows the dye exhaustion percentage corresponding to UV irradiation time for the different samples. It can be seen that the dye has a high affinity for wool as the exhaustion value in the untreated sample is quite high. However, after UV irradiation, the dye uptake (exhaustion) increased significantly. After 20 min of dyeing, the exhaustion percentage increased from 72.4 % in the untreated sample to 89.1 % in the 1 min UV-treated sample in nitrogen atmosphere with 16.7 % more dye uptake. With increasing UV irradiation time, the dye exhaustion percentage increased further to 97.9 % in the 15 min irradiated sample. In the untreated sample, dye uptake slowly increased with dyeing cycle time due to the availability of dye sites. In contrast in the UV-irradiated samples, the exhaustion percentage reached the maximum value within 20 min of dyeing cycle.
Effect of UV irradiation time on dye uptake of wool fabric in N2 atmosphere [3]
Further dyeing had happened at equilibrium condition except for the 1 min treated sample. It was interesting to observe that in the untreated sample, the dye uptake after 120 min of dyeing was 85.2 %, whereas the dye uptake was in the range of 89.1–97.9 % in 1, 5, and 15 min irradiated samples. This signifies that in the UV-irradiated samples, the dyeing cycle could be reduced by 100 min to achieve a similar uptake. This will help in saving a significant amount of energy in the textile coloration process.
It can be seen from Fig. 6 that with increasing dyeing time, the K/S value increases, mainly due to the increase in dye uptake. It can also be seen that with increased UV irradiation time, K/S also increases. The 15 min–irradiated sample in the presence of N2 atmosphere showed a darker shade compared to 5 and 1 min–treated samples [4]. This improvement in K/S value is mainly because of higher dye exhaustion in the fiber structure with increasing irradiation time. The increase in dye uptake was also attributed to the decrease in number of disulphide (S–S) linkages in keratin that act as an inhibiter for fiber welling and dye uptake. The other reasons for higher dye uptake are the destruction of the lipid surface barrier film and formation of more amine groups, in addition to an increase in surface roughness, which helps faster penetration of dye molecules. Upon UV exposure, degradation of cystine linkages leads to the formation of cysteic acid and some other intermediate products of cystine oxidation such as cystine monoxide and cystine dioxide were also formed. It provides a suitable site for introducing agents, such as dyes carrying a reactive group [33]. However, oxygen-rich groups might have been introduced at lipid carbon sites as well [9]. It was observed that in the 10 min UV-treated sample, the surface oxygen percentage increased to 27.5 from 10–12 % in the untreated sample. As discussed above, it might be due to the oxidation of disulphide sulphur (S–S) in the control sample to sulphonic acid (SO3H). The untreated wool fabric showed major peaks at 285, 286.5, and 288 eV due to the presence of C–C/C–H bonds in the lipid layer; C–N, C–S, C–O groups and N=C=O of cystine groups, respectively. On the other hand, in the 9 min UV-treated wool fabric the unresolved peaks were observed at 287 and 289 eV due to the presence of the N–C–O group and carboxylic acid or ester group. These unresolved peaks are generated in the treated sample as a consequence of surface oxidation by high-energy UV photons [8, 74]. The increase in amine groups and different oxygen-containing polar groups, in addition to the removal of the hydrophobic fatty acid layer and decrease in disulphide linkage cumulatively helped in rapid dye exhaustion even at a lower temperature. The wool fabric surface was modified using 253.7 nm UV irradiation for 40 and 60 min [74]. The XPS showed the sulphonate peak at 168.2–168.7 eV increased at the expense of the disulphide (S–S) peak at 163.6–164.0 eV upon UV irradiation due to surface oxidation of cystine. Dyeing of the samples was done at 45, 50, 55, and 60 °C using CI Acid Blue 7, and it was found that the dye uptake in the treated samples was always greater than the untreated sample. As discussed above, the reason for the increase in dye uptake was due to the decrease in the number of disulphides (S–S) in the keratin that acts as a crosslinking barrier near the fiber surface. The UV treatment could improve the dye uptake of CI Acid Blue 7 significantly by 30 and 15 % even at lower dyeing temperatures of 45 and 50 °C, respectively, and at short dyeing times of 30 min or less. The UV-treated and untreated samples had almost the identical adsorption isotherms as the number of free amino groups on wool remained unchanged, leaving the absorption properties unaffected. The diffusion coefficient of the UV-treated fibers was three times over the untreated fibers. The K/S value of the untreated fiber was 16.1 and it increased to 23.6 in the treated sample after 50 min of dyeing.
Effect of UV irradiation time on K/S value of the wool fabric in N2 atmosphere [5]
Effect of Atmosphere
Figure 7 shows the effect of different gaseous environments on exhaustion of dye in the untreated and the 15 min UV-irradiated wool samples [5]. It can be seen that in all the irradiated samples, the dye uptake was much more compared to the untreated samples. The dye uptake was only 72.7 % in the untreated sample and it increased to 91.3, 91.3, and 97.9 % in the air, O2, and N2 plasma-treated samples, respectively, after 20 min of dyeing. The sample treated in the N2 environment showed a better result compared to the other gases. However, at the end of the dyeing cycle (after 120 min) all the irradiated samples showed a similar level of exhaustion. The improvement in dye uptake was highest in the N2-irradiated sample and the lowest in the O2-irradiated sample. The air-irradiated sample showed an intermediate result, as a major component of air is nitrogen only. When the samples were treated in the presence of oxygen and air atmosphere, the improvement in the K/S values were similar and were a little lower compared to the nitrogen-treated samples. This is because the dyeing of wool using acid dye depends on the number of amine groups present in the fiber structure. Possibly, the surface modification in nitrogen has helped in the formation of more amine groups that act as new dye sites. Similar findings have also been reported in other literature [70, 71].
Effect of different gaseous environments on dye exhaustion [4]
On the other hand, in the O2-treated sample, the oxygen containing functional groups, such as C–O, C=O, O–C=O, and COOH were formed predominantly over amine groups, resulting in a lower dye uptake. Although these polar groups help in wetting and wicking, they do not have an active role in dyeing. Dye uptake represents the amount of dye taken up by the fibers from the solution, whereas diffusion represents the migration of dyes from the surface to the core of the fiber. The diffusion coefficient (DC) is related to the bulk property and was calculated using the Hills equation. It was found that the DC increased from 0.11 in the treated sample to 0.21–0.35 in the different plasma-treated samples. It was slightly higher in the O2-treated sample than N2 and air-treated samples. This may be due to a significant increase in the O/C atomic ratio after the UV treatment in O2 atmosphere.
The SEM micrographs of the untreated sample showed a well-defined contour of scales and seem to have a smoother surface morphology. On the other hand, the treated fibers’ surface appeared to be relatively less waxy irrespective of the gaseous environment used. Surface morphology of the treated samples in nitrogen atmosphere appeared to have a distinct feature compared to other irradiated samples. The typical scale geometry of wool was not visible and there were deep striations on the surface. Micropores were visible when the samples were exposed to O2 atmosphere. Appearance of such surface features was due to the etching of the UV photon. These new features in the treated samples might have also helped in increasing surface area and a new pathway of dye molecule penetration.
6.2.2 Dyeing with Reactive Dye
The UV-irradiated samples were also dyed using reactive dyes in air atmosphere. It was observed that after UV irradiation, the K/S value significantly improved from 12.8 in the untreated sample to 25.8 in the treated sample. The effect of UV treatment in the different gaseous environments for Ramazol Black B reactive dye has also been reported [5]. The rate of dyeing or the dye uptake for the air, O2, and N2 treated wool fabrics were faster than that of the untreated sample. After 120 min of dyeing, the dye exhaustion was 85–89 % in the different UV-treated samples, whereas it was only 55 % in the untreated sample. As expected, with increasing dyeing time, the dye exhaustion was found to increase linearly. The UV treatment appears to cause etching associated with chemical modification and formation of C–O, C=O, O–C=O, C–O–O, and –OH groups that are responsible for enhanced dye uptake and dye–fiber interaction [36]. In a N2 atmosphere, additional formation of –NH2 groups also acted in favor of dyeing with anionic reactive dyes. It was observed that the loss in color strength upon washing was negligible in the both the untreated and treated samples. Excellent dye fixation was observed for all the treated samples (air, N2, and O2) and it was found satisfactory throughout the dyeing cycles. The additional dye taken by the fiber was covalently bonded to the protein molecule of wool rather than being deposited on the surface.
6.2.3 Dyeing with Basic Dye
The effect of UV irradiation in air, O2, and N2 atmosphere on wool fabric for 15 min on coloration using basic (cationic) dye (Methylene Blue) is plotted in Fig. 8. As expected, the dye exhaustion trend is different in comparison to dyeing with acid and reactive dyes. It can be seen that the rate of dye exhaustion and the total dye uptake by the irradiated samples in air and oxygen atmospheres were higher than the untreated sample. The sample treated in a N2 atmosphere took less dye compared to the untreated sample. This implies that the exposure of the sample in nitrogen environment leads to formation of cationic amino groups that repel the positively charged basic dye molecules. Higher dye uptake in air- and O2-treated samples was due to the formation of anionic groups that attracted the positive dye molecules.
Dye uptake in the untreated and UV-irradiated samples in different atmospheres [3]
The UV pretreatment for 10 min in air atmosphere followed by grafting with acrylic acid was found to improve the basic dye uptake in microdenier polyester fabrics [16]. The depth of shade on microfibers, otherwise very difficult to dye, increased with the irradiation time. The untreated sample was nearly colorless and the 10 min UV-treated and -dyed samples showed a deep blue shade (K/S = 11.51). The negative charge on the fiber surface attracts the positively charged dye molecules, which then get attached to the fiber by forming ionic bonds. Shade depth and K/S value were found to increase gradually with increasing UV irradiation time possibly due to the formation of free radicals on the fiber surface, etching of the fiber surface, and formation of more anionic polar –COOH groups. A similar study using UV excimer laser treatment on polyester microfibers followed by dyeing with disperse dyes (Dispersol Red DB and Dispersol C2R) has been reported [73]. Laser irradiation at 248 nm with 1 Hz repetition rate and laser facility of 70 mJ/cm2 developed ripple-like structures and also enhanced the surface area. Initial faster dyeing rate and dye uptake in the treated samples (7 % more at equilibrium) indicated that the dye molecules could diffuse relatively faster and there was more availability of dye sites. The enhancement in surface areas upon laser treatment would have also facilitated swelling of the fiber that might have helped in better dye diffusion. Due to the surface physical changes, the treated samples gave lower spectral reflectance implying deeper dyeing.
7 Antistatic Finishing of Textiles
In the past, significant effort has been made to design suitable personal protective clothing to ensure workers’ health, hygiene, and safety in hazardous work environments. In this regard, protection from static electricity is an important aspect, as it causes accidental ignition and fire in the paper, oil, gas, and plastic industries. The static charge buildup also adversely affects a garment’s fall by clinging to the wearer’s body and increases soil and lint pickup by the fabric. The term “static electricity” refers to the phenomena associated with accumulation of electrical charge in contact with and/or rubbing of the two objects. Synthetic fibers, such as polyester and nylon as well as natural fibers, such as wool do not have any conducting/wet layer on the fiber surface due to their hydrophobic character resulting in generation and accumulation of static charge. Many physical and chemical formulations for antistatic finishing of textiles have been developed, such as (i) grafting of the fiber surface, (ii) modifying the polymer (fiber) by copolymerization with a suitable monomer, (iii) use of metal particles and metal coatings on textiles, (iv) chemical finishes of fiber/fabric, and (v) and use of metallic fiber in the yarn or fabric structures [1, 12, 23]. These processes are water and energy intensive and cause environmental pollution. In contrast, cold plasma or UV eximer lamps can effectively be used for environmentally friendly antistatic finishing of textiles.
It was observed that after the plasma treatment both nylon and polyester fabrics became antistatic. The untreated nylon produced a 2.8 kV static charge and after 60 s the He plasma treatment sample produced only 2.1 kV static charge, that is, a 25 % reduction in static charge development as shown in Table 3. The static charge and half-decay (½) were measured using a STATIC HONESTMETER (Type H-0110-B, Shishido Electrostatic Ltd., Japan). In the air plasma-treated nylon samples, the reduction in development of the static charge was much more noticeable [56]. The 60 s air plasma-treated sample produced only 1.5 kV static charge, resulting in a 46 % reduction. It can be seen that with increasing plasma treatment time, the antistatic properties also improved. The antistatic performance of the samples could be measured by two methods, namely development of static charge and half-decay time. Half-decay time is the time that is required to dissipate half magnitude of the developed static charge. In the plasma-treated samples, the half-decay time (½ decay) decreased exponentially with plasma treatment time from 8.9 s in the untreated sample to 2.8 and 4.6 s in the 10 s air and He plasma-treated samples, respectively. After 60 s He-plasma treatment, ½ decay time was as low as 1.1 s, amounting to a reduction of ~88 % and in the air–plasma treated sample, it was lowest at 0.63 s, that is, a 93 % reduction. Polyester is more susceptible to charge buildup due to the absence of polar groups in the polymer backbone and low moisture regain (0.4 %). In such a sample, the developed static charge decreased from 1.53 kV in the untreated sample to 1.42 kV in the 60 s He–plasma treated sample. The half-decay time also was found to reduce significantly from 107 to 19.8 s in the untreated to plasma-treated samples, respectively. Air plasma could impart a better antistatic property compared to He–plasma. The improvement in antistatic property was due to the improvement in hydrophilic property, and increase in surface energy and surface area. The surface energy of the nylon fabric was found to increase from 49.5 to 63 mJ/m2 in the untreated to plasma-treated samples, respectively. In polyester samples, it increased from 55 to >71 mJ/m2. This happened due to the formation of various oxygen-containing hydrophilic groups as discussed in Sect. 5.1. It was also observed that in both plasma-treated nylon and polyester fabrics, there was formation of nanosized channels (SEM and AFM analysis). Due to the increase in the surface energy and the formation of nanosized channels, water spreading time decreased significantly as discussed in Sect. 5.1. AFM micrographs showed the plasma induced horizontal and vertical nanosized channels with dimensions of <100–200 nm. Due to the formation of such channel-like features in the treated samples, the surface area increased from 16.27 to 19.27 µm2 in the nylon (18.3 % increase) and from 16.01 to 17.18 µm2 in the polyester (7.3 % increase) compared to their respective untreated samples. The increase in surface area provided more area for charge dissipation resulting in lower ½ decay time (Table 3).
Low-temperature oxygen plasma (LTP) was employed on polyester fabric to improve the moisture content and the rate of static charge dissipation [32]. The effects of discharge power, treatment time, and system pressure were evaluated in this study. The optimum condition for improving the antistatic property was discharge power of 200 W, system pressure of 25 Pa, and 3 min of plasma treatment. The untreated polyester showed half-decay time of 1675.5 s and after plasma treatment it significantly reduced to 286 s, which is a bit more than commercially antistatic finished fabric (157.5 s). After plasma treatment, the moisture content of the sample improved to 5 % from 1 % in the untreated sample, whereas it was 1.41 % for the commercial finished sample. The SEM images showed the untreated sample had a smooth surface, and after plasma treatment, the surface became rougher (plasma etching) allowing moisture capture from air. The improvement in moisture content and antistatic property were associated with an increase in O/C ratio from 0.29 in the untreated sample to 0.58 in the plasma-treated sample due to the generation of –OH and –COOH species. In a similar study, it was observed that with increasing plasma discharge power, the half-life decay time decreased linearly [28]. In higher plasma discharge power, the ions and active species of plasma interact actively with textiles, resulting in better antistatic, physical, and chemical properties. With decreasing plasma pressure and treatment time, the half-life decay time increased, thus adversely affecting the antistatic property due to the smaller amount of interactions between the plasma species and textile. Similarly, plasma treatment of O2, N2, and a mixture of hydrogen–nitrogen (25 % H and 75 % N2) on wool was carried out to impart an antistatic property [31]. The samples were treated in low-pressure glow plasma for 5 min and it was found that surface resistivity decreased from 263.25 × 1011 Ω to 182.5 × 1011 Ω in the untreated to H–N2 plasma-treated samples (warp direction). It was also noticed that on plasma treatment, the resistance decreased in the following order: untreated > nitrogen–plasma-treated > oxygen–plasma-treated > H–N2 plasma-treated samples. Similar results were also observed for both in the warp and weft directions of the fabric. Plasma etching makes the wool surface hydrophilic, decreases the crosslinking on surface layers, and increases the sorption behavior of wool by enhancing the hydrogen bond forming capacity between the fiber and water molecules. Thus, water on the fiber surface forms a continuous film allowing free movement of ions resulting in an increase in conductivity and decrease in discharge time. Upon plasma treatment, the wetting time was found to reduce from 900 s in the untreated sample to less than 1 s in the plasma-treated sample. The breaking load was found to increase marginally in the plasma-treated samples due to increases in interyarn and interfiber friction.
8 Hydrophobic Finishing of Textiles
Hydrophobic textiles are important in many applications, because liquids are abundant in the form of rainwater, food, beverages, chemicals, and pesticides. Unavoidable interaction of these liquids with textiles causes unwanted wetting, staining, or contamination. Hydrophobic textiles help to protect textiles as well as their users from staining or enabling the liquid droplets to roll off leaving the underlying materials unchanged. There are three important aspects in hydrophobic finishing of textiles (i) surface energy of the base materials, (ii) surface energy of the coated materials, and (iii) surface energy of the liquid. All the solid surfaces have a distinct surface energy, which is a function of surface area and the molecules that are present on the surface. Similarly each liquid has a specific surface tension, which is a measure of interaction energies between the liquid molecules. In hydrophobic finishing of textiles, the surface energy of the base materials is reduced by coating or grafting with a material of low surface energy. Mainly hydrocarbon-, fluorocarbon-, and silicone-based compounds are used for such modification. Hydrocarbon and silicone help in producing water-repellent hydrophobic textiles. On the other hand, fluorocarbon chemicals are used for oil-repellent finishing of textiles in addition to water repellency. Hydrophobic finishing of textiles using plasma is carried out by plasma polymerization, plasma coating, or plasma deposition of silicone, hydrocarbon, or fluorocarbon liquid or gaseous precursor. Glow plasma was generated at an atmospheric pressure in the mixture of 1,3-butadiene (hydrocarbon monomer) and He gas for hydrophobic finishing of cellulosic textiles [57]. After 1.5 min of plasma reaction, the water absorbency time was found to significantly increase to 28.5 min from <1 s in the untreated sample. With increasing plasma treatment time, water absorbency time increased to >3,600 s in the 12 min plasma-treated sample. In this sample, the water contact angle increased to 142 from ~0° in the untreated sample. The hydrophobic finish was found to be durable to soap washing. In the untreated sample, the carbon atomic percentage was 57.1 % and it increased to 85.2 and 90.9 % in 1.5 and 12 min plasma-treated and soap-washed samples, respectively. A similar reduction in oxygen percentage was observed due to the depletion of −OH bond of cellulose. The increase in carbon percentage helped in formation of −CHx containing hydrophobic species, such as −CH, −CH2, and –CH3. SIMS analysis showed a similar result, where the ratio of total hydrophilic molecules to total hydrophobic molecules reduced from 1.87 in the untreated sample to 0.64 in the 12 min plasma-treated sample. GC-MS analysis revealed that mainly two types of species were formed: based on a dimer of 1, 3 butadiene (110 and 108 amu) and species with seven carbon atoms (96 amu). Based on the XPS, SIMS, GC-MS, and other analysis, a possible mechanism of plasma reaction of 1, 3-butadiene with cellulose has been reported. After the plasma reaction, the individual fibers in the yarn structure were visible and there was no blockage of interfiber spacing observed under SEM. This had happened possibly due to the nanoscale surface modification of fibers.
Atmospheric pressure helium–fluorocarbon (He/FC) cold plasma has been used to impart a high degree of hydrophobic functionality in cotton textiles [58]. After the plasma reaction for 3–8 min, it was observed that hydrophilic cotton turned into a highly hydrophobic one. As a result of this, a water droplet was not absorbed by the fabric even after 1,800 s, whereas in the untreated sample it was absorbed within 4 s as shown in Fig. 9. The water contact angle in the plasma-treated sample was as high as 140º compared to ~0º in the untreated sample. FTIR analysis of the treated sample showed the presence of different fluorocarbon species and EDX analysis showed ~4 % fluorine atoms present on the fabric surface, which were responsible for the development of hydrophobicity in the otherwise hydrophilic cotton. Similar finishing of textiles by the traditional wet chemical process requires ~10 L of water and takes 20–30 min time, besides being energy intensive. On the other hand, plasma processing of textiles, being a water-free dry process, can save a large quantity of water and energy. Vinogradov ans Lunk in [69] studied the deposition of the fluorocarbon layer on the surface of Si wafers and technical textiles in the presence of DBD plasma using CF4, C2F6, C3HF7, C2H2F4, C3F8, and C4F8 fluorocarbon molecules. It was found that fluorocarbon polymer films can be deposited, if the ratio of F/C atoms in the starting fluorocarbon molecules is smaller than three (3). The deposition of the fluorocarbon layer was confirmed using FTIR and XPS analysis. The effect of CF4 and C3F6 plasmas on the surface properties of cotton fabric was studied by McCord et al. in [37]. The hydrophobic functionality was reported to increase after treatment with both the plasma gases. XPS analysis showed an increase in surface fluorine content to ~1–2 and 2.3–7.8 %, respectively, in CF4 and C3F6 plasma-treated cotton fabrics. Based on the XPS analysis, the possible mechanism of plasma reaction with cellulose has been proposed. The effect of SF6 plasma treatment on the hydrophobic property of silk fabric was reported by Kamlangkla et al. in [26]. The improvement in the hydrophobic property was attributed to the attachment of –CF, –CF2, and –CF3 molecules to silk fabric, and it also depended on the pressure of the plasma reactor and power.
Conversion of hydrophilic cotton into hydrophobic by plasma reaction [58]
9 Hydrophobic/Hydrophilic Finishing of Textile
Multifunctional wool and silk fabrics having the hydrophobic property on one side and the hydrophilic property on the other side is an interesting area of research in the development of smart textiles. This kind of special fabric, that is, with both hydrophilic and hydrophobic functionalities in one fabric has not been produced by plasma treatment thus far. Periyasami et al. in [47] functionalized one side of mulberry silk fabric by 172 nm UV excimer lamp. It has been elucidated that after one side irradiation for 5 min in air atmosphere, wetting and wicking properties could be improved significantly on the irradiated side. The UV irradiation showed an average wetting time of 7.2 s, which was 60 % lower than the control silk fabric. However, the other side, that is, the nonirradiated side of the fabric showed behavior similar to the control silk. In the treated surface, the wetting/wicking of water was better due to the improvement in the hydrophilic property in addition to formation of nanopores of 100 nm × 10 nm, and the surface morphology of the other side (not treated) remaining unchanged. Later on based on this concept, in 2012 Basak et al. in [4] developed hydrophilic–hydrophobic wool fabric by 172 nm UV excimer lamp. Scoured wool fabric was initially treated with a fluorocarbon chemical to make the sample hydrophobic (both sides). Then one side of the hydrophobic sample was exposed to UV irradiation for 5–30 min. It was found that with increasing UV irradiation time, hydrophilic properties in terms of wetting, wicking, and contact angle improved significantly. The contact angle of the scoured wool (control sample) was 90° and it increased to 140° in the fluorocarbon-treated sample. It was seen that the contact angle on the irradiated side of the fabric decreased exponentially with increasing irradiation time. The contact angle decreased to 60° within 5 min of UV exposure and it was as low as 10° after 30 min of irradiation. The other side of the fabric, that is, the unexposed side remained completely hydrophobic (water-repellent) and there was no visible change in contact angle. It might be because the high-energy UV irradiation (7.2 eV) on the fluorocarbon finished fabric caused photo-induced oxidation involving defluorination of the surface and incorporation of oxygen by forming CF–O–CF2, CF2–O–CF2, and CF–O–CF3 moieties [29, 48].
10 Antifelting Finishing of Wool
Wool fabric is felted in an aqueous medium by mechanical agitation due to the presence of scale on the fiber surface. The bombardment of high-energy plasma species on wool has been explored for antifelting finishing. It has been reported that plasma treatment damaged the scales and oxidized the upper fatty layer to produce shrinkproof wool [19]. The untreated wool fabric showed 12.3 % shrinkage compared to only 2 % shrinkage in the plasma-treated wool [30]. The SEM micrograph showed that the O2 plasma treatment could etch the fiber surface and break the part of the surface scales that helped in imparting antifelting characteristic. For further improvement in shrink resistance properties, the plasma-pretreated sample was treated with 5 % aminofunctional poly-dimethyl siloxane. It showed excellent antifelting properties with almost no change in the fabric handling [40]. Recently, plasma-pretreated wool has been grafted with chitosan polymer and significant improvement in the antifelting property has been demonstrated [61].
In Dood et al. [11] reported that UV treatment is also effective for shrinkproofing of wool fabric treated with silicone polymer. In this process, UV treatment helps to achieve adequate shrinkproofing (only 3 % shrinkage) of wool fabric treated with only 3 % silicone-containing polymer compared to the 20 % shrinkage of untreated wool fabric. UV treatment helps to adhere silicone polymer on the wool surface better so that it can maintain its shrink-resistance properties even after washing cycles. There was only a marginal change in tensile strength and weight loss properties. Sayed and Khatib in [60] reported that the UV treatment can modify wool fabric to enhance its felting shrinkage and pilling resistance properties. In this study, freshly scoured wool fabric was first irradiated with 254 nm UVC radiation for 10 to 60 min followed by treatment with an eco-friendly oxidizing agent such as H2O2 or proteolytic enzyme. It was found that UV/H2O2-treated wool fabric showed lower area shrinkage of 13.7 % compared to 32.6 % in the control wool fabric. Shrinkage and pilling resistance results of the treated fabric were compared with harsh and non-eco-friendly chlorination treatment. The results were attributed to the fact that UVC radiation could partially oxidize wool fiber surface (–S–S into –SO3H), oxidize the thin fatty lipid layer of the upper epicuticular, and break the waxy smooth scales responsible for felting. These physicochemical surface changes facilitate the adhesion and penetration of H2O2 inside the modified wool fabric. Treatment of wool fabric with these systems was found to be effective in reducing pilling and shrinkage without severe loss in weight and strength of the fabric.
11 Conclusion
The chemical processing of textiles is important, as it imparts the highest value to the textile. However, during wet chemical processing of textiles, the industry causes significant water and air pollution in operations such as padding, drying, curing, and postwashing. The cost of the final product also increases due to the multiple numbers of drying operations and effluent treatment. There is a need to have two approaches to address these issues: reduce the consumption of water in wet chemical processing either partially or fully, and modify the surface of the fibers or the fabric to reduce consumption of chemicals and energy. Among the various techniques of surface modification, plasma and UV treatment are the most promising emerging technologies for engineering of fiber surfaces at a nanometer level, while avoiding the usage of water. Only low-temperature (cold) plasma either at atmospheric pressure or at low pressure is suitable for treatment of heat-sensitive textile substrates. Similarly, high-energy UV photons (7.2 eV) with wavelength <200 nm are mostly used for textile modification. Plasma and UV treatment of textiles using nonpolymerizing gases (small molecule), such as oxygen (O2), nitrogen (N2), air, argon (Ar), helium (He), and the like, brought both physical and chemical changes to the substrates due to surface cleaning, activation, oxidation, etching, radical formation, polymerization, and creation of nanostructures.
Due to the chemical changes, there was an increase in surface oxygen and nitrogen percentage. This leads to formation of the amine group (silk, wool, nylon), –COOH and C=O groups (polyester, wool, cotton), –OH (wool, cotton), sulphonic acid (–SO3H) group (wool), and the carbonyl group (cotton). Similarly, due to the physical changes there was a formation of vertical channels with dimensions of <200 nm (nylon), horizontal channel with size of 100 nm (polyester), nanopores of 100 nm × 10 nm (silk), and microcrack (cotton). The above physical and chemical changes result in significant improvement in water absorbency time, wetting time, wicking time, oil absorbency time, and surface energy. It is interesting to observe that in nylon fabric, oil spreading time decreases from 152 s in the untreated sample to 52 s in the 60 s He–plasma-treated sample. In the plasma-treated samples, there was an increase in surface area from 16.27 to 19.27 µm2 (nylon), from 16.01 to 17.18 µm2 (polyester) and from 0.1 to 0.35 m2/gm (wool) in the untreated to plasma-treated samples, respectively. This increase in surface area in addition to the chemical changes helped the textile to produce not only less static charge, but also to dissipate the same at a faster rate. The 60 s air–plasma-treated sample produced only a 1.5 kV static charge compared to 2.8 kV in the untreated sample. Similarly, the half-decay time was found to reduce sharply from 8.9 s in the untreated sample to 0.63 s in the 60 s air–plasma-treated sample.
Chemical changes play a more active role in the improvement of dyeing of plasma- and UV-treated wool, silk, cotton, and polyester fabrics than the physical changes. In wool fiber, in addition to formation of amine groups, removal and/or oxidation of the fatty hydrophobic layer on the epicuticle, and reduction in the disulphide cystine linkage helps in better dye exhaustion. Removal or partial damage of scale upon plasma and UV irradiation leads to the development of antifelting woolen textiles. Cotton and silk can made hydrophobic without using water by plasma reaction of hydrophobic precursor (butadiene) and fluorocarbon precursors (CF4, C2F6, C3HF7, C2H2F4, C3F8, C–C4F8, and SF6). Specialty wool and silk textiles, such as one side being hydrophilic and other side hydrophobic can be produced by UV excimer treatment. The treated fabric surface is hydrophilic in nature with a water contact angle of 10° compared to the untreated surface with a contact angle of 140°, which is highly hydrophobic. Plasma- and UV-induced surface modification of textiles occur at the nanometer level, hence the bulk physical and chemical properties remain unaltered. As, these processes are carried out in a dry state, adoption of such emerging technologies will help to develop superior product quality in textiles at a lower cost, while addressing the negative environmental issues.
References
Bajaj P, Sengupta AK (1992) Protective clothing. Text Prog 22(2–4):1–110
Banchero M, Sicardi S, Ferri A et al (2008) Super critical dyeing of textiles-from the laboratory apparatus to pilot plant. Text Res J 78(3):217
Basak S (2009) Process development of wool fabric by 172 nm VUV Excimer lamp, M.Tech. thesis, Indian Institute of Technology (IIT)-Delhi, India
Basak S (2012) Process development of wool fabric by 172 nm VUV Excimer lamp. Lap lambert publishing, Germany
Basak S, Gupta D (2010) Functionalisation of wool fabric using 172 nm VUV Excimer lamp. Text Excellent 29:45–49
Basak S, Gupta D (2012) Double hydrophilic/hydrophobic wool fabric by using 172 nm Excimer lamp. Text Excellent 23:25–29
Bhat NV, Netravali AN, Gore AV et al (2011) Surface modification of cotton fabrics using plasma technology. Text Res J 81(10):1014–1026
Bradley RH, Mathieson I (1997) Chemical interactions of ultraviolet light with wool fiber surfaces. J Colloid Interfacial Sci 194:338–343
Bradley RH, Mathieson I, Byrene KM (1997) Spectroscopic studies of modified wool fiber surfaces. J Mater Chem 67:2477–2482
Cireli A, Kutlu B, Mutlu M (2007) Surface modification of polyester and polyamide fabrics by low frequency plasma polymerization of acrylic acid. J Appl Polym Sci 104(4):2318–2322
Dood KJ, Carr CM, Byrne K (1993) An investigation into the application of a UV-curable silicone for the shrink proofing of wool fabric. J Text Inst 84:620–625
Friedman LA, Faulkner JD, King AD Jr (1979) Method and Composition for Neutralizing Static Charge. US Patent, 4152288. Lester Laboratory Inc., Atlanta, Ga
Guglani R (2002) Recent developments in textile dyeing techniques. http://www.fibre2fashion.com/industryarticle/12/1171/recent-developments-in-textile-dyeing-techniques11.asp
Guo L, Campagne C, Perwuelz A et al (2009) Zeta potential and surface physico-chemical properties of atmospheric air-plasma-treated polyester fabrics. Text Res J 79(15):1371–1377
Gupta D, Basak S (2010) Surface functionalisation of wool using 172 nm UV Excimer lamp. J Appl Polym Sci 117:223–227
Gupta D, Periyasamy S, Banerjee A (2007) Basic dyeable polyester: a new approach using a VUV excimer lamp. Color Technol 123:248–251
Hegemann D (2006) Plasma polymerization and its application in textiles. Indian J Fib Tex R 31(1):99–115
Hegemann D, Hossain MM, Balazs DJ (2007) Nanostructured plasma coatings to obtain multifunctional textile surfaces. Prog Org Coat 58:237–240
Hocker H (2002) Plasma treatment of textile fibers. Pure Appl Chem 74(3):423–427
Hossain MM, Herrmann AS, Hegemann D (2006) Plasma hydrophilization effect on different textile structures’. Plasma Process Polym 3(3):299–307
Iriyama Y, Mochizuki T, Watamabe M et al (2003) Preparation of silk film and its plasma treatment for better dyeability. J Photopolym Sci Technol 16(1):75–80
Jahagirdar CJ, Tiwari LB (2004) Study of plasma polymerization of dichloromethane on cotton and polyester fabrics. J Appl Polym Sci 94(5):2014–2021
Joshi VK (1996) Antistatic fibers and fabrics. Manmade Text India 7:245–251
Judd DB, Wysezki G (2002) UV treatment of textile fibers. Text Res J 72:425–427
Kale K, Palaskar S (2010) Studies on atmospheric pressure plasma treatment of polyester/cotton blended fabric’. In: Paper presented in 51st Joint Technological Conference of ATIRA, BTRA, SITRA and NITRA (NITRA, Ghaziabad), 29 June 2010
Kamalangkla K, Hodak SK, Grutzmacher JL (2011) Multifuctional silk fabrics by means of the plasma induced graft polymerisation process. Surf Coat Technol 205:3755–3762
Kan CW (2007) Effect of low temperature plasma on different wool dyeing systems. Autex Res J 8(4):132–139
Kan CW (2007) Evaluating antistatic performance of plasma-treated polyester. Fiber Polym 8(6):629–634
Kan CW, Chan K, Yuen CWM et al (1999) Study on the influence of scouring on the wettability of keratin fibers. Text Res J 69(6):407–416
Kan CW, Chan K, Yuen CWM (2004) A study of the oxygen plasma treatment on the serviceability of a wool fabric. Fiber Polym 5(3):213–218
Kan CW, Chan K, Yuen CWM et al (1997) Physico-chemical study on the surface properties of physically and chemically treated wool fiber. JHKITA 32:33–47
Kan CW, Yuen CWM (2008) Nuclear instruments and methods. Phys Res 266:127–132
Kan CW (2006) Dyeing behavior of low temperature plasma treated wool. Fiber Polym 7:262–269
Kang ET, Neoh KG (2009) Surface modification of polymers. Encycl Polym Sci Technol 115:167. (Wiley, New York)
Karmakar SR (1999) Chemical Technology in the pre-treatment processes of textiles. Text Sci Technol 12:1243–1249
Maclaren JA, Kirkpatrick A (1968) Partially oxidised disulphide groups in oxidised wool- reactivity with thiols. J Soc Dyers Colour 84:564–567
McCord MG, Hwang YJ, Qiu Y et al (2003) Surface analysis of cotton fabrics fluorinated in radio-frequency plasma. J Appl Polym Sci 88(8):2038–2047
Molina R, Canal C, Bertran E et al (2004) Low temperature plasma modified wool fabrics: Surface study by S.E.M. Micros Res Edu 23:242–249
Muthukumar M, Sargunamani D, Selvakumar N et al (2004) Statistical analysis of the effect of aromatic, azo and sulphonic acid groups on decolouration of acid dye effluents using advanced oxidation process. Dyes Pigm 63:199–304
Naebe M, Cookson PG, Denning R et al (2011) Use of low level plasma for enhancing the shrink resistance of wool fabric treated with a silicon polymer. J Text Inst 102(11):942–956
Nasadil P, Benešovsky P (2008) Plasma in textile treatment. II central european symposium on plasma chemistry. Chem Listy 102:1486–1489
Nehra V, Kumar A, Dwivedi HK (2008) Atmospheric non-thermal plasma sources. Int J Eng 2:53–67
Özdogan E, Saber R, Ayhan H et al (2002) A new approach for dyeability of cotton fabrics by different plasma polymerization methods. Color Technol 118(3):100–103
Panda PK, Rastogi D, Jassal M, Agrawal AK (2012) Effect of atmospheric pressure helium plasma on felting and low temperature dyeing of wool. J App Poly Sci 124(5):4289–4297
Parida D (2010) Functionalization of textiles using atmospheric pressure plasma reaction technology, M.Tech thesis, Indian Institute of Technology (IIT)-Delhi, India
Periyasamy S, Gulrajani ML, Gupta D (2007) Preparation of a multifunctional mulberry silk fabric having hydrophobic and hydrophilic surfaces using VUV excimer lamp. Surf Coat Technol 201:7286–7291
Periyasamy S, Gupta D, Gulrajani ML (2007) Nanoscale surface roughening of mulberry silk by monochromatic VUV Excimer lamp. J Appl Polym Sci 103:4102–4106
Periyasamy S, Gupta D, Gulrajani ML (2007) Modification of one side of mulberry silk fabric by monochromatic VUV Excimer lamp. Euro Polym J 43:4573–4581
Prabhakara HR, Sangappa S, Roy S (2009) The application of low pressure plasma treatment to silk yarn and fabric. National workshop on eco-friendly plasma applications in textile, Institute for plasma research, Bhat, Gandhinagar, India
Ratnapandian S, Wang L, Fergusson SM et al (2011) Effect of atmospheric pressure plasma treatment on pad dyeing of natural dyes on wool. J. Fiber Bioeng Inform 4(3):267–276
Rombaldoni F, Montarsolo A, Mossotti R et al (2010) Oxygen plasma treatment to reduce the dyeing temperature of wool fabrics. J Appl Polym Sci 118(2):1173–1183
Samanta KK (2010) Surface functionalization of textile substrates using atmospheric pressure glow plasma, Ph.D. thesis. Indian Institute of Technology (IIT)-Delhi, India
Samanta KK, Jassal M, Agrawal AK (2008) Formation of nano-sized channels on polymeric substrates using atmospheric pressure glow discharge cold plasma. Nanotrends: J Nanotechnol Appl 4(1):71–75
Samanta KK, Jassal M, Agrawal AK (2009) Improvement in water and oil absorbency of textile substrate by atmospheric pressure cold plasma treatment. Surf Coat Technol 203:1336–1342
Samanta KK, Jassal M, Agrawal AK (2006) Atmospheric pressure glow discharge plasma and its applications in textile. Indian J Fib Tex Res 31(1):83–98
Samanta KK, Jassal M, Agrawal AK (2010) Antistatic effect of atmospheric pressure glow discharge cold plasma treatment on textile substrates. Fiber Polym 11(3):431–437
Samanta KK, Joshi M, Amish G et al (2012) Study of hydrophobic finishing of cellulosic substrate using He/1, 3-butadiene plasma at atmospheric pressure. Surf Coat Technol 213:65–76
Samanta KK, Saxena S, Arputharaj A et al (2013) Value-addition to cotton textiles using water-free plasma technology, ICAR News: A Sci Technol Newsl 19(3):23–25
Sarmadi AM, Ying TH, Denes F (1993) Surface modification of polypropylene fabrics by acrylonitrile cold plasma. Text Res J 63(12):697–705
Sayed H, Khatib EI (2005) Modification of wool fabric using ecologically acceptable UV-assisted treatments. J Chem Technol Biotechnol 80:1111–1117
Shahidi S, Ghoranneviss M, Sharifi SD (2013) Effect of atmospheric pressure plasma treatment follwed by chitosan grafting on antifelting and dyeability of wool fabric. J Fusion Energy 33:177–183
Skurat VE, Samsonov PV (2004) Yellowing of wool in Oxygen atmosphere. High Perform Polym 2:339–343
Souto AP, Oliveira FR, Fernandes M et al (2012) Influence of DBD plasma modification in the dyeing process of polyamide. J Text Eng 19(85):20–26
Sparavigna A (1993) Laser treatment of textiles. J Mater Sci Technol 68:242–247
Spitzl S, Hildegard M (2003) Plasma pre-treatment of textiles for improvement of dyeing processes. Inter Dyer 188(5):20–25
Sun D, Stylios GK (2004) Effect of low temperature plasma treatment on the scouring and dyeing of natural fabrics. Text Res J 74(9):751–756
Tsai PP, Wadsworth LC, Roth JR (1997) Atmospheric pressure plasma treatment textiles using non-polymerising gases. Text Res J 67:359–365
Vesel A, Mozetic M (2009) Surface functionalization of organic materials by weakly ionized highly dissociated oxygen plasma. J Phy: Conf Series 162:012015
Vinogradov IP, Lunk A (2005) Structure and chemical composition of polymer films deposited in a DBD in Ar/Fluorocarbon mixtures. Surf Coat Technol 200(5):660–663
Wai C, Wong CK, Chuan-W MU (2003) Nanosols and textiles. Autex Res J 3(4):195–200
Wai KC (2007) Effect of low temperature plasma on different wool dyeing. Autex Res J 8(4):258
Wakida T, Tokino S, Niu S et al (1993) Characterization of wool and polyethylene terephtalate fabrics and film treated with low temperature plasma under atmospheric pressure. Text Res J 63:433–438
Wong K, Chan KW, Lau KS (2000) A potential textile application of UV excimer laser irradiation on polyester fabrics. Res J Text Apparel 3(2):1–10
Xin JH, Zhu R, Shen J (2002) Surface modification and low temperature dyeing properties of wool treated by UV radiation. Color Technol 118:169–174
Yasuda T (1982) Mukogawa Joshi Daigaku Kiyo Shokumotsu-Hen 30, A9
Yue CY, Hong L, Yu R et al (2004) Study of bombyx mori silk treated by oxygen plasma. J Zhejiang Univ Sci 5(8):918–922
Zawhary MM, Ibrahim NA, Eid MA (2006) The impact of nitrogen plasma treatment on physical chemical and dyeing properties of wool fabric. Polym Plastics Technol Eng 45:1123–1132
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Samanta, K.K., Basak, S., Chattopadhyay, S.K. (2014). Environment-Friendly Textile Processing Using Plasma and UV Treatment. In: Muthu, S. (eds) Roadmap to Sustainable Textiles and Clothing. Textile Science and Clothing Technology. Springer, Singapore. https://doi.org/10.1007/978-981-287-065-0_6
Download citation
DOI: https://doi.org/10.1007/978-981-287-065-0_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-287-064-3
Online ISBN: 978-981-287-065-0
eBook Packages: EngineeringEngineering (R0)