Abstract
Although heart diseases continue to be the leading cause of death worldwide, advances performed in recent decades have facilitated a decrease in the mortality rate related to severe heart diseases. This is due to the recognition that has been acquiring the role of the immune system and its contribution to the progression of heart disease. Recent studies have shown that there is a close relationship between cardiac disturbances and inflammatory mediators produced by immune system cells since there is a close interaction between the innate and adaptive immune response in the pathophysiology of heart diseases. Regarding innate immune response, macrophages are the leading cells, which play a fundamental role in a wide variety of cardiac disorders. These cells produce a variety of cytokines that open up a wide range of therapeutic possibilities in the treatment of heart diseases. However, under certain circumstances, it is known that immune system cells can cause irreparable damage that contributes to heart failure. Therefore, it is essential to study the crosstalk between innate and adaptive response in order to better understand the mechanism of action of the different cardiac disturbances. In this sense, biotechnology emerges as a pioneering tool that allows on the one hand to effectively detect the various cardiovascular and inflammatory diseases, and on the other, to develop innovative therapies that result in effective treatments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 The Heart
The human heart is a void muscle, shaped as an inverted blunt cone, where the base forms a flat part, larger than its apex. The apex is the inferior end which tapers to a blunt, rounded point. It is positioned in the space between the lungs (mediastinum cavity), where the base lies posteriorly and superiorly, extending itself up to the level of the second rib. The apex lies anteriorly and inferiorly, resting on the central tendon of the diaphragm. Posteriorly, the heart rests against the vertebral bodies of the fifth to eighth thoracic vertebrae and is located behind the sternum (Durward 1950).
This important organ is composed of three layers of tissue, all involved by the pericardium (pericardial sac) which is a double-layered closed sac composed of a fibrous outer layer. Inside this sac, there is a viscous liquid known as pericardial fluid, which helps to lubricate the external surfaces involved in the heartbeat and to prevent friction between the fibrous and serous layers of the pericardium. From the outside in, the first layer is called epicardium. Some authors consider it an inner tier of the pericardium. It is composed of connective tissue fused with the muscular tissue on one side and the serous pericardium on the other. The second layer is the myocardium, composed of a muscle layer. It is considered the thickest layer and is where the contractions take place. This tissue is striated like skeletal muscle; however, it responds to involuntary stimulus of the autonomic nervous system. The third layer, and the most internal one, is the endocardium. It forms the layer which barks all cardiac chambers and is directly connected to all internal cardiac appendages (Durward 1950).
The main cell types that constitute the cardiac tissue are cardiomyocytes (or cardiac myocytes), cardiac fibroblasts, smooth muscle cells, and endothelial cells. Even though classically only those cell types were considered part of the heart, now we know that immune cells, mainly macrophages, also play a vital role in cardiac function (Zohman 1964). It can be seen in Fig. 1.1. Although cardiomyocytes are the ones which respond to electrical stimuli and correspond to approximately 75% of the total volume of the myocardium, cardiac fibroblasts are the predominant cells in this tissue, reaching about 2/3 of the total number of cells, having an essential role in the production of collagen I and III, the main constituents of the cardiac extracellular matrix (Bongartz et al. 2005).
The main cell types that constitute the cardiac tissue. They are cardiomyocytes (or cardiac myocytes), cardiac fibroblasts, and endothelial cells. There are also immune cells populating the cardiac tissue such as macrophages, dendritic cells (DC), B cells, T cells, mast cells, monocytes, and neutrophils
The heart is divided into four chambers (two atria and two ventricles). The atriums are the upper ones, while the ventricles are the lower ones (both right and left). An important concept to consider is that the blood flow is unidirectional: the blood flows from the atrium to the ventricles → exits the ventricles out of the heart (lungs or body) → flows back to the atrium. This is only possible since the heart has two main kinds of valves: atrioventricular (AV) and semilunar. The AV valves (mitral—left ventricle, and tricuspid—right ventricle) separate the atriums from the ventricles. They are composed of thin wires of connective tissue (chordae tendineae) attached by papillary muscles to the heart wall. This tissue prevents the valves from opening upwards because of the high pressures achieved during some conditions. The semilunar valves (pulmonary and aortic) are located between the ventricles and the arteries. The pulmonary valve is located between the right ventricle and the pulmonary artery while the aortic valve is situated between the left ventricle and aorta (Tortora and Derrickson 2009). It can be better visualized in Fig. 1.2a.
As commented before, blood flow is unidirectional, and is divided into two types: systemic and pulmonary. Systemic circulation consists of oxygenated (arterial) blood being released from the left ventricle through the aorta to the body, where the cells will consume the oxygen in their processes. The blood returns to the heart. It is pumped from the ventricle to the lungs through the pulmonary artery. Gas exchange occurs in the millions of lungs’ alveoli and capillary vessels that surround them, and it is called pulmonary hematosis. Re-oxygenated blood returns to the heart through the 4 pulmonary veins to the left atrium, and then it goes to the left ventricle passing through the left AV valve (mitral), restarting the route (Durward 1950).
This cardiac cycle happens because an electrical depolarizing and repolarizing cycle in the cardiomyocytes occurs. It is well described as a specialized electrical conduction system in the heart. The sinoatrial (SA) node is the heart’s pacemaker and is located superior to the terminal groove of the right atrium, close to the opening of the superior vena cava. This group of special cardiac cells propagate electrical stimulus. From the SA the electrical stimulus goes to the atrioventricular (AV) node, which is also an area of specialized conduction tissue located between the atriums and ventricles, which conducts electrical impulse from the atrium towards the ventricles. It can delay the passage of the electrical impulse, forcing the ventricles to contract later than the atriums.
The electrical impulse previously quoted is propagated through the membrane of each cardiomyocyte via action potentials (AP). The AP during the cardiac cycle includes two main steps: depolarization and repolarization of the cardiomyocyte. This process arises in T tubules, which are invaginations of the cardiomyocytes’ plasmatic membrane containing voltage-sensitive receptors. Its depolarization is initiated after the stimulus from the sinus node is transmitted cell–cell by specific ion channel named connexin, mainly connexin-43. Afterward, some sodium (Na+) channels open up and its ions move into the cell, resulting in the inside of the cell being positively charged. After the gradual depolarization of the cell, until the threshold is reached for triggering the next AP, the voltage-dependent L type Ca2+ channels open. This Ca2+ is sufficient to induce the opening of ryanodine receptors (RYRs) located in the sarcoplasmic reticulum (SR) membrane, the main calcium store in cardiac cells. Ca2+ ions diffuse out of the SR to interact with the contractile machinery. This process also is known as “calcium induce calcium release”—CICR. The contraction of cardiac myocytes is facilitated by myofilaments organized in sarcomeres, located along the long axis of the cell. The sarcomere is composed of actin and regulatory units: troponin (Tn) and tropomyosin (Tm). The binding of Ca2+ with TnC induces a conformational change allowing Tn/Tm to slide in the groove between the actin monomers, allowing the thick filament of the myosin to bind to the actin thus forming a cross-bridge. Through repeated and transient interactions of actin–myosin and, using energy from ATP hydrolysis, these two filaments slide in relation to each other, shortening the cell. The coordinated shortening of the entire cardiomyocyte population by the spread of the AP leads to cardiac contraction. During systole, the main mechanism of Ca2+ efflux in cardiac myocytes is SERCA2A (sarcoplasmic reticulum Ca2+-ATPase) which uses energy from ATP hydrolysis to pump Ca2+ back to SR; and the sodium-calcium exchanger (NCX), located in the cardiomyocyte membrane which uses the electrochemical gradient in the sarcolemma to translocate three Na+ ions into the cytosol and expel a Ca2+ ion (Bers 2002). The groups of these changes in cardiac AP and consequently membrane potential occurs in each cardiac beat, generating a cardiac cycle. The cardiac cycle consists of stages that occur in the interval of a heartbeat (called cardiac systole and diastole), which are the atrial systole, isovolumetric contraction, ventricular systole, isovolumetric relaxation, and ventricular filling (Patterson et al. 1914).
The SA node initiates an electrical impulse that flows over the right and left atriums causing their depolarization. Consequently, an atrial contraction happens (atrium systole). The blood will immediately be displaced into the ventricles through the opening of the AV valves, while the semilunar valves remain closed to avoid blood reflux from the great vessels. On an electrocardiogram (ECG), atrial depolarization is represented by the P wave. The AV and semilunar valves are closed. At that point, the ventricles begin to contract and, although the ventricular myocardial fibers shorten only a little, the intraventricular pressure increases rapidly. The electrical impulse spreads along the tissue reaching the AV node that conducts the stimulus through the bundle of the nerve fibers (His and Purkinje fibers) down the ventricle myocardium. Unlike atrial systole, during ventricular ejection the semilunar valves are opened while the AVs remain closed. This happens once the pressure in the ventricles exceeds the pressure in the arterial trunks and the valves are forced to open. It starts the ventricular systole, allowing blood to escape out of the heart. The left ventricle ejects blood to the body as well as right one ejects to lung circulation. During isovolumetric relaxation all cardiac valves close (Fig. 1.2b) (Tortora and Derrickson 2009; Farley et al. 2012).
All the phenomena described above work orchestrated to maintain the body function. It is known that a progressive decrease in cardiac function could be due to changes in the downgrading of sympathetic nervous system, in the calcium handling, in the reduction of myofilament function, in the heart anatomy, or as a result of a combination of these factors. Several pathologies may be considered when talking about abnormal calcium waves, among them are heart failure, myocardial infarction, ventricular tachycardia, and hypertrophy (González et al. 2015). It is known that inflammation is one of the most important point of convergence and can alter several cardiac conditions.
1.2 Immune System and Cardiovascular Diseases
Recent studies have explored the mechanisms involved in the progress of cardiovascular diseases from those related to the rupture of the atheromatous plaque to those observed in acute myocardial infarction, the process of self-repair, and the development of heart failure. Results indicate that the occurrence of cardiovascular diseases depend largely on either inflammatory mediator produced by the cells of the immune system and by the immune system’s cells themselves (Chiale et al. 2001; Thomas et al. 2017).
Immune system cells are numerous and diverse in form and function and are classified into: leukocytes of the innate immune system, responsible for the identification and removal of foreign substances present in organs, tissues, blood and lymph as neutrophils, monocytes, macrophages, dendritic cells, and NK cells; and adaptive immune system cells such as T and B lymphocytes, which eliminate or prevent pathogen threats.
The immune system plays a pivotal role in the heart’s response to injury, but until recently, the amount of confusing data made it difficult to distinguish immune factors that promote recovery of the heart after a heart attack from those that lead to greater damage, for example.
1.2.1 Inflammation and Heart
There are several factors that affect the proper functioning of the heart leading to heart failure or even death. Myocardial infarction deprives a part of the heart of oxygen and can lead to ischemic injuries that can be fatal; myocarditis, that can occur due to a viral infection and generate autoimmune disease; endocarditis, mainly due to bacterial infections; and arrhythmia. Most of these conditions are associated with a complex immune response that can either spread or defend against the disease.
The immune system response is therefore divided functionally, into two types: innate and adaptive. The innate immune response acts as the first line of defense against infectious agents, and most pathogens can be controlled before they produce an infection. Besides, the adaptive system keeps memory of the infectious agent and can prevent it from causing later disease. Both responses play a fundamental role and, on several times, an interplay in the physiopathology of heart disease.
1.2.1.1 Innate Response
At the time it is well accepted that not only microorganisms are able to activate innate response thus also several diseases also can activate this kind of immune response, like lifestyle diseases, cancer, and heart disease (Swirski and Nahrendorf 2018).
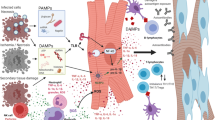
One of the innate immune system’s cellular populations that attract greater attention of researchers in the biomedical area are macrophages, which are responsible for phagocytosing damaged or infected cells or cell waste, as well as secreting various substances such as cytokines. Macrophages are involved in a wide variety of pathologies, such as heart failure, acute myocardial infarction, atherosclerosis, and obesity.
In the last decade, several studies have pointed out the macrophages as a key player in different cardiac diseases. In this regard, Epelman et al. demonstrated for the first time the presence of different resident macrophage populations in cardiac tissue (Epelman et al. 2014). After that, the group led by Dr. Medei was the first to demonstrate that cardiac macrophages can increase cardiac arrhythmic susceptibility (Monnerat et al. 2016). In the same line, the group led by Dr. Nahrendorf described a key role of cardiac macrophages consistently demonstrating that these cells also could be involved in the cardiac conduction system and that these cells actively participate in several cardiac mechanisms of repairs (Hulsmans et al. 2017).
The main action of macrophages is usually mediated by a high cytokine production such as IL-1β, TNF-α, and IL-6 (Duncan et al. 2010). One of the molecules that play a key role in the function of the innate immune system is interleukin 1β (IL-1β) (Cossio et al. 1974). The synthesis and maturation of IL-1β mainly depend on two signals: signal 1, classically mediated by the activation of Toll-like receptors (TLR’s) and signal 2 that depends on the activation/formation of inflammasome. In the cell membrane, the most important receptors involved in the synthesis of IL-1β in macrophages and monocytes are TLR’s, which are pattern recognizers (Maenhaut and Van de Voorde 2011). In the last two decades, at least 13 molecules of the TLR’s family have been described.
Signal 2 which results in the maturation of IL-1β involves a cytoplasmic protein molecular complex important in the inflammatory response, called inflammasome. Sensors that activate the inflammasome include the nucleotide-binding oligomerization (NOD) domains, receptors containing Leucine-rich repeats (NLRs), receptors such as those absent in melanoma-2 (ALRs), and proteins that contain a tripartite motif (TRIM) (Cossio et al. 1974; Malik and Kanneganti 2017).
It has been reported that in cardiomyocytes of patients with paroxysmal atrial fibrillation (AF) and with chronic AF, NLRP-3 inflammasome activity was increased (Yao et al. 2018). Moreover, patients with AF present increased circulating levels of inflammatory cytokines such as IL-1β, IL-18, and TNF-α. Inflammatory response mediators can also alter atrial electrophysiology and structural substrates, leading to increased vulnerability to AF (Hu et al. 2015). In this direction, it is currently known that NLRP-3 inflammasome plays a pivotal role in the development of cardiac disorders such as atherosclerosis (Grebe et al. 2018), coronary heart diseases (Libby et al. 2014), or cardiac arrhythmias related to renal ischemia-reperfusion (Alarcon et al. 2019). This points out, once again, the importance of NLRP-3 inflammasome as a target for the prevention or even the treatment of cardiovascular diseases.
1.2.1.2 Adaptive Response
The adaptive immune system develops as we are exposed to pathogens and other potentially harmful substances throughout our lives. This system comprises B and T lymphocytes and their products, including antibodies. In general terms, B lymphocytes are responsible for the antibody-mediated immune system, while T lymphocytes are responsible for the cell-mediated immune system.
Since 1976 it has been studied the role of G-Type immunoglobulins (Ig-G) from chronic chagasic patients, which are able to present agonist activity upon cardiac beta-adrenergic receptors (Cossio et al. 1974; Sterin-Borda et al. 1976). Neumann et al. described the presence of similar antibodies, but in patients with idiopathic dilated cardiomyopathy (Neumann et al. 1990). In this regard, our group has demonstrated that Ig-G from chronic chagasic patients is able to induce cardiac arrhythmias and AV conduction defects when perfused in isolated rabbit hearts (Farias de Oliveira et al. 1997). Besides, when studied in detail, it has been demonstrated that either Ig-G that activates beta-one adrenergic receptors or Ig-G that activates type-2 muscarinic receptors are involved in the mechanism of cardiac arrhythmias in chronic Chagas disease cardiomyopathy, modulating ventricular repolarization parameters (Medei et al. 2007). In 2006, Escobar et al. presented consistent data showing that Ig-G from chagasic patients was also able to activate beta-2 adrenergic receptors that induce cardiac conduction disturbances (Escobar et al. 2006). Several works were carried out to determine the clinical implication of the presence of these “functional autoantibodies” being Dr. Wallukat and his group in Germany the pioneer in this line. Wallukat’s group began the challenge of the specific removal of beta1-adrenergic autoantibodies from patients with idiopathic dilated cardiomyopathy by extracorporeal immunoadsorption (Wallukat et al. 2002). Preliminary clinical results of this therapy demonstrated a long-term benefit; however, at the time, different practical and methodological limitations clearly limit the use of this method. In fact, strategies aimed at directly suppressing the generation of pathogenic autoantibodies and/or their activity in the patients’ blood as intravenous Ig-G treatment (IVIG) or B cell depletion therapies could potentially be useful.
1.2.1.3 Adaptative-Innate Immune Response Crosstalk and Heart
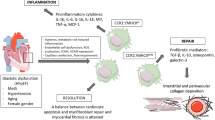
In recent years, crosstalk between lymph and monocytes/macrophages emerged as a new mechanism to explain several diseases. Myocardial infarction (MI) is one of the most prevalent heart diseases in Western countries. MI occurs when blood flow decreases or stops in one part of the heart. Consequently, several cardiac cells die impairing the cardiac ventricular function. The left ventricular dysfunction is usually a consequence of the replacement of cardiac cells by fibroblast creating a “scar” area in the myocardium. In this sense, scar extension will determine cardiac function. Therefore, all clinical efforts are focused on preserving the ventricular mass by limiting the scar extension. In the last decade, several works consistently demonstrated the key role played by the immune system in the repair of cardiac mass after injury.
Immediately after the injury, takes place the clearance of cell debris and digestion of extracellular matrix, which is mediated by macrophages and neutrophils. It was demonstrated that this first instance, when exacerbated, could induce a higher scar region in the infarction zone. Conversely, some works remarked that the lack of these cells in the first moment of the MI could contribute to worse cardiac prognostic (Frantz et al. 2013) (Fig. 1.3).
Chronologically, the first immune cells to act after a MI are the mast cells and 1 day later, monocytes and Ly6G+ neutrophils play an important role. It has been observed that monocytes depicted a biphasic response after MI in mice. Thus, Ly-6Chigh and Ly-6Clow monocytes peak is about 3 days and 5–7 days after MI, respectively (Swirski and Nahrendorf 2018). In humans however circulating inflammatory CD14+CD16− monocytes expanded first (peak on day 2.6), followed by CD14+CD16+ monocytes (peak on day 4.8) (Tsujioka et al. 2009). Between days 4–7 post-MI, resident macrophages appear as the most important immune cells, and after 7 days of MI, B and T lymphocytes take a leading role. Thus, a higher concentration of these cells was documented in the mediastinal lymph node and also in the cardiac tissue. In addition, a shift from neutrophils to resident macrophages was observed in this phase. All these changes consistently help with cardiac healing. In this scenario, an elegant work from Stefan Frantz’s Laboratory demonstrated the important interaction between Foxp3+CD4+ T cells/macrophage signaling crosstalk (Weirather et al. 2014). Thus, the authors demonstrated the important modulation of Foxp3+CD4+ T cells through IL-10, IL-13, and TGF-β1 on monocytes inducing their differentiation to macrophages. Additionally, TGF-β1 and IL-13 were shown to play a pivotal role on myofibroblast.
So, both extremes exist on the one hand the exacerbation and on the other the lack of immune response, which are deleterious for cardiac wound healing. Nowadays, the study of the equilibrium points of this orchestrated immune response, involving innate/adaptive crosstalk, is one of the most fascinating topics in the field of cardiology.
1.3 Biotechnological Tools Applied to the Treatment of Cardiovascular Diseases: New Insights
Biotechnology brings enormous benefits to many areas of knowledge, from agriculture to health sciences. It can increase productivity in crops and improve treatments of previously irremediable diseases. In the health area, biotechnology finds some of its most beneficial and comprehensive applications. When targeting cardiovascular and inflammatory diseases, biotechnology can be applied in two main ways: detection (through biomarkers and molecular diagnosis) and treatment. Innumerable techniques have been used in both scopes.
A biomarker is a characteristic molecule or substance that when measured and evaluated indicates physiological or pathological alterations. Classical biomarkers are measurable in plasma, serum, or urine. Specific ones leading to alterations of cell DNA or RNA, metabolite, and protein level are called molecular biomarkers. The biomarkers can track a disease progression over time or simply indicate a division or an endpoint in clinical studies. In other words, a biomarker reflects the state of some disease, being used for diagnosis or monitoring (Jain 2011a). Regarding cardiovascular diseases, many biomarkers have already been described (Table 1.1).
As heart cells die, their intercellular proteins are released out being exposed or degraded. Here lies the importance of the biotechnological techniques: capture this change or exposure of proteins that physiologically alter the cardiovascular system. Therefore, the most sensitive markers are those more abundant in the cell, whereas the ones involved in contraction are the most detectable in blood during heart diseases, as well as those markers involved in cytoskeleton (troponins, natriuretic peptide, ryanodine receptors, and others), enzymes responsible for cell energy (myoglobin and creatinine kinases), inflammatory cytokines, muscular tonus, cellular adhesion molecules, acute-phase reactants, rupture biomarker, and others. If such proteins have cardiac-specific forms, then specificity might be achievable as well as sensitivity (Lam et al. 2016).
In order to determine the number of biomarkers found in blood, many techniques were created. Basic technologies of molecular diagnostics are the Southern blot, DNA probes, pulsed-field gel electrophoresis, and polymerase chain reaction (PCR) (Jain 2011b). PCR has revolutionized the molecular diagnostics of heart diseases since it can be performed on even a few cells from body fluids or blood and is a key tool for genomics. Apart from that, we have proteomics, which has a fundamental role in the discovery of new and useful biomarkers of heart diseases. Proteomics comes to supplement the base already given by traditional genomics and traditional approaches. Proteomics investigates the protein alterations associated with the etiology of heart disease and its progression, outcome, and response to therapy. This is a systemic and sophisticated analysis of all protein profiles produced by a species in a determinate tissue. The term cardioproteomics is used for proteomic technologies applied to the cardiovascular system, providing a large-scale set of tools to study heart alterations, allowing the identification of pathological outcomes as well as new target treatments as in personalized medicine, the twenty-first century recent medicine goal (Jain 2011a).
Years of studies have led to many classic cardioprotective mechanisms, many of them pharmacological. There does not appear to be a repair of the heart tissue itself but control of the risk factors in order to restore health. The most used therapeutic agents nowadays for the treatment of many heart disorders are the beta-blockers, the angiotensin-converting enzyme (ECA) inhibitors, and the calcium channels blockers, all particularly indicated as antiarrhythmic, antihypertensive, and cardioprotective after myocardial infarction. The beta blocker drugs reduce blood pressure, decrease heart rate, and soften irregular heartbeats. These drugs can also help relieve congestive heart failure and prevent secondary heart attacks and are atenolol, carvedilol, labetalol, metoprolol, and propranolol (among others). The ACE inhibitors work to lower high blood pressure by blocking ACE that creates a hormone that can cause blood vessels to compress (angiotensin II). When the vessels start to relax, blood pressure will drop. Some ACE inhibitors also help to relieve the symptoms of congestive heart failure as captopril, enalapril, lisinopril, quinapril, ramipril, benazepril, fosinopril, trandolapril or moexipril (Jain 2011a; Panico et al. 2019; Kumar et al. 2019).
The calcium channels blockers reduce blood pressure by opening blood vessels. They can also relieve angina by lowering the heart rate. They are amlodipine, diltiazem, nifedipine, nicardipine and verapamil. Many other types can be used in order to improve cardiovascular disorders as diuretics, nitrates, statins, and anti-inflammatories. Still in a pharmacological approach, some drugs have been used in a not so classic way. New strategies are being used to better understand the development of heart diseases. One of them is resveratrol. This molecule is considered an effective antioxidant by increasing the nitric oxide (NO) synthase, as well as the maintenance of intracellular redox. Its performance has already been shown in models of myocardial infarction, arrhythmias, hypertension, cardiomyopathies, fibrosis, atherosclerosis, thrombosis, and diabetes, proving to be very effective in reducing reactive oxygen species, improving vasorelaxation and angiogenesis, preventing inflammation and apoptosis, and delaying atherosclerosis as well as decreasing cardiovascular remodeling in those models (Dyck et al. 2019).
As mentioned above, NO plays an important role, mainly cardioprotective. This has been already discussed and it appears to be a common denominator among various causes of cardiovascular disease, serving as the main factor of the related treatments, pharmacological and nonpharmacological. Nitric oxide synthase (NOS) plays a very controversial role since some forms of iNOS seem to be deleterious and others as eNOS cardioprotective. Furthermore, NO interacts not only with nuclear factors but also with the electron transport chain in mitochondria, providing us with fundamental considerations about its molecular role during the mechanism of cardioprotection (Pieretti et al. 2020).
Besides that, new cell-based therapies are being developed for heart diseases. Cell therapy aims to treat some diseases by restoring or altering certain sets of cells, or by using the cells themselves to deliver treatment to the body. In cell therapy, cells are cultured or modified outside the body before being injected into the patient. Cells can come from the patient itself (autologous cells) or from a donor (allogeneic cells). The first type hinders the possibility of rejection since the patient’s own cells are used. One technique well-used nowadays is the reprogramming of fibroblasts into pluripotent stem cells (iPSCs). This application allows cell reprograming using transcriptional factors (Gata4, Mef2c, and Tbx5). Another option is to use the bone marrow (BM) cells or endothelial cells to induce reprograming to cardiomyocytes. These BM-derived progenitor cells have been observed to play an important role in vasculogenesis, already described to remodel this tissue. Despite these findings, critical points are being raised for the improvement of techniques. It is necessary to determine if the characteristics of cardiomyocyte will persist over time. It has been raised by investigators that the efficiency of the generation of functional and contractible cardiomyocytes through these techniques is about 1%. The outcomes of these trials have been ambiguous, and no robust result has been stipulated, mainly because of the variation in cell population and differentiation. Although cell therapy initiatives exist in the experimental context, there is no large-scale and productive clinical approach. The results in basic science are being now discussed and tested rigorously to stipulate the worthiest way to get closer to clinical research and medical industry (Jain 2011a; Ieda et al. 2010).
Not far from it, we can find some models of 3D contractile bioengineered heart muscle (BEHM) using cardiac myocytes from rats. Since about 2007, researchers are working on in order to develop potential 3D functional models that replace dead cardiac cells. It puts the researchers one step closer to the goal of growing a whole new heart for heart-injured rats. A 4-day experiment already showed the ability of myocytes from a heart tissue patch to contract with an active force of 800 mN. These myocytes spontaneously organize and begin to contract and appear to respond to external forces such as calcium. Experiments injuring this formed tissue already show us also the ability of regeneration and remodeling, and a significant finding of response to inflammation (Mohamed et al. 2017; Huang et al. 2007). Further studies will expose the BEHM tissue to more nutrients and other conditions that are present in the body. This methodology has several advantages when compared to the other approaches however still far from being the human failure treatment, accelerating the goal to the moment when we will be able to replace a whole heart constructed from the patient’s own cells.
Another application of great importance for advances in the treatment of cardiovascular diseases is nanobiotechnology. Nanocardiology is the application of nanobiotechnology to cardiovascular diseases. Recent advances in nanotechnology and nanoscience offer a range of new opportunities for the diagnosis and therapy of cardiovascular and pulmonary diseases. Nanoparticles are an ample field and have strongly redefined molecular imaging for diagnosis and also the treatment of heart diseases. Magnetic nanoparticles are being used to target images of vascular inflammation, using conjugated nanoparticles with green fluorescent protein which reveals the inflammation location once it is injected in mice, as well as in different treatments as drug dealers. The dual role of these particles offers an individualized therapy since image-based treatments are selective and verify whenever the drug is reaching the target and point to the molecular effect that is occurring (Fan et al. 2020).
In general, nanobiotechnology facilitates the repairing and replacement of blood vessels, myocardium, and myocardial valves. It also can be used to stimulate regenerative processes such as therapeutic angiogenesis for ischemic heart disease. Within these technologies, we can name the nano-scaffolds and the nanofibers that guide tissue repair and replacement of blood vessels and cardiac tissue. They are polymers that have drug-release properties. Using nanofibers, it is possible to produce biomimetic scaffolds that can mimic the extracellular matrix for tissue engineering, as nanofibers can guide cell growth along their direction. Combining factors like fiber diameter, alignment, and chemicals offers new ways to control tissue engineering. Scaffolds capable of mimicking cellular matrices should be able to stimulate the growth of new cardiac tissue and direct revascularization. New advances in electrospinning, especially in drug delivery, support the massive potential of these nanomaterials. Inside these materials, encapsulated, we can find cytokines, growth factors, or angiogenic factors, for example (Ashammakhi et al. 2009).
The nanoparticles offer many solutions for cardiac therapies such as the improvement of the solubilization of the drugs, the use of noninvasive administration routes, improvement of the instabilities of absorption of the compounds, improvement of the bioavailability and release rates, control of the particle size and surface’s morphology, direct drug coupling to target restricted ligands, within others (Jain 2011a; Park et al. 2020). In addition to it, new strategies have been improved to better understand the role of many pathways including inflammation and immune system.
1.4 Final Considerations
The history of cardiology is marked by great names in science, art, and technology. Like other sciences, we are going through periods of progress and stagnation. However, it is inevitable to recognize that advances were greater and today, we can say that modern cardiology permeates areas that go beyond physiology. Interdisciplinarity has provided greater achievements since heart disease is the result of several other causes. The immune system has been shown to be a great ally in the understanding of cardiovascular diseases since it is the great systemic connector of the human organism. The breaking of barriers between the areas makes us think of medicine as being inter- and trans-disciplinary, turning technological studies to discover new treatments and disease prevention in a much more integrated way.
References
Alarcon MML, Trentin-Sonoda M, Panico K, Schleier Y, Duque T, Moreno-Loaiza O, de Yurre AR et al (2019) Cardiac arrhythmias after renal I/R depend on IL-1β. J Mol Cell Cardiol 131:101–111. https://doi.org/10.1016/j.yjmcc.2019.04.025
Ashammakhi N, Wimpenny I, Nikkola L, Yang Y (2009) Electrospinning: methods and development of biodegradable nanofibres for drug release. J Biomed Nanotechnol 5(1):1–19. https://doi.org/10.1166/jbn.2009.1003
Bers DM (2002) Cardiac excitation-contraction coupling. Nature 415(6868):198–205. https://doi.org/10.1038/415198a
Bongartz LG, Cramer MJ, Doevendans PA, Joles JA (2005) The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J 25(1):11–17. https://doi.org/10.1093/eurheartj/ehi020
Chiale PA, Ferrari I, Mahler E, Vallazza MA, Elizari MV, Rosenbaum MB, Levin MJ (2001) Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation 103(13):1765–1771. https://doi.org/10.1161/01.CIR.103.13.1765
Cossio PM, Diez C, Szarfman A, Kreutzer E, Candiolo B, Arana RM (1974) Chagasic cardiopathy. Circulation 49(1):13–21. https://doi.org/10.1161/01.CIR.49.1.13
de Oliveira SF, Pedrosa RC, Nascimento JHM, Campos de Carvalho AC, Masuda MO (1997) Sera from chronic Chagasic patients with complex cardiac arrhythmias depress electrogenesis and conduction in isolated rabbit hearts. Circulation 96(6):2031–2037. https://doi.org/10.1161/01.CIR.96.6.2031
Duncan DJ, Yang Z, Hopkins PM, Steele DS, Harrison SM (2010) TNF-α and IL-1β increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium 47(4):378–386. https://doi.org/10.1016/j.ceca.2010.02.002
Durward A (1950) Anatomy and physiology, 8th edn. McGraw Hill, Boston
Dyck G, Raj P, Zieroth S, Dyck J, Ezekowitz J (2019) The effects of resveratrol in patients with cardiovascular disease and heart failure: a narrative review. Int J Mol Sci 20(4):904. https://doi.org/10.3390/ijms20040904
Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T et al (2014) Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40(1):91–104. https://doi.org/10.1016/j.immuni.2013.11.019
Escobar AL, Fernández-Gómez R, Peter J-C, Mobini R, Hoebeke J, Mijares A (2006) IgGs and Mabs against the β2-adrenoreceptor block AV conduction in mouse hearts: a possible role in the pathogenesis of ventricular arrhythmias. J Mol Cell Cardiol 40(6):829–837. https://doi.org/10.1016/j.yjmcc.2006.03.430
Fan C, Joshi J, Li F, Xu B, Khan M, Yang J, Zhu W (2020) Nanoparticle-mediated drug delivery for treatment of ischemic heart disease. Front Bioeng Biotechnol 8:687. https://doi.org/10.3389/fbioe.2020.00687
Farley A, McLafferty E, Hendry C (2012) The cardiovascular system. Nurs Stand 27(9):35–39. https://doi.org/10.7748/ns2012.10.27.9.35.c9383
Frantz S, Hofmann U, Fraccarollo D, Schäfer A, Kranepuhl S, Hagedorn I, Nieswandt B et al (2013) Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J 27(3):871–881. https://doi.org/10.1096/fj.12-214049
González GE, Rhaleb N-E, D’Ambrosio MA, Nakagawa P, Liu Y, Leung P, Dai X et al (2015) Deletion of interleukin-6 prevents cardiac inflammation, fibrosis and dysfunction without affecting blood pressure in angiotensin II-high salt-induced hypertension. J Hypertens 33(1):144–152. https://doi.org/10.1097/HJH.0000000000000358
Grebe A, Hoss F, Latz E (2018) NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res 122(12):1722–1740. https://doi.org/10.1161/CIRCRESAHA.118.311362
Hu Y-F, Chen Y-J, Lin Y-J, Chen S-A (2015) Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol 12(4):230–243. https://doi.org/10.1038/nrcardio.2015.2
Huang Y-C, Khait L, Birla RK (2007) Contractile three-dimensional bioengineered heart muscle for myocardial regeneration. J Biomed Mater Res Part A 80(3):719–731. https://doi.org/10.1002/jbm.a.31090
Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ et al (2017) Macrophages facilitate electrical conduction in the heart. Cell 169(3):510–522. https://doi.org/10.1016/j.cell.2017.03.050
Ieda M, Fu J-D, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D (2010) Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142(3):375–386. https://doi.org/10.1016/j.cell.2010.07.002
Jain KK (2011a) Nanobiotechnology: applications, markets and companies. Jain PharmaBiotech, Basel
Jain KK (2011b) Applications of biotechnology in cardiovascular therapeutics. Springer Science & Business Media, New York
Kumar U, Wettersten N, Garimella PS (2019) Cardiorenal syndrome: pathophysiology. Cardiol Clin 37(3):251–265. https://doi.org/10.1016/j.ccl.2019.04.001
Lam MPY, Ping P, Murphy E (2016) Proteomics research in cardiovascular medicine and biomarker discovery. J Am Coll Cardiol 68(25):2819–2830. https://doi.org/10.1016/j.jacc.2016.10.031
Libby P, Tabas I, Fredman G, Fisher EA (2014) Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res 114(12):1867–1879. https://doi.org/10.1161/CIRCRESAHA.114.302699
Maenhaut N, Van de Voorde J (2011) Regulation of vascular tone by adipocytes. BMC Med 9(1):25. https://doi.org/10.1186/1741-7015-9-25
Malik A, Kanneganti T-D (2017) Inflammasome activation and assembly at a glance. J Cell Sci 130(23):3955–3963. https://doi.org/10.1242/jcs.207365
Medei E, Pedrosa RC, Benchimol Barbosa PR, Costa PC, Hernández CC, Chaves EA, Linhares V et al (2007) Human antibodies with muscarinic activity modulate ventricular repolarization: basis for electrical disturbance. Int J Cardiol 115(3):373–380. https://doi.org/10.1016/j.ijcard.2006.03.022
Mohamed MA, Islas JF, Schwartz RJ, Birla RK (2017) Electrical stimulation of artificial heart muscle: a look into the electrophysiologic and genetic implications. ASAIO J 63(3):333–341. https://doi.org/10.1097/MAT.0000000000000486
Monnerat G, Alarcón ML, Vasconcellos LR, Hochman-Mendez C, Brasil G, Bassani RA, Casis O et al (2016) Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat Commun 7(1):13344. https://doi.org/10.1038/ncomms13344
Neumann DA, Lynne Burek C, Baughman KL, Rose NR, Herskowitz A (1990) Circulating heart-reactive antibodies in patients with myocarditis or cardiomyopathy. J Am Coll Cardiol 16(4):839–846. https://doi.org/10.1016/S0735-1097(10)80331-6
Panico K, Abrahão MV, Trentin-Sonoda M, Muzi-Filho H, Vieyra A, Carneiro-Ramos MS (2019) Cardiac inflammation after ischemia-reperfusion of the kidney: role of the sympathetic nervous system and the renin–angiotensin system. Cell Physiol Biochem 53(4):587–605. https://doi.org/10.33594/000000159
Park JH, Dehaini D, Zhou J, Holay M, Fang RH, Zhang L (2020) Biomimetic nanoparticle technology for cardiovascular disease detection and treatment. Nanoscale Horizons 5(1):25–42. https://doi.org/10.1039/C9NH00291J
Patterson SW, Piper H, Starling EH (1914) The regulation of the heart beat. J Physiol 48(6):465–513. https://doi.org/10.1113/jphysiol.1914.sp001676
Pieretti JC, Junho CVC, Carneiro-Ramos MS, Seabra AB (2020) H2S- and NO-releasing gasotransmitter platform: a crosstalk signaling pathway in the treatment of acute kidney injury. Pharmacol Res 161:105121. https://doi.org/10.1016/j.phrs.2020.105121
Sterin-Borda L, Cossio PM, Gimeno MF, Gimeno AL, Diez C, Laguens RP, Meckert PC et al (1976) Effect of Chagasic sera on the rat isolated atrial preparation: immunological, morphological and functional aspects. Cardiovasc Res 10(6):613–622. https://doi.org/10.1093/cvr/10.6.613
Swirski FK, Nahrendorf M (2018) Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol 18(12):733–744. https://doi.org/10.1038/s41577-018-0065-8
Thomas B, Matsushita K, Abate KH, Al-Aly Z, Ärnlöv J, Asayama K, Atkins R et al (2017) Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol 28(7):2167–2179. https://doi.org/10.1681/ASN.2016050562
Tortora G, Derrickson B (2009) Principles of anatomy and physiology: organization, support and movement, and control systems of the human body, 12th edn. Wiley, New York
Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, Kitabata H et al (2009) Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol 54(2):130–138. https://doi.org/10.1016/j.jacc.2009.04.021
Wallukat G, Müller J, Hetzer R (2002) Specific removal of β1-adrenergic autoantibodies from patients with idiopathic dilated cardiomyopathy. N Engl J Med 347(22):1806. https://doi.org/10.1056/NEJM200211283472220
Weirather J, Hofmann UDW, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G et al (2014) Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 115(1):55–67. https://doi.org/10.1161/CIRCRESAHA.115.303895
Yao C, Veleva T, Scott L, Cao S, Li L, Chen G, Jeyabal P et al (2018) Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation 138(20):2227–2242. https://doi.org/10.1161/CIRCULATIONAHA.118.035202
Zohman BL (1964) Medical physiology, vol 5. Elsevier, Amsterdam
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Junho, C.V.C., de Yurre, A.R., Medei, E., Sorelli Carneiro-Ramos, M. (2023). Cardioimmunology: An Interdisciplinary Approach. In: Ribeiro de Araujo, D., Carneiro-Ramos, M. (eds) Biotechnology Applied to Inflammatory Diseases. Interdisciplinary Biotechnological Advances. Springer, Singapore. https://doi.org/10.1007/978-981-19-8342-9_1
Download citation
DOI: https://doi.org/10.1007/978-981-19-8342-9_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8341-2
Online ISBN: 978-981-19-8342-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)