Abstract
Cervical cancer appeared to be the fourth most common cancer among women worldwide and Human Papillomavirus (HPV) is identified as the primary causal factor for cervical cancer pathogenesis. Recently, application of synbiotics is reported to play a beneficial role in the treatment of many cancers and some studies also identified its beneficial effects on cervical cancer and HPV infection. The possible molecular mechanisms of synbiotics for the treatment of cervical cancer include reduction of inflammatory response due to HPV infection, promotion of apoptosis, and inhibition of cellular proliferation and metastasis. Sometimes, conventional anti-infective drugs along with synbiotics exhibited better prognosis for cervical cancer patients. Most importantly, some clinical trials confirmed that most common gastrointestinal side effects due to cervical cancer therapies were reduced by administration of synbiotics. However, the precise molecular mechanisms for synbiotics mediated cervical cancer therapies should be evaluated in future along with the side effects of synbiotics in cervical cancer patients. The current study is focused on synbiotics as a promising anticancer agent for the prevention and treatment of cervical cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
The rapid increases of oncological diseases are now the main global burden which is multifactorial and caused due to genetic as well as environmental factors, such as dietary and lifestyle habits. Some environmental factors significantly change the host gut microbial community, which leads to induce changes in host physiology and contributes to the development of numerous diseases such as cancer (Marta et al. 2020). In current scenario, for the management of various diseases including cancers, synbiotics are used.
17.2 Synbiotics: An Overview
The concept of synbiotics was introduced firstly by Glenn R. Gibson and Marcel B. Roberfroid in 1995. They defined it as “a mixture of probiotics and prebiotics that beneficially affects the host by improving the survival and implantation of live microbial dietary supplements in the gastrointestinal tract, by selectively stimulating the growth and/or by activating the metabolism of one or a limited number of health-promoting bacteria, and thus improving host welfare” (Gibson and Roberfroid 1995). However, along with time, as the definitions of prebiotics and probiotics were changed, the definition of synbiotics has also been updated. In May 2019, the panel gathered by the International Scientific Association for Probiotics and Prebiotics (ISAPP) defined synbiotics as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host.” They also clarified the two types of synbiotics (Fig. 17.1). Complementary synbiotics are defined as a combination of prebiotics and probiotics having heath benefit(s) but functioning independently. In synergistic synbiotics, the substrate is designed, so that the co-administered microorganisms can selectively utilize it (Swanson et al. 2020).
Several positive effects of synbiotics on human nutrition and health have been reported. It was reported that activation of digestive enzymes like sucrase, lactase, isomaltase along with the reduction of coliform bacteria were observed after the application of synbiotics and they significantly increased the number of probiotic bacteria in the fecal sample (Yang et al. 2005; Yadav et al. 2022). Application of synbiotics also reported to reduce the risk of various metabolic disorders like type 2 diabetes, cardiovascular disease, and cancer (Cicero et al. 2021; Yadav et al. 2022). Several therapeutic potentials of synbiotics were also observed. A positive effect of synbiotics on diseases like sepsis in early infancy, hepatic conditions, obesity, type 2 diabetes, insulin resistance, irritable bowel syndrome, and cancer were also observed (Yadav et al. 2022). Ongoing clinical trials of synbiotics on Sars-Cov2 infected patients is nowadays also suggested a reduction of its gastrointestinal symptoms (Xavier-Santos et al. 2022).
17.3 Effects of Synbiotics on Cancer
One of the major probiotic bacteria such as Lactobacillus sp. can utilize the prebiotics for their own growth in synbiotic food products (Yadav et al. 2022). Interestingly, this combination of pro- and prebiotics has a greater effect than individual pre- or probiotic administration (Fotiadis 2008). Symbiotics are not only modifying the host microbiome, but they can also act as antimutagens. These synbiotics play a vital role in scavenging and eliminating carcinogens. Growing evidence suggested that the symbiotics modulate the adverse effects of chemotherapy in cancer patients, thus it is widely used for the treatment of cancer (Qiu et al. 2019; Tian et al. 2019). Many anticancer drugs are designed for the treatment of malignancies and most of them are generally toxic for healthy cells with numerous side effects and some of which are life-threatening also. In past several years, chemotherapy and immunotherapy are used for cancer treatment. But there are many limitations in these types of the treatment procedure. It is reported that these anticancer therapies affect the microbiota profile in patients and induce high toxicity (Panebianco et al. 2018). Current studies also reveal that synbiotics have many beneficial effects on human health as well as they have a very limited side-effect profile. It is observed that cancer patients are often in a state of immunocompromised due to property of cancer cell itself or by the treatment regime. In recent years, many studies are oriented towards the administration of synbiotics as a principal therapy in regard to cancer with minimal side effects. By different mechanisms, synbiotics show their oncosuppressive effects by preventing of host cell proliferation, maintaining intestinal barrier function, and immunomodulation. There are some strong evidences which suggested that the human microbiota plays an important role in carcinogenesis. A large proportion of cancer patients usually consume antibiotics for their therapeutic perspective, but use of these antibiotics has a large impact on host–microbiome composition and function (Francino 2015). It was reported that antibiotic-treated patients had worse overall survival when compared with those patients treated with synbiotics. Thus, in comparison to negative manipulation of microbiota with antibiotics, synbiotics currently represent the alternative therapy towards the positive manipulation of host microbiome and thus now it is used for potential therapeutic treatment in cancer (Scott et al. 2018). A meta-analysis also confirms that symbiotics can minimize the adverse effects associated with surgery, chemotherapy, radiotherapy, and antibiotics (Marta et al. 2020). Another important role of synbiotics is to prevent the conversion of non-toxic pro-carcinogens to harmful carcinogens, resulting in reducing the carcinogenic effects (Marta et al. 2020). Some other evidences suggested that use of particular synbiotics results in reduced levels of chemotherapy- and radiotherapy-related diarrhea and post-surgery infectious complications.
17.4 Cervical Cancer: A Major Global Burden Among Women
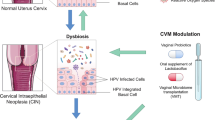
According to GLOBOCAN, 2021, cervical cancer appeared to be the fourth most common cancer among women in the world. Oncogenic Human Papillomavirus (HPV) was identified as primary causal factor for cervical cancer. It is already known that in cervical cancer cases, HPV E6 and E7 oncoprotein interact with p53 and pRB tumor suppressor genes and suppress their expression for the development of cervical cancer. In recent years, multidisciplinary approaches are used for the treatment of cervical cancer. It was observed that symbiotic supplementation can reduce the adverse gastrointestinal side effects of various cancer patients including patients of cervical cancer (Jahanshahi et al. 2020). Now different studies tried to understand the molecular mechanistic pathways of synbiotics in the treatment of various cancers including cervical cancer. Studies on cervical cancer also suggested that the presence and enrichment of some specific bacterial species may resist HPV infection in the cervix, and these beneficial bacterial communities also help to clear off the HPV infection and reduce the risk of the development of cervical cancer. Thus, in the future, for better and safer oncological treatment, synbiotics can be used, which provide a great opportunity as an alternative therapeutic strategy.
The use of these therapies, both chemo and radiation with surgery effectively abolish the growth of cancerous cells in cervix. But these therapies induce several short- and long-term effects on the patients and thereby lead to several side effects. The adverse side effects are pain, nausea, vomiting, and fatigue (Cho and Blaser 2012). The chemoradiotherapy are generally applied on patients with locally advanced cervical cancer which are restricted to the pelvis (Eifel 2006). Other patients who are treated with concurrent chemotherapy in addition to the radiotherapy have increased gastrointestinal side effects (Eifel 2006). This side effect of nausea and vomiting could lead to severe diarrhea and weight loss. In recent years, the understanding of overall importance of microbiome in our lives has increased, also its role in cancer. Disturbances in the vaginal microbiota composition may play an important role in cervical cancer pathogenesis. Therefore, microbiota-based therapy can serve as a better option for cervical cancer prevention and treatment (Nelson et al. 2015). The beneficial effects of synbiotics on cervical cancer therapy are reported by various studies, and application of these class of therapy can reduce the risk of gastrointestinal side effects by the conventional chemotherapeutic strategies.
17.5 Synbiotics as Therapeutic Strategy in Cervical Cancer
The two categories of synbiotics may help to understand the correlation between prebiotics and probiotics and ultimately the formulation of synbiotic products for the beneficial effect on cervical cancer and therapeutic application.
In synbiotics, probiotics serve as the major component. Probiotics are living microorganisms, which have health beneficial effects when consumed or applied to the system. It may contain diverse microorganisms. The most common one in probiotics are Bifidobacterium and Lactobacillus and some yeasts like Saccharomyces boulardii, etc. Probiotics have diverse characteristics which are summarized in Fig. 17.2 (Morelli and Capurso 2012; Han et al. 2021; Krishnamoorty et al. 2022).
It was already established that, probiotics can modulate cancers via induction of apoptosis, inhibition of mutagenic and kinase activity, downregulation of oncogenic expression, induction of autophagy, activation of tumor suppressors, and inhibition of metastasis (Kim et al. 2010; Motevaseli et al. 2017; Jahanshahi et al. 2020). Various studies demonstrated the effect of probiotics on cervical cancer therapy. Some studies on cervical cancer cell lines like HeLa, Caski, and SiHa reveals the effect of some probiotics which are summarized in Table 17.1.
Based on these in vitro studies, it was identified that probiotics have amazing abilities to prevent or regress cervical cancer by reduction of cellular proliferation, metastasis and inflammatory response, and induction of apoptosis. Not only that, probiotics with other chemotherapeutic drugs exert better results (Kim et al. 2015; Jahanshahi et al. 2020; Negi et al. 2020). Administration of probiotics confers prevention against gastrointestinal side effects caused by cervical cancer therapies in combination with conventional anti-infective drugs. Some studies proposed that in reduction of incidence of diarrhea, the probiotics have a beneficial role (Liu et al. 2017; Jiang et al. 2021). A study identified that supplementation of probiotics reduces radiation-induced diarrhea among cervical cancer patients effectively (Linn et al. 2019). Other studies also reported modest reduction in incidence of diarrhea of cervical cancer patient undergoing chemoradiotherapy by using probiotic liquid yogurt (Giralt et al. 2008; Liu et al. 2017; Linn et al. 2019). The most common used probiotics are Lactobacillus and Bifidobacteria in these studies. Lactobacillus-based treatment can enhance p21 tumor suppressor expression in cervical cancer cell lines (Wang et al. 2018). Lactobacillus plantarum are cultured from vaginal secretions of young adult and adolescent women, and it exhibited probiotic and anticancer features in HeLa cervical cancer line (Nami et al. 2014). Another study also revealed that Lactobacillus strains that were isolated from human milk have remarkable antioxidant activity, resistance to low pH and high level of bile salts, antibiotic susceptibility, and probiotic characteristics (Rajoka et al. 2018). Exopolysaccharides of L. gasseri strains in lyophilized state induce apoptosis in HeLa cells in relation to Bax and Caspase3 upregulation (Sungur et al. 2017). L. gasseri also reduces TNF-α and increases IL-10, which leads to their anti-inflammatory impact on HPV-induced cervical cancer. Supernatants of Lactobacillus crispatus and Lactobacillus rhamnosus also reduced the expression of matrix metalloproteases like MMP2 and MMP9 along with CASP3 and eventually metastasis in HeLa cell line (Nouri et al. 2016). In terms of management of gastrointestinal symptoms in cervical cancer patients, it is reported that administering a probiotic with live Bifidobacterium animalis subsp. lactis BB-12 and Lactobacillus acidophilus LA-5 associated with reduced development of severe diarrhea after beam pelvic radiotherapy (Linn et al. 2019). In another study, a probiotic drink consisting of Lactobacillus casei was employed on cervical cancer patients who had undergone radiotherapy and cisplatin-mediated therapy, and this application is proven to be beneficial for improving stool consistency (Giralt et al. 2008). Microbiome also serves as a biomarker for diagnosis of cervical cancer. A study using Lactobacillus rhamnosus and Lactobacillus reuteri serves as a promising biomarker for detection of cervical malignancies (Perisic et al. 2011). A study on 228 stage IIIB cervical cancer patients, combination therapy with heat-killed Lactobacillus casei (LC9018) with radiotherapy significantly improved the response pattern of the patients (Okawa et al. 1993). It was also reported that LC9018 can be used as adjuvant and associated with longer disease-free survival among patients who had undergone radiotherapy alone. Another study also reported that the pessaries containing both cisplatin and probiotic biomass can be utilized as better therapeutic method for cervical cancer patients, and they are reported as good scavenger for free radicals (Negi et al. 2020).
Prebiotics serve as another major component in synbiotics. They are basically compounds in food, which can promote the proliferation or activity of beneficial microorganisms including bacteria and fungi. Normally, dietary prebiotics is nondigestible food ingredients that travel undigested through the upper part of the intestine and stimulate the activity and growth of beneficial microorganisms by acting as a substrate for them (Markowiak and Slizewska 2017). Cereals, vegetables, and fresh fruits serve as the good sources of prebiotics. Specifically, green vegetables, garlic, onion, tomatoes, artichokes, bananas, asparagus, berries, chicory, green vegetables, legumes, as well as oats, linseed, barley, and wheat are potential sources of prebiotics (Crittenden and Playne 2008; Markowiak and Slizewska 2017).
Some artificial prebiotics are also reported such as lactulose, maltooligosaccharides, galactooligosaccharides (GOS), and lactosaccharose. Fructans, like inulin and oligofructose, have an effective relationship with various types of probiotics (Markowiak and Slizewska 2017). Like probiotics, there are many reports regarding the beneficial effects of prebiotics on malignancy. Some in vitro studies on human colorectal cancer cell lines (L97 and HT29) demonstrated that inulin fractions on plasma supernatant reduced growth and promote apoptosis in human colorectal carcinoma cell lines (Munjal et al. 2009; Markowiak and Slizewska 2017). This study supports that prebiotic has an impact on cancer. Not only colorectal cancer, but some in vitro studies also observed that employment of inulin and oligofructose (dose 5–15%) exerts beneficial effect on breast cancer and resists metastases to the lungs (Markowiak and Slizewska 2017; Taper and Roberfroid 2002). Studies between prebiotics and cervical cancer are now unclear but previous reports of prebiotics on various types of cancer justified that there is a close relationship between cervical cancer and prebiotics.
It was already known that a synbiotic is a mixture of probiotics and prebiotics which significantly affects the host by improving the growth and activity of beneficial gut microbiota.
Table 17.2 represents the combination of some popularly used probiotics and prebiotics used as a synbiotics (Crittenden and Playne 2009; Olveira and Gonzalez-Molero 2016; Saez-Lara et al. 2016).
It was found that, the application of a symbiotic product containing blended probiotics (Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus bulgaricus, Lactobacillus acidophilus, Bifidobacterium breve, Streptococcus thermophilus, Bifidobacterium longum) and fructooligosaccharides as prebiotic resulted in the downregulation of nuclear factor B and decreased expression of TNF-a (Markowiak and Slizewska 2017; Eslamparast et al. 2014). Findings demonstrated that patients who were given synbiotic containing Bifidobacterium lactis Bi-07 1 × 106 CFU/g biogel, 1 × 107 (CFU)/g biogel of L. acidophilus NCFM, and inulin reduced levels of fecal calprotectin and less incidence of intense vomiting in cervical cancer patients (De Loera Rodriguez et al. 2018). Thus, synbiotic supplementation may be beneficial for reducing gastrointestinal side effects of cervical cancer patients.
17.6 Influence of Synbiotics on HPV Infection in Cervical Cancer
It is established that use of synbiotics can reform and maintain a healthy balance of bacterial species. Also, it is seen that the use of oral probiotics has effectively treated gastrointestinal diseases such as irritable bowel syndrome, traveler’s diarrhea, gastroenteritis, and others (Champer et al. 2018). Interestingly, it has been shown that synbiotics such as Lactobacilli-based treatment results in the downregulation of cyclin A, CDK2, and HPV oncogenes E6 and E7 (Wang et al. 2018; Yim and Park 2005). The first report of the use of Lactobacillus rhamnosus that could prevent diarrhea induced by radiotherapy was reported by two studies (Delia et al. 2002; Wang et al. 2019). Similar result was reported in the study by Urbancsek. The study reported that the use of this bacteria helps in reducing the need of anti-diarrheal drug (Urbancsek et al. 2001; Linn et al. 2019). Rauch and their co-workers suggested that risk of gastrointestinal cancer could be decreased by regular intake oral probiotics (Rauch and Lynch 2012; Champer et al. 2018). This similar effect could be achieved by using vaginal probiotics which could reduce the rate of HPV infection and also increase the rate of clearance of the HPV (Champer et al. 2018). The rate of relapse of bacterial vaginosis can also be reduced by using probiotics (Champer et al. 2018). Lactobacillus iners is generally associated with high-risk HPV infections. Other lactobacilli, including L. jensenii, L. gasseri, and L. crispatus, present preferably in the healthiest part of the cervix. They can produce antimicrobial substances such as bacteriocin, lactic acid, and hydrogen peroxide. They also compete with the pathogenic bacteria and form barriers to prevent their colonization and adherence on cervix. E6 and E7 are two oncogenes that are encoded by high-risk HPV (Yim and Park 2005). These two genes can suppress p53 and pRB tumor suppressors which is prerequisite for cervical cancer pathogenesis. Lactobacillus supernatants (LS), L. jensenii, L. crispatus, and L. gasseri, treatment leads to downregulation of cyclin A, CDK2, and HPV oncogenes (E6 and E7) which may be beneficial for cervical cancer patients (Wang et al. 2018). Earlier studies reported that Bifidobacterium adolescentis exerts an antiviral effect on SiHa cervical cell line (Cha et al. 2012). Treating cells with this bacteria strain are reported to reduce the E6 mRNA and protein levels expression. It was also reported that L. gasseri has a smaller inhibitory impact on the E6 gene alone and L. crispatus has an inhibitory effect on the expression of E6 and E7 oncogene at the mRNA level (Li et al. 2019). A study identified the impacts of probiotic strains on the cytological quality of cervical smears and clearance of high-risk human papillomavirus in cervix (Ou et al. 2019). Study also reported the anti-inflammatory role of Lactobacillus plantarum NK3 and Bifidobacterium longum NK49 which suppress NF-κB that was induced by HPV infection in the mice vagina and uterus (Kim et al. 2019).
17.7 Conclusion
It can be postulated that the use of synbiotic therapy with other conventional treatments of cancer can help in reducing the side effects of those treatments. Synbiotics clearly represent a novel and popular therapeutic approach to cervical cancer prevention because they are cost-effective, with little side effects, easier to administer unlike the current complicated treatment regime for high-grade cervical cancer, which involves a surgical method that carries significant risk to future reproductive side effects. Thus, it appears that modulation of vaginal microbiota with the application of synbiotics can prevent HPV and such application would be a safe and cost-effective way to protect the reproductive health of women.
References
Cha MK, Lee DK, An HM, Lee SW, Shin SH, Kwon JH et al (2012) Antiviral activity of Bifidobacterium adolescentis SPM1005-a on human papillomavirus type. BMC Medicine 10:72
Champer M, Wong AM, Champer J, Brito IL, Messer PW, Hou JY, Wright JD (2018) The role of the vaginal microbiome in gynaecological cancer. BJOG 125(3):309–315
Cho I, Blaser MJ (2012) The human microbiome: at the interface of health and disease. Nat Rev Genet 13:260–270
Cicero A, Fogacci F, Bove M, Giovannini M, Borghi C (2021) Impact of a short-term synbiotic supplementation on metabolic syndrome and systemic inflammation in elderly patients: a randomized placebo-controlled clinical trial. Eur J Nutr 60(2):655–663
Crittenden R, Playne MJ (2008) Nutrition news. Facts and functions of prebiotics, probiotics and synbiotics. In: Handbook of probiotics and prebiotics. Wiley, USA. 535–582.
Crittenden R, Playne MJ (2009) Prebiotics. In: In handbook of probiotics and prebiotics. John Wiley & Sons, USA, pp 535–561
De Loera Rodriguez LH, Ortiz GG, Rivero Moragrega P, Velazquez Brizuela IE, Santoscoy Gutierrez JF, Rincon Sanchez AR et al (2018) Effect of symbiotic supplementation on fecal calprotectin levels and lactic acid bacteria, Bifidobacteria, Escherichia coli and Salmonella DNA in patients with cervical cancer. Nutr Hosp 35:1394–1400
Delia P, Sansotta G, Donato V, Messina G, Frosina P, Pergolizzi S, De Renzis C, Famularo G (2002) Prevention of radiation-induced diarrhea with the use of VSL#3, a new high-potency probiotic preparation. Am J Gastroenterol 97(8):2150–2152
Eifel PJ (2006) Chemoradiotherapy in the treatment of cervical cancer. Semin radiat oncol 16(3):177–185
Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hetmatdoost A (2014) Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr 99:535–542
Fotiadis CI (2008) Role of probiotics, prebiotics and synbiotics in chemoprevention for colorectal cancer. World J Gastroenterol 14(42):6453
Francino MP (2015) Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front Microbiol 6:1543
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125(6):1401–1412
Giralt J, Regadera JP, Verges R, Romero J, Fuente IDL, Biete A, Villoria J, Cobo JM, Guarner F (2008) Effects of probiotic Lactobacillus casei DN-114 001 in prevention of radiation-induced diarrhoea: results from multicenter, randomized, placebo-controlled nutritional trial. Int J Radiat Oncol Biol Phys 71(4):1213–1219
Han S, Lu Y, Xie J, Fei Y, Zheng G, Wang Z, Liu J, Lv L, Ling Z, Berglund B et al (2021) Probiotic gastrointestinal transit and colonization after Oral administration: a long journey. Front Cell Infect Microbiol 11:102
Jahanshahi M, Maleki DP, Badehnoosh B, Asemi Z, Hallajzadeh J et al (2020) Anti-tumor activities of probiotics in cervical cancer. J Ovarian Res 13(1):68
Jiang L, Li B, Zhang Y, Ma S, Liu C, Liang F, Wei Z, Huang T, Wang R (2021) Influence of pelvic intensity-modulated radiation therapy with concurrent cisplatin-based chemotherapy of cervical cancer on the vaginal microbiome. Front Oncol 23(11):615439
Kim Y, Oh S, Yun HS, Oh S, Kim SH (2010) Cell-bound exopolysaccharide from probiotic bacteria induces autophagic cell death of tumour cells. Lett Appl Microbiol 51:123–130
Kim SN, Lee WM, Park KS, Kim JB, Han DJ, Bae J (2015) The effect of Lactobacillus casei extract on cervical cancer cell lines. Contemp Oncol 19:306–312
Kim DE, Kim JK, Han SK, Jang SE, Han MJ, Kim DH (2019) Lactobacillus plantarum NK3 and Bifidobacterium longum NK49 alleviate bacterial vaginosis and osteoporosis in mice by suppressing NF-kappa B-linked TNF-alpha expression. J Med Food 22:1022–1031
Li C, Jia L, Yu Y, Jin L (2019) Lactic acid induced micro RNA-744 enhances motility of SiHa cervical cancer cells through targeting ARHGAP5. Chem Biol Interact 298:86–95
Linn YH, Thu KK, Win NHH (2019) Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study. Probiotics Antimicrob Proteins. 11(2):638–647
Liu MM, Li ST, Shu Y, Zhan HQ (2017) Probiotics for prevention of radiation-induced diarrhea: a meta-analysis of randomized controlled trials. PLoS One 12(6):e0178870
Markowiak P, Slizewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9(1021):1–30
Marta M, Maria R, Francesc S (2020) The role of pro-, pre-and symbiotics in cancer: a systematic review. J Clin Pharma Ther 13292:1–16
Morelli L, Capurso L (2012) FAO/WHO guidelines on probiotics: 10 years later. J Clin Gastroenterol. 46:S1–S2
Motevaseli E, Dianatpour A, Ghafouri-Fard S (2017) The role of probiotics in cancer treatment: emphasis on their in vivo and in vitro anti-metastatic effects. Int J Mol Cell Med 6:66–76
Munjal U, Glei M, Pool-Zobel BL, Scharlau D (2009) Fermentation products of inulin-type fructans reduce proliferation and induce apoptosis in human colon tumour cells of different stages of carcinogenesis. Br J Nutr. 27:1–9
Nami Y, Abdullah N, Haghshenas B, Radiah D, Rosli R, Khosroushahi AY (2014) Assessment of probiotic potential and anticancer activity of newly isolated vaginal bacterium Lactobacillus plantarum 5BL. Microbiol Immunol 58:492–502
Negi D, Singh A, Joshi N, Mishra N (2020) Cisplatin and probiotic biomass loaded pessaries for the management of cervical cancer. Anticancer Agents Med Chem. 20(5):589–598
Nelson MH, Diven MA, Huff LW, Paulos CM (2015) Harnessing the microbiome to enhance cancer immunotherapy. J Immunol Res 2015:368736
Nouri Z, Karami F, Neyazi N, Modarressi MH, Karimi R, Khorramizadeh MR et al (2016) Dual anti-metastatic and anti-proliferative activity assessment of two probiotics on HeLa and HT-29 cell lines. Cell J 18:127–134
Okawa T, Niibe H, Arai T, Sekiba K, Noda K, Takeuchi S et al (1993) Effect of LC9018 combined with radiation therapy on carcinoma of the uterine cervix. A phase III, multicenter, randomized, controlled study. Cancer 72:1949–1954
Olveira G, Gonzalez-Molero I (2016) An update on probiotics, prebiotics and symbiotics in clinical nutrition. Endocrinol Nutr 63:482–494
Ou YC, Fu HC, Tseng CW, Wu CH, Tsai CC, Lin H (2019) The influence of probiotics on genital high-risk human papillomavirus clearance and quality of cervical smear: a randomized placebo-controlled trial. BMC Womens Health 19:103
Panebianco C, Andriulli A, Pazienza V (2018) Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome. 6(1):92
Perisic Z, Perisic N, Golocorbin Kon S, Vesovic D, Jovanovic AM, Mikov M (2011) The influence of probiotics on the cervical malignancy diagnostics quality. Vojnosanit Pregl. 68:956–960
Qiu G, Yu Y, Wang Y, Wang X (2019) The significance of probiotics in preventing radiotherapy-induced diarrhea in patients with cervical cancer: a systematic review and meta-analysis. Int J Surg. 65:61–69
Rauch M, Lynch SV (2012) The potential for probiotic manipulation of the gastrointestinal microbiome. Curr Opin Biotechnol 23:192–201
Rajoka MSR, Zhao H, Lu Y, Lian Z, Li N, Hussain N et al (2018) Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct 9:2705–2715
Saez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A (2016) Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: A review of human clinical trials. Int J Mol Sci 17:928
Scott AJ, Merrifield CA, Younes JA, Pekelharing EP (2018) Pre-, pro- and synbiotics in cancer prevention and treatment-a review of basic and clinical research. Ecancermedicalscience. 12:869
Sungur T, Aslim B, Karaaslan C, Aktas B (2017) Impact of exopolysaccharides (EPSs) of Lactobacillus gasseri strains isolated from human vagina on cervical tumor cells (HeLa). Anaerobe 47:137–144
Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, Sanders ME (2020) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17(11):687–701
Taper HS, Roberfroid MB (2002) Inulin/Oligofructose and anticancer therapy. Br J Nutr. 87:283–286
Tian Y, Li M, Song W, Jiang R, Li YQ (2019) Effects of probiotics on chemotherapy in patients with lung cancer. Oncol Lett. 17(3):2836–2848
Urbancsek H, Kazar T, Mezes I, Neumann K (2001) Results of a double-blind, randomized study to evaluate the efficacy and safety of Antibiophilus® in patients with radiation-induced diarrhoea. Eur J Gastroenterol Hepatol. 13:391–396
Wang KD, Xu DJ, Wang BY, Yan DH, Lv Z, Su JR (2018) Inhibitory effect of vaginal Lactobacillus supernatants on cervical cancer cells. Probiotics Antimicrob Proteins. 10:236–242
Wang Z, Wang Q, Wang X et al (2019) Gut microbial dysbiosis is associated with development and progression of radiation enteritis during pelvic radiotherapy. J Cell Mol Med. 23:3747–3756
Xavier-Santos D, Padilha M, Fabiano GA, Vinderola G, Gomes Cruz A, Sivieri K, Costa Antunes AE (2022) Evidences and perspectives of the use of probiotics, prebiotics, synbiotics, and postbiotics as adjuvants for prevention and treatment of COVID-19: a bibliometric analysis and systematic review. Trends Food Sci Technol. 120:174–192
Yadav MK, Kumari I, Singh B, Sharma KK, Tiwari SK (2022) Probiotics, prebiotics and synbiotics: safe options for next-generation therapeutics. Appl Microbiol Biotechnol. 106(2):505–521
Yang SC, Chen JY, Shang HF, Cheng TY, Tsou SC, Chen JR (2005) Effect of synbiotics on intestinal microflora and digestive enzyme activities in rats. World J Gastroenterol. 11(47):7413–7417
Yim EK, Park JS (2005) The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat. 37:319–324
Acknowledgment
The authors acknowledge the Department of Zoology, the University of Burdwan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
There are none.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Goswami, A., Ghosh, S., Chandra, S., Sadhu, A., Mandal, P. (2023). Understanding the Role of Synbiotics in Prevention and Management of Cervical Cancer. In: Mishra, N., Bhatt, S., Paudel, K.R., M Hansbro, P., Dua, K. (eds) Synbiotics for the Management of Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-19-7550-9_17
Download citation
DOI: https://doi.org/10.1007/978-981-19-7550-9_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7549-3
Online ISBN: 978-981-19-7550-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)