Abstract
Chronic pulmonary diseases often include inflammation in the upper or lower respiratory tract causing severe/extreme discomfort. To subside inflammation, currently, small molecules are administered to patients. Nano-based therapies have the potential to replace drugs by significantly increasing efficacy and decreasing dosage for long-term relief. In this chapter, we discuss the various nanotherapeutics being employed in preclinical and clinical settings to ameliorate inflammation. We also describe different nano-drug delivery systems used for diverse modes of treatment. To specifically target pulmonary inflammation, nanoparticles developed and tested via inhalation are also discussed. Recent developments summarizing the last 20 years for a variety of pulmonary diseases are explored. Although these nano-based studies are promising, long-term toxicity and clearance strategies are still debated and must be investigated.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction to Inflammation in the Respiratory System
It is hard to imagine life without respiration. Yet, millions of lives are lost to respiratory ailments each year. Science has progressed enough to identify the causes and symptoms of respiratory diseases. The real challenge is to eliminate these pathological conditions and alleviate the suffering of a patient. Understanding respiratory diseases in a better way take us to history; many pieces of evidence point to the existence of these illnesses as far back as 10,000 years ago (Comas et al. 2013). The long history of humanity has allowed pathogens to coexist in our immediate community. However, infection or affliction by disease does not always explain discomfort during respiration. The human body’s evolved response against a pathological disturbance may create emergent symptoms in an individual. The host immune response against a pathological disorder within the pulmonary system has been well characterized over several decades of medical history. Inflammation is one of the many articulated and cascaded pathways among these. Still, due to pathogen-induced subversion of inflammation, prior genetic conditions, smoking, hypersensitivity, and coexisting infections, it is often dysregulated in individuals, leading to more severe disorders accompanying the original (Herbst et al. 2008). The presence of lifestyle diseases such as obesity, hypertension, and type 2 diabetes mellitus in modern times exacerbates inflammation in the human body, leading to extended periods of discomfort and multiple disease complications (Sharma et al. 2019; Rodríguez-Hernández et al. 2013).
Inflammation is primarily mediated by an imbalance in the Th1 or Th2 immune response against a pathological condition, elevated levels of pro-inflammatory cytokines such as TNFα and IL-1β result in increased susceptibility to diseases such as tuberculosis (Piergallini and Turner 2018). To combat inflammation in the lungs arising due to a pathological condition, drugs must be delivered in a manner that does not affect homeostasis and causes fewer or no side effects. Nanotechnology has previously been employed for purposes of better deliverability of drugs to the target site, sustained release, better solubility of hydrophilic drugs, and many others (Alam et al. 2014; Azarmi et al. 2008). The variety and easy manipulation offered by nanotechnology in therapeutics make it an application-based science, which is essential for therapeutics in today’s world. In this chapter, we discuss and elaborate on the inflammatory mechanisms in the lung correlated to some of the most common afflictions in humans, along with a detailed description of nanotechnology developments to tackle unwanted inflammation and the pathological condition itself (Fig. 11.1). Further, we address knowledge gaps and important discoveries that can aid nanoscience in the construction of newer, multipurpose devices that help in the elimination of disease and reduce lung inflammation.
11.2 Asthma: Improving Nanotherapeutics for Longer Relief
Asthma is a chronic, non-communicable respiratory disease originating from the downstream effects of several genetic polymorphisms interacting with a reaction-inducing environment (Wang et al. 2019). A comprehensive study conducted from 1990 to 2010 noted that asthma is prevalent in over 330 million people worldwide (Vos et al. 2012). Smoking, increase in air pollution, and frequent occurrence of smog-like weather with increased particulate matter in the lower strata of the atmosphere are important risk factors for asthma after infancy, especially in genetically susceptible individuals (Ober and Vercelli 2011). The term “asthma” is used for a set of clinically diagnosable characteristics, which includes inflammation of the bronchial passages (Wenzel 2012). Conventionally, early-onset asthmatic individuals are given symptomatic relief by inhalable vaporized β2-adrenoreceptor agonists using a metered-dose inhaler. Newer drugs targeting inflammatory symptoms via systemic or inhalation routes have also been developed (Keil et al. 2020).
Nanotherapeutics in the context of asthma is an oxymoron. There are many pieces of evidence of inhaled environmental and occupational nanoparticles creating a chronic inflammatory response within the pulmonary system (Lu et al. 2014; Ferreira et al. 2013; Qiao et al. 2015). In 2005, PM10 was closely correlated with daily hospital admissions for asthma, acute or chronic bronchiolitis, and lower respiratory tract infections. However, in the same study, it was also noted that nanoparticles (NPs) have a larger surface area than bigger particles and penetrate deep into the respiratory tract (Inoue et al. 2005). Therefore, studies aim to target inflammation using micro- and nano-scale particles, devices, and medicine. Initially, experiments focused on micro-particle delivery to the pulmonary system. Shifting from metered-dose inhalers to dry powder inhalers provides benefits of ease-of-use, multidose capability, and greater chemical stability of the drug (Porta et al. 2005). These rapidly gave way to the development of new nano-based therapeutic technologies against pulmonary inflammation in asthma.
11.2.1 Nucleic Acid Supplementation
Recent advances in nanotechnology have made direct pulmonary delivery possible. Nucleic acid supplementation is an important and upcoming field in this respect (Kaczmarek et al. 2017). Gene therapy technology is known for its longer effectiveness. To develop NPs for alleviating asthmatic inflammation, cationic lipid NPs were conjugated with locked nucleic acid oligonucleotides to target miR-154, a sequence identified in regulating asthma of mouse models. Conclusively, the study correlates miR-154 nanoparticle treatment of asthmatic mice with the downregulation of multiple cytokine and chemokine genes associated with inflammation (Ramelli et al. 2020). Targeting transcription factors provides yet another route. Keil et al. attempted downregulation of GATA3, which is essential for the control of inflammatory processes. To stably transport a negatively charged GATA3-silencing siRNA to activated T cells, polyethyleneimine (PEI), a positively charged polymer, was conjugated with transferrin (Tf) for uptake to the T cells. However, due to the inability of PEI to cross the mucus barrier, these nano-in-micro particles are being tested in vivo with oligospermine instead for biological compatibility, as well as spray-drying characteristics to develop a bench-to-bedside formulation (Keil et al. 2020). da Silva and colleagues developed murine models to test the efficiency of thymulin gene-loaded biodegradable NPs for therapeutic relief from asthma. Mice treated with the NPs indicated a reversal of inflammatory processes and a significant reduction of eosinophil counts, CXCL1, CCL11 (BALF chemokines), and M2 macrophage counts. However, discontinuous mouse exposure to the allergen as compared to human asthma may play a critical role in demonstrating effectiveness in humans (da Silva et al. 2020).
11.2.2 Plant-Derived Nanotherapeutics
An alternative approach to the development of nanotherapeutics for asthma is the introduction of plant products. Quercitin and celastrol are examples of plant-derived hydrophobic molecules introduced into liquid crystalline NPs and their in vitro characteristics have been studied. Effects on cytokine suppression were observed to conclude that these NPs could be a potential nano-based drug therapy to reduce inflammation in asthma (Cherk Yong et al. 2019; Chan et al. 2020). Other plant-derived products such as the extract of Eriobotrya japonica leaves and Hyssopus cuspidatus Boriss can be employed in the NPs to alleviate inflammation (Kim et al. 2020; Yuan et al. 2019).
11.2.3 Drug Delivery Systems
NPs alone may not be the solution. Deposition of particles into the deep lungs requires a diameter of 1–5 μm, which can be achieved by encapsulating NPs in a microgel easily dissolvable in the lower lung environment, targeting cells by introduction of enzyme-responsive crosslinkers on their surface. These nano-in-microgel particles were designed as a drug delivery system and had a retention time of several hours followed by clearance in a murine model (Mejías and Roy 2019). NPs as drug delivery systems were previously coupled with salbutamol sulfate, the current mainstay for asthmatic relief. Newer technologies include liposomal delivery vehicles, which are biodegradable, safe, and sustainable as inhalable technology. Synthetic liposomal carriers show enhanced persistence in the lungs of mice (up to 18 h), whereas free salbutamol sulfate was retained for about 8 h (Yang et al. 2012). Liposome bilayers shield less soluble molecules such as curcumin from the pulmonary environment and provide a mechanism for sustained release in vitro (Ng et al. 2018). Curcumin was also part of hydrogel microspheres encapsulating PLGA NPs to be used as a delivery system to the lungs. El-Sherbiny and Smyth concluded that these particles could evade the rapid phagocytosis by macrophages, although in vivo studies were not carried out (El-Sherbiny and Smyth 2012).
As an example of a prescription drug going back to the bench, montelukast is a leukotriene receptor antagonist that binds to the CysLT1 receptor. Nanostructured-lipid carriers encapsulating montelukast can be an alternative to conventional montelukast tablets for temporary relief. These nanostructures were tested to be safe in lung epithelial cell line A549; increased bioavailability, higher lung deposition, greater residence time, and slow release of the drug by the nanocarrier were reported (Patil-Gadhe et al. 2014). Another drug delivery system utilized the tea-based compounds theophylline or budesonide for long-term relief from asthma. A potent bronchodilator, theophylline, is commonly recommended to asthma patients for late-stage management; however, it has a narrow therapeutic range. Buhecha et al. studied the loading efficiency of budesonide and theophylline into mono-encapsulated and co-encapsulated PLA NPs as well as their sustained release to lung cell lines (Buhecha et al. 2019). Prior studies also evaluated the role of cyclosporine A as an option for inhalation therapy (Sato et al. 2016).
11.2.4 Peptide Nanoparticles
Peptides and other biologics are increasingly employed as first-line drugs. Similarly, nanotechnology is increasingly employing peptides and/or whole proteins. For example, the overactive Th2 response in asthma induces a higher concentration of IL-4 and IL-13. Both IL-4 and IL-13 share the IL-4α subunit in their structures which presents itself as a target for nanotherapeutic development. Halwani et al. synthesized an anti-IL-4α NP formulated using superparamagnetic iron-oxide NPs that could control inflammation in ovalbumin-sensitized mice (Halwani et al. 2016). Athari et al. developed a PLGA nano delivery system for the anti-inflammatory vasoactive intestinal peptide. Their study was limited to in vitro observations and in vivo characteristics of the particles remain to be tested (Athari et al. 2016). However, peptide mimetics can be associated with adverse effects on high doses of administration. Gene therapy of specific peptides might be useful in this respect. To combat Th2-mediated airway inflammation, a chitosan NP incorporating the IFN-g gene into plasmid DNA was administered intranasally to mice models. By promoting a Th1-type response and balancing the immune reaction, airway inflammation was successfully reduced in mice via a STAT4 signaling pathway (Kumar et al. 2003). The challenges accompanying asthma treatments are slowly being overcome by nanotechnological advances. The development of newer systems to counteract inflammatory reactions in asthma patients due to an imbalanced Th2 response to allergens is encouraging. A greater number of microbubble- and hydrogel-based technologies incorporating natural products also provide a positive outlook (Corthésy and Bioley 2017; Secret et al. 2014).
11.3 Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD), a combination of chronic bronchitis and emphysema, is a chronic inflammatory lung disease that results in obstructed airflow to and from the lungs and difficulty in breathing. According to the Global Burden of Disease Study, 251 million cases of COPD were reported globally in 2016, and around 3.17 million fatalities were estimated in 2015 (Soriano et al. 2020). Characterized by an abnormal inflammatory response, COPD patients suffer from chronic inflammation of the airways and damaged alveoli of the lungs (Hogg et al. 2004). Being a progressive disease that worsens over time, COPD is among the leading causes of death due to respiratory illnesses. Cigarette smoking (CS) is known to be the primary risk factor for COPD, and the immune response is mainly driven by inflammatory cells such as macrophages, neutrophils, and T cells (Zuo et al. 2014). Smoking elevates lavage iron and ferritin levels in the lungs which produce oxidative stress leading to inflammation (Ghio et al. 2008). Other secondary risk factors, including long-term exposure to inhaled noxious particles, chemicals, or gases, also contribute to COPD pathogenesis (Boschetto et al. 2006).
11.3.1 No Smoke without Fire: Inflammatory Aspect
COPD is associated with inflammatory mucus accumulation, disruption of the epithelial barrier, widespread damage to the bronchial epithelium, and lung parenchymal tissue destruction. The small airway morphology in a COPD patient shows thickened airway wall, narrowed lumen along with mucus, and cellular debris (Baraldo et al. 2012). This obstruction occurs due to the surge of tissue volume of the bronchial walls, which occurs due to the infiltration of macrophages, neutrophils, CD4, CD8, and B lymphocytes. Alveolar macrophages show impaired phagocytosis of non-typeable Haemophilus influenzae, which increases the severity of the disease by complementing bacterial colonization (Berenson et al. 2013). A complex network of inflammatory cytokines, reactive oxygen species (ROS), and proteases are produced by phagocytes and epithelial cells damaging lung tissues. Cigarette smoking elevates the expression of TNFα, IL-1, IL-6, and reduces the expression of the anti-inflammatory cytokine IL-10 (King 2015) (Fig. 11.2).
Schematic representation of a diseased COPD alveolus. The bronchial epithelium begins to slough off, while the cavity of each alveolus reduces due to wall thickening. The alveolus itself becomes a host for pro-inflammatory processes resulting in bacterial infections and severe blockage to gaseous exchange
Recent research has shown that autoimmunity linked to emphysema is also among the most commonly associated factors with inflammation in COPD. Deficiency of alpha 1-antitrypsin (A1AD) due to a mutation in the SERPINA1 gene resulted in increased release of proteases like neutrophil elastase and decreased production of inhibitors (alpha 1-antitrypsin), leading to disruption of lung tissues (Alvarado 2018). Current therapeutic options with long-term adherence can control the symptoms but do not cure the underlying disease. The most commonly followed treatment includes antioxidant therapeutic agents like N-acetyl-l-cysteine (NAC), and Nrf2 activators to reduce oxidative stress in the body during COPD. Other treatment strategies include corticosteroids, bronchodilator inhalers, anticholinergics, mucolytics, bronchodilator tablets, and even gene therapy (Vogelmeier et al. 2017). Nano-based therapeutics such as drug delivery systems overcome major challenges like low diffusion rate, mucociliary clearance, acute inflammation, and blocked airways which are generally encountered by conventional drug delivery treatments (da Silva et al. 2017).
11.3.2 Optimal Nanoparticle Characteristics
Nanocarriers for efficient drug release need to be optimized, like conjugation with specific ligands, to enhance the targeted delivery and therapeutic effect of nanomedicine. AuNPs have shown enhanced epithelial targeting in mice with COPD/emphysema and can be utilized for targeting alveolar epithelial cells and macrophages (Geiser et al. 2013). Similar to cystic fibrosis, nanoparticles to be used as nanocarriers should be small-sized with a negative surface charge along with surface modifications that facilitate easy penetration through a highly thick and viscoelastic mucus layer. Mucus also acts as a barrier to inhaled gene therapy for respiratory illnesses like cystic fibrosis and COPD which can be overcome with the involvement of nanoparticles (Duncan et al. 2016). Li et al. used black phosphorus quantum dots (BPQDs) along with PEGylated chitosan nanospheres which facilitate the delivery of antibiotic amikacin through the mucus layer. BPQDs, being biocompatible and biodegradable, play a significant role in enhancing amikacin delivery to the lungs (Li et al. 2020). Cerium oxide nanoparticles mimic the activity of superoxide dismutase (SOD) and catalase due to their tendency to coexist either in a reduced or oxidized state and act as a potential nanozyme to treat oxidative stress in COPD (Passi et al. 2020). Bhushan et al. synthesized biocompatible cerium oxide nanoparticles encapsulated in albumin nanoparticles that subsided intracellular ROS (Bhushan and Gopinath 2015).
In an ex vivo model of COPD, the antioxidant and anti-inflammatory activity of N-acetylcysteine (NAC) was demonstrated (Cazzola et al. 2017). NAC acts as a mucolytic agent by loosening thick mucus in airways in patients with COPD or cystic fibrosis, but it displays low bioavailability (6–10%), limiting its therapeutic potential. To refine its therapeutic effect, nanoparticles that can stabilize it inside the body, increasing its bioavailability are being developed. Lancheros et al. accomplished the NAC-loaded PLGA nanoparticles by nanoprecipitation method, which is simple, economical, and results in the increased load capacity and efficient entrapment of the compound (Lancheros et al. 2018). Muralidharan et al. reported for the first time the use of therapeutic dry powder inhalers carrying micro or nanoparticulate powders of dimethyl fumarate (DMF) that can be administered using a DPI device. DMF is a nuclear transcription factor, Nrf2 activator, and has shown both antioxidant and anti-inflammatory properties targeting the lung Nrf2/Keap1 pathway to treat pulmonary inflammation. Nrf2 (Transcription factor nuclear factor erythroid 2-related factor) triggers cellular protection against inflammatory or oxidative stress in lung cells. Their administration via DPIs has shown excellent aerosol dispersion performance and enhanced penetration to lower airways (Muralidharan et al. 2016). NPs have also been employed for early and better diagnosis of COPD. In a study by Faraj et al., MR imaging coupled with antibody-conjugated superparamagnetic nanoparticles was developed for targeting a specific macrophage subpopulation which offers an attractive approach for timely diagnosis (Al Faraj et al. 2014). The narrow safety range of theophylline can cause adverse systemic side effects if administered in high doses hence nano-based sensors are also developed for detection in drug analysis. One such electrochemical sensor was developed by the fabrication of poly-sulfosalicylic acid on carbon fibers to detect theophylline level (Duan et al. 2021).
11.3.3 Inhalation Therapy
Administration of pharmaceuticals for the treatment of lung disease by inhalation show certain advantages over orally given drugs in terms of targeted delivery, decreased side effects, and higher retention and their incorporation with nanotechnology can further enhance the efficacy of treatment (Kuzmov and Minko 2015). Novel nano-based theranostics that can offer a real-time diagnosis of COPD along with drug delivery can be an enticing invaluable approach for tackling COPD (Vij 2011). Furthermore, nanoparticles can also be incorporated into dry powder microparticles (NCMPs) to facilitate their deposition in the lungs. NCMPs have been developed for carrying miR-146a with PFA-co-PDL nanoparticles. Micro RNAs are short, regulatory, non-coding RNAs involved in the pathogenesis of COPD (Ebrahimi and Sadroddiny 2015). A study indicated the role of miR-146a in the severity of disease and differences observed in miRNA expression in COPD patients versus healthy individuals (Pottelberge et al. 2011). The expression level of miRNA was found to be 2.5-fold lower in patients, which results in overexpression of the cyclooxygenase 2 gene and in turn, prostaglandin E2 production in fibroblasts contributing to chronic inflammation of the pulmonary system (Sato et al. 2010). Hence, the administration of miR-146a by spray drying of NCMPs has emerged as an attractive therapy in the management of COPD (Mohamed et al. 2019). Differential expression analysis of other miRNAs such as miR-223/1274a, miR-1, miR-150, and let-7c have also been tested, but a more vigorous understanding of disease complexity and relevance of these miRNAs is required (Ezzie et al. 2012; Fujita et al. 2013). In a recent finding, it was found that miR-155 expression is increased in lung tissues and alveolar macrophages in CS-induced inflammation and COPD, which can also serve as a new therapeutic tool for COPD treatment (De Smet et al. 2020).
Recent studies by Beyeler et al. have revealed that nanomaterials like multi-walled carbon nanotubes are responsible for the polarization of alveolar macrophages toward pro-inflammatory M1 phenotype and affect the pulmonary mucosal immune cells in mouse model studies (Beyeler et al. 2020). This can cause increased susceptibility to adverse side effects in COPD patients and therefore marks the importance of clinical assessment for the toxicity of nanomaterials. COPD is estimated to be the world’s third leading cause of death; hence, it should be among the prime focuses of the scientific community (Soriano et al. 2020). Efficient drugs with high bioavailability, increased stability, and strongly targeted to the site of action need to be developed to combat this complication. Nanotechnology is a potent tool to accomplish the shortcomings faced by conventional drugs. More than a billion people smoke globally on a daily basis and cigarette smoking is the primary risk factor that poses a significant risk for airway blockage and the development of COPD.
11.4 Crossing Mucus in Cystic Fibrosis
Cystic fibrosis (CF) is a chronic genetic disease affecting nearly 70,000 patients worldwide (Cystic Fibrosis Foundation 2018; Velino et al. 2019). Characterized by the build-up of thick, sticky mucus that can damage many of the body’s organs, CF is an autosomal recessive disease caused by defects in the CFTR gene, encoding the cystic fibrosis transmembrane conductance regulator (CFTR) protein (Turcios 2005). A membrane protein, CFTR forms a chloride channel for regulating transport across the membrane of epithelial cells in pulmonary, gastrointestinal, renal, and male reproductive tissues (Vankeerberghen et al. 2002). It regulates the secretion of chloride ions out of cells producing mucus, sweat, tears, saliva, and digestive enzymes thereby controlling fluid flow across epithelial cell membranes (Rey et al. 2019). More than 1900 mutations are described since the discovery of CFTR in 1989 (Kerem 1989; Rowe and Verkman 2013). Among all the mutations, the most common is a 3-bp deletion, F508del, preventing protein movement to the cell surface (Kälin et al. 1999). 84.7% of individuals in the CF Foundation Patients Registry have at least one copy of this mutation (Cystic Fibrosis Foundation 2018).
Mutations of the CFTR gene lead to impaired Cl− ions secretion and overabsorption of Na+ ions, resulting in an imbalance of ion concentration across the cell membrane. As a result, cells lining lung passageways produce mucus that is usually thick, sticky, and difficult to clear, and in the end, ducts become plugged and atrophic. Chronic airway infection, progressing to bronchiectasis, persistent high-intensity inflammation in lung epithelium, gas trapping, hypoxemia, and hypercarbia, ultimately damage leading to respiratory failure are the hallmarks of CF lung disease. Neutrophils are the first cells migrating into the pulmonary compartment, releasing oxidants and proteases like elastase, which eventually overwhelm the antiprotease capacity of the lungs, leading to bronchiectasis (Cantin et al. 2015). IL-10, anti-inflammatory cytokine production by bronchial epithelium cells, is downregulated in CF airways, which may contribute to enhancing local inflammation and tissue damage (Bonfield et al. 1995). On cell death, DNA released by neutrophils also increases mucus viscosity leading to airway obstruction (Elizur et al. 2008). CF causes breathing difficulties, recurrent lung infections, persistent bacterial infections (particularly Pseudomonas aeruginosa), edema, progressive impairment of lung airways, malnutrition, chronic endobronchial inflammation, pancreatitis, and death (O’Sullivan and Freedman 2009).
11.4.1 Nano-Based Therapies
Presently, treatment strategies used include CFTR modulators (potentiators, correctors, and amplifiers) that restore CFTR functions, mucociliary clearance, antibiotics, anti-inflammatory, gene therapy, etc. (Edmondson and Davies 2016). The disadvantage of current treatments is their inability to reach the site of action due to thick mucus (Velino et al. 2019). The use of NPs for CF creates new perspectives to counter mucus formed within the alveolus and eliminate resulting bacterial infections. The mucus layer is a mesh structure composed of highly cross-linked mucin-fibers, cytoskeletal fragments of actin, and DNA along with other macromolecules creating hydrophobic and electrostatic barriers, reducing drug delivery efficacy (Duncan et al. 2016). Viscoelasticity is increased dramatically due to changes in mucus structure and composition in CF patients with mesh size reduced to 100–400 nm in size as compared to >500 nm in healthy individuals (Yaakov et al. 2007). Small NPs bypass steric hindrance effects and diffuse through the mucus network. Electrostatic interactions can be countered by coating NPs with electrostatically neutral molecules or muco-inert polymers such as polyethylene glycol (PEG) or by using mucolytic agents like N-acetylcysteine (NAC) (Ong et al. 2019). Suk et al. illustrated that PEGylation for 200 nm particles increased penetration into the mucosal barrier in CF sputum pretreated with NAC and also increased NP mobility in Burkholderia multivorans and Pseudomonas aeruginosa biofilms (Suk et al. 2011).
Mucus penetrating particles (MPPs), used as vehicles for drug delivery, possess nonadhesive coatings to rapidly penetrate mucus through pores in the mesh at rates similar to pure water. Highly compacted DNA NPs with block copolymers of poly-l-Lysine and PEG have been shown to mediate effective gene transfer (Ensign et al. 2012). The molecular weight of PEG should be sufficiently less to avoid adhesive interactions with mucins, and PEG coating density should be appropriate to effectively shield or protect the hydrophobic NP core. The conformation should also be taken into account; brush-like PEG facilitates penetration while mushroom conformation increases the time of an adhered fraction of particles on the mucus layer (citation). Another approach is the formation of nano-embedded microparticles (NEMs). In an interesting article, Porsio et al. produced PEGylated, and Transactivating transcriptional activator peptide (Tat)-decorated FNPs linked to PHEA-PLA to deliver Ivacaftor to the pulmonary epithelial cells across the mucus barrier and promote lung cellular uptake of the drug. FNPs showed proper nanometric sizes (~70 nm), slightly negative ζ potential (~ −12 mV), and high cytocompatibility. Tat, a cell-penetrating peptide (CPP), strongly enhanced the uptake of FNPs by lung epithelial cells. Moreover, nano into micro strategy was applied by encapsulating NPs into Matryoshka microparticles, inhalable by dry powder inhalers (DPI) devices to achieve an inhalator therapy (Porsio et al. 2018).
The rapid progress of nanomedicine enhances the efficacy of inhalation treatment for CF. Alton et al. conducted a randomized, placebo-controlled Phase2b trial (clinicaltrials.gov ID NCT01621867) for the application of pGM169/GL67A gene therapy formulations to CF patients. The GL67A, a cationic lipid mixed with equal amounts of pGM169 drug, was given to patients once a month for a year with a nebulizer. The administration of non-viral CFTR gene therapy gave statistically significant results with improvements in Forced expiratory volume (FEV1), forced vital capacity, and gas trapping (Alton et al. 2015). Over the last decade, different types of NPs like liposomes, polymeric NPs, dendrimers, solid and lipid NPs have been designed as nanocarriers for drug and gene delivery in CF treatment (Table 11.1) (Upadhyay and Ganguly 2015; Mansour et al. 2009).
LIPOSOMES are the most widely used and best characterized lipid-based drug delivery systems, especially for pulmonary applications, as it is prepared primarily from phospholipids, which are inherent in the lungs. Liposomes can entrap both lipophilic and hydrophobic drugs due to their amphiphilic nature. Studies have shown that drugs encapsulated within cationic liposomes exhibit greater antibacterial efficacy since they target bacterial biofilms via electrostatic interactions thus allowing drug release close to the pathogen (Messiaen et al. 2013). But they were found to be toxic to human lung cells and can introduce genetic aberrations (Shah et al. 2013). Liposomes have been used to develop antibiotics. Inhalation formulation of Amikacin liposome consisting of neutral liposomes (DPPC:Chol) completed Phase III of clinical trials and was approved by FDA in 2018 (ClinicalTrials.gov ID NCT03905642) (Paranjpe and Müller-Goymann 2014).
SOLID LIPID NANOPARTICLES (SLNs) are lipophylic particulate colloidal drug delivery systems comprised of solid lipids with mean diameters ranging in size between 50 and 1000 nm. Various drugs for CF have been encapsulated within SLNs as it provides physical stability and low cytotoxicity. Amikacin-loaded SLNs were developed previously (Varshosaz et al. 2013), and the results showed sustained release and increased efficacy of the drug with respect to free drugs. Another interesting approach for the treatment of Pseudomonas aeruginosa infection in CF utilizes SLN loaded with quorum sensing inhibitor. The SLN penetrated artificial sputum and also demonstrated a sevenfold higher anti-virulent effect in comparison to free compounds (Nafee et al. 2014).
DENDRIMERS are ordered, highly branched structures synthesized and studied for biomedical applications. Dendrimers for pulmonary therapies are developed as both inhalable suspensions and dry powders. Considering gene therapy, Agnoletti et al. prepared dendrimer-siRNA nanocomplexes, processed into microparticle-based dry powders for inhalation, which showed enhanced cellular uptake and gene silencing efficiency (Agnoletti et al. 2017). Brockman et al. developed a polyamidoamine (PAMAM) dendrimer with terminal groups modified to obtain Cysteamine-like structure (Cysteamine is an FDA-approved drug with antioxidant, anti-biofilm, and mucolytic properties) to reduce Pseudomonas aeruginosa infection in addition to preventing delF508-CFTR sequestration to aggresome bodies (Brockman et al. 2017).
POLYMERIC NANOPARTICLES, a major class of nanotherapeutics widely used as drug delivery systems, are highly versatile, having the ability to a prolonged and controlled drug release, stabilize encapsulated drugs and promote cellular uptake (Kuzmov and Minko 2015). PLA and PLGA are widely used because of their biocompatibility and biodegradability. Developing efficient non-viral delivery systems for gene therapy had been a major challenge for CF. Guan et al. developed synthetic peptides able to self-assemble to poloxamines and nucleic acids to form compact and monodisperse NPs. This led to increased expression of both mRNA and plasmid DNA expression in the lungs of CF mice with negligible toxicity thus providing a new strategy for the development of non-viral gene delivery (Guan et al. 2019).
11.5 Tuberculosis: A New Approach
Tuberculosis (TB) is an ancient illness associated with humans (Comas et al. 2013), leading to its description as a “heritage disease” (Cambier et al. 2014a). The causative agent, Mycobacterium tuberculosis (Mtb), has developed strategies to avoid the hosts’ immune responses by removing the need for colonization before infection. Instead, M. tuberculosis manipulates the hosts’ immune system deeper inside the respiratory tract (Cambier et al. 2014b). Although a significant fraction of the population is infected with pulmonary tuberculosis, most of the population is asymptomatic, harboring a very low bacterial load and resulting in a latent disease period. Poor health triggers active infection in 5–10% of the infected individuals, allowing transmission of disease (Piergallini and Turner 2018). The active infection however is often fatal, with an estimated 1.7 million deaths from TB in 2019 (World Health Organization 2019).
11.5.1 Subverted Inflammation in Tuberculosis
Initial latent infection of the Mtb bacteria occurs ca. 80% in the lungs, serving as the entry and exit points of transmission of the disease (Kaufmann and Dorhoi 2013). On contact with the immune system, controlled inflammation is the first line of response. Macrophages are the primary target of Mtb, where it lives in a modified membrane-bound vacuole—altered Rab GTPase composition, increased pH, and presence of the protein TACO are primary characteristics (Glickman and Jacobs 2001). Current knowledge indicates that Mtb can modulate processes such as the production of the pro-inflammatory cytokines IL-1, TNFα, and interferons. IL-1 production requires caspase-1 and inflammasome complex activity, while also regulating TNFα synthesis and TNFR expression. TNFα, on the other hand, can be modulated directly or indirectly via eicosanoids by Mtb. A positive feedback loop between TNFα and IL-1 works to the benefit of the bacterium, creating an intensely pro-inflammatory environment (Kaufmann and Dorhoi 2013). Further, IFNγ forces T cells into apoptosis while lymphocyte activation is downregulated (Cooper et al. 2002). Inflammatory granuloma formation in tuberculosis is a disease hallmark, with the observation of the granuloma replacing functional tissues, while also being necrotic and damaging surrounding cells (Cooper et al. 2002). It is evident that inflammatory responses in tuberculosis should be controlled to surmount a significant response.
11.5.2 Emerging Nanotherapeutics
Although antitubercular drugs have been available via prescription for the last two decades, patient noncompliance due to extended treatment is a common cause of treatment failure. Biocompatible NPs encapsulating current drugs showed promise with improved bioavailability. Other advances include the altered route of administration, compatibility with hydrophilic and hydrophobic drugs, increased dosage capacity, and better stability (Gelperina et al. 2005). As an example, Pandey et al. described a poly-(dl-lactide-co-glycolide) (PLG) nanoparticle system containing rifampicin, isoniazid, or pyrazinamide for inhalable therapy against tuberculosis (Pandey 2003; Azarmi et al. 2008). Sustained release is also one of the main goals for scientists currently developing better treatments (Gelperina et al. 2005). Considering this, the study detected the presence of rifampicin in the circulation for 4 days and isoniazid and pyrazinamide for 9 days. They also reported a reduction in the treatment period (Pandey 2003). In a similar light, wheat germ agglutinin lectin coated or conjugated onto PLG NPs was used to deliver isoniazid, pyrazinamide, and rifampicin to Mtb-infected guinea pigs. The study noted severe necrosis in the lungs of untreated guinea pigs, while the treated animals had no necrotic regions and no observable hepatotoxicity (Sharma et al. 2004).
11.5.3 Tuberculosis and Drug Resistance
Multidrug treatment is usually prescribed to patients with tuberculosis infections for the prevention of drug resistance. However, long periods of intensive care (6–9 months) and high doses of drugs resulting in health and economic issues often cause patients to opt out of the treatment course. An increase in drop-out rates and drug resistance eventually led to the rise of multidrug-resistant tuberculosis (MDR-TB) (Blasi et al. 2009). A separate category of drugs developed to treat MDR-TB suffers from low effectiveness and higher toxicity. Other problems that mark the treatment of patients include higher doses (125–200 up to 1000 mg/day), even longer administration periods (up to 2 years), low cure rates (60%), and daily intramuscular injections (Blasi et al. 2009). A compilation of several newer formulations against tuberculosis is presented previously (Hussain et al. 2019).
Liposomal carriers easily take up hydrophobic drugs and exhibit low toxicity. In a study, Le Conte et al. described capreomycin liposomes as being effective against Mycobacterium avium infection (Le Conte et al. 1994). A study to increase the content of capreomycin within liposomes was also reported (Ricci et al. 2006). Another report by Adams et al. used clofazimine in liposomes for the treatment of acute and chronic tuberculosis in mice. Their experiments conclusively indicated that treatment with liposome-encapsulated clofazimine reduced mononuclear cell infiltration significantly by localizing the granuloma formation (Adams et al. 1999). Rifabutin is a broad-spectrum anti-mycobacterial agent, particularly in use for its low resistance development against Mtb. To increase the bioavailability of the drug to the lungs, liver, and spleen on intravenous administration, multilamellar liposomal vehicles were developed by Gaspar et al. In vivo studies in BALB/c mice infected with Mtb indicated an increase in drug efficiency and a significant reduction in the lung inflammatory response. Although the liposomes were not effective in reducing bacterial load within the lungs, the liver and spleen sections indicated improvement in colony-forming units as compared to free rifabutin (Gaspar et al. 2008).
11.5.4 Alveolar Macrophages: Aiming for the Heart
The symptoms and duration of active disease widely vary person-to-person in tuberculosis. The activation of inflammatory responses depends on bacterial ligand expression and host cell type. Primarily mediated via MyD88 and TLR2 as observed in murine tuberculosis, the production of pro-inflammatory cytokines is beneficial to the bacterium by inhibition of the expression of major histocompatibility complex class II on the surface of antigen-presenting cells (Sasindran and Torrelles 2011). To this effect, alveolar macrophages should be targeted; they are most modulated by Mtb, making them a critical component of infection. A step in this direction was the treatment of macrophages with PLGA NPs containing rifampicin to treat BCG infection. Although the loading of rifampicin was insufficient, appropriate loading enabled the clearing of infection from the cells (Kalluru et al. 2013).
Targeting lung macrophages has also been achieved by the development of Stealth® liposomes coated using O-stearyl amylopectin, the liposomes increase their affinity toward mice lung tissue (Deol and Khuller 1997). The development of other nanocarriers for sustained drug release by targeting macrophages may be of interest in newer studies. For example, O-palmitoyl mannan and O-palmitoyl pullulan can be coated on the drug vehicle to assist in eradicating the bacterium from its host (Vyas et al. 2005). Depending on the inherent design of NPs, such as the charge, size, composition, and coating, they can be directed to not just the correct cell type, but also the right cellular sub-compartment. This would increase the possibility of the drug delivery system ending up together with the bacteria during infection and aid in the targeted killing of the bacteria (Lawlor et al. 2011).
In this context, nonstructured lipid carriers containing linezolid were developed and studied for their characteristics in vitro as well as in vivo. These drug carriers exhibited phagocytosis by macrophages and were able to cross the mucus barrier in the lungs. The particles showed effectivity as aerosols for inhalation therapy by spray drying. This study could be used as a framework for the development of more patient-friendly tuberculosis treatments using nanomedicine (Makled et al. 2020). In summary, TB remains a major respiratory disease, affecting millions each year. Although the prescribed treatments exhibit efficiency in pathogen clearance, severe inflammatory response generation, and side effects often make patients discontinue their treatment. To combat the growing drug resistance of Mtb, it is imperative to develop newer nanomedicines that directly target macrophages, if not the site of the residence of latent bacteria in order to eradicate the disease.
11.6 A Balancing Act: Battling Coronaviruses
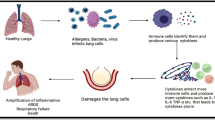
COVID-19 or the Coronavirus Disease 2019 began with a coronavirus (SARS-CoV-2) infecting several visitors in a seafood market in Wuhan, China. The virus binds to the host cell’s ACE2 (angiotensin-converting enzyme 2) receptors present on the epithelial cells of alveoli, trachea, bronchi, and serous bronchial glands of the lower respiratory tract, rapidly producing new viral particles inside the host cell and infecting more cells as the disease progresses (Shereen et al. 2020). Transmission occurs by droplets of viral particles released on coughing, sneezing, or respiratory distress (Rothan and Byrareddy 2020). Clinical features indicated by chest imaging are pneumonia, RNAemia, acute respiratory distress syndrome, and incidence of ground-glass opacities that led to death (Zhu et al. 2020).
11.6.1 Cytokine Storms: Battling Inflammation
Similar behavior was previously observed in the SARS virus, an epidemic that affected the human population in 2003 (Nile et al. 2020). Pathogenetic similarities do not end there. The host response against SARS-CoV-2 includes aggressive inflammation, which is conducive to causing increased damage to the airways (Tay et al. 2020). Inflammation is typically caused by pro-inflammatory cytokine activation in epithelial cells, endothelial cells, and alveolar macrophages induced by the identification of damage-associated molecular patterns, including ATP and nucleic acids released by pyroptotic host cells (Tay et al. 2020). Primary mediators of cytokine-related inflammation include IL-6, IL-10, IP-10 (Laing et al. 2020), macrophage inflammatory protein 1α (MIP-1α), MIP1β, and MCP1. These proteins establish a chemoattractive gradient and a positive feedback loop of inflammation is generated by the incoming monocytes, macrophages, and T cells at the site of infection (Tay et al. 2020).
11.6.2 Potential Nanotherapy: Lessons from SARS and MERS
Previously encountered coronaviruses SARS and MERS (Middle East Respiratory Syndrome) exhibited quite a similar pathophysiology (Channappanavar et al. 2016). Therefore, strategic targeting to cure infections can be derived from studies that report effectiveness against SARS or MERS, albeit with some differences. By modulating the immune response, Wiley et al. induced the formation of bronchus-associated lymphoid tissue (iBALT) by the use of protein cage NPs in the lung parenchyma. In subsequent experiments, they indicated how iBALT in the lungs could help prevent the alleviation of SARS infection. Although it is unknown how the NPs enhanced the host immune response, where B cell and/or CD4+ T cell-dependent mechanisms are involved (Wiley et al. 2009).
In an attempt to synthesize an immunogen, a study in 2009 carried out a step-wise assembly of SARS-CoV subunit virus-like NPs exhibiting properties of easy protein expression, purification, and high stability. These NPs were conformation-specific, had neutralization activity toward the virus, and did not infect any host cells (Pimentel et al. 2009). A different approach to synthesizing vaccines could be the DNA route, as in a report using polyethyleneimine NPs coated with spike protein-encoding DNA to intranasally immunize mice from SARS (Shim et al. 2010). Similarly, nucleocapsid protein-encoding DNA of SARS was loaded into biotinylated chitosan NPs and intranasally administered to mice to target mucosal dendritic cells. The NPs elicited a humoral immune response, exhibiting elevated levels of IgG in mice (Raghuwanshi et al. 2012). The development of spike protein NPs can be of interest to target multiple coronaviruses, as predicted in Coleman et al. They synthesized spike protein NPs for SARS and MERS and depicted that these particles could generate an immune response in mice. However, none of these NPs provided specificity to both diseases, leaving a potential gap for multiple-coronavirus targeting NPs (Coleman et al. 2014). Peptide mimics that interact with the viral particles in place of cellular receptors could be therapeutic in nature. Huang et al. developed a peptide mimic conjugated with gold nanorods against MERS, increasing its inhibitory effects by ten times and preventing membrane fusion of the virus both in vitro and in vivo (Huang et al. 2019).
11.6.3 Current Drug Repurposing and Nanotherapeutics
In severe cases of COVID-19, different drug cocktails are being used such as chloroquine (antimalarial drug), lopinavir/ritonavir (from AIDS treatments), favipiravir (against influenza), and ribavirin (from Hepatitis C treatments) (Bhavana et al. 2020). Better clinical outcomes by prevention of viral replication within the host are required, either by aiding the immune response in capturing viral particles or preventing their attachment to the ACE2 protein (Alphandéry 2020). Alphandery et al. review several options available for modification and specific targeting of NPs to SARS-CoV-2 (Alphandéry 2020). The majority of the antiviral nanotherapies involved silver NPs; this may be due to their previously established role in ameliorating other viral infections (Bhavana et al. 2020).
Additionally, Zhou et al. developed a GSH-ZnS NP that could effectively target multiple viruses, including both RNA and DNA viruses. The observed action of NPs against the porcine epidemic diarrhea virus belonging to the Coronaviridae family implied their probable use against SARS-CoV, SARS-CoV-2, and MERS (Zhou et al. 2020). Interferons, critical components of the antiviral immune response, could be induced to reduce the viral load and enable the strengthening of defenses. Therefore, nanoceria or cerium oxide NPs are hypothesized to be a possible agent in curbing inflammation—they attenuate cytokine signaling by modulation and inhibition of MAP kinase/NF-κB, p65-NF-κB, and Nrf2/NF-κB pathways, significantly disrupting and halting disease progression (Allawadhi et al. 2020). Targeting viral proteins such as proteases (3CLpro and PLpro), RNA polymerase (RdRp), and the spike protein involved in cellular uptake is critical to the development of any therapeutic against coronaviruses. Although these proteins do not directly undertake immunomodulation, their role in the viral life cycle is indispensable, and inhibition will substantially prevent inflammation. To this end, neutralizing antibodies coated on the surface of NPs might be an effective nanotherapeutic approach available (Chauhan et al. 2020).
11.7 Conclusion and Future Prospects
Chronic lung diseases are often driven by the inflammatory state due to the complex microenvironment involving close communication between the respiratory and immune systems. Any dysregulation of the immune half results in inflammatory processes sabotaging the exchange of gases. Action against inflammation is now being explored through the use of nanotechnology, which promises better drug availability, safer delivery, and efficacy. Although many preclinical studies have been conducted for the diseases mentioned, clinical studies using nanotechnological advances to improve patient rehabilitation are still far from reality. This may be due to the unknown impact of nanodevices or vehicles as a systemic supplement. To avoid this, several dry powder inhaler technologies have been developed to specifically target the lung and avoid contact with other organs for maximal retention and safety. Newer developments in drug technology, among other investigations, revealed that drug delivery via oral or inhalation or IV route by nanovehicles is a feasible strategy for application in pulmonary therapy. Further, liposomal and dendrimer carriers are noted as drug vehicles gaining importance to improve efficacy, targeting, and safety. To explore the full potential of nano-based drug carriers and delivery systems, further studies and clinical trials are vital to test the toxicity and long-term effects. Several natural derivatives can be employed as anti-inflammatory agents in disease and the potential for combination with existing treatments is noted as well.
References
Adams LB, Sinha I, Franzblau SG, Krahenbuhl JL, Mehta RT (1999) Effective treatment of acute and chronic murine tuberculosis with liposome-encapsulated clofazimine. Antimicrob Agents Chemother 43(7):1638–1643. https://doi.org/10.1128/AAC.43.7.1638
Agnoletti M, Bohr A, Thanki K, Wan F, Zeng X, Boetker JP, Yang M, Foged C (2017) Inhalable siRNA-loaded nano-embedded microparticles engineered using microfluidics and spray drying. Eur J Pharm Biopharm 120:9–21. https://doi.org/10.1016/j.ejpb.2017.08.001
Al Faraj A, Sultana Shaik A, Afzal S, Al Sayed B, Halwani R (2014) MR imaging and targeting of a specific alveolar macrophage subpopulation in LPS-induced COPD animal model using antibody-conjugated magnetic nanoparticles. Int J Nanomedicine 9:1491–1503. https://doi.org/10.2147/IJN.S59394
Alam F, Naim M, Aziz M, Yadav N (2014) Unique roles of nanotechnology in medicine and cancer. Indian J Cancer 51(4):506. https://doi.org/10.4103/0019-509X.175320
Allawadhi P, Khurana A, Allwadhi S, Joshi K, Packirisamy G, Bharani KK (2020) Nanoceria as a possible agent for the management of COVID-19. Nano Today 35:100982. https://doi.org/10.1016/j.nantod.2020.100982
Alphandéry E (2020) The potential of various nanotechnologies for coronavirus diagnosis/treatment highlighted through a literature analysis. Bioconjug Chem 31(8):1873–1882. https://doi.org/10.1021/acs.bioconjchem.0c00287
Alton EWFW, Armstrong DK, Ashby D, Bayfield KJ, Bilton D, Bloomfield EV, Boyd AC, Brand J, Buchan R, Calcedo R, Carvelli P, Chan M, Cheng SH, Collie DDS, Cunningham S, Davidson HE, Davies G, Davies JC, Davies LA, Wolstenholme-Hogg P (2015) Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med 3(9):684–691. https://doi.org/10.1016/S2213-2600(15)00245-3
Alvarado A (2018) Autoimmunity in chronic obstructive pulmonary disease: un update. Clin Res Trials 4(3):222. https://doi.org/10.15761/CRT.1000222
Athari SS, Mortaz E, Pourpak Z, Moin M, Moazzeni SM (2016) VIP-loaded PLGA as an anti-asthma nanodrug candidate. Comp Clin Pathol 25(4):791–796. https://doi.org/10.1007/s00580-016-2265-6
Azarmi S, Roa WH, Löbenberg R (2008) Targeted delivery of nanoparticles for the treatment of lung diseases. Adv Drug Deliv Rev 60(8):863–875. https://doi.org/10.1016/j.addr.2007.11.006
Baraldo S, Turato G, Saetta M (2012) Pathophysiology of the small airways in chronic obstructive pulmonary disease. Respiration 84(2):89–97. https://doi.org/10.1159/000341382
Berenson CS, Kruzel RL, Eberhardt E, Sethi S (2013) Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J Infect Dis 208(12):2036–2045. https://doi.org/10.1093/infdis/jit400
Beyeler S, Steiner S, Wotzkow C, Tschanz SA, Adhanom Sengal A, Wick P, Haenni B, Alves MP, von Garnier C, Blank F (2020) Multi-walled carbon nanotubes activate and shift polarization of pulmonary macrophages and dendritic cells in an in vivo model of chronic obstructive lung disease. Nanotoxicology 14(1):77–96. https://doi.org/10.1080/17435390.2019.1663954
Bhavana V, Thakor P, Singh SB, Mehra NK (2020) COVID-19: pathophysiology, treatment options, nanotechnology approaches, and research agenda to combating the SARS-CoV2 pandemic. Life Sci 261:118336. https://doi.org/10.1016/j.lfs.2020.118336
Bhushan B, Gopinath P (2015) Antioxidant nanozyme: a facile synthesis and evaluation of the reactive oxygen species scavenging potential of nanoceria encapsulated albumin nanoparticles. J Mater Chem B 3(24):4843–4852. https://doi.org/10.1039/C5TB00572H
Bielski E, Zhong Q, Mirza H, Brown M, Molla A, Carvajal T, da Rocha SRP (2017) TPP-dendrimer nanocarriers for siRNA delivery to the pulmonary epithelium and their dry powder and metered-dose inhaler formulations. Int J Pharm 527(1–2):171–183. https://doi.org/10.1016/j.ijpharm.2017.05.046
Blasi P, Schoubben A, Giovagnoli S, Rossi C, Ricci M (2009) Fighting tuberculosis: old drugs, new formulations. Expert Opin Drug Deliv 6(9):977–993. https://doi.org/10.1517/17425240903130577
Bonfield TL, Konstan MW, Burfeind P, Panuska JR, Hilliard JB, Berger M (1995) Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol 13(3):257–261. https://doi.org/10.1165/ajrcmb.13.3.7544594
Boschetto P, Quintavalle S, Miotto D, Lo Cascio N, Zeni E, Mapp CE (2006) Chronic obstructive pulmonary disease (COPD) and occupational exposures. J Occup Med Toxicol 1(1):11. https://doi.org/10.1186/1745-6673-1-11
Brockman SM, Bodas M, Silverberg D, Sharma A, Vij N (2017) Dendrimer-based selective autophagy-induction rescues δF508-CFTR and inhibits Pseudomonas aeruginosa infection in cystic fibrosis. PLoS One 12(9):1–17. https://doi.org/10.1371/journal.pone.0184793
Buhecha MD, Lansley AB, Somavarapu S, Pannala AS (2019) Development and characterization of PLA nanoparticles for pulmonary drug delivery: co-encapsulation of theophylline and budesonide, a hydrophilic and lipophilic drug. J Drug Deliv Sci Technol 53:101128. https://doi.org/10.1016/j.jddst.2019.101128
Cambier CJ, Falkow S, Ramakrishnan L (2014a) Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell 159(7):1497–1509. https://doi.org/10.1016/j.cell.2014.11.024
Cambier CJ, Takaki KK, Larson RP, Hernandez RE, Tobin DM, Urdahl KB, Cosma CL, Ramakrishnan L (2014b) Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 505(7482):218–222. https://doi.org/10.1038/nature12799
Cantin AM, Hartl D, Konstan MW, Chmiel JF (2015) Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros 14(4):419–430. https://doi.org/10.1016/j.jcf.2015.03.003
Caretti A, Bragonzi A, Facchini M, De Fino I, Riva C, Gasco P, Musicanti C, Casas J, Fabriàs G, Ghidoni R, Signorelli P (2014) Anti-inflammatory action of lipid nanocarrier-delivered myriocin: therapeutic potential in cystic fibrosis. Biochim Biophys Acta Gen Subj 1840(1):586–594. https://doi.org/10.1016/j.bbagen.2013.10.018
Cartiera MS, Ferreira EC, Caputo C, Egan ME, Caplan MJ, Saltzman WM (2010) Partial correction of cystic fibrosis defects with PLGA nanoparticles encapsulating curcumin. Mol Pharm 7(1):86–93. https://doi.org/10.1021/mp900138a
Cazzola M, Calzetta L, Facciolo F, Rogliani P, Matera MG (2017) Pharmacological investigation on the anti-oxidant and anti-inflammatory activity of N-acetylcysteine in an ex vivo model of COPD exacerbation. Respir Res 18(1):26. https://doi.org/10.1186/s12931-016-0500-y
Chan Y, Ng SW, Chellappan DK, Madheswaran T, Zeeshan F, Kumar P, Pillay V, Gupta G, Wadhwa R, Mehta M, Wark P, Hsu A, Hansbro NG, Hansbro PM, Dua K, Panneerselvam J (2020) Celastrol-loaded liquid crystalline nanoparticles as an anti-inflammatory intervention for the treatment of asthma. Int J Polym Mater Polym Biomater 70:65350. https://doi.org/10.1080/00914037.2020.1765350
Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S (2016) Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 19(2):181–193. https://doi.org/10.1016/j.chom.2016.01.007
Chauhan G, Madou MJ, Kalra S, Chopra V, Ghosh D, Martinez-Chapa SO (2020) Nanotechnology for COVID-19: therapeutics and vaccine research. ACS Nano 14(7):7760–7782. https://doi.org/10.1021/acsnano.0c04006
Cherk Yong DO, Saker SR, Wadhwa R, Chellappan DK, Madheswaran T, Panneerselvam J, Tambuwala MM, Bakshi HA, Kumar P, Pillay V, Gupta G, Oliver BG, Wark P, Hsu A, Hansbro PM, Dua K, Zeeshan F (2019) Preparation, characterization and in-vitro efficacy of quercetin loaded liquid crystalline nanoparticles for the treatment of asthma. J Drug Deliv Sci Technol 54:101297. https://doi.org/10.1016/j.jddst.2019.101297
Coleman CM, Liu YV, Mu H, Taylor JK, Massare M, Flyer DC, Glenn GM, Smith GE, Frieman MB (2014) Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine 32(26):3169–3174. https://doi.org/10.1016/j.vaccine.2014.04.016
Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, Parkhill J, Malla B, Berg S, Thwaites G, Yeboah-Manu D, Bothamley G, Mei J, Wei L, Bentley S, Harris SR, Niemann S, Diel R, Aseffa A, Gagneux S (2013) Out-of-Africa migration and neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet 45(10):1176–1182. https://doi.org/10.1038/ng.2744
Cooper AM, Adams LB, Dalton DK, Appelberg R, Ehlers S (2002) IFN-γ and NO in mycobacterial disease: new jobs for old hands. Trends Microbiol 10(5):221–226. https://doi.org/10.1016/S0966-842X(02)02344-2
Corthésy B, Bioley G (2017) Therapeutic intranasal instillation of allergen-loaded microbubbles suppresses experimental allergic asthma in mice. Biomaterials 142:41–51. https://doi.org/10.1016/j.biomaterials.2017.07.019
Cystic Fibrosis Foundation (2018) Patient registry annual data report. Cystic Fibrosis Foundation, Bethesda, p 92
da Silva AL, Cruz FF, Rocco PRM, Morales MM (2017) New perspectives in nanotherapeutics for chronic respiratory diseases. Biophys Rev 9(5):793–803. https://doi.org/10.1007/s12551-017-0319-x
da Silva AL, de Oliveira GP, Kim N, Cruz FF, Kitoko JZ, Blanco NG, Martini SV, Hanes J, Rocco PRM, Suk JS, Morales MM (2020) Nanoparticle-based thymulin gene therapy therapeutically reverses key pathology of experimental allergic asthma. Sci Adv 6(24):eaay7973. https://doi.org/10.1126/sciadv.aay7973
De Smet EG, Van Eeckhoutte HP, Avila Cobos F, Blomme E, Verhamme FM, Provoost S, Verleden SE, Venken K, Maes T, Joos GF, Mestdagh P, Brusselle GG, Bracke KR (2020) The role of miR-155 in cigarette smoke-induced pulmonary inflammation and COPD. Mucosal Immunol 13(3):423–436. https://doi.org/10.1038/s41385-019-0241-6
Deol P, Khuller GK (1997) Lung specific stealth liposomes: stability, biodistribution and toxicity of liposomal antitubercular drugs in mice. Biochim Biophys Acta Gen Subj 1334(2–3):161–172. https://doi.org/10.1016/S0304-4165(96)00088-8
Duan Y, Wang A, Ding Y, Li L, Duan D, Lin J, Yu C, Liu J (2021) Fabrication of poly-sulfosalicylic acid film decorated pure carbon fiber as electrochemical sensing platform for detection of theophylline. J Pharm Biomed Anal 192:113663. https://doi.org/10.1016/j.jpba.2020.113663
Duncan GA, Jung J, Hanes J, Suk JS (2016) The mucus barrier to inhaled gene therapy. Mol Ther 24(12):2043–2053. https://doi.org/10.1038/mt.2016.182
Ebrahimi A, Sadroddiny E (2015) MicroRNAs in lung diseases: recent findings and their pathophysiological implications. Pulm Pharmacol Ther 34:55–63. https://doi.org/10.1016/j.pupt.2015.08.007
Edmondson C, Davies JC (2016) Current and future treatment options for cystic fibrosis lung disease: latest evidence and clinical implications. Ther Adv Chronic Dis 7(3):170–183. https://doi.org/10.1177/2040622316641352
Elizur A, Cannon CL, Ferkol TW (2008) Airway inflammation in cystic fibrosis. Chest 133(2):489–495. https://doi.org/10.1378/chest.07-1631
El-Sherbiny IM, Smyth HDC (2012) Controlled release pulmonary administration of curcumin using swellable biocompatible microparticles. Mol Pharm 9(2):269–280. https://doi.org/10.1021/mp200351y
Ensign LM, Schneider C, Suk JS, Cone R, Hanes J (2012) Mucus penetrating nanoparticles: biophysical tool and method of drug and gene delivery. Adv Mater 24(28):3887–3894. https://doi.org/10.1002/adma.201201800
Ezzie ME, Crawford M, Cho J-H, Orellana R, Zhang S, Gelinas R, Batte K, Yu L, Nuovo G, Galas D, Diaz P, Wang K, Nana-Sinkam SP (2012) Gene expression networks in COPD: microRNA and mRNA regulation. Thorax 67(2):122–131. https://doi.org/10.1136/thoraxjnl-2011-200089
Ferreira AJ, Cemlyn-Jones J, Robalo Cordeiro C (2013) Nanoparticles, nanotechnology and pulmonary nanotoxicology. Rev Port Pneumol 19(1):28–37. https://doi.org/10.1016/j.rppneu.2012.09.003
Fujita Y, Takeshita F, Kuwano K, Ochiya T (2013) RNAi therapeutic platforms for lung diseases. Pharmaceuticals 6(2):223–250. https://doi.org/10.3390/ph6020223
Gaspar MM, Cruz A, Penha AF, Reymão J, Sousa AC, Eleutério CV, Domingues SA, Fraga AG, Filho AL, Cruz MEM, Pedrosa J (2008) Rifabutin encapsulated in liposomes exhibits increased therapeutic activity in a model of disseminated tuberculosis. Int J Antimicrob Agents 31(1):37–45. https://doi.org/10.1016/j.ijantimicag.2007.08.008
Geiser M, Quaile O, Wenk A, Wigge C, Eigeldinger-Berthou S, Hirn S, Schäffler M, Schleh C, Möller W, Mall MA, Kreyling WG (2013) Cellular uptake and localization of inhaled gold nanoparticles in lungs of mice with chronic obstructive pulmonary disease. Part Fibre Toxicol 10(1):19. https://doi.org/10.1186/1743-8977-10-19
Gelperina S, Kisich K, Iseman MD, Heifets L (2005) The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med 172(12):1487–1490. https://doi.org/10.1164/rccm.200504-613PP
Ghio AJ, Hilborn ED, Stonehuerner JG, Dailey LA, Carter JD, Richards JH, Crissman KM, Foronjy RF, Uyeminami DL, Pinkerton KE (2008) Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect. Am J Respir Crit Care Med 178(11):1130–1138. https://doi.org/10.1164/rccm.200802-334OC
Glickman MS, Jacobs WR (2001) Microbial pathogenesis of Mycobacterium tuberculosis: dawn of a discipline. Cell 104(4):477–485. https://doi.org/10.1016/S0092-8674(01)00236-7
Guan S, Munder A, Hedtfeld S, Braubach P, Glage S, Zhang L, Lienenklaus S, Schultze A, Hasenpusch G, Garrels W, Stanke F, Miskey C, Johler SM, Kumar Y, Tümmler B, Rudolph C, Ivics Z, Rosenecker J (2019) Self-assembled peptide–poloxamine nanoparticles enable in vitro and in vivo genome restoration for cystic fibrosis. Nat Nanotechnol 14(3):287–297. https://doi.org/10.1038/s41565-018-0358-x
Günday Türeli N, Torge A, Juntke J, Schwarz BC, Schneider-Daum N, Türeli AE, Lehr CM, Schneider M (2017) Ciprofloxacin-loaded PLGA nanoparticles against cystic fibrosis P. aeruginosa lung infections. Eur J Pharm Biopharm 117:363–371. https://doi.org/10.1016/j.ejpb.2017.04.032
Halwani R, Sultana Shaik A, Ratemi E, Afzal S, Kenana R, Al-Muhsen S, Al Faraj A (2016) A novel anti-IL4Rα nanoparticle efficiently controls lung inflammation during asthma. Exp Mol Med 48(10):e262. https://doi.org/10.1038/emm.2016.89
Haque AKMA, Dewerth A, Antony JS, Riethmüller J, Schweizer GR, Weinmann P, Latifi N, Yasar H, Pedemonte N, Sondo E, Weidensee B, Ralhan A, Laval J, Schlegel P, Seitz C, Loretz B, Lehr CM, Handgretinger R, Kormann MSD (2018) Chemically modified hCFTR mRNAs recuperate lung function in a mouse model of cystic fibrosis. Sci Rep 8(1):1–14. https://doi.org/10.1038/s41598-018-34960-0
Herbst RS, Heymach JV, Lippman SM (2008) Lung cancer. N Engl J Med 359(13):1367–1380. https://doi.org/10.1056/NEJMra0802714
Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD (2004) The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350(26):2645–2653. https://doi.org/10.1056/NEJMoa032158
Huang X, Li M, Xu Y, Zhang J, Meng X, An X, Sun L, Guo L, Shan X, Ge J, Chen J, Luo Y, Wu H, Zhang Y, Jiang Q, Ning X (2019) Novel gold nanorod-based HR1 peptide inhibitor for middle east respiratory syndrome coronavirus. ACS Appl Mater Interfaces 11(22):19799–19807. https://doi.org/10.1021/acsami.9b04240
Hussain A, Singh S, Das SS, Anjireddy K, Karpagam S, Shakeel F (2019) Nanomedicines as drug delivery carriers of anti-tubercular drugs: from pathogenesis to infection control. Curr Drug Deliv 16(5):400–429. https://doi.org/10.2174/1567201816666190201144815
Inoue K, Takano H, Yanagisawa R, Sakurai M, Ichinose T, Sadakane K, Yoshikawa T (2005) Effects of nano particles on antigen-related airway inflammation in mice. Respir Res 6(1):106. https://doi.org/10.1186/1465-9921-6-106
Kaczmarek JC, Kowalski PS, Anderson DG (2017) Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med 9(1):60. https://doi.org/10.1186/s13073-017-0450-0
Kälin N, Claaß A, Sommer M, Puchelle E, Tümmler B (1999) ΔF508 CFTR protein expression in tissues from patients with cystic fibrosis. J Clin Investig 103(10):1379–1389. https://doi.org/10.1172/JCI5731
Kalluru R, Fenaroli F, Westmoreland D, Ulanova L, Maleki A, Roos N, Paulsen Madsen M, Koster G, Egge-Jacobsen W, Wilson S, Roberg-Larsen H, Khuller GK, Singh A, Nystrom B, Griffiths G (2013) Poly(lactide-co-glycolide)-rifampicin nanoparticles efficiently clear Mycobacterium bovis BCG infection in macrophages and remain membrane-bound in phago-lysosomes. J Cell Sci 126(14):3043–3054. https://doi.org/10.1242/jcs.121814
Kaufmann SH, Dorhoi A (2013) Inflammation in tuberculosis: interactions, imbalances and interventions. Curr Opin Immunol 25(4):441–449. https://doi.org/10.1016/j.coi.2013.05.005
Keil TWM, Baldassi D, Merkel OM (2020) T-cell targeted pulmonary siRNA delivery for the treatment of asthma. WIREs Nanomed Nanobiotechnol 12(5):e1634. https://doi.org/10.1002/wnan.1634
Kerem BS (1989) Identification of the cystic fibrosis gene: genetic analysis. Trends Genet 245(4922):1073. https://doi.org/10.1016/0168-9525(89)90156-X
Kim T, Paudel KR, Kim D-W (2020) Eriobotrya japonica leaf extract attenuates airway inflammation in ovalbumin-induced mice model of asthma. J Ethnopharmacol 253:112082. https://doi.org/10.1016/j.jep.2019.112082
King PT (2015) Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin Transl Med 4(1):0068. https://doi.org/10.1186/s40169-015-0068-z
Kumar M, Kong X, Behera AK, Hellermann GR, Lockey RF, Mohapatra SS (2003) Chitosan IFN-γ-pDNA nanoparticle (CIN) therapy for allergic asthma. Genet Vaccines Ther 1(1):3. https://doi.org/10.1186/1479-0556-1-3
Kuzmov A, Minko T (2015) Nanotechnology approaches for inhalation treatment of lung diseases. J Control Release 219:500–518. https://doi.org/10.1016/j.jconrel.2015.07.024
Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, Muñoz-Ruiz M, McKenzie DR, Hayday TS, Francos-Quijorna I, Kamdar S, Joseph M, Davies D, Davis R, Jennings A, Zlatareva I, Vantourout P, Wu Y, Sofra V, Hayday AC (2020) A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med 26(10):1623–1635. https://doi.org/10.1038/s41591-020-1038-6
Lancheros R, Guerrero CA, Godoy-Silva RD (2018) Improvement of N-acetylcysteine loaded in PLGA nanoparticles by nanoprecipitation method. J Nanotechnol 2018:1–11. https://doi.org/10.1155/2018/3620373
Lawlor C, Kelly C, O’Leary S, O’Sullivan MP, Gallagher PJ, Keane J, Cryan SA (2011) Cellular targeting and trafficking of drug delivery systems for the prevention and treatment of MTb. Tuberculosis 91(1):93–97. https://doi.org/10.1016/j.tube.2010.12.001
Le Conte P, Le Gallou F, Potel G, Struillou L, Baron D, Drugeon HB (1994) Pharmacokinetics, toxicity, and efficacy of liposomal capreomycin in disseminated Mycobacterium avium beige mouse model. Antimicrob Agents Chemother 38(12):2695–2701. https://doi.org/10.1128/AAC.38.12.2695
Li Z, Luo G, Hu W, Hua J, Geng S, Chu PK, Zhang J, Wang H, Yu X (2020) Mediated drug release from nanovehicles by black phosphorus quantum dots for efficient therapy of chronic obstructive pulmonary disease. Angew Chem Int Ed 59(46):20568–20576. https://doi.org/10.1002/anie.202008379
Lu X, Zhu T, Chen C, Liu Y (2014) Right or left: the role of nanoparticles in pulmonary diseases. Int J Mol Sci 15(10):17577–17600. https://doi.org/10.3390/ijms151017577
Makled S, Boraie N, Nafee N (2020) Nanoparticle-mediated macrophage targeting—a new inhalation therapy tackling tuberculosis. Drug Deliv Transl Res 11(3):1037–1055. https://doi.org/10.1007/s13346-020-00815-3
Mansour HM, Rhee Y-S, Wu X (2009) Nanomedicine in pulmonary delivery. Int J Nanomedicine 4:299–319
Meers P, Neville M, Malinin V, Scotto AW, Sardaryan G, Kurumunda R, Mackinson C, James G, Fisher S, Perkins WR (2008) Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother 61(4):859–868. https://doi.org/10.1093/jac/dkn059
Mejías JC, Roy K (2019) In-vitro and in-vivo characterization of a multi-stage enzyme-responsive nanoparticle-in-microgel pulmonary drug delivery system. J Control Release 316:393–403. https://doi.org/10.1016/j.jconrel.2019.09.012
Messiaen AS, Forier K, Nelis H, Braeckmans K, Coenye T (2013) Transport of nanoparticles and tobramycin-loaded liposomes in Burkholderia cepacia complex biofilms. PLoS One 8(11):9220. https://doi.org/10.1371/journal.pone.0079220
Mohamed A, Pekoz AY, Ross K, Hutcheon GA, Saleem IY (2019) Pulmonary delivery of nanocomposite microparticles (NCMPs) incorporating miR-146a for treatment of COPD. Int J Pharm 569:118524. https://doi.org/10.1016/j.ijpharm.2019.118524
Mugabe C, Halwani M, Azghani AO, Lafrenie RM, Omri A (2006) Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother 50(6):2016–2022. https://doi.org/10.1128/AAC.01547-05
Muralidharan P, Hayes D, Black SM, Mansour HM (2016) Microparticulate/nanoparticulate powders of a novel Nrf2 activator and an aerosol performance enhancer for pulmonary delivery targeting the lung Nrf2/Keap-1 pathway. Mol Syst Des Eng 1(1):48–65. https://doi.org/10.1039/C5ME00004A
Nafee N, Husari A, Maurer CK, Lu C, de Rossi C, Steinbach A, Hartmann RW, Lehr C-M, Schneider M (2014) Antibiotic-free nanotherapeutics: ultra-small, mucus-penetrating solid lipid nanoparticles enhance the pulmonary delivery and anti-virulence efficacy of novel quorum sensing inhibitors. J Control Release 192:131–140. https://doi.org/10.1016/j.jconrel.2014.06.055
Ng ZY, Wong J-Y, Panneerselvam J, Madheswaran T, Kumar P, Pillay V, Hsu A, Hansbro N, Bebawy M, Wark P, Hansbro P, Dua K, Chellappan DK (2018) Assessing the potential of liposomes loaded with curcumin as a therapeutic intervention in asthma. Colloids Surf B Biointerfaces 172:51–59. https://doi.org/10.1016/j.colsurfb.2018.08.027
Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G (2020) COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev 53:66–70. https://doi.org/10.1016/j.cytogfr.2020.05.002
O’Sullivan BP, Freedman SD (2009) Cystic fibrosis. Lancet 373(9678):1891–1904. https://doi.org/10.1016/S0140-6736(09)60327-5
Ober C, Vercelli D (2011) Gene–environment interactions in human disease: nuisance or opportunity? Trends Genet 27(3):107–115. https://doi.org/10.1016/j.tig.2010.12.004
Okusanya ÓO, Bhavnani SM, Hammel J, Minic P, Dupont LJ, Forrest A, Mulder GJ, Mackinson C, Ambrose PG, Gupta R (2009) Pharmacokinetic and pharmacodynamic evaluation of liposomal amikacin for inhalation in cystic fibrosis patients with chronic pseudomonal infection. Antimicrob Agents Chemother 53(9):3847–3854. https://doi.org/10.1128/AAC.00872-08
Ong V, Mei V, Cao L, Lee K, Chung EJ (2019) Nanomedicine for cystic fibrosis. SLAS Technol 24(2):169–180. https://doi.org/10.1177/2472630318824334
Pandey R (2003) Poly(dl-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J Antimicrob Chemother 52(6):981–986. https://doi.org/10.1093/jac/dkg477
Paranjpe M, Müller-Goymann CC (2014) Nanoparticle-mediated pulmonary drug delivery: a review. Int J Mol Sci 15(4):5852–5873. https://doi.org/10.3390/ijms15045852
Passi M, Shahid S, Chockalingam S, Sundar IK, Packirisamy G (2020) Conventional and nanotechnology based approaches to combat chronic obstructive pulmonary disease: implications for chronic airway diseases. Int J Nanomedicine 15:3803–3826. https://doi.org/10.2147/IJN.S242516
Patil-Gadhe A, Kyadarkunte A, Patole M, Pokharkar V (2014) Montelukast-loaded nanostructured lipid carriers: part II pulmonary drug delivery and in vitro–in vivo aerosol performance. Eur J Pharm Biopharm 88(1):169–177. https://doi.org/10.1016/j.ejpb.2014.07.007
Piergallini TJ, Turner J (2018) Tuberculosis in the elderly: why inflammation matters. Exp Gerontol 105:32–39. https://doi.org/10.1016/j.exger.2017.12.021
Pimentel TAPF, Yan Z, Jeffers SA, Holmes KV, Hodges RS, Burkhard P (2009) Peptide nanoparticles as novel immunogens: design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chem Biol Drug Des 73(1):53–61. https://doi.org/10.1111/j.1747-0285.2008.00746.x
Porsio B, Craparo EF, Mauro N, Giammona G, Cavallaro G (2018) Mucus and cell-penetrating nanoparticles embedded in nano-into-micro formulations for pulmonary delivery of Ivacaftor in patients with cystic fibrosis. ACS Appl Mater Interfaces 10(1):165–181. https://doi.org/10.1021/acsami.7b14992
Porta GD, De Vittori C, Reverchon E (2005) Supercritical assisted atomization: a novel technology for microparticles preparation of an asthma-controlling drug. AAPS PharmSciTech 6(3):E421–E428. https://doi.org/10.1208/pt060352
Pottelberge GRV, Mestdagh P, Bracke KR, Thas O, van Durme YMTA, Joos GF, Vandesompele J, Brusselle GG (2011) MicroRNA expression in induced sputum of smokers and patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 183(7):898–906. https://doi.org/10.1164/rccm.201002-0304OC
Qiao H, Liu W, Gu H, Wang D, Wang Y (2015) The transport and deposition of nanoparticles in respiratory system by inhalation. J Nanomater 2015:1–8. https://doi.org/10.1155/2015/394507
Raghuwanshi D, Mishra V, Das D, Kaur K, Suresh MR (2012) Dendritic cell targeted chitosan nanoparticles for nasal DNA immunization against SARS CoV nucleocapsid protein. Mol Pharm 9(4):946–956. https://doi.org/10.1021/mp200553x
Ramelli SC, Comer BS, McLendon JM, Sandy LL, Ferretti AP, Barrington R, Sparks J, Matar M, Fewell J, Gerthoffer WT (2020) Nanoparticle delivery of anti-inflammatory LNA oligonucleotides prevents airway inflammation in a HDM model of asthma. Mol Ther Nucleic Acids 19:1000–1014. https://doi.org/10.1016/j.omtn.2019.12.033
Rey MM, Bonk MP, Hadjiliadis D (2019) Cystic fibrosis: emerging understanding and therapies. Annu Rev Med 70(2018):197–210. https://doi.org/10.1146/annurev-med-112717-094536
Ricci M, Giovagnoli S, Blasi P, Schoubben A, Perioli L, Rossi C (2006) Development of liposomal capreomycin sulfate formulations: effects of formulation variables on peptide encapsulation. Int J Pharm 311(1–2):172–181. https://doi.org/10.1016/j.ijpharm.2005.12.031
Robinson E, MacDonald KD, Slaughter K, McKinney M, Patel S, Sun C, Sahay G (2018) Lipid nanoparticle-delivered chemically modified mRNA restores chloride secretion in cystic fibrosis. Mol Ther 26(8):2034–2046. https://doi.org/10.1016/j.ymthe.2018.05.014
Rodríguez-Hernández H, Simental-Mendía LE, Rodríguez-Ramírez G, Reyes-Romero MA (2013) Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. Int J Endocrinol 2013:678159. https://doi.org/10.1155/2013/678159
Rothan HA, Byrareddy SN (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 109:102433. https://doi.org/10.1016/j.jaut.2020.102433
Rowe SM, Verkman AS (2013) Cystic fibrosis transmembrane regulator correctors and potentiators. Cold Spring Harb Perspect Biol 5(8):a009761
Sasindran SJ, Torrelles JB (2011) Mycobacterium tuberculosis infection and inflammation: what is beneficial for the host and for the bacterium? Front Microbiol 2:2. https://doi.org/10.3389/fmicb.2011.00002
Sato T, Liu X, Nelson A, Nakanishi M, Kanaji N, Wang X, Kim M, Li Y, Sun J, Michalski J, Patil A, Basma H, Holz O, Magnussen H, Rennard SI (2010) Reduced miR-146a increases prostaglandin E2 in chronic obstructive pulmonary disease fibroblasts. Am J Respir Crit Care Med 182(8):1020–1029. https://doi.org/10.1164/rccm.201001-0055OC
Sato H, Suzuki H, Yakushiji K, Wong J, Seto Y, Prud’homme RK, Chan HK, Onoue S (2016) Biopharmaceutical evaluation of novel cyclosporine A nano-matrix particles for inhalation. Pharm Res 33(9):2107–2116. https://doi.org/10.1007/s11095-016-1949-6
Secret E, Kelly SJ, Crannell KE, Andrew JS (2014) Enzyme-responsive hydrogel microparticles for pulmonary drug delivery. ACS Appl Mater Interfaces 6(13):10313–10321. https://doi.org/10.1021/am501754s
Shah V, Taratula O, Garbuzenko B, O., L. Patil, M., Savla, R., Zhang, M., & Minko, T. (2013) Genotoxicity of different nanocarriers: possible modifications for the delivery of nucleic acids. Curr Drug Discov Technol 10(1):8–15. https://doi.org/10.2174/1570163811310010003
Sharma A, Sharma S, Khuller GK (2004) Lectin-functionalized poly(lactide-co-glycolide) nanoparticles as oral/aerosolized antitubercular drug carriers for treatment of tuberculosis. J Antimicrob Chemother 54(4):761–766. https://doi.org/10.1093/jac/dkh411
Sharma JK, Gupta A, Khanna P (2019) Diabetes and respiratory system including tuberculosis—challenges. Indian J Tuberc 66(4):533–538. https://doi.org/10.1016/j.ijtb.2019.11.006
Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R (2020) COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res 24:91–98. https://doi.org/10.1016/j.jare.2020.03.005
Shim B-S, Park S-M, Quan J-S, Jere D, Chu H, Song M, Kim D, Jang Y-S, Yang M-S, Han S, Park Y-H, Cho C-S, Yun C-H (2010) Intranasal immunization with plasmid DNA encoding spike protein of SARS-coronavirus/polyethylenimine nanoparticles elicits antigen-specific humoral and cellular immune responses. BMC Immunol 11(1):65. https://doi.org/10.1186/1471-2172-11-65
Soriano JB, Kendrick PJ, Paulson KR, Gupta V, Abrams EM, Adedoyin RA, Adhikari TB, Advani SM, Agrawal A, Ahmadian E, Alahdab F, Aljunid SM, Altirkawi KA, Alvis-Guzman N, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Antó JM et al (2020) Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 8(6):585–596. https://doi.org/10.1016/S2213-2600(20)30105-3
Suk JS, Lai SK, Boylan NJ, Dawson MR, Boyle MP, Hanes J (2011) Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedicine 6(2):365–375. https://doi.org/10.2217/nnm.10.123
Tagalakis AD, Munye MM, Ivanova R, Chen H, Smith CM, Aldossary AM, Rosa LZ, Moulding D, Barnes JL, Kafetzis KN, Jones SA, Baines DL, Moss GWJ, O’Callaghan C, McAnulty RJ, Hart SL (2018) Effective silencing of ENaC by siRNA delivered with epithelial-targeted nanocomplexes in human cystic fibrosis cells and in mouse lung. Thorax 73(9):847–856. https://doi.org/10.1136/thoraxjnl-2017-210670
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP (2020) The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20(6):363–374. https://doi.org/10.1038/s41577-020-0311-8
Turcios NL (2005) Cystic fibrosis: an overview. J Clin Gastroenterol 39(4):307–317. https://doi.org/10.1097/01.mcg.0000155140.63510.cd
Upadhyay S, Ganguly K (2015) Wonders of nanotechnology in the treatment for chronic lung diseases. J Nanomed Nanotechnol 06(06):337. https://doi.org/10.4172/2157-7439.1000337
Vankeerberghen A, Cuppens H, Cassiman JJ (2002) The cystic fibrosis transmembrane conductance regulator: an intriguing protein with pleiotropic functions. J Cyst Fibros 1(1):13–29. https://doi.org/10.1016/S1569-1993(01)00003-0
Varshosaz J, Ghaffari S, Mirshojaei SF, Jafarian A, Atyabi F, Kobarfard F, Azarmi S (2013) Biodistribution of amikacin solid lipid nanoparticles after pulmonary delivery. Biomed Int Res 2013:136859
Velino C, Carella F, Adamiano A, Sanguinetti M, Vitali A, Catalucci D, Bugli F, Iafisco M (2019) Nanomedicine approaches for the pulmonary treatment of cystic fibrosis. Front Bioeng Biotechnol 7:406. https://doi.org/10.3389/fbioe.2019.00406
Vij N (2011) Nano-based theranostics for chronic obstructive lung diseases: challenges and therapeutic potential. Expert Opin Drug Deliv 8(9):1105–1109. https://doi.org/10.1517/17425247.2011.597381
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DMG, López Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Agustí A (2017) Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med 195(5):557–582. https://doi.org/10.1164/rccm.201701-0218PP
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Murray CJ (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2163–2196. https://doi.org/10.1016/S0140-6736(12)61729-2
Vyas S, Quraishi S, Gupta S, Jaganathan K (2005) Aerosolized liposome-based delivery of amphotericin B to alveolar macrophages. Int J Pharm 296(1–2):12–25. https://doi.org/10.1016/j.ijpharm.2005.02.003
Wang L, Feng M, Li Q, Qiu C, Chen R (2019) Advances in nanotechnology and asthma. Ann Transl Med 7(8):20. https://doi.org/10.21037/atm.2019.04.62
Wenzel SE (2012) Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 18(5):716–725. https://doi.org/10.1038/nm.2678
Wiley JA, Richert LE, Swain SD, Harmsen A, Barnard DL, Randall TD, Jutila M, Douglas T, Broomell C, Young M, Harmsen A (2009) Inducible bronchus-associated lymphoid tissue elicited by a protein cage nanoparticle enhances protection in mice against diverse respiratory viruses. PLoS One 4(9):e7142. https://doi.org/10.1371/journal.pone.0007142
World Health Organization (2019) Global tuberculosis report 2019. World Health Organization, Geneva
Yaakov Y, Kerem E, Yahav Y, Rivlin J, Blau H, Bentur L, Aviram M, Picard E, Bdolah-Abram T, Wilschanski M (2007) Reproducibility of nasal potential difference measurements in cystic fibrosis. Chest 132(4):1219–1226. https://doi.org/10.1378/chest.06-2975
Yang Z, Chen X, Huang W, Wong BC, Yin L, Wong I, Xu M (2012) Liposomes prolong the therapeutic effect of anti-asthmatic medication via pulmonary delivery. Int J Nanomedicine 7:1139. https://doi.org/10.2147/IJN.S28011
Yuan F, Liu R, Hu M, Rong X, Bai L, Xu L, Mao Y, Hasimu H, Sun Y, He J (2019) JAX2, an ethanol extract of Hyssopus cuspidatus Boriss, can prevent bronchial asthma by inhibiting MAPK/NF-κB inflammatory signaling. Phytomedicine 57:305–314. https://doi.org/10.1016/j.phymed.2018.12.043
Zhou Y, Tong T, Jiang X, Fang L, Wu Y, Liang J, Xiao S (2020) GSH-ZnS nanoparticles exhibit high-efficiency and broad-spectrum antiviral activities via multistep inhibition mechanisms. ACS Appl Bio Mater 3(8):4809–4819. https://doi.org/10.1021/acsabm.0c00332
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382(8):727–733. https://doi.org/10.1056/NEJMoa2001017
Zuo L, He F, Sergakis GG, Koozehchian MS, Stimpfl JN, Rong Y, Diaz PT, Best TM (2014) Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am J Physiol Lung Cell Mol Physiol 307(3):L205–L218. https://doi.org/10.1152/ajplung.00330.2013
Acknowledgements
A. K. and R. S. are thankful to the Department of Biotechnology, Government of India for the fellowship (DBT-2729). GP acknowledges the Department of Biotechnology, Government of India (BT/PR 25095/NER/95/1011/2017), Science & Engineering Research Board (SERB), Government of India (Grant number: TAR/2019/000245) and Shastri Institutional Collaborative Research Grant (SICRG) 2020–2021. IKS is supported by the R01 HL142543 from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, USA as well as the University of Kansas Medical Center, School of Medicine, Internal Medicine Start-Up Funds (I.K.S.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kaur, A., Sharma, R., Sundar, I.K., Packirisamy, G. (2023). Nanomedicine Applied to Inflammatory and Infectious Pulmonary Diseases. In: Ribeiro de Araujo, D., Carneiro-Ramos, M. (eds) Biotechnology Applied to Inflammatory Diseases. Interdisciplinary Biotechnological Advances. Springer, Singapore. https://doi.org/10.1007/978-981-19-8342-9_11
Download citation
DOI: https://doi.org/10.1007/978-981-19-8342-9_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8341-2
Online ISBN: 978-981-19-8342-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)