Abstract
Diabetes, a metabolic chronic disease characterized by hyperglycemia, has dire consequences for health and well-being if left uncontrolled. In recent years, there are some studies about the effects of static magnetic fields (SMFs) on diabetes and its complications, but the reported effects are highly inconsistent, especially for glycemia levels. The aim of this chapter is to compare and analyze reported effects of multiple parameter SMFs on glycemia and insulin levels, as well as diabetic complications. It is interesting that although the reported effects of SMFs on glycemia and insulin levels are variable due to the differences in SMF parameters and experimental subjects, SMFs have consistently shown beneficial effects on diabetic complications including wound healing. Mechanistic studies indicate that SMFs may play an important role in insulin secretion by affecting membrane proteins, hormone levels, and reactive oxygen species. This not only contributes to a better understanding of SMF effects on diabetes and its complications, but also lays the foundation for more systematic and in-depth studies to develop potential applications of SMFs in the clinical setting of diabetes in the future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Magnetic field (MF)
- Static magnetic field (SMF)
- Glycemia
- Insulin
- Diabetes
- Diabetic complications
- Mechanisms
10.1 Introduction
Diabetes mellitus is a serious chronic condition with hyperglycemia, mostly because the body cannot generate enough insulin or cannot efficiently utilize insulin. There are two main types of diabetes, including type 1 diabetes mellitus (T1D, or T1DM) and type 2 diabetes mellitus (T2D, or T2DM). But there are also some specific forms of diabetes mellitus, for example, diabetes that occur during pregnancy, or mediated by drugs or chemicals, viral infections, etc. (Forbes and Cooper 2013; Magliano et al. 2021). Among the various reasons for triggering diabetes, the main causes include autoimmune destruction of pancreatic islet cells, insulin resistance, and insufficient insulin secretion (American Diabetes Association 2010). Besides hyperglycemia, diabetes can also cause a series of complications, including dysfunctions in the kidney, retina, cardiovascular system, neurons, and liver, which are the major causes of morbidity and mortality in diabetic patients (Morrish et al. 2001; Demir et al. 2021).
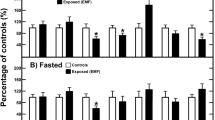
In recent years, there are multiple studies that have reported the effects of magnetic fields on diabetes and its complications. For example, Carter et al. performed multiple mice experiments to demonstrate that a combined static magnetic field (SMF) and static electric field can effectively improve glycemia, insulin resistance, and glucose intolerance in T2D (Carter et al. 2020) (Fig. 10.1). Our group compared four types of moderate SMFs, with different SMF flux, directions, and distributions, and found that a ~100 mT vertically downward direction SMF could effectively alleviate the development of hyperglycemia, fatty liver, and weight gain in T2D (Yu et al. 2021) (Fig. 10.2). Both of these two studies have showed beneficial effects on T2D and both of them have pointed out that the oxidative stress regulation plays an essential role. In this chapter, we will focus on the effects and mechanisms of various types of SMF treatments on glycemia, diabetes and its complications.
A combined static magnetic field and static electric field can alleviate T2D. [Reprinted from reference with permission (Carter et al. 2020).]
A ~100 mT downward direction static magnetic field improves iron metabolism and prevents high-fat diet/streptozotocin-induced T2D. [Reprinted from reference with permission (Yu et al. 2021).]
10.2 Effects of Static Magnetic Fields on Glycemia Levels in Diabetic Animals
Currently, the effects of various SMFs on glycemia, the key indicator for diabetes diagnosis, in diabetic model animals are still inconsistent (Table 10.1), which is largely due to the SMF parameter differences in different experiments. Some studies have reported that SMF can raise glycemia levels. For example, Carter et al. reported that 3 mT horizontal SMF exposure for 7 h/day for consecutive 25 days significantly increased glycemia (Carter et al. 2020). Conversely, studies have also reported that SMF can decreased glycemia levels. Li et al. reported that alternating pole SMFs (400 and 600 mT) exposure for 24 h can also induce glycemia reduction (Li et al. 2020). In addition, there are also studies that found no effect of SMF on glycemia. For example, our group found no statistically changes in glycemia levels in db/db mice after exposure to ~15 mT inhomogeneous SMF (Feng et al. 2022). Zhang et al. used a 4 mT equipment to treat with diabetic rats for 16 weeks and did not observe significant changes in glycemia levels either (Zhang et al. 2018). We found that T2D mice treated with ~100 mT upward direction SMF for consecutive 12 weeks increased the glycemia level, while the downward SMF decreased glycemia (Yu et al. 2021). These demonstrate that SMF parameter, especially SMF direction, is critical for the SMF effects on glycemia.
10.3 Effects of Static Magnetic Fields on Insulin Levels in Diabetic Animals
Generally speaking, increased insulin levels usually correspond to the decreased glycemia. However, it is not always the case because insulin resistance is another important feature of diabetes, which results in reduced sensitivity of the body to insulin. As far as we know, there are only three studies that have reported the effects of SMFs on insulin levels in diabetic mice, and their results are also variable (Table 10.2). However, Carter et al. found that although the combined static magnetic and electric fields decreased insulin secretion, they still can decrease glycemia by increasing the insulin sensitivity in mice (Carter et al. 2020). Therefore, people are recommended to also measure the insulin sensitivity in their studies to get a more comprehensive understanding of how SMFs affect the glucose metabolism. In our study, we found that the ~100 mT downward direction SMF not only increased the insulin levels, but also improved the insulin sensitivity in high-fat diet (HFD)/streptozotocin (STZ)-induced T2D mice (Yu et al. 2021).
10.4 Effects of Static Magnetic Fields on Diabetic Complications
The hyperglycemia of diabetes produces glucotoxicity that cause damage to the macrovasculature system (cardiovascular disease), microvasculature system (diabetic nephropathy, diabetic retinopathy, and neuropathy), and other tissues (diabetic bone, diabetic foot, and diabetic encephalopathy), resulting in various complications (Ceriello 2005; Cole and Florez 2020).
Diabetes significantly impairs bone formation, reduces the mechanical strength of bone, and ultimately leads to osteoporosis (Hofbauer et al. 2022). It also accelerates the degeneration of skeletal structures (Rabe et al. 2021), makes diabetic patients more prone to fractures (Janghorbani et al. 2007; Wang et al. 2019) and difficult to heal after fractures (Retzepi and Donos 2010), which make the mortality rate due to fractures significantly higher than that of the non-diabetic population (Gulcelik et al. 2011). In 2018, Zhang et al. showed that a 4 mT SMF treatment (2 h/day, 16 weeks) can improve bone stiffness, increase the expression of osteogenesis-related genes, and improve symptoms associated with diabetic osteoarthropathy (Zhang et al. 2018). Although it is the only report so far that has investigated on the SMF effects on diabetes osteoarthropathy (Zhang et al. 2018) as far as we know, there are actually a large number of studies demonstrated that SMFs can exhibit positive effects on the skeletal system of non-diabetic animals, which has been reviewed (Zhang et al. 2014) and discussed in the Chap. 11 of this book. Moreover, our group found that a ~100 mT downward direction SMF increased the number of trabecular osteoblasts in the tibia of T1D mice, but not in 0.5 T upward SMF (unpublished data).
Moreover, it should be noted that at least 50% of diabetic patients suffer from diabetic neuropathy, a set of clinical syndromes caused by damage to the peripheral and autonomic nervous systems, which causes allodynia, spontaneous pain, burning, and numbness (Feldman et al. 2019). Similar to the above-mentioned effect of SMFs on bone, there are also many studies of SMFs on nervous system in non-diabetic animals, which will be discussed in Chap. 13 of this book. However, there are only two studies so far that have investigated the effects of SMFs on diabetic neuropathy and the results are still inconclusive. László et al. examined the STZ-induced CD1 mice treated with 2.8–476.7 mT inhomogeneous SMF for 0.5 h/day for 6 weeks and found no significant effect (László et al. 2011). However, Weintraub et al. found that shoe insole of 45 mT alternating pole SMF (24 h/day, 4 months) can play a mitigating role in patient feet with symptoms associated with diabetic neuropathy (Weintraub et al. 2003).
Lastly, it is well known that one of the most prevalent complications in diabetic patients is diabetic wounds (Bowling et al. 2015), which are usually hard to heal and can lead to infection, amputation, and even death (Falanga 2005; Lavery et al. 2010; Lipsky et al. 2012). It is interesting that although various SMFs have inconsistent effects on glycemia, insulin levels, and diabetic neuropathy, all four reported studies of SMFs on wound healing in diabetic mice we got from the literature showed very consistently positive effects (Table 10.3). In fact, in 2021, Lv et al. have reviewed about the effects of multiple types of magnetic fields, including time-varying magnetic fields, on diabetic wounds, which show that all types of magnetic fields have positive effects in promoting diabetic wound healing, according to the literature (Lv et al. 2021). This is interesting and promising, but the reasons for this phenomenon are varied and still unclear.
10.5 Effects of Static Magnetic Fields on Glycemia and Insulin Levels in Cells and Non-Diabetic Animals
Besides the studies of SMFs on diabetic animals, there are actually quite a few studies performed on non-diabetic animals (Table 10.4). Similar to that of diabetic animals, the results in non-diabetic animals are also inconsistent. However, it is interesting that there are no studies reporting decreased glycemia levels in non-diabetic animals so far. Gorczynska et al. found that blood glucose in Wistar rats can be elevated by 1 mT and 10 mT SMFs (Gorczynska and Wegrzynowicz 1991). Meanwhile, several works by Lahbib et al. also found that 128 mT SMF increased glycemia levels in Wistar rats (Lahbib et al. 2010, 2015a, b). In addition, some studies have also shown no effect of SMF on glycemia levels. Currently, we cannot make an accurate conclusion or explanation because of the differences in mice strains, SMF parameters, and SMF treatment methods.
Moreover, insulin levels were also investigated in many studies in cells and non-diabetic mice (Table 10.5). We found that treatment of INS-1 cells with 400 mT SMF for more than 6 h can increase insulin expression and secretion (Mao et al. 2015, 2017), and the exposure of INS-1 cells with 6 T SMF for 1 h can also increase insulin secretion (Sakurai et al. 2009). Interestingly, studying the effects of SMFs on islet cells isolated from Sprague-Dawley rats, Hayek et al. found that the SMF increases insulin levels in a magnetic flux density-dependent manner at magnetic flux density of 0.1–1 mT and lower initial glucose concentrations (5.4 mmol/L) (Hayek et al. 1984). In contrast, these effects were not significant at higher (16.7 mmol/L) initial glucose concentration conditions (Hayek et al. 1984). From the above results, we speculate that the influence of SMFs on insulin is related to the magnetic flux density, exposure time, and the initial glucose level.
10.6 Analysis of Inconsistent Effects of Static Magnetic Fields on Glycemia or Insulin
It is obvious that SMFs have generated very variable effects on most aspects of diabetes and complications, except for the diabetic wound healing. We think there are multiple factors that contribute to these inconsistencies, which are discussed below.
First of all, the major factor is the SMF parameters, including distributions (direction and gradient, etc.) and flux densities generated by the different devices (Fig. 10.3), especially the SMF direction. Our group has previously reported on the SMF direction-induced differential bioeffects (Tian et al. 2018; Yang et al. 2020, 2021) and has also systematically summarized them in Chap. 2 of this book. Moreover, we have side-by-side compared four different SMF settings and different exposure times on HFD/STZ-induced T2D mice. We found that different magnetic flux densities, distributions, directions, and treatment time could produce totally differential effects on glycemia (Yu et al. 2021). More specifically, we found that neither the 400 mT, 600 mT alternating pole SMFs (Figs. 10.3a, b), nor the ~100 mT upward direction SMF (Fig. 10.3c) reduced blood glucose levels, while the ~100 mT downward direction SMF (Fig. 10.3d) could reduce blood glucose. Furthermore, most of studies showing elevated glycemia levels and reduced insulin levels in non-diabetic animals have used a 128 mT SMF exposure system (Fig. 10.3g) by the Lake Shore electromagnets device manufactured by Lake Shore Cryotronics, Inc. (Tables 10.4 and 10.5). Interestingly, the direction of SMF generated by their device is vertically upward, which reinforce our hypothesis that the upward direction SMF has a tendency to increase glycemia. Moreover, the magnetic flux density also matters because Hayek et al. found that the release of insulin is dose-dependent with magnetic flux density (Hayek et al. 1984).
Examples of apparatus that have different static magnetic field settings. (a, b) Experimental setup and magnetic field distribution for mice exposed to 0.4 T and 0.6 T inhomogeneous SMFs provided by alternating pole magnets (Yu et al. 2021); (c, d) Experimental setup and magnetic field distribution for mice exposed to upward and downward quasi-uniform SMFs (Yu et al. 2021); Figures were adapted from (Yu et al. 2021), open access. (e) A water-cooled magnet (water-cooled magnet #4) in the Chinese High Magnetic Field Laboratory that can provide vertical SMFs up to 27.5 T; (f) A superconducting magnet in Xin Zhang lab that can provide vertical SMF up to 10 T; (g) The Lake Shore device (picture was from the public website: https://www.lakeshore.com/products/categories/overview/discontinued-products/discontinued-products/em4-em7-electromagnets); (h) Magnetic plate contains 8 cylindrical permanent magnets of 0.5 T. [Figure was adapted from (Feng et al. 2022), open access]
Secondly, the biological sample differences contributed to the experimental inconsistencies. This point has also been brought up and reviewed in Chaps. 1 and 3 of this book. As far as we know from Tables 10.1 and 10.2, several types of diabetic animal models have been used to evaluate the effects of SMFs on glycemia or insulin. Some studies use chemical-induced diabetic models, for example, STZ or alloxan, while others use genetic diabetic animals of different strains. For example, by analyzing the results of Yu et al. and Li et al. we found that they used the same magnetic field parameters (alternating pole SMFs of 400 mT and 600 mT), but the glycemia level of T2D mice was different (Li et al. 2020; Yu et al. 2021). We speculate that the mice strain and the modeling methods of the diabetic mice are also important factors. Yu et al. used C57BL/6J mice, whereas Li et al. used ICR mice. And Yu et al. used high-fat chow to feed mice for 6 weeks and then injected 45 mg kg−1 of STZ, while Li et al. used high-fat chow to feed mice for 2 weeks and then injected 80 mg kg−1 of STZ. In addition, our recent studies found that SMFs also have different effects on glycemia in mild and severe forms of type 1 diabetes (unpublished data). Therefore, since there are multiple diabetes subtypes, and the same type of diabetes also varies based on severity, the exact effects of SMFs are also different.
The third factor is the SMF treatment method, including the duration of exposure, whether to use pretreatment. It has been shown that exposure time is a key factor that contributes to the differential effects of magnetic fields on biological samples. We exposed diabetic mice to SMF for different time points and found that the effects on glycemia are time dependent. After 8 weeks SMF exposure, the glycemia of diabetic mice was not reduced, but after 9 weeks SMF exposure, the glycemia of diabetic mice was significantly reduced compared with the sham control group (Yu et al. 2021). According to László et al. and Li et al. we also found different effects of SMFs exposure time on glycemia (László et al. 2011; Li et al. 2020). In addition, SMF pretreatment may also be an important factor contributing to differences in experimental results. We pretreated the mice with SMF for 6 weeks before they were induced for T2D, whereas Li et al. treated the mice with SMF after they were induced for T2D, which may have contributed to the difference in their results. Finally, whole-body and targeted exposure were also categorized as SMF treatment method, which could also be a potential factor for inconsistencies. From Tables 10.1, 10.2, 10.4, and 10.5, although there is no report using targeted exposure in experiments, the possibility that researchers will not use targeted exposure in the future cannot be ruled out. And we advocate that the effects of SMFs on specific organs, such as the pancreas and liver, should also be explored to discover the specific biological effects of SMFs on specific organs.
Therefore, in order to promote the standardization of related research, we recommend that investigators should carefully design their experiments and accurately describe the experimental details. This includes but not limited to the relevant parameters of the magnetic fields in the experiment (the distance of magnet surface from tissue, exposure time, magnetic flux density, direction, and distribution), and treatment procedure. Besides the basic parameters including body weight, diet, and glycemic change profile in diabetic mice, other assays are also recommended, such as insulin levels and sensitivity, bone mineral density, and angiogenesis markers. The animal sex, age, species, and other key factors should also be clearly recorded.
10.7 Potential Mechanisms for the Effects of Static Magnetic Fields on Glycemia or Insulin
Some preliminary investigations of the potential mechanisms underlying the effects of SMFs on glycemia and insulin have been performed (Fig. 10.4). For example, it was shown that pancreatic islet β-cells can release insulin to reduce glycemia, and SMFs may affect transcription factors and transport channels in pancreatic islet β-cells to regulate insulin secretion (Gorczynska and Wegrzynowicz 1991; Lahbib et al. 2015a; Mao et al. 2017). Some other mechanisms have been proposed, such as iron metabolism, norepinephrine, insulin conformation, cell membrane conformation.
Potential mechanisms for the static magnetic field effects on glycemia or insulin proposed by reported studies. 1. SMF exposure may change the conformation of insulin, reducing its affinity for the insulin receptor and impairing its function (Elferchichi et al. 2011). 2. After SMF exposure, GLUT2 expression in pancreatic islet β-cells is reduced, which prevents pancreatic islet β-cells from sensing extracellular glucose concentrations, resulting in cells being unable to release insulin (Lahbib et al. 2015a). 3. SMF exposure can evoke an effect resembling interleukin-1 receptor antagonist, which may be equivalent to insulin intake reducing interleukin-1β level, suppressing the recruitment of innate immune cells, and thus diminishing glycemia (László et al. 2011). 4. The soluble N-ethylmaleimide-sensitive factor attachment protein receptors protein complex can help insulin transport out of cells, and exposure to SMF promotes mRNA expression of synaptosomal-associated protein 25 and synaptotagmin 1, components of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors protein complex, to facilitate insulin release (Mao et al. 2017). 5. SMF promotes insulin release by increasing cortisol levels (Gorczynska and Wegrzynowicz 1991). 6. SMF inhibits insulin release and increases glycemia by raising norepinephrine levels (Abdelmelek et al. 2006; Elferchichi et al. 2011). 7. SMF elevates adrenaline levels (stimulate pancreatic islet B cell α receptors) to decrease insulin release and promote glycemia elevation (Gorczynska and Wegrzynowicz 1991). 8. SMF increases intracellular ROS levels to reduce insulin secretion (Elferchichi et al. 2010). 9. SMF restores the abundance of iron complex outer membrane receptor genes in gut microbiota, thus probably allowing dietary iron to enter microbes, reducing iron storage in cells, decreasing oxidative stress caused by excess iron accumulation, and finally restoring insulin secretion (Yu et al. 2021). 10. SMF promotes insulin gene expression by inducing the expression of multiple transcription factors that bind to the promoter regions of insulin genes (Mao et al. 2017). 11. SMF contributes to insulin-related mRNA expression and insulin secretion by increasing intracellular calcium concentration (Sakurai et al. 2009). 12. SMF of defined intensity can change the lipid layer of the cell into the nematic phase, thus constituting a barrier to the diffusive movement of glucose, which is not beneficial to glucose transport to the interior of the cell (Gorczynska and Wegrzynowicz 1991). 13. SMF elevates thyroxine and triiodothyronine levels (enhance the absorption of glucose in the digestive tract) to induce hyperglycemia (Gorczynska and Wegrzynowicz 1991). 14. SMF reduces insulin levels by inducing sympathetic hyperactivity (Lahbib et al. 2010). 15. SMF decreases glycemia by reducing the activity of glycogen phosphorylase and diminishing glycogen breakdown in the liver (Li et al. 2020). 16. SMF can reduce glycemia by promoting the regeneration and repair of pancreatic islet B cells, protecting pancreatic islet cells, and improving insulin secretion (Li et al. 2020). 17. SMF can repair the injury of the pancreas and improve the function of pancreatic islet cells to promote insulin secretion (Li et al. 2020). The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license
However, it should be mentioned that although these mechanistic study results are listed in Fig. 10.4, it is clear that there is still no consensus model so far. Moreover, most of them are hypothesis-based, and the direct molecular evidence, or more importantly, the physical mechanism is still lacking. In addition, due to differences in the SMF parameters, treatments, and subjects used in these studies, the mechanisms by which SMFs affect glycemia and insulin levels are very diverse. Therefore, in the future, we should systematically study their mechanism and focus more on a biophysical perspective.
10.8 Conclusion
In conclusion, although the regulation of glycemia and insulin levels by SMFs is inconclusive so far due to the SMFs parameter and biological sample difference, it is clear that multiple SMFs treatment modalities have shown significant beneficial effects on diabetic complications, especially the consistently improving effects on diabetic wound healing. In addition, based on current experimental evidences, we have also revealed some clues to optimize SMF parameters to achieve better anti-diabetic effects, including SMF flux density, direction, and distribution. We believe that more systematic and in-depth investigations will definitely help us to unravel the detailed mechanisms of SMF regulation on diabetes and its complications, both biologically and physically, so that we can eventually take the best advantages of SMFs and apply them in the clinical treatment of diabetes.
References
Abbasi M, Nakhjavani M, Hamidi S, Tarafdari AM, Esteghamati A (2007) Constant magnetic field of 50 mT does not affect weight gain and blood glucose level in BALB/c mice. Med Sci Monit 13(7):Br151–Br154
Abdelmelek H, Molnar A, Servais S, Cottet-Emard JM, Pequignot JM, Favier R, Sakly M (2006) Skeletal muscle hsp72 and norepinephrine response to static magnetic field in rat. J Neural Transm 113(7):821–827
American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33:S62–S69
Bowling FL, Rashid ST, Boulton AJM (2015) Preventing and treating foot complications associated with diabetes mellitus. Nat Rev Endocrinol 11(10):606–616
Carter CS, Huang SC, Searby CC, Cassaidy B, Miller MJ, Grzesik WJ, Piorczynski TB, Pak TK, Walsh SA, Acevedo M, Zhang Q, Mapuskar KA, Milne GL, Hinton AO, Guo D-F, Weiss R, Bradberry K, Taylor EB, Rauckhorst AJ, Dick DW, Akurathi V, Falls-Hubert KC, Wagner BA, Carter WA, Wang K, Norris AW, Rahmouni K, Buettner GR, Hansen JM, Spitz DR, Abel ED, Sheffield VC (2020) Exposure to static magnetic and electric fields treats type 2 diabetes. Cell Metab 32(6):1076
Ceriello A (2005) Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 54(1):1–7
Chater S, Abdelmelek H, Pequignot JM, Sakly M, Rhouma KB (2006) Effects of sub-acute exposure to static magnetic field on hematologic and biochemical parameters in pregnant rats. Electromagn Biol Med 25(3):135–144
Cole JB, Florez JC (2020) Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol 16(7):377–390
Demir S, Nawroth PP, Herzig S, Ekim Üstünel B (2021) Emerging targets in type 2 diabetes and diabetic complications. Adv Sci 8(18):2100275
Elferchichi M, Mercier J, Coisy-Quivy M, Metz L, Lajoix AD, Gross R, Belguith H, Abdelmelek H, Sakly M, Lambert K (2010) Effects of exposure to a 128-mT static magnetic field on glucose and lipid metabolism in serum and skeletal muscle of rats. Arch Med Res 41(5):309–314
Elferchichi M, Mercier J, Bourret A, Gross R, Lajoix AD, Belguith H, Abdelmelek H, Sakly M, Lambert K (2011) Is static magnetic field exposure a new model of metabolic alteration? Comparison with Zucker rats. Int J Radiat Biol 87(5):483–490
Falanga V (2005) Wound healing and its impairment in the diabetic foot. Lancet 366(9498):1736–1743
Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V (2019) Diabetic neuropathy. Nat Rev Dis Prim 5(1):41
Feng CL, Yu B, Song C, Wang JJ, Zhang L, Ji XM, Wang Y, Fang YW, Liao ZC, Wei M, Zhang X (2022) Static magnetic fields reduce oxidative stress to improve wound healing and alleviate diabetic complications. Cells 11(3):443
Forbes JM, Cooper ME (2013) Mechanisms of diabetic complications. Physiol Rev 93(1):137–188
Gorczynska E, Wegrzynowicz R (1991) Glucose-homeostasis in rats exposed to magnetic-fields. Investig Radiol 26(12):1095–1100
Gulcelik NE, Bayraktar M, Caglar O, Alpaslan M, Karakaya J (2011) Mortality after hip fracture in diabetic patients. Exp Clin Endocrinol Diabetes 119(7):414–418
Hashish AH, El-Missiry MA, Abdelkader HI, Abou-Saleh RH (2008) Assessment of biological changes of continuous whole body exposure to static magnetic field and extremely low frequency electromagnetic fields in mice. Ecotoxicol Environ Saf 71(3):895–902
Hayek A, Guardian C, Guardian J, Obarski G (1984) Homogeneous magnetic fields influence pancreatic islet function in vitro. Biochem Biophys Res Commun 122(1):191–196
Hofbauer LC, Busse B, Eastell R, Ferrari S, Frost M, Müller R, Burden AM, Rivadeneira F, Napoli N, Rauner M (2022) Bone fragility in diabetes: novel concepts and clinical implications. Lancet Diabetes Endocrinol 10(3):207–220
Janghorbani M, Van Dam RM, Willett WC, Hu FB (2007) Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 166(5):495–505
Jing D, Shen GH, Cai J, Li FJ, Huang JH, Wang YQ, Xu QL, Tang C, Luo EP (2010) Effects of 180 mT static magnetic fields on diabetic wound healing in rats. Bioelectromagnetics 31(8):640–648
Lahbib A, Elferchichi M, Ghodbane S, Belguith H, Chater S, Sakly M, Abdelmelek H (2010) Time-dependent effects of exposure to static magnetic field on glucose and lipid metabolism in rat. Gen Physiol Biophys 29(4):390–395
Lahbib A, Ghodbane S, Louchami K, Sener A, Sakly M, Abdelmelek H (2015a) Effects of vitamin D on insulin secretion and glucose transporter glut2 under static magnetic field in rat. Environ Sci Pollut Res Int 22(22):18011–18016
Lahbib A, Ghodbane S, Maaroufi K, Louchami K, Sener A, Sakly M, Abdelmelek H (2015b) Vitamin D supplementation ameliorates hypoinsulinemia and hyperglycemia in static magnetic field-exposed rat. Arch Environ Occup Health 70(3):142–146
László JF, Szilvási J, Fényi A, Szalai A, Gyires K, Pórszász R (2011) Daily exposure to inhomogeneous static magnetic field significantly reduces blood glucose level in diabetic mice. Int J Radiat Biol 87(1):36–45
Lavery LA, Hunt NA, Ndip A, Lavery DC, Van Houtum W, Boulton AJM (2010) Impact of chronic kidney disease on survival after amputation in individuals with diabetes. Diabetes Care 33(11):2365–2369
Li Q, Fang YW, Wu NZ, Gu LL, Li HX, Liao ZC, Liu MY, Fang ZC, Zhang XY (2020) Protective effects of moderate intensity static magnetic fields on diabetic mice. Bioelectromagnetics 41(8):598–610
Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJG, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E (2012) 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 54(12):e132–e173
Lv HH, Liu JY, Zhen CX, Wang YJ, Wei YP, Ren WH, Shang P (2021) Magnetic fields as a potential therapy for diabetic wounds based on animal experiments and clinical trials. Cell Prolif 54(3):e12982
Magliano DJ, Boyko EJ, Balkau B, Barengo N, Barr E, Basit A, Bhata D, Bommer C, Booth G, Cariou B, Chan J, Chen H, Lei ChenDodd S (2021) IDF diabetes atlas. International Diabetes Federation, Brussels
Mao LB, Guo ZX, Wang HQ, Wu QY, Zhang TC (2015) Exposure to static magnetic fields affects insulin secretion in INS cells. Springer, Singapore
Mao LB, Wang HQ, Ma FH, Guo ZX, He HP, Zhou H, Wang N (2017) Exposure to static magnetic fields increases insulin secretion in rat INS-1 cells by activating the transcription of the insulin gene and up-regulating the expression of vesicle-secreted proteins. Int J Radiat Biol 93(8):831–840
Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H (2001) Mortality and causes of death in the who multinational study of vascular disease in diabetes. Diabetologia 44(2):S14
Rabe OC, Winther-Jensen M, Allin KH, Svendsen OL (2021) Fractures and osteoporosis in patients with diabetes with Charcot foot. Diabetes Care 44(9):2033–2038
Retzepi M, Donos N (2010) The effect of diabetes mellitus on osseous healing. Clin Oral Implant Res 21(7):673–681
Sakurai T, Terashima S, Miyakoshi J (2009) Effects of strong static magnetic fields used in magnetic resonance imaging on insulin-secreting cells. Bioelectromagnetics 30(1):1–8
Shang WL, Chen GL, Li YX, Zhuo YJ, Wang YH, Fang ZC, Yu Y, Ren HW (2019) Static magnetic field accelerates diabetic wound healing by facilitating resolution of inflammation. J Diabetes Res 2019(2):1–11
Sihem C, Hafedh A, Mohsen S, Marc PJ, Khmais BR (2006) Effects of sub-acute exposure to magnetic field on blood hematological and biochemical parameters in female rats. Turk J Hematol 23(4):182–187
Tian XF, Wang DM, Zha M, Yang XX, Ji XM, Zhang L, Zhang X (2018) Magnetic field direction differentially impacts the growth of different cell types. Electromagn Biol Med 37(2):114–125
Wang H, Ba Y, Xing Q, Du JL (2019) Diabetes mellitus and the risk of fractures at specific sites: a meta-analysis. BMJ Open 9(1):e024067
Weintraub MI, Wolfe GI, Barohn RA, Cole SP, Parry GJ, Hayat G, Cohen JA, Page JC, Bromberg MB, Schwartz SL (2003) Static magnetic field therapy for symptomatic diabetic neuropathy: a randomized, double-blind, placebo-controlled trial. Arch Phys Med Rehabil 84(5):736–746
Yang X, Li Z, Polyakova T, Dejneka A, Zablotskii V, Zhang X (2020) Effect of static magnetic field on DNA synthesis: the interplay between DNA chirality and magnetic field left-right asymmetry. FASEB Bioadv 2(4):254–263
Yang X, Song C, Zhang L, Wang J, Yu X, Yu B, Zablotskii V, Zhang X (2021) An upward 9.4 T static magnetic field inhibits DNA synthesis and increases ros-p53 to suppress lung cancer growth. Transl Oncol 14(7):101103
Yu B, Liu JJ, Cheng J, Zhang L, Song C, Tian XF, Fan YX, Lv Y, Zhang X (2021) A static magnetic field improves iron metabolism and prevents high-fat-diet/streptozotocin-induced diabetes. Innovations 2(1):100077
Zhang J, Ding C, Ren L, Zhou YM, Shang P (2014) The effects of static magnetic fields on bone. Prog Biophys Mol Biol 114(3):146–152
Zhang H, Gan L, Zhu XQ, Wang J, Han LC, Cheng P, Jing D, Zhang XD, Shan QS (2018) Moderate-intensity 4 mT static magnetic fields prevent bone architectural deterioration and strength reduction by stimulating bone formation in streptozotocin-treated diabetic rats. Bone 107:36–44
Zhao J, Li YG, Deng KQ, Yun P, Gong T (2017) Therapeutic effects of static magnetic field on wound healing in diabetic rats. J Diabetes Res 2017:6305370
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Feng, C., Yu, B., Zhang, X. (2023). Effects of Static Magnetic Fields on Diabetes and Its Complications. In: Zhang, X. (eds) Biological Effects of Static Magnetic Fields. Springer, Singapore. https://doi.org/10.1007/978-981-19-8869-1_10
Download citation
DOI: https://doi.org/10.1007/978-981-19-8869-1_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8868-4
Online ISBN: 978-981-19-8869-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)