Abstract
Human microbiota has gained significant interest in the prevention and treatment of cancer. Synbiotics are profound examples of how microbiota can be manipulated for therapeutic purpose and their importance in the therapeutic management of cancer. Lung cancer is considered to be the second largest death-causing cancer worldwide. One-third of the cancer pathology can be prevented by maintaining a healthy nutritious diet and tailor-made supplements of probiotics and prebiotics. Synbiotics are the combination of both prebiotics and probiotics, whose synergistic effect results in an adverse change in gut microbial flora. The microbial content in the gastrointestinal tract of lung cancer patients differs hugely from the normal healthy person. Based on the gut-lung axis, it has been proposed that the imbalance in the gut microbial flora may cause lung cancer. Reduced composition of Firmicutes and Bacteroides leads to lung cancer. These microbes can serve as a biomarker for the detection of lung cancer. Gut microbial flora decreases further during antibiotic-associated chemotherapy. Hence, administration of appropriate synbiotics could either restore these depleted microorganisms or introduce new microorganisms to our gut which could enhance anti-cancer activity and confer health benefits on the host. Ultimately, understanding the gut-lung axis and the influence of the microbiome involved in lung cancer may help decide the therapeutic interventions. This chapter summarizes the types, causative agents, and treatment strategies of lung cancer, an overview of synbiosis highlighting the gut microbiome, with a prime focus on synbiosis and dysbiosis of lung cancer patients. The onco-suppressive effects of synbiotics by modulating the immune responses and maintaining the intestinal barrier function have been discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Among the cancer types, lung cancer is considered to be the most common with a high mortality rate. The lung is an organ that is constantly connected with the external environment so there is a high risk of microbiome harboring and carcinogen attack. Active or second handed smoking is the major reason for lung cancer (Lukeman 2015). Lung cancer is generally categorized into two major types. One is less common and it is known as small cell lung cancer. In lung cancer, the tumors can grow and spread faster than the second type, hence it responds well to chemotherapy (Escalera et al. 2021). The second type is known as non-small cell lung cancer, it is the major type of lung carcinoma. There are three major subtypes of non-small cell lung cancer, they are adenocarcinoma where oncogenes are triggered in cells producing mucus. Squamous cell carcinoma occurs in the inner linings of the lung and large cell carcinoma where cancer can develop in any part of the lung (López et al. 2021). It is considered that the number of microbiomes present in our body is 10 times greater than the number of cells in our body. Of which, the colon contains 70% of the microbial flora. Some of the commonly found gut microbiota are Bacteroidetes, Firmicutes, and Actinobacteria (Sagar et al. 2015). Imbalance in this gut microbial flora results in dysbiosis. This results in various diseases like bowel inflammation, lung cancer, hypercholesterolemia, etc. Scientists have figured out a plan to cure this disease using synbiotics. Synbiosis is the synergistic effect of both probiotics (live microorganisms) and prebiotics (substrate for the microorganisms) that positively affect the growth of gut microbial flora. When cancer patients undergo chemotherapy the inner mucosal layer of their intestine gets degraded. The microbial flora present in the gut produces a small chain fatty acid called butyrate which induces mucin secretion that helps to bring back the normal mucosal layer of the intestine (Sagar et al. 2015). It was reported that the saliva of the person who is affected with lung cancer contains the strains of Neisseria, Streptococcus, and Porphyromonas. This serves as the biomarker to detect lung cancer. Scientists believe that there is a strong connection between the gut and lung which is known as the gut-lung axis (Liu et al. 2021). The communication between the lung and gut is bidirectional. The metabolites produced by this microbial flora serve as an immune response to the host, thus maintaining systemic inflammation and immune homeostasis. Dysbiosis of Firmicutes and Bacteroidetes increases the risk of lung cancer. The presence of Blautia obeum, La. Salivarius, Akkermansia muciniphila, and Coriobacteriaceae indicate metastatic lung cancer (De Maria et al. 2021). Metastasis is a condition in which cancer cells migrate as packets from the primary tumor site to the secondary tumor site via blood or lymphatic vessels. Hence, synbiotics and fecal microbiota transplantation help to prevent and treat pulmonary disorders.
9.2 Lung Cancer
Lung cancer or lung cell carcinoma is a malignant tumor characterized by the uncontrolled growth of cells in lung tissues. It is the most common cancer that causes death in men and the second most common cancer in women, which is preceded by breast cancer (Mustafa et al. 2016). Lung cancer is the rarest disease during the First World War, and which popularized after the Second World War. The article which was published in the British Medical Journal in 1950, by Sir Richard Doll and Austin Hill, suspected that smoking can cause lung cancer and is an eye opener for all researchers. As suspected, people who smoke have the greatest risk of lung cancer, while people who never smoked are also susceptible to cancer. In 1985, the total death rate for lung cancer was 9,21,000 which is 17% more than the lung cancer death rate calculated in 1980 (Spiro and Silvestri 2005). By the data from Global Statistics 2020, lung cancer has occurred in 2.21 million people and resulted in 1.8 million deaths (Sung et al. 2021).
9.2.1 Causative Agents
The major reason for lung cancer is smoking. A lifetime smoker has a 20–30 times increased risk of lung cancer than non-smokers contributing to nearly 85% of the lung cancers (Minna et al. 2002). Cigarettes contain 73 known carcinogens which include polycyclic aromatic compounds like benzo-alpha-pyrene, NNK [Nitrosamine 4(methylnitrosamino) 1-(3pyridyl)-1-butanone], a radioactive isotope of Polonium 210, etc. (Mustafa et al. 2016). Micro dissection of epithelial tissues of a former smoker and current smoker with lung cancer showed thousands of lesions than the lung of a non-smoker (Spiro and Silvestri 2005). So far smoking cannabis is not proven as a risk factor for lung cancer (Mustafa et al. 2016). The remaining 10–15% of lung cancer patients are non-smokers. They are either inherited genetically or when they are exposed to carcinogens including asbestos, nitrogen dioxides, sulphate aerosols, indoor and outdoor air pollution containing 2.5 PM (Particulate Matter). Polymorphism of chromosomes 5,6 and 15 can increase the risk of lung cancer. Miscellaneous substances like metals (aluminum, cadmium, chromium (VI), beryllium, etc.), ionizing radiation (X-rays, gamma rays, plutonium), soot resulting due to incomplete combustion, MOPP from paint and toxic gases [methyl ether, bis(chloromethyl) ether, etc.] can also cause lung cancer (Mustafa et al., 2016).
9.2.2 Mode of Action
When the carcinogens enter our body, they bind directly to the DNA. These adducts cause mutation like guanosine to thymine transversion which may turn a normal cell into a cancer cell (Spiro and Silvestri 2005). The pathogenicity of lung cancer is mediated by the activation of proto-oncogenes and the inhibition of tumor suppressor genes. C-myc, cyclin 1, BCL2 gene mutations can trigger proto-oncogenes. Mutation in signaling pathways, especially K ras mutation has a 10–30% chance of converting proto-oncogene into an oncogene. The tumor suppressor gene gets inactivated by methylation, histone tail modification, and micro-RNA regulation. Overexpression of Endothelial Growth Factor Receptor (EGFR) plays a major role in cancer development. It induces cell proliferation, angiogenesis, tumor invasion (metastasis), and resistance to apoptosis. Mutations in genes like C-MET, NKX2–1, LKBI, PIK3CA, and BRAF exhibit similar kinds of activity (Mustafa et al. 2016). The presence of telomerase RNA and catalytic component (hTERT) in cancer cells makes them immortal (Minna et al., 2002).
9.2.3 Symptoms
People with lung cancer have the following clinical manifestations. Respiratory and systemic symptoms like cough, shortness of breath, and chest pain that occur due to cancer mass in the lungs, pressing adjacent structures. Ectopic ADH (antidiuretic hormone) and ACTH (adrenocorticotrophic hormone), and other symptoms like hypercalcemia, invading of Pancoast tumor (tumor on top of the lung) in the sympathetic nervous system, Horner syndrome (dropping of the eyelid and small pupil) can be observed in lung cancer patients (Mustafa et al. 2016). One of the major drawbacks of lung cancer treatment is late detection since most of these symptoms are shown during III B or IV stages (Spiro and Silvestri 2005).
9.2.4 Diagnosis
People with such symptoms are diagnosed through sputum cytology, and they are histologically diagnosed by the following technologies. One of the earliest methods of diagnosis was chest radiography which was performed from 1970 to 1980. This method failed to show a reduction in lung cancer after chemotherapy, and it is rejected as it causes contamination. Followed by which people started using and still rely on computed tomography (CT scan) that is sensitive to visualize pulmonary nodules and non-calcified nodes in the lungs. But there are some chances of getting false-positive results which may be due to increased time length and overdiagnosis. The non-surgical biopsy technology is Positron Emission Tomography (PET); it is also one of the diagnostic tools that are based on the biological activity of a neoplastic cell. Tumor cells uptake more glucose and perform extensive glycolysis than normal cells, so PET uses radiolabeled glucose analog 2-[F-18]-fluoro-2-deoxy-D-glucose (FDG) to detect cancer cells for both intra and extra-thoracic analysis (Spiro and Silvestri 2005).
9.2.5 Types of Lungs Cancer
Based on treatment methods, lung cancer is majorly divided into two types. They are non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). The NSCLC is the majority type of lung cancer (80–85%). It is subdivided into three sub-classes. They are adenocarcinoma that is developed in the periphery of the lung (Lukeman 2015). These adenocarcinomas arise from the progenitor cells of bronchioles (Clara cells), alveoli (type II pneumocytes), and mucin-producing cells. Next is squamous cell carcinoma which arises from the central part of the lung, that is from the segments of the bronchi, and it is extended to the main stem bronchus and the lobes of the lung. In well-developed stages, intracellular bridging, squamous pearl formation, and individual cell keratinization are clearly seen. Large cell lung cancer is the rarest form of NSCLC which is found in the periphery although it is centrally located. It is a large necrotic tumor that contains sheets and nests of polygonal cells with vesicular nuclei and prominent nucleoli. The next type of lung cancer is small cell lung cancer where 30,000 new cases are reported every year. It is usually located in the peri-bronchial region. This SCLC compresses the circumference of the inner lining of the bronchus, and it is generally metastasized through the external lymph node. These tumors are white-tan, soft, and friable. It also has three subtypes. They are oat cell lung carcinoma, intermediate cell type, and combined oat cell lung carcinoma (Travis 2011).
9.2.6 Treatment Strategies
Depending on the intensity of cancer and the performance status of cancer patients, lung cancer can be categorized into different stages. The treatment of lung cancer includes palliative care, surgery, chemotherapy, and radiotherapy. The first surgery for lung cancer was made in 1821 by an American “Milton Anthony” who surgically removed ½ pound of lung tissue along with 2 ribs just for 1-year survival. Lung cancer patients undergo different types of surgery including wedge resection having a high chance of recurrence, and contralateral resection which has a 55% of survival chance for 5 years (Spiro and Silvestri 2005). Our right lung has 3 lobes and the left has 2 lobes (Lukeman 2015). When patients are diagnosed with a small tumor on these lobes, it is removed through lobar excision or lobectomy. Usually, it is omitted because of concomitant disease risks (Spiro and Silvestri 2005). Radiotherapy is preferred to control tumor growth and reduce the chance of recurrence. Radiotherapy is compromised between adequate doses to minimize lung toxicity. The British Medical Research Council has devised Continuous High fractioned Accelerated Radio Therapy (CHART), where 1.5 Gy (Gray) is administered at eight-hour intervals to a total of 54 Gy, along with conventional daily treatment had striking effects in squamous lung carcinoma patients. 60 Gy is the standard radical dose for NSCLC patients. Radiotherapy has an 80% success rate for palliative lung cancer symptoms that include bone pain, hemoptysis, cough, and superior vena cava syndrome (Spiro and Silvestri 2005). Chemotherapy is given based on the patient’s age, response, and willingness to take chemotherapy. Modern chemotherapy with carbo or cisplatin along with vinorelbine, gemcitabine, and taxane has shown easy administration with lesser side effects like nausea, vomiting, hair loss, and increased survival rates. Mutation in EGFR could be prevented by two anti-tumor oral inhibitors: gefitinib and erlotinib which are small anti-tumor agents that selectively inhibit the intracellular tyrosine kinase activity of EGFR. The antiangiogenic drug known as thalidomide is used to stop lung cancer progression (Spiro and Silvestri 2005). Sometimes a combination of one or more treatments is suggested based on the condition of the patient. For instance, to treat NSCLC either surgery resection in mediastinum involving lymph node is associated with neoadjuvant chemotherapy or chemotherapy is combined with radiography. A combination of one or more chemotherapeutic drugs is also suggested, for example, a patient with an extensive stage of SCLC is treated with the combination of drugs such as cisplatin, etoposide, irinotecan, topotecan, or paclitaxel whose action shrink the tumor, reduce symptoms, and extended rate of survival from 10 to 16 months (Minna et al. 2002).
9.3 Synbiosis
9.3.1 Gut Microbiome
The gastrointestinal tract of our body contains 1014 bacterial cells which are 10 times greater than the number of cells in our body. Hence, it is known as the second genome of humans. Usually, a newly born baby has a sterile gut, but when it is exposed to the external environment, mother’s milk, and solid foods their gut is harbored with microbes. Even though our body contains many microbes, nearly 70% of them are harbored in the gastrointestinal tract because of the availability of nutrients. These microbes that coexist with the human body (host) are termed gut microbial flora. This microbial flora confers many health benefits to the host like increased oral health, decreased respiratory infections, increased healing process in acute distal radial fracture, and an increase in the mental health of dementia and meningitis patients (Ale and Binetti 2021). Some common examples of these gut microbiota are Lactobacillus and Bifidobacterium (LAB); sometimes Firmicutes are added to this list. The imbalance in this microbial content (dysbiosis) can cause diseases like inflammatory bowel syndrome, antibody-associated diarrhea, colon cancer, hypercholesterolemia, etc. (Vyas and Ranganathan 2012). Dysbiosis of microbial flora also leads to metabolic disorders. For example, a decrease in Bacteroides viable count in the gut reduces the digestion of polysaccharides entering our gut. Likewise, decrease in fibro lytic microbes like Eubacteria, Bifidobacterium, and Faecalibacteria reduce starch and sucrose metabolism, pyruvate and galactose metabolism, and metabolic processes like glycolysis and gluconeogenesis (Ale and Binetti 2021). So, it is important to maintain these microbes in our gut. This can be achieved through supplements like probiotics and prebiotics.
9.3.2 Probiotics
According to WHO, probiotics are non-pathogenic live microbe(s) that when administrated in adequate amounts confer health benefits to the host (Vyas and Ranganathan 2012). When Vergin and co-workers were studying about the detrimental effects of antibiotics, he coined the term probiotics from probiotika (a substance that survived antibiotics) (Pandey et al. 2015). The microbes that are viable, stable, storable, and which are GRAS (Generally Regarded as Safe) certified are selected as probiotics. Some examples of these health conferring microbes are Bifidobacterium, Lactobacillus sp. like Lactobacillus rhamnosus, L. reuteri, L. casei, L. acidophilus, Bacillus coagulans, Enterococcus faecium, and Saccharomyces boulardii (Pandey et al. 2015).
9.3.3 Prebiotics
Prebiotics are non-digestible fibers (an oligomer made up of 4–6 monomeric hexose units) that are fermented by microorganisms in the gut as the source of nutrition (Vyas and Ranganathan 2012). Some other prebiotics includes breast milk, soybean, raw oat, unrefined wheat or barley, chicory like inulin, and non-digestible carbohydrates like trans-galactooligosaccharide (GOS), fructooligosaccharide (FOS), and xylooligosaccharide (XOS). Prebiotics can prevent diarrhea, constipation, flatulence, etc. Prebiotics should be resistant to bile salts, gastric acid, and hydrolyzing enzymes. The microbiome in the gut should be able to ferment them easily. Generally, probiotics are active in the small intestine and prebiotics are active in the large intestine (Cerezuela et al. 2011).
9.3.4 Benefits of Gut Microbiome
Some highlighting functions of gut microbial flora, in the presence of probiotics and prebiotics, are as follows. Gut microbial flora can synthesize Small Chain Fatty Acid (SCFA) like acetate, propionate, butyrate, etc. which is involved in mucin production, growth, and differentiation of other microbes, regulation of hepatic lipogenic enzyme, accessing genes to transcriptional factors by acetylating histone tail and reversal of cells from neoplastic to non-neoplastic phenotype (Vyas and Ranganathan 2012). It can synthesize derivatives of vitamin B and amino acids, and it is involved in the biotransformation of bile salts which is important for glucose and cholesterol metabolism (Sagar et al. 2015). This microbial flora competes with pathogens for nutrients and attachment to alimentary canals either by producing antimicrobial compounds against them or by producing inhibitory compounds like hydrogen peroxide which may block or degrade the toxin receptors on the pathogen. It increases anti-inflammatory responses like enhanced IgA secretion and increased lymphocyte and leucocyte secretion in GALT and periphery blood vessels to destroy the invaded pathogen (Pandey et al. 2015). This function varies from person to person since the microbial composition varies (Vyas and Ranganathan 2012). It differs based on various factors like age, sex, diet, lifestyle, location, and functions of the immune system (Ale and Binetti 2021). Some of these functions are commonly observed in synergetic conditions prevalent during the therapeutic management using probiotics and prebiotics in a synbiotic manner.
9.3.5 Synbiotics
Synbiosis (syn means together and biotic means for life) is defined as the synergistic beneficial effect of both probiotics and prebiotics. This idea was proposed by Gibson to improve the implantation and survival rate of live microorganisms in the gastrointestinal tract. When two supplements (probiotics and prebiotics) are combined, their synergistic effect is greater than the sum of individually administered supplements (Cerezuela et al. 2011). Synbiosis is introduced to overcome the survival difficulties faced by prebiotics. Administration of synbiotics increases the concentration of Lactobacillus and Bifidobacterium, and it helps to maintain the gut microbial balance. It improves the liver function of the liver cirrhotic patient. It increases immuno-modulating ability, prevents bacterial translocation, and reduces nosocomial infection caused during hospitalization or surgery (Pandey et al. 2015). The genome sequence and annotation of probiotics used in synbiosis should be publicly available for safety, identity, and purity. The synbiotics should commonly satisfy both autochthonous (resident/colonizing inside host) and allochthonous (microbes from an external source such as probiotics) microbes (Swanson et al. 2020). Synbiosis can be divided into two types, namely complementary synbiotics and synergistic synbiotics. In complementary synbiotics, the effects of probiotics and prebiotics are independent of each other. For example, the microorganisms which are used as probiotics adhere to the intestinal walls to confer health benefits, and the prebiotic aids in the growth of autochthonous microorganisms (Ale and Binetti 2021). Hence, they work separately. For example, the probiotic strain of Lactobacillus acidophilus increases the abundance of beneficial microbial flora such as Akkermansia spp. and Lactobacillus spp. in our gut. But in the case of synergistic synbiotics, the prebiotics is designed in such a way that they can provide nutrients to the co-administered probiotics. So, their effect of conferring health benefits is increased (Swanson et al. 2020). Some of the well-known functions of synbiotics are listed below. B.coagulans + inulin, when administered for 6 weeks reduces the c-reactive protein (a marker for inflammatory signs in our body) and increases glutathione (an antioxidant) levels. (Lactobacillus and Bifidobacterium + 10% FOS) can suppress systemic and intestinal inflammations. (L. acidophilus + rice bran) regulate hypercholesterolemia. Synbiotics prevent azoxymethane-mediated suppression of NK cell activity in Peyer’s patches. Synbiotics are widely used to suppress cancer cells or to inhibit the recurrence of various types of cancers. B. longum + FOS and inulin inhibit pre-neoplastic lesion formation, and it suppresses mammary and colon cancer (Pandey et al. 2015). Probiotic L. casei is used to prevent the recurrence of bladder cancer. Prebiotics like inulin/FOS are used to reduce precancerous lesions and increase defense functions like secretion of IL-10, NK cells, etc. (Vyas and Ranganathan 2012).
9.4 Synbiotics in Lung Cancer
9.4.1 Gut Lung Axis
The complex interconnection between our gut and lung is known as the “gut-lung” axis. The cross talk between gut and lung occurs via microbiome and immune response. Though the communication between them is bidirectional, the cross talk between the gut to the lung is most common. (Liu et al. 2021) The gut harbors different bacteria like Firmicobacteria, Bacteroidetes, Eubacteria, Ruminococcus, Faecalibacterium, etc. When a pathogen enters the small intestines, the cell wall fragments or protein parts of the microbes escape cytokines/chemokines and travel to cisterna chyli via the mesenteric lymphatic system and from there it reaches the lungs by entering the circulatory system (Liu et al. 2019). When it reaches the lung region, an immune response like dendritic cells, macrophages, and T cells are produced and get differentiated. This pathway can be altered in a different way also, that is, in the mesenteric lymphatic system antigen-presenting cells recruits naive B cells and T cells. These B cells get matured (plasma B cells) and synthesize immunoglobulins which leave the lymph node and enter into mucosal tissues to spread immune information. This process switches off the innate ability to produce IL-10 and anti-inflammatory molecules. This initiates dendritic cells to migrate to local lymph nodes to differentiate T cells. Later these differentiated T cells migrate to GALT (Gut Associated Lymphoid Tissue), which distributes them to the mucosal (bronchus) and periphery non-mucosal layer, thus improving immune response against the pulmonary pathogen. Similarly, an immune response from the lungs can travel to the gut as well. The lungs, which are considered sterile organs, harbor air-borne microbes below the vocal cord and in bronchoalveolar lavage fluid. The upper respiratory tract (nostrils) contains Firmicutes and Actinobacteria. The lower respiratory tract (lungs) harbors Proteobacteria, Bacteriodetes, and Firmicobacteria. During the cross talk between lung and gut, the dendritic cells present in the lungs imprint the expression of gut homing integrin (α4β7 and CCR9) on T cells, which initiate T cells migrate to the gut to elicit an immune response (Bingula et al. 2017).
9.4.2 Dysbiosis of Microbiome in Lung Cancer Patients
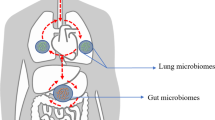
Since gut and lungs are interconnected, the dysbiosis of gut microbial flora may increase the chance of lung disease. The dysbiosis of the gut microbiome leads to the progression of lung cancer through genotoxicity, systemic inflammation, and defective immune surveillance. Imbalance in Firmicutes and Bacteroides increases the risk of lung cancer. In general, the gut microbiome of lung cancer patients shows increased Bacteroides, Enterococcus, Fusobacterium, Prevotella, Ruminococcus, Veillonella, and decreased Bifidobacteria, Blautia, Coprococcus, Dialister, Escherichia, Enterobacter, Faecalibacterium, Firmicutes, Lachnospiraceae, Shigella, and phylum of Actinobacteria. Microbes like Blautia obeum, Lactobacillus salivarius, Akkermansia muciniphila, and Coriobacteriaceae are overgrown in lung cancer subtypes and metastatic patients (Liu et al. 2021). An experimental study, which was conducted on 16 healthy individuals and 30 lung cancer patients based on the three tumor biomarkers (CYFRA 21-1, NSE, and CEA) showed the following results. There was a decrease in Firmicutes, Bacteroides, and Actinobacteria in adenocarcinoma patients. Similarly, squamous epithelial lung cancer patients showed decreased Prevotella spp. The NSCLC patients showed increased Proteobacteria and Verrucomicrobia and recurrent adenocarcinoma patients show increased Fusobacteria and Streptococcus. Certain microbes are commonly found in all groups of lung cancer they include Bifidobacterium, Blautia, Dialister, Lachnospiraceae, and Ruminococcaceae (Liu et al. 2019). Hence, the composition of certain gut microbial flora serves as a biomarker for the diagnosis of different types of lung cancer. It helps to identify different stages of tumor, and it also helps to monitor the recurrence, prognosis, and metastatic condition of lung cancer patients after treatment. It is suspected that the tumor cells might synthesize certain metabolic products or create differential profiles that may favor selective adherence of some members of microflora to the gut (Liu et al. 2019) (Fig. 9.1).
Illustration of lung dysbiosis and homeostasis: In the lung dysbiosis, SCFA (Small Chain Fatty Acids) takes the lead in mediating the pro-inflammatory responses resulting in loss of immune-homeostasis. In a healthy state, the immune cells take over the production of anti-inflammatory markers ensuring a steady state of immune-homeostasis
9.4.3 Probiotic Effect on Lung Cancer
Restoration of these depleted microbes back into the gut can either resist or reduce the effects caused by lung diseases (Liu et al. 2021). This can be achieved through probiotics, whose administration has positive effects on lung cancer patients. The evidence of probiotic influence on lung cancer was found in 1985, and it has recently emerged due to increased results. When mice C57BL16 is injected with Bifidobacterium infantis, it increases the necrosis rate of lung cancer cells, thus prolonging the survival period (Zhou et al. 2022). When the Lewis lung cancer model is treated with cisplatin and ABX (an abiotic cocktail of vancomycin, ampicillin, and neomycin), it increased the tumor size and decreased the survival rate. Here the ABX upregulates VEGF (Vascular Endothelial Growth Factor) that induces angiogenesis in tumor cells and downregulates apoptotic promoters BAX and CDKNIB. But when it is treated along with probiotic Lactobacillus acidophilus, it resulted in reduced tumor size due to upregulation of IFN gamma and granzyme B, thus increasing the survival rate (Gui et al. 2015). Similarly, when the mouse melanoma model is treated with a Bifidobacterium cocktail (B. bifidum, B. longum, B. lactis, B. breve), it showed control against tumor which is as same as PD-L1 (Programmed Death Ligand 1)-specific antibody therapy. A probiotic strain of Parabacteroides and Methanobrevibacter results in the downregulation of cancer cell proliferation and thus increases the survival rate of lung cancer patients.
9.4.4 Synbiotic Approach to Reduce the Effect of Chemotherapy
Increased use of antibiotics like penicillin, cephalosporin, and quinolones can increase the risk of lung cancer. When lung cancer patients undergo antibiotic-associated chemotherapy, their gut microbial flora gets degraded along with their intestinal walls, which results in severe diarrhea. When these patients are supplemented with a probiotic strain of Clostridium butyricum, the patient showed a reduced effect of diarrhea and other inflammatory bowel diseases. In general, the depletion of Firmicutes and Bacteroides colonies in the gut reduces the production of SCFA (Liu et al. 2021). SCFA are Small Chain Fatty Acids, synthesized either by autochthonous or allochthonous microbes by degrading natural dietary fibers present in the colon and caecum or by degrading prebiotic fibers like inulin, FOS, etc. These SCFAs are essential for our body because it nurtures intestinal epithelial cells and exhibits an anti-inflammatory effect (Zhou et al. 2022). Some common examples of this SCFA include acetate, propionate, and butyrate. The NSCLC patients show dysbiosis of butyrate-producing microorganisms like Faecalibacterium prausnitzii, Clostridium leptem, Clostridial cluster I, Clostridial cluster XIVA, Rumminococcus sp., and Roseburia sp. (Gui et al. 2020). These butyrate- producing microorganisms synthesize a compound called mucin which helps in the regeneration of the ruptured mucosal layer of the intestine. Variations in this SCFA like sodium butyrate induce apoptosis in tumor cells or act as histone deacetylase inhibitors (Gui et al. 2020). Hence, synbiotic administration of these depleted microbes along with a suitable substrate can reduce the risk of lung cancer. For example, Bifidobacterium (probiotic) supplied with inulin or oligo fructose (prebiotic) restores Bifidobacterium (Liu et al. 2020). Sometimes a mismatched selection of probiotics or prebiotics for the growth of microbes can cause adverse effects. For example, synbiotics with Bacillus strain is harmful because of their ability to generate multiple toxins as it harbors mobile antimicrobial resistance gene. So, care should be taken while designing synbiotics for lung cancer patients. However, the interaction between gut and lung is undefined. Acute exposure of a single dose of intra-tracheal LPS in the airways of mice has been transported into the bloodstream within 24 h. Similarly, when mice are subjected to inhalation of a non-absorbable tracer, the gastrointestinal tract of mice showed a trace amount of this non-absorbable element within a few hours of inhalation (Bingula et al. 2017). Hence, there is always a debate whether lung cancer is the product of gut microbial variation or vice versa. Scientists are on their way to discover genetically modified microorganisms to enhance the efficacy of anti-cancer treatments. It can also be used as a delivery vehicle to administer chemotherapeutic agents (Scott et al. 2018).
9.5 Conclusion
Synbiotics have a promising potential for the prevention and treatment of cancer. Yet the research studies are still not sufficient to substantiate the significance of synbiotics. There is evidence to showcase the beneficial effects of probiotics and prebiotics. But it is still difficult to conclude the role of these agents in the treatment of cancer. On the other hand, the use of prebiotics and probiotics as supplementary therapy in cancer treatment has been demonstrated in several reports. Research on lung microbe population in in vivo experiments dealing with the complex interaction between the microbes and the host may provide insights into future studies. Synbiotics and the metabolic products of probiotics can be produced economically and has fewer side effects, as there is no evidence or reports stating that ingested probiotics can pose risk to the patients. Probiotics in the balanced diet were also found to be beneficial in the prevention of cancer, but worldwide people prefer foods containing high fat and low fiber, which is always a threat to cancer-associated risk factors including obesity and diabetes. However, the effect of pro- and prebiotics to combat lung cancers concerning the gut-lung axis is more likely to depend on the host microbiota, duration of pro- and prebiotic consumption (Minimum of 6 days and maximum of 1 year), and the age of the patients. Further clinical studies investigating the role of synbiotics and their impact on lung cancer will pave way for the therapeutic management of cancer.
References
Ale EC, Binetti AG (2021) Role of probiotics, prebiotics, and synbiotics in the elderly: insights into their applications. Front Microbiol 12:631254. https://doi.org/10.3389/fmicb.2021.631254
Bingula R, Filaire M, Radosevic-robin N, Bey M, Berthon J, Bernalier-donadille A, Vasson M, Filaire E (2017) Desired turbulence? Gut-lung Axis, immunity, and lung cancer. J Oncol 2017:5035371
Cerezuela R, Meseguer J, Esteban MA (2011) Current knowledge in synbiotic use for fish aquaculture: a review. J Aquac Res Dev 1:1–7. https://doi.org/10.4172/2155-9546.S1-008
De Maria YNLF, Barbosa DA, Menegidio FB, Santos KBNH, Humberto AC, Alencar VC, Silva JFS, De Oliveira RC, Batista ML, Nunes LR, Jabes DL (2021) Analysis of mouse fecal dysbiosis, during the development of cachexia, induced by transplantation with Lewis lung carcinoma cells. Microbiology 167(10). https://doi.org/10.1099/mic.0.001088
Escalera E, Bellido L, del Barco E, Cigarral B, Barrios B, Casado D, Claros J, Figuero L, Olivares A, Terán E, López A, Cruz JJ (2021) Small cell lung cancer. Medicine 13(25). https://doi.org/10.1016/j.med.2021.02.001
Gui QF, Lu HF, Zhang CX, Xu ZR, Yang YM (2015) Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet Mol Res 14(2):5642–5651. https://doi.org/10.4238/2015.May.25.16
Gui Q et al (2020) The association between gut butyrate-producing bacteria and non-small-cell lung cancer. J Clin Lab Anal 34:E23318
Liu F, Li J, Guan Y, Lou Y, Chen H, Xu M, Deng D, Chen J, Ni B, Zhao L, Li H, Sang H, Cai X (2019) Dysbiosis of the gut microbiome is associated with tumor biomarkers in lung cancer. Int J Biol Sci 15(11):2381–2392. https://doi.org/10.7150/ijbs.35980
Liu NN, Ma Q, Ge Y, Yi CX, Wei LQ, Tan JC, Chu Q, Li JQ, Zhang P, Wang H (2020) Microbiome dysbiosis in lung cancer: from composition to therapy. NPJ Precis Oncol 4(1). https://doi.org/10.1038/s41698-020-00138-z
Liu X, Cheng Y, Zang D, Zhang M, Li X, Liu D, Gao B, Zhou H, Sun J, Han X, Lin M, Chen J (2021) The role of gut microbiota in lung cancer: from carcinogenesis to immunotherapy. Front Oncol 11:1–13. https://doi.org/10.3389/fonc.2021.720842
López A, Escalera E, del Barco E, Bellido L, Cigarral B, Barrios B, Casado D, Claros J, Figuero L, Olivares A, Terán E, Cruz JJ (2021) Non-small cell lung cancer. Medicine 13(25). https://doi.org/10.1016/j.med.2021.02.002
Lukeman JM (2015) What is lung cancer? Perspectives in Lung Cancer:30–40. https://doi.org/10.1159/000400400
Minna JD, Roth JA, Gazdar AF (2002) Focus on lung cancer. Cancer Cell 1:49–52
Mustafa M, Jamalulazizi AR, Iiizam EL, Nazirah A, Abbas SA (2016) Lung cancer: risk factors, management, and prognosis lung cancer : risk factors, management, and prognosis. IOSR J Dent Med Sci. https://doi.org/10.9790/0853-15100494101
Pandey KR, Naik SR, Vakil BV (2015) Probiotics, prebiotics and synbiotics–a review. J Food Sci Technol 52(12):7577–7587. https://doi.org/10.1007/s13197-015-1921-1
Sagar S, Folkerts G, Garssen J (2015) Prebiotics, probiotics, and synbiotics for the management of respiratory disease. In: Bagchi D, Preuss HG, Swaroop A (eds) Nutraceuticals and functional foods in human health and disease prevention. https://doi.org/10.1201/b19308
Scott AJ, Merrifield CA, Younes JA, Pekelharing EP (2018) Pre-, pro- and synbiotics in cancer prevention and treatment—a review of basic and clinical research. Ecancermedicalscience 12:1–11. https://doi.org/10.3332/ecancer.2018.869
Spiro SG, Silvestri GA (2005) Centennial review one hundred years of lung cancer. Am J Respir Crit Care Med 172:523–529. https://doi.org/10.1164/rccm.200504-531OE
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, Sanders ME (2020) The international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17(11):687–701. https://doi.org/10.1038/s41575-020-0344-2
Travis WD (2011) Pathology of lung cancer. Clin Chest Med 32(4):669–692. https://doi.org/10.1016/j.ccm.2011.08.005
Vyas U, Ranganathan N (2012) Probiotics, prebiotics, and synbiotics: gut and beyond. Gastroenterol Res Pract 2012:872716. https://doi.org/10.1155/2012/872716
Zhou A et al (2022) Gut microbiota: the emerging link to lung homeostasis and disease. J Bacteriol 203(4):e00454–e00420
Acknowledgment
The authors express their gratitude to SASTRA-Deemed-to-be-University, Tamil Nadu, India for infrastructure and financial support. Authors also extend their appreciation for the contribution of Biopharmaceutical research lab members, SASTRA-Deemed-to-be-University.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Diwahar Prakash: Writing—Original Draft, Writing—Review and Editing Collection of data; Suresh Krishna Venkataramanan: Writing—Original Draft, Writing—Review and Editing, Collection of data; Gayathri G: Writing—Original Draft, Review and Editing; Shibi Muralidar: Writing—Original Draft, Review, and Editing; Senthil Visaga Ambi: Conceptualization, Visualization, Supervision, and Writing—Review and Editing.
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Prakash, D., Venkataramanan, S.K., Gopal, G., Muralidar, S., Ambi, S.V. (2023). Synbiotics in Lung Cancer. In: Mishra, N., Bhatt, S., Paudel, K.R., M Hansbro, P., Dua, K. (eds) Synbiotics for the Management of Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-19-7550-9_9
Download citation
DOI: https://doi.org/10.1007/978-981-19-7550-9_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7549-3
Online ISBN: 978-981-19-7550-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)