Abstract
Acute on chronic liver failure is a serious ailment which is associated with rapid development of liver failure which progresses to multiorgan failure in the absence of liver transplantation. The incidence of acute on chronic liver failure (ACLF) has been steadily increasing secondary to excessive alcohol use, usage of over-the-counter hepatotoxic drugs, complementary and alternative medicines, and the rising epidemic of non-alcoholic fatty liver disease. ACLF is defined differently by the European association for the study of Liver disease and the Asian Pacific Association for the Study of Liver (APASL). Conceptually liver failure is a driver of extrahepatic organ failures according to the APASL definition while EASL defines ACLF based on the presence of two or more organ failures which should include the kidneys and the brain. Sepsis is a consequence of liver failure according to the APASL while it can predispose to development of ACLF according to the EASL definition. However, both definitions converge on the concept of high 28-day mortality and a potential for reversibility and presence and severity of systemic inflammatory response syndrome as a key factor causing secondary organ failures. Therapeutic strategies using anti-inflammatory drugs, artificial liver support are an unmet need in patients with ACLF. At the same time, strategies for potentiating liver regeneration and modulation of gut dysbiosis are an area of active research in these patients. Prognostic models to identify patients who need an emergency liver transplant and those who would achieve reversibility are required which could lead to appropriate stratification of these patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The incidence of acute on chronic liver failure (ACLF) has been steadily increasing secondary to excessive alcohol use, usage of over-the-counter hepatotoxic drugs, complementary and alternative medicines, and the rising epidemic of non-alcoholic fatty liver disease [1,2,3]. Almost one in four outpatients with decompensated cirrhosis patients develop ACLF [4]. There are different definitions for ACLF but the two most widely accepted and validated are the one proposed by the Asian Pacific Association for the Study of Liver (APASL) [1,2,3] and the second by the European Association for the Study of Liver (EASL) Chronic Liver Failure (EASL-CLIF) consortium [5]. Following this, the world gastroenterology organization had combined the two definitions stratifying ACLF patients into three types [6] based on the underlying severity of chronic liver disease. It is challenging to have a unified definition of ACLF to develop treatment protocols, prognostic scores as well as stratification for an emergency liver transplantation. Research exploring liver regenerative therapies, artificial liver support systems, strategies targeting systemic inflammation, and management of bacterial infections which are a key driver of extrahepatic organ failures is an unmet need [1]. Until, these therapies are able to conclusively improve transplant-free survival, liver transplant remains the only definitive treatment option for these patients [1,2,3,4,5,6].
2 Definitions of ACLF
The Asian pacific association for the study of liver (APASL) defines ACLF as an acute hepatic insult manifesting as jaundice (serum bilirubin ≥5 mg/dL) and coagulopathy (INR ≥1.5 or prothrombin activity <40%) complicating within 4 weeks by clinical ascites and or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease and is associated with high 28-day mortality [1,2,3]. Conceptually, the APASL definition of ACLF specifies the syndrome wherein there is liver failure precipitated by an acute hepatic insult in a patient with compensated chronic liver disease. The acute insults include hepatitis B reactivation as the commonest cause in the Asia Pacific, followed by alcohol and drugs [1, 7,8,9]. Alcohol is the most common cause of acute insult in several Asian countries for instance in the Indian subcontinent. Super-infection with hepatitis E virus is also an important cause in the Indian subcontinent [1,2,3, 9]. Hepatotoxic drugs and complementary and alternative medicines (CAM) are other important contributing causes of acute insult causing the syndrome of ACLF. Drugs used for treatment of tuberculosis are next most important cause of drug induced acute liver failure especially reported from the Indian subcontinent [7]. The definition of ACLF excludes non-hepatic causes as acute insult for instance acute variceal bleed and particularly sepsis. According to APASL, sepsis is a consequence and not a cause for liver failure. The common causes of underlying chronic liver disease include alcohol, NASH, and hepatitis B and C [1,2,3].
The second most popular definition of ACLF is that proposed by the Chronic Liver Failure (CLIF) acute-on-Chronic Liver Failure in Cirrhosis (CANONIC) definition of ACLF [5, 10, 11]. According to this definition, ACLF is defined as “an acute deterioration of pre-existing chronic liver disease, usually related to a precipitating event and associated with increased mortality at 3 months due to multisystem organ failure.” ACLF is defined and graded as ACLF grade 0 if patients had single non-kidney organ failure [5] or had no kidney dysfunction defined as serum creatinine level 1.5 mg/dL and absence of hepatic encephalopathy. Patients with ACLF grade 1 included patients with either single kidney failure (serum creatinine ≥2 mg/dL) or patients with single failure of either liver, coagulation, circulation, or respiration defined according to the CLIF-SOFA score. Patients with kidney dysfunction (serum creatinine between 1.5 and 1.9 mg/dL) and/or mild to moderate hepatic encephalopathy and patients with single cerebral failure (grade III or IV hepatic encephalopathy) and kidney dysfunction were classified as ACLF grade 1. ACLF grade 2 included patients with any two organ failures and ACLF grade 3 included patients with 3 organ failures. The 28-day and 90-day mortality rates increased with ACLF grades and were highest for ACLF grade 3, i.e., 76.7% and 79.1%, respectively. The 28-day and 90-day mortality rates of ACLF grade 1 were 22.1% and 40.7%, respectively and for ACLF grade 2 were 32.0% and 52.3%, respectively [5, 10, 11].
The North-American consortium has defined ACLF based on two or more organ failures. They define renal as requirement of dialysis, respiratory as requirement of mechanical ventilation, cerebral as grade III or IV hepatic encephalopathy and circulatory as requirement of vasopressors [12]. The way ACLF is defined based on these definitions is quite heterogenous and has generated confusion across the world. The context has become a bit more confused by inclusion of terms like hepatic and extrahepatic ACLF and infection related ACLF- iACLF. A unifying definition of ACLF is an unmet need to have a clarity for the syndrome and to differentiate it from patients with decompensated cirrhosis with organ failures. The APASL recommends for homogeneity by avoiding extrahepatic organ failures and sepsis in the definition of ACLF [1]. A comparison of the different definitions is given in Table 43.1 and a summary of existing studies on ACLF has been highlighted in Table 43.2.
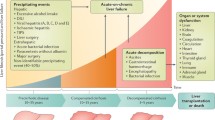
3 Pathogenetic Basis of ACLF (Fig. 43.1)
3.1 Systemic Inflammation
The presence of low-grade systemic inflammation in patients with stable or decompensated cirrhosis is considered to cause or augment relevant clinical signs and symptoms such as hyperdynamic circulation, fatigue, or minimal hepatic encephalopathy (MHE) [13, 14]. The etiology of cirrhosis could be chronic infections secondary to viruses, drugs, alcohol or autoimmune diseases. The progression of liver damage, fibrogenesis, and sinusoidal portal hypertension results in production of damage associated molecular patterns (DAMPS) which could be derived from the nucleus, i.e., high-mobility group protein B1 (HMGB1), histones, ATP, derived from cytoplasmic membrane, i.e. glypican and syndecan, from mitochondria or endoplasmic reticulum like calreticulin [15,16,17]. These DAMPs could initiate sterile inflammation and result in activation of the innate and adaptive immune system. At the same time, cirrhosis is characterized by gut dysbiosis, increase in gut permeability and enhancement of local intestinal inflammation with endogenous endotoxemia, and impairment of local intestinal defenses [15,16,17]. In animal models of liver cirrhosis, endotoxin-mediated tumor-necrosis-factor-alpha (TNF-α) is implicated in other organ dysfunction, worsening of systemic vasodilatation with impairment of cardiac contractility. All these effects could be abrogated by fecal microbial transplantation [18, 19]. In a study from EASL-CLIF consortium it was demonstrated that higher grades of systemic inflammation in ACLF were associated with higher incidence of organ failures which also differentiated them from patients with acute decompensation of cirrhosis [20]. Trebicka et al. evaluated baseline plasma levels of 15 cytokines, chemokines, and oxidized albumin) in 161 patients with ACLF which were compared to 40 healthy controls, 39 patients with stable compensated cirrhosis, and 342 patients with acute decompensation of cirrhosis. They observed that these markers were significantly elevated in patients with ACLF and in those patients with acute decompensation who finally succumbed at 28 days of systemic inflammation [21]. Considering systemic inflammation as the key driver of organ failures, the concept of “golden-window” has been proposed by the APASL (Fig. 43.2). In a study by Chowdhury and colleagues the relevance of SIRS was shown in patients with ACLF [22]. It was seen that presence, persistence, and development of new SIRS was associated with worse outcomes in patients with ACLF while resolution was associated with improved outcomes. Therefore, dynamicity of SIRS has an important prognostic implication in patients with ACLF. Altogether, SIRS in patients with ACLF can lead to a state of immunodysfunction which is a harbinger of sepsis and multiorgan failure. SIRS and or infection in these patients results in cell-death by causing deprivation of oxygen and energy from the tissues [23].
The PIRO concept of ACLF. The PIRO concept i.e. (Predisposition, Injury, Response, Organ Failure) which has been used for stratifying patients of any acute illness can well be used for patients with ACLF. PIRO incorporates assessment of pre-morbid baseline susceptibility (predisposition) factors which includes the underlying hepatic reserve and gut dysbiosis which have an influence on the course of the disease. The injury includes the specific factor causing acute illness (insult) which includes viral, drugs, alcohol in patients with acute on chronic liver failure (ACLF). The acute insult in turn incites the host response. In patients with ACLF, the acute insult activates the Kupffer cells localized to the hepatic sinusoids, through toll-like receptor 4 (TLR4), complement receptors (C3R and C5R), and damage-associated-molecular-patterns (DAMPs) which results in increased release of proinflammatory and anti-inflammatory cytokines, endotoxin, prostaglandins, bile acids, lysosomal, and proteolytic enzymes. The activation of the hepatic stellate cells by the Kupffer cells produces vasoactive mediators like endothelin-1, thromboxane A2, nitric oxide, and prostaglandins which lead to a perturbed hepatic microcirculatory function, endothelial dysfunction, and an acute increase in the portal pressure. The response of the host to the inciting insult which is measured as systemic inflammatory response syndrome which is commonly assessed by the physiological variables and can progress to a compensatory anti-inflammatory response syndrome (CARS) causes infections and secondary organ failures. The last component of the PIRO incorporates organ failures which includes organs of utility, i.e. the brain and kidneys and organs of futility that is circulation and respiration which contraindicate a liver transplant. The PIRO concept is especially useful in diseases like ACLF where the clinicians have limited therapeutic options in their armamentarium and therefore a stratification system enables identification of a possible clinical trajectory, to predict outcome much early, allowing allocation of the best treatment options to the patients before the development of organ failure what is called as the “golden window” of therapeutic intervention. ACLF acute-on-chronic liver failure, SIRS systemic inflammatory response syndrome, DAMP damage-associated molecular pattern, DCs dendritic cells, KC kupffer cells, eNOS endothelial nitric oxide synthase, LPS lipopolysaccharide, MODS multiorgan dysfunction syndrome, PV portal vein, TGFβ transforming growth factor beta, TH17 cell type 17T helper cell, TREG cell regulatory T cell
The “Golden-Window” of therapeutic intervention in patients with ACLF. Systemic inflammation as the key driver of infection and multiorgan failure in patients with ACLF. The first 2 weeks provide the “golden-window” of targeted strategies for combating systemic inflammation using liver support therapies, immunomodulation, and potentiation of liver regeneration using granulocyte colony stimulating factor (G-CSF) or modulation of gut dysbiosis using fecal microbial transplant for patients with ACLF as a possible bridge toward spontaneous recovery or liver transplant
3.2 Immunodysfunction in Patients with ACLF
Patients with ACLF not only have state of systemic inflammation but at the same time a state of prolonged and suppressed state of immune exhaustion has been well-described in these patients. These patients characteristically have increased concentrations of ant-inflammatory cytokines, i.e., interleukin-10 (IL-10) or IL-1 receptor antagonist (IL-1RA) [24]. The cells of the innate immune system, for instance, the monocytes are even though increased in frequency and display an activated phenotype but have failure to respond to stimulation with bacterial lipopolysaccharide (LPS). An increase in the number of peripheral blood monocytes expressing the tyrosine-protein kinase Mer (encoded by MERTK) which has anti-phagocytic functions has also been shown [25]. Changes in the adaptive immune system i.e. a lower frequency of naïve helper and suppressor T-cells while the number of activated T-cells is inappropriately noted in patients with ACLF. The state of cirrhosis-associated immune dysfunction is further exaggerated in patients with ACLF which is characterized by defects in phagocytosis, complement presentation, defects in innate and adaptive immunity, and defects in clearance of intestinal and bacterial pathogens [26]. Continuous exposure of bacterial derived pathogen-associated molecular patterns (PAMPs) and DAMPs amidst a state of sustained inflammation results concomitantly to state of immunosuppression in these patients [27].
3.3 Intestinal Inflammation and Gut Dysbiosis
Patients with cirrhosis have loss of gut barrier integrity secondary to an increase in the inflammatory mediators which downregulate the tight junctions causing leaky gut and associated bacterial translocation. Intestinal dysbiosis is a hallmark of patients with ACLF [28,29,30,31,32]. There is alteration of the gut microbial environment which is characterized by a shift to pathogenic bacterial species (e.g. Enterococcus spp.) and a decrease in the number of beneficial bacterial species (e.g. Bifidobacterium spp.) Concomitant to this, these patients have alteration in the gut motility, a reduction in the antimicrobial proteins, altered composition of bile salts and reduction in the gastric acid which gets exacerbated by the use of proton pump inhibitors. All this results in an increase in the translocation of gut-derived pathogens, i.e. LPS, flagellin, etc. which exacerbates systemic inflammatory response syndrome and leads to the development of bacterial infections. Amongst all etiologies, patients with alcohol have the highest gut associated dysbiosis and altered permeability because of the direct effects of alcohol itself in these changes [18, 19, 28,29,30,31,32].
3.4 Infections
Patients with ACLF develop an increased frequency of infections which are both community-acquired and nosocomial infections. Spontaneous bacterial peritonitis, gastrointestinal hemorrhage or hepatic encephalopathy are known risk factors for development of infections in patients with ACLF [1,2,3,4,5,6]. Patients of ACLF frequently develop both bacterial and fungal infections [33, 34]. Prophylactic antibiotics are therefore recommended in these situations to lower the risk of bacterial infections. The diagnosis of bacterial infection, however, remains a challenge. Currently, there are no rapid diagnostic methods for the diagnosis of occult infections and culture methods are the only definite proof of the presence of infections in these patients. Serum procalcitonin and C-reactive protein (CRP) in combination have a positive predictive value of more than 90% for the diagnosis of bacterial infection. A cut-off level of CRP of more than 24.7 ng/mL and serum procalcitonin of more than 0.47 μg/L is used for recommending prophylactic antibiotics [35]. The degree of systemic inflammation is could also be determined by the white cell counts and the use of neutrophil to lymphocyte ratio [5, 36]. A number of other pro-inflammatory markers, i.e. tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-8 have been evaluated in these patients other markers, i.e., caspase-cleaved keratin18 (CK18) and keratin 18 (K18) are reflective of apoptotic and total cell death, respectively, and cK18: K18 ratio is known to increase with the severity of ACLF [37, 38]. However, currently none of the biomarkers can reliably differentiate sterile inflammation from infection. Infections are important triggers for the development of ACLF by causing organ failures which is associated with high mortality in the absence of liver transplantation. It is recommended to consider the site and acquisition of infection as well as the local microbiological profile to decide the choice of prophylactic antibiotics in patients with ACLF. In a large multicentric-multinational study global study performed to capture the data on bacterial infections across the globe regional differences were observed in the spectrum of infections in patients with cirrhosis. In the asian countries, particularly India a predominance of multidrug resistant infections was observed which was were associated with a higher incidence of organ failures, prolonged ICU stay, and higher mortality [39]. Choice of appropriate empirical antibiotics was associated with improved outcomes. As a protocol, the patients should be reassessed at 48–72 h for de-escalation of antibiotics after the culture report.
4 The Concept of Tolerance in ACLF
Sepsis is defined as the host response to a bacterial pathogen. Infections in patients with ACLF can directly impact or damage the tissues or cause stimulation of the immune system resulting in the release of pro-inflammatory cytokines which cause end-organ dysfunction or failure. The host response is related to the intrinsic tolerance. It has been well-documented that as compared to patients with decompensated cirrhosis, who are exposed to repeated prior episodes of bacterial infection and chronic endotoxemia, patients with ACLF respond poorly to containment of bacterial infections because of failure of protective mechanisms of tolerance [40].
4.1 Assessment of Liver and Extrahepatic Organs in Patients with ACLF
4.1.1 Liver Failure
According to the APASL definition the liver remains at the core of the entire syndrome of ACLF [1,2,3]. All patients therefore have liver failure which is manifested by jaundice, coagulopathy and/or ascites, and hepatic encephalopathy. Majority of patients with ACLF have ascites which is a consequence of underlying chronic liver disease, hemodynamic alterations secondary to systemic inflammation, and the development of acute portal hypertension. The severity of liver failure therefore is determined by the degree of jaundice, coagulation impairment, and the degree and severity of ascites [1,2,3]. Development of any grade of hepatic encephalopathy and its persistence is associated with worse clinical outcomes. Assessment of hepatic reserve would be worthwhile to determine the potential of spontaneous liver regeneration in patients with ACLF.
4.1.2 Coagulation Failure
Assessment of coagulation can be performed by standard tests, i.e., the international normalized ratio, platelet counts, and serum fibrinogen levels. In patients with decompensated cirrhosis, an intricate balance is noted between coagulation and fibrinolysis and is usually procoagulant [41]. The state of coagulation in patients with ACLF should be assessed by thromboelastography (TEG) or rotational thromboelastometry (ROTEM) [42, 43]. In a single-center prospective study consecutive patients of ACLF without sepsis were recruited and assessed by TEG and other specific assays (Factor VIII, von Willebrand factor, protein C and antithrombin III and followed for development of sepsis, bleeding events and overall outcomes [44]. A hypocoagulable TEG at baseline was an independent predictor of not only bleeding events but also mortality. The global coagulation index, lower levels of protein C, anti-thrombin III, and tissue plasminogen activator levels predicted 28-day mortality after adjusting for patient demographics and the MELD scores. Furthermore, during bleeding correction of coagulation using either ROTEM or TEG could also limit transfusion related adverse effects in patients with AC LF and may result in targeted coagulation correction.
4.1.3 Kidney Dysfunction or Failure
Kidneys are one of the most frequent extrahepatic organs that are affected in patients with ACLF. Acute kidney injury is reported in 22.8–34% of patients with ACLF [45]. Kidneys in patients with ACLF should be assessed using the relative changes in serum creatinine or by measuring urine output in hospitalized patients rather than relying on serum creatinine. This is because various factors influence the serum creatinine estimation which might result in underdiagnosis of renal dysfunction [45]. Use of biomarkers like serum cystatin C could be helpful in early detection of AKI in patients with ACLF. The AKI spectrum has also not been well-studied in patients with ACLF. These patients have predominance of structural AKI secondary to a higher prevalence of bacterial infections, systemic inflammation, high serum bilirubin, and predominance of circulatory dysfunction [46, 47].
4.1.4 Spectrum of AKI in ACLF
Patients with ACLF have acute portal hypertension, the main abnormality causing renal dysfunction in these patients is severe systemic and splanchnic vasodilatation which leads to decreased effective arterial blood volume and activation of the renin–angiotensin aldosterone (RAAS), the sympathetic nervous system and non-osmotic release of antiduiuretic hormone which causes salt and water retention. The pathogenetic basis of renal dysfunction in ACLF is quite different from that of patients with decompensated cirrhosis. Majority of patients have structural kidney damage as assessed by microscopic urinalysis and renal biomarkers. Severity of systemic inflammation, bacterial infections, cholemic nephropathy are most common reasons for structural kidney damage [1,2,3, 45,46,47,48].
4.1.5 Prediction of AKI in ACLF
In a large multicenter multinational prospective study of patients with ACLF from the Asia Pacific, a predictive score was developed for identification of the development or progression of AKI in patients with ACLF. The score was developed on the concept of PIRO, i.e. predisposition, injury, response and organ failure which was initially developed for patients with sepsis. Components of the predisposition component included high urea, serum creatinine, potassium, and serum bilirubin. In the injury component, the use of nephrotoxic drugs was identified as an important predictor, response component included presence of systemic inflammatory response syndrome, and organ failure included presence of low mean arterial pressure. Patients of ACLF could be risk stratified for AKI using the PIRO score for additional therapeutic interventions targeting the components of PIRO [48].
4.1.6 Diagnosis of AKI in Patients with ACLF
Considering the limitations of serum creatinine in patients with ACLF and especially in context of intensive care unit stay retention of urine output criteria may be relevant in the diagnosis of AKI in these patients [49]. However, this needs validation in patients with ACLF. The data from the AARC database suggested a lower value of serum creatinine is more relevant in patients with ACLF. Serum creatinine above 0.7 mg/dL (as derived from the AARC score) has a sensitivity of 78% and specificity of 36% for prediction of 30-day mortality in patients with ACLF. For the diagnosis of kidney failure, the conventional cut-off of 1.5 mg/dL even though had a low sensitivity of 48% but had a specificity of 99.8% for 30-day mortality [3]. The revised consensus criteria for AKI in patients with ACLF lead down by the international club of ascites suggest diagnosis of AKI using the AKIN criteria. In patients with stage 1 AKI or those with serum creatinine less than 1.5 mg/dL should be managed by removal of the precipitating cause and conservative measures. Patients who have stage 2 or 3 AKI and those with serum creatine above 1.5 mg/dL should undergo volume expansion with intravenous albumin. Kidney failure (serum creatinine ≥1.5 mg/dL) was seen in 22% of ACLF patients at baseline and developed in another 30% within a month [50]. The majority of patients of ACLF developed new episodes of AKI in the first 2 weeks (11%). Apart from the severity, the course of AKI was seen to be an important predictor of clinical outcomes. Patients with AKI resolution have improved outcomes while those with either AKI progression or persistence have worse outcomes [3].
5 Role of Biomarkers
5.1 Biomarkers of Glomerular Injury
5.1.1 Cystatin C
Cystatin C is a nonglycosylated protein with low molecular weight (13 kDa), has a constant rate of production and concentration of cystatin C is determined by glomerular filtration. It is, therefore, considered as an early marker of glomerular dysfunction. We have demonstrated the role of serum cystatin C in a large prospective cohort study in patients with cirrhosis, wherein it has been shown as a marker of renal reserve to predict development of new AKI episode and chronic kidney disease [47, 51]. In patients with hepatitis-B virus (HBV) related ACLF CysC was shown to accurately predict AKI even in patients with normal serum creatinine [52].
5.2 Biomarkers of Proximal Tubular Damage
5.2.1 Kidney Injury Molecule (KIM-1)
Kidney injury molecule-1 is a type 1 transmembrane glycoprotein which is comprised of an immunoglobulin and mucin domain. Under normal conditions, KIM-1 protein is only minimally expressed in kidney tissue or urine but is shed from the proximal tubules with tubular dysfunction wherein it can be detected in the urine by immunoassay. It is known to be upregulated in response to renal ischemia or nephrotoxic insult and is also believed to participate in the regeneration process after epithelial injury [53].
5.2.2 Liver Fatty Acid Binding Protein (L-FABP)
Fatty-acid protein bindings (FABPs) facilitate transfer of fatty acids between intra and extracellular membranes. They also have a role in the amelioration of cellular oxidative stress by inhibition of the toxic effects of oxidative intermediates on cellular membranes. In the normal healthy state, urinary L-FABP is undetectable; however, under states of renal ischemia there is decreased proximal tubular reabsorption of L-FABP which is detected as increased excretion in urine [53].
5.2.3 Interleukine-18
Interleukine-18 (IL-18) is a proinflammatory cytokine which is synthesized in renal proximal tubular epithelial cells as well as monocytes and macrophages. The concentrations of IL-18 have also been demonstrated to be increased in postischemic AKI following renal hypoxia. It can therefore be considered as an early biomarker of AKI in critically ill patients. It has also been shown to correlate with poor clinical outcomes (death or requirement of renal replacement therapy) in patients with sepsis [53].
5.3 Biomarkers of Distal Tubular Damage
5.3.1 Neutrophil Gelatinase-Associated Lipocalin
Neutrophil gelatinase-associated lipocalin (NGAL) NGAL belongs to the lipocalin superfamily (lipocalin 2, siderocalin). Both plasma and urine NGALs are increased after an episode of AKI. Elevated urine NGAL originates from both proximal and distal nephron after a nephrotoxic insult. Injury to proximal renal tubules precludes NGAL reabsorption and/or increase denovo NGAL synthesis secondary to upregulation of NGAL mRNA in the distal nephron segments (especially in the thick ascending limb of Henle’s loop and the collecting ducts) [54].
5.4 Studies Assessing Markers of Tubular Injury in Patients with ACLF
The major challenge in patients with ACLF is to differentiate HRS associated with bacterial infections from ATN as it evolves through a continuous spectrum. In fact, HRS patients who are non-responders to vasoconstrictors are known to have tubular dysfunction requiring prolonged RRT [45]. In another prospective study in patients with cirrhosis and bacterial infections, measurement of urinary NGAL at infection diagnosis was reported to be useful in predicting clinical outcomes, persistent AKI and type of AKI [55]. Interestingly, N-GAL also accurately predicted development of a second infection and 3-month mortality. In this study significantly higher uNGAL was noted in patients who developed persistent AKI and amongst these patients was able to discriminate type-1 HRS from other causes of AKI with accuracy. In another study done in 55 patients with an acute decompensation of cirrhosis a panel of 12 biomarkers was studied to differentiate ATN from other causes of AKI. In this study also, NGAL was identified as the best biomarker, others being IL-18, albumin, trefoil-factor-3 (TFF-3) and glutathione-S-transferase-π (GST-π) [53]. In a large prospective study performed in 716 patients with ACLF, urine and plasma NGAL levels were analyzed. The authors noted that the levels of urine NGAL were markedly elevated in patients with ACLF (108(35–400) vs. 29 (12–73) μg/g creatinine; p < 0.001) and independently predicted 28-day mortality [54]. The authors proposed urine NGAL as a biomarker for patients with ACLF. In another study performed in patients with HBV-ACLF 280 patients were compared to 132 patients with HBV-related decompensated cirrhosis (DC). The authors studied the levels of five urinary tubular injury including neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), liver-type fatty acid binding protein (L-FABP), cystatin C (CysC), and kidney injury molecule-1 (KIM-1). This was correlated to patient demographics, development and progression of AKI, and response to terlipressin therapy were recorded. The levels of urinary biomarkers (NGAL, CysC, L-FABP, IL-18) were significantly elevated in patients with HBV-ACLF and AKI (ACLF-AKI), compared with that in patients with HBV-DC and AKI (DC-AKI) or those without AKI [56].
5.4.1 Management of AKI
According to the new consensus by the ICA for AKI, a new algorithm for the management of AKI based on the revised criteria has been proposed. Based on this algorithm it is recommended that patients with initial AKI stage 1 should be managed by removal of all precipitants (careful review of medications, diuretics, nephrotoxic drugs, vasodilators or non-steroidal anti-inflammatory drugs). Second step is to consider plasma volume expansion in patients with hypovolemia (the choice of fluid could either be a crystalloid or albumin or even blood as indicated) along with identification and early treatment of bacterial infections. Patients who respond with a decrease in serum creatinine value of 0.3 mg/dL of the baseline value should be subsequently followed up for any new episodes of AKI. Patients who have progression, should be managed as ICA-AKI stage 2 and 3. In this group of patients, along with the institution of all measures as recommended for patients with stage 1 AKI a work up for the differential diagnosis should be done on an immediate basis to identify whether it is HRS-AKI, intrinsic AKI or post-renal cause. It was further decided by the panel of experts that for patients with stage 1 AKI who do not improve but have no progression further management can be decided based on the absolute value of serum creatinine and if the serum creatinine is more than 1.5 mg/dL it was recommended to consider the same protocol as for management for stage 2 and 3 AKI. Patients with HRS-AKI are recommended to be managed with early use of vasoconstrictors based on the revised criteria for HRS-AKI (either with terlipressin or norepinephrine or midodrine plus octreotide). Management of non-responders to vasoconstrictors which constitute a large group of patients therefore still remains an ongoing challenge. There is paucity of data on dialysis in patients with cirrhosis therefore there are no specific recommendations regarding the dose, the intensity, duration and time of initiation of dialysis in these patients [45]. We propose different management algorithm with incorporation of antioxidants and anti-inflammatory strategies, early initiation of vasoconstrictors and extracorporeal support therapies considering a higher incidence of structural AKI and poor response to vasoconstrictors [45, 46, 48].
5.4.2 Cerebral Failure
Development and persistence of hepatic encephalopathy is associated with a grim prognosis in patients with ACLF. The pathophysiology of HE is multifactorial and complex important factors include hyperammonemia, systemic inflammation, gut dysbiosis, genetic factors, bacterial infection, and insulin resistance [1,2,3]. Alcohol use and hyponatremia are other factors implicated in brain dysfunction in patients with ACLF. Contrary to patients with acute liver failure, cerebral oedema is rare and is observed in 5% of the patients with hepatic encephalopathy as reported in imaging studies [57]. Ammonia induces oxidative and cellular stress and in patients with ACLF. Whether higher levels of ammonia correlate with more severe grades of HE has not been studied in patients with ACLF [58]. Management involves identification and correction of precipitating factors should be identified and treated as required. Use of lactulose for bowel cleansing, non-absorbable antibiotics, novel ammonia lowering drugs, such as glycerol phenylbutyrate and ornithine phenylacetate, have shown some promise but are still experimental. Use of liver dialysis for refractory hepatic encephalopathy has shown some benefits. Abstinence of alcohol, strategies for systemic inflammation, use of antibiotics for infection, and treatment of diabetes may also improve hepatic encephalopathy by combating systemic inflammation [59].
5.4.3 Circulatory and Respiratory Failure
The revised consensus of APASL defined organs of utility and futility in patients with ACLF. Among the extrahepatic organ failures, brain and kidneys are considered as organs of utility because even though dysfunction or failure of these organs is associated with worse prognosis but these do contraindicate liver transplant. On the contrary, data from Europe and America has suggested that protocols of excluding patients with severe circulatory or respiratory failure. In patients wherein transplant is performed dysfunction or circulation or respiration is associated with worse outcomes as compared to patients who did not have these organ failures [60].
6 Management of Patients with ACLF (Fig. 43.3)
6.1 Albumin
Albumin has an important role in the treatment of ACLF. Normal liver synthesizes 11–15 g of albumin, however, this capacity is reduced by 60–80% in patients with ACLF. Albumin has colloid osmotic functions, is an important carrier of different substances, has anti-inflammatory and anti-oxidant property as well as maintenance of capillary permeability. Recent data has suggested utility of albumin in combating systemic inflammation and resolution of uncomplicated ascites [61,62,63,64]. Albumin is recommended for management for HRS-AKI, prevention of renal dysfunction in patients with spontaneous bacterial peritonitis (SBP), and prevention of paracentesis induced circulatory dysfunction (PICD). In a single-center randomized controlled trial in patients of ACLF who underwent modest-volume paracentesis the incidence of PICD and its associated complications was significantly reduced as compared to standard medical treatment [65].
6.2 Renal Replacement Therapy
The indications of renal replacement therapy are the same as those for other conditions, i.e. metabolic acidosis, volume overload, uremic complications, and electrolyte abnormalities. It should be considered in patients who are candidates for orthotopic liver transplantation (OLT) or those with acute tubular necrosis (ATN), hypovolemia related renal failure or where renal functions are likely to be reversible. The leading indication identified in these patients has been volume overload. Continuous renal replacement therapy (CRRT) is better tolerated than intermittent hemodialysis because of improved cardiovascular stability, clear ammonia and pro-inflammatory cytokines, and improved cerebral oedema. Complications such as hypotension, bleeding secondary to coagulopathy, and catheter-related sepsis are commonly encountered with renal replacement therapy when used in patients with advanced liver disease. Hence, a multidisciplinary approach involving a hepatologist, a nephrologist, and an intensive care specialist is needed to decide the exact timing and modality of renal replacement therapy in patients with ACLF. Considering an extremely poor response to vasoconstrictors in only 35% of patients with HRS in patients with ACLF and higher prevalence of structural AKI in patients with ACLF, the utility of RRT remains to be explored [66, 67]. There is paucity of data on dialysis in patients with ACLF and decompensated cirrhosis therefore there are no specific recommendations regarding the dose, the intensity, and the duration of dialysis in these patients. In a recent multicentric prospective study from North American Consortium for the Study of End-Stage Liver Disease (NACSELD) database for cirrhotic patients hospitalized with an infection (I-ACLF) where RRT was not identified as an independent predictor of survival when it was done as a bridging therapy to liver transplantation [12]. There is emerging data to suggest that initiation of RRT early may attenuate both kidney-specific and non-kidney specific organ dysfunction as well as counteract systemic inflammation in critically ill patients. However, unfortunately complications such as hypotension, coagulopathy-related bleeding, and catheter-related sepsis are frequently encountered with RRT in patients with cirrhosis and therefore in the absence of absolute indications it is a daunting task for the clinicians to decide initiation of early RRT in such a severely sick group of patients. Randomized controlled trials are therefore needed to decide the timing of initiation of RRT (that is, “early” versus “late”) in patients of ACLF who have structural kidney damage or have non-response to vasoconstrictors awaiting liver transplantation.
6.3 Extracorporeal Liver Support Systems
These can be non-cell based or cell-based systems. Non-cell based systems do not incorporate tissue and provide only detoxification functions using membranes and adsorbents which allow removal of both water-soluble and protein bound substances as against conventional hemodialysis which removes only water-soluble toxins. These newer developing therapies have demonstrated benefits in biochemical parameters, hemodynamic, hepatic encephalopathy and also renal functions but are expensive and still considered experimental in patients with ACLF [68, 69]. Currently, they are considered as an option in patients as a bridge to liver transplantation or clinical recovery. The Molecular Adsorbent Recirculatory System (MARS), single-pass albumin dialysis (SPAD), and the Fractionated Plasma Separation and Adsorption (FPSA or Prometheus) have shown limited efficacy in improving transplant-free survival in patients with ACLF. In the HELIOS trial survival of patients with type 1 HRS when treated with FPSA was better compared to SMT (28-day survival 62% vs. 39%, 90-day survival probability, 42% vs. 6%, respectively; log-rank test, P = 0.04). Similarly in the RELIEF trial with MARS it was seen that the proportion of patients with a serum creatinine below 1.5 mg/dL at day 4 in patients with HRS at baseline tended to be higher in patients who were treated with MARS (p = 0.07). Considering a higher prevalence of structural AKI and cholemic nephropathy in patients with ACLF, the utility of MARS remains to be explored [45, 70, 71]. Larger randomized controlled trials are required for patients with ACLF as the patient populations enrolled in the large trials in Europe were performed using heterogenous definitions of ACLF [68,69,70,71]. Case reports and series have suggested beneficial effects of plasma-exchange in patients with ACLF [72,73,74,75]. In the large European multicentric trial performed in patients with ALF, plasma-exchange was shown to improve survival by dampening the immune response [76]. The results from the AARC database suggested a beneficial role of plasma-exchange in patients with ACLF in preventing multiorgan failure and ameliorating SIRS. Currently, a specific device (DIALIVE) with an aim to remove dysfunctional albumin and endotoxin and replacing it with functional albumin is being evaluated in ACLF patients [35]. Table 43.2 summarizes the studies on artificial liver support therapies in patients with ACLF.
6.4 Therapeutic Strategies Targeting Liver Regeneration in ACLF
Initial randomized controlled clinical trials from India suggested encouraging data for G-CSF. An impressive survival benefit was observed in these studies, however most of them were mono-centric [77,78,79,80,81]. Hence, the broad application of G-CSF in ACLF has not been routinely recommended outside clinical trials. The large multicentric trial performed in Europe, the. GRAFT-Study, did not Lreplicate the observed benefits observed in Asian trials. However, the differences in the definitions used to define ACLF may be a key factor explaining the observed differences [35].
6.5 Role of Anti-Oxidants in ACLF
Oxidative stress is hypothesized to play a crucial role in liver disease with the generation of advanced oxidative protein products (AOPP) playing a primary role in active inflammation. AOPP have been found to be in higher concentration in the serum in patients with viral hepatitis, diabetics, and advanced age. AOPP levels have also found to be higher in liver biopsies taken from severe ACLF secondary to alcohol compared to stable alcoholic cirrhosis, indicating role in ongoing damage [82].
Treatment with N-acetyl cysteine (NAC) in non-acetaminophen liver failure has been shown to improve survival in multiple studies. Nabi et al. showed that treatment of 40 patients with intravenous NAC for 72 h was associated with a decrease in mortality to 28% as compared to 53% in the control group [83]. These findings corroborated results of Mumtaz et al. study in 47 patients given oral NAC that showed a survival of 47% in treatment group and 27% in the non-treatment group [84]. Baniasadi et al. also showed benefit of NAC in antitubercular drug induced liver injury [85]. A meta-analysis of four prospective studies including 331 patients also showed that NAC was safe in non-acetaminophen liver disease and improved survival in both liver transplant and native liver patients [86]. However, studies are limited and NAC is not the standard of care for ACLF.
6.6 Liver Transplantation in ACLF
ACLF as a disease entity is characterized by dynamic course during hospital admission, with the course between day 3 and day 7 being the most integral in determining long-term management. An improvement in overall health opens the doors to other therapies such as bioartificial liver support (as summarized in Table 43.3), granulocyte colony stimulating factors, and stem cell transplant. These are in early phases of development and liver transplant is the only definite management option. Unlike acute liver failure (ALF), ACLF does not qualify for enlistment in the high urgency list. Furthermore, evaluation time is limited by the rapid evolution of disease with age, multiorgan failure, and recidivism forming key barriers to inclusion to the transplant list. Additionally, among those patients present on the waiting list, the incidence of mortality is high and exceeds that of ALF patients on the waiting list [87]. The key studies are summarized in Table 43.3.
Current data indicates that <50% ACLF patients are listed for transplant and < 20% ACLF patients actually successfully undergo transplantation. The 5-year survival in the patient that undergo successful transplantation is 74–90% [88]. This data highlights the necessity to validate prognostic tools to allow prioritization of patients with ACLF on the transplant list. Such patients should also be aggressively managed in the intensive care unit (ICU) with early management of known triggers of downward cascade such as infection and bleeding. The multiorgan failure seen as a defining feature of ACLF should be supported with vasopressors, mechanical ventilation, and continuous renal replacement as needed. It is notable that the highest quality of care can be provided with a well-balanced multidisciplinary team and early ICU admission [89].
The other options for these patients are living donor liver transplantation (LDLT) which has shown reasonable success, specifically with the use of right lobe liver grafts including the middle hepatic vein that ensures adequate venous drainage and speedy recovery. The 5-year survival rate with LDLT is also over 90% in patients with high MELD score at admission [90].
6.7 Assessing Futility in Patients with ACLF
In patients with deteriorating clinical course over the first week, a goals of care discussion should be undertaken. This patient population has shown to have the highest mortality in the second week of ICU admission. The CLIF-C ACLF score, designed to predict short-term mortality over 28 days in ACLF patients, has a 100% specificity in predicting mortality when the score is ≥70 has, despite all supportive treatment. The cumulative rate of survival in the ICU with MELD >28.2 is estimated to be 28.2% and SOFA greater than 10.5 is 10.5% [91]. Cirrhotic patients are prone to infection with higher risk of mortality as compared to non-cirrhotic, and the presence of septic shock is estimated to predict mortality independently (OR 50.3, 95% CI 8.99–281) [92]. Additionally, multiorgan failure involving >3 organs requiring support (i.e. ionotropic support, mechanical ventilation, and continuous renal replacement therapy) is independently associated with increased mortality [93].
6.8 Need of Dynamic Prognostic Models
Patients with ACLF rapidly develop infections, organ failures leading to high mortality in the absence of liver transplant. Currently, there is no universal prognostic model for deciding the liver transplant in patients with ACLF. The model for end stage liver disease score (MELD) is validated for patients with decompensated cirrhosis and King’s College Hospital Criteria (KCH) for acute liver failure [94]. In patients with severe alcoholic hepatitis, the Lille’s score has shown the need of an emergency liver transplant [95, 96]. In patients with autoimmune hepatitis, failure to improve the MELD scores at day 7 has been shown to be associated with worse outcomes and need for liver transplant [97, 98]. The AARC score has been developed from the large AARC database which is a composite of five variables [99]. The score includes bilirubin, creatinine, international normalized ratio (INR), arterial lactate, and hepatic encephalopathy. The score incorporates measures of liver failure (i.e., bilirubin, INR and lactate) and organs of utility, i.e., kidneys and brain. Kidneys are one of the most frequent extrahepatic organ failure in patients with ACLF and also have prognostic implication. Similarly, akin to ALF, brain involvement is an ominous sign and necessitates need of emergency liver transplantation. The AARC score additionally is dynamic and performed superior to other prognostic scores in predicting the outcome of ACLF patients. The score could therefore determine the need of emergency liver transplant in these patients, however, has not been validated in this context. Apart from these, the CLIF-C ACLF score developed by the EASL-CLIF consortium can be used in prognostication in ACLF patients admitted to the intensive care unit. A score above 70 has been shown to have a 100% specificity in predicting mortality in patients who are critically ill [100, 101]. However, considering the differences in the definitions, the score needs to be evaluated in ACLF patients defined according to the APASL.
7 Conclusion
ACLF is a distinct entity characterized by the development of liver failure on a background of chronic liver disease usually precipitated by an acute insult. Systemic inflammation is a key event in the pathogenesis of the syndrome. The management of the syndrome is a composite of identification and treatment of the etiological insult, systemic inflammation, and potentiation of liver regeneration. Development of infection and extrahepatic organ failure is a key event with a prognostic implication. The role of liver support therapies needs to be explored both as a bridge to transplant and to spontaneous recovery. Dynamic prognostic models for deciding transplant, reversibility, and futile ICU care are an unmet need in patients with ACLF.
Highlights
-
Acute on Chronic Liver Failure (ACLF) is characterized by high 28-day mortality.
-
Liver failure drives extrahepatic organ failures in patients with ACLF.
-
ACLF occurs in the context of gut dysbiosis and systemic inflammation.
-
The syndrome is characterized by a dynamic course and the rapidity of progression to organ failures providing the first 2 weeks as the “golden-window” for therapeutic interventions.
-
Liver transplant is the ultimate savior in patients with ACLF.
-
The syndrome of ACLF is a clinical challenge and an area of unwavering research for clinicians.
Abbreviations
- ACLF:
-
Acute on chronic liver failure
- AKI:
-
Acute kidney injury
- AKIN:
-
Acute kidney injury network
- ALF:
-
Acute liver failure
- AOPP:
-
Advanced oxidative protein products
- APASL:
-
Asian Pacific Association for the study of liver
- ATN:
-
Acute tubular necrosis
- ATP:
-
Adenosine triphosphate
- CAM:
-
Complementary and alternative medicines
- CK18:
-
Caspase-cleaved keratin18
- CLD:
-
Chronic liver disease
- CLIF:
-
Chronic liver failure
- CRP:
-
C-reactive protein
- CRRT:
-
Continuous renal replacement therapy
- CysC:
-
Cystatin C
- DAMP:
-
Damage associated molecular patterns
- DDLT:
-
Deceased donor liver transplant
- EASL:
-
European Association for the study of liver
- eGFR:
-
Estimated glomerular filtration rate
- FPSA:
-
Fractionated plasma separation and adsorption
- G-CSF:
-
Granulocyte colony stimulating factor
- HBV:
-
Hepatitis-B virus
- HCC:
-
Hepatocellular carcinoma
- HE:
-
Hepatic encephalopathy
- HIV:
-
Human immunodeficiency virus
- HMGB1:
-
High mobility group protein B1
- HRS:
-
Hepatorenal syndrome
- iACLF:
-
Infection related
- ICA:
-
International club of ascites
- ICU:
-
Intensive care unit
- IL:
-
Interleukin
- IL-1RA:
-
IL-1 receptor antagonist
- INR:
-
International normalized ratio
- K18:
-
Keratin 18
- KCH:
-
King’s College Hospital criteria
- KIM-1:
-
Kidney-Injury molecule
- LDLT:
-
Living donor liver transplant
- L-FABP:
-
Liver fatty acid binding protein
- LPS:
-
Lipopolysaccharide
- LT:
-
Liver transplant
- MAP:
-
Mean arterial pressure
- MARS:
-
Molecular adsorbents recirculatory system
- MELD:
-
Model for end-stage liver disease
- MERTK:
-
Mer tyrosine-protein kinase
- MHE:
-
Minimal hepatic encephalopathy
- NAC:
-
N-acetyl cysteine
- NACSELD:
-
North American consortium for the study of end-stage liver disease
- NASH:
-
Non alcoholic steatohepatitis
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- OLT:
-
Orthotopic liver transplantation
- PAMPs:
-
Pathogen-associated molecular patterns
- PICD:
-
Paracentesis induced circulatory dysfunction
- PIRO:
-
Predisposition, injury, response, organ failure
- RAAS:
-
Rennin–Angiotensin aldosterone
- ROTEM:
-
Rotational thromboelastometry
- SBP:
-
Spontaneous bacterial peritonitis
- SIRS:
-
Systemic inflammatory response syndrome
- SOFA:
-
Sequential organ failure assessment
- SPAD:
-
Single-pass albumin dialysis
- TEG:
-
Thromboelastography
- TNF-α:
-
Tumor necrosis factor alpha
References
Sarin SK, Chandan K, Zaigham A, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2014;8:453–71.
Sarin SK, Kumar A, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL). Hepatol Int. 2009;3(269):282.
Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13(4):353–90. [published correction appears in Hepatol Int. 2019 Nov;13(6):826-828].
Piano S, Tonon M, Vettore E, et al. Incidence, predictors and outcomes of acute-on-chronic liver failure in outpatients with cirrhosis. J Hepatol. 2017;67(6):1177–84.
Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–37.
Jalan R, Yurdaydin C, Bajaj JS, et al. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147(1):4–10.
Devarbhavi H, Choudhury AK, Sharma MK, et al. Drug-induced acute-on-chronic liver failure in Asian patients. Am J Gastroenterol. 2019;114(6):929–37.
Chen T, Yang Z, Choudhury AK, et al. Complications constitute a major risk factor for mortality in hepatitis B virus-related acute-on-chronic liver failure patients: a multi-national study from the Asia-Pacific region. Hepatol Int. 2019;13(6):695–705.
Gawande A, Gupta GK, Gupta A, et al. Acute-on-chronic liver failure: etiology of chronic and acute precipitating factors and their effect on mortality. J Clin Exp Hepatol. 2019;9(6):699–703.
Arroyo V, Moreau R, Kamath PS, et al. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2:16041. Published 2016 Jun 9. https://doi.org/10.1038/nrdp.2016.41.
Arroyo V, Jalan R. Acute-on-chronic liver failure: definition, diagnosis, and clinical characteristics. Semin Liver Dis. 2016;36(2):109–16.
Bajaj JS, O'Leary JG, Reddy KR, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60(1):250–6.
Clària J, Stauber RE, Coenraad MJ, et al. Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64(4):1249–64.
Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63(5):1272–84.
Arroyo V, Moreau R, Jalan R, Ginès P, EASL-CLIF Consortium CANONIC Study. Acute-on-chronic liver failure: a new syndrome that will re-classify cirrhosis. J Hepatol. 2015;62(1 Suppl):S131–43.
Shi S, Verstegen MMA, Mezzanotte L, de Jonge J, Löwik CWGM, van der Laan LJW. Necroptotic cell death in liver transplantation and underlying diseases: mechanisms and clinical perspective. Liver Transpl. 2019;25(7):1091–104.
Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev Pathol. 2020;15:493–518.
Posteraro B, Paroni Sterbini F, Petito V, et al. Liver injury, endotoxemia, and their relationship to intestinal microbiota composition in alcohol-preferring rats. Alcohol Clin Exp Res. 2018;42(12):2313–25.
Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33(10):1836–46.
Claria J, Stauber RE, Coenraad MJ, et al. Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249–64.
Trebicka J, Amoros A, Pitarch C, Titos E, Alcaraz-Quiles J, Schierwagen R, et al. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis. Front Immunol. 2019;10:476.
Choudhury A, Kumar M, Sharma BC, et al. Systemic inflammatory response syndrome in acute-on-chronic liver failure: relevance of ‘golden window’: a prospective study. J Gastroenterol Hepatol. 2017;32(12):1989–97.
Gustot T. Multiple organ failure in sepsis: prognosis and role of systemic inflammatory response. Curr Opin Crit Care. 2011;17:153–9.
Albillos A, Lario M, Alvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–96.
Bernsmeier C, Pop OT, Singanayagam A, et al. Patients with acute-on-chronic liver failure have increased numbers of regulatory immune cells expressing the receptor tyrosine kinase MERTK. Gastroenterology. 2015;148(3):603–615.e14.
Hensley MK, Deng JC. Acute on chronic liver failure and immune dysfunction: a mimic of sepsis. Semin Respir Crit Care Med. 2018;39(5):588–97.
Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006.
Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–24.
Wang L, Fouts DE, Starkel P, et al. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe. 2016;19:227–39.
Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64.
Chen Y, Guo J, Qian G, et al. Gut dysbiosis in acuteon-chronic liver failure and its predictive value for mortality. J Gastroenterol Hepatol. 2015;30:1429–37.
Giannelli V, Di Gregorio V, Iebba V, et al. Microbiota and the gut-liver axis: bacterial translocation, inflammation and infection in cirrhosis. World J Gastroenterol. 2014;20:16795–810.
Fernández J, Acevedo J, Wiest R, et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67(10):1870–80.
Verma N, Singh S, Taneja S, et al. Invasive fungal infections amongst patients with acute-on-chronic liver failure at high risk for fungal infections. Liver Int. 2019;39(3):503–13.
Bechstein WO, Zeuzem S. Acute-on-chronic liver failure. Visc Med. 2018;34(4):243–4.
Moreau N, Wittebole X, Fleury Y, Forget P, Laterre PF, Castanares-Zapatero D. Neutrophil-to-lymphocyte ratio predicts death in acute-on-chronic liver failure patients admitted to the intensive care unit: a retrospective cohort study. Shock. 2018;49(4):385–92.
Kerbert AJC, Verspaget HW, Navarro ÀA, et al. Copeptin in acute decompensation of liver cirrhosis: relationship with acute-on-chronic liver failure and short-term survival. Crit Care. 2017;21(1):321.
Macdonald S, Andreola F, Bachtiger P, et al. Cell death markers in patients with cirrhosis and acute decompensation. Hepatology. 2018;67(3):989–1002.
Piano S, Singh V, Caraceni P, et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology. 2019;156(5):1368–1380.e10.
Moreau R. The pathogenesis of ACLF: the inflammatory response and immune function. Semin Liver Dis. 2016;36(2):133–40.
Stravitz RT. Algorithms for managing coagulation disorders in liver disease. Hepatol Int. 2018;12(5):390–401.
Goyal S, Jadaun S, Kedia S, et al. Thromboelastography parameters in patients with acute on chronic liver failure. Ann Hepatol. 2018;17(6):1042–51.
Blasi A, Calvo A, Prado V, et al. Coagulation failure in patients with acute-on-chronic liver failure and decompensated cirrhosis: beyond the international normalized ratio. Hepatology. 2018;68(6):2325–37.
Premkumar M, Saxena P, Rangegowda D, et al. Coagulation failure is associated with bleeding events and clinical outcome during systemic inflammatory response and sepsis in acute-on-chronic liver failure: an observational cohort study. Liver Int. 2019;39(4):694–704.
Maiwall R, Sarin SK, Moreau R. Acute kidney injury in acute on chronic liver failure. Hepatol Int. 2016;10(2):245–57.
Maiwall R, Kumar S, Chandel SS, et al. AKI in patients with acute on chronic liver failure is different from acute decompensation of cirrhosis. Hepatol Int. 2015;9(4):627–39.
Maiwall R, Pasupuleti SSR, Bihari C, et al. Incidence, risk factors, and outcomes of transition of acute kidney injury to chronic kidney disease in cirrhosis: a prospective cohort study. Hepatology. 2020;71(3):1009–22.
Maiwall R, Sarin SK, Kumar S, et al. Development of predisposition, injury, response, organ failure model for predicting acute kidney injury in acute on chronic liver failure. Liver Int. 2017;37(10):1497–507.
Amathieu R, Al-Khafaji A, Sileanu FE, et al. Significance of oliguria in critically ill patients with chronic liver disease. Hepatology. 2017;66(5):1592–600.
Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71(4):811–22.
Maiwall R, Kumar A, Bhardwaj A, Kumar G, Bhadoria AS, Sarin SK. Cystatin C predicts acute kidney injury and mortality in cirrhotics: a prospective cohort study. Liver Int. 2018;38(4):654–64.
Wan ZH, Wang JJ, You SL, et al. Cystatin C is a biomarker for predicting acute kidney injury in patients with acute-on-chronic liver failure. World J Gastroenterol. 2013;19(48):9432–8.
Ariza X, Solà E, Elia C, et al. Analysis of a urinary biomarker panel for clinical outcomes assessment in cirrhosis. PLoS One. 2015;10(6):e0128145.
Ariza X, Graupera I, Coll M, et al. Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis. J Hepatol. 2016;65(1):57–65.
Fagundes C, Pépin MN, Guevara M, et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57(2):267–73.
Jiang QQ, Han MF, Ma K, et al. Acute kidney injury in acute-on-chronic liver failure is different from in decompensated cirrhosis. World J Gastroenterol. 2018;24(21):2300–10.
Joshi D, O'Grady J, Patel A, et al. Cerebral oedema is rare in acute-on-chronic liver failure patients presenting with high-grade hepatic encephalopathy. Liver Int. 2014;34(3):362–6.
Sawhney R, Holland-Fischer P, Rosselli M, Mookerjee RP, Agarwal B, Jalan R. Role of ammonia, inflammation, and cerebral oxygenation in brain dysfunction of acute-on-chronic liver failure patients. Liver Transpl. 2016;22(6):732–42.
Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62(2):437–47.
Michard B, Artzner T, Lebas B, et al. Liver transplantation in critically ill patients: preoperative predictive factors of post-transplant mortality to avoid futility. Clin Transpl. 2017;31(12):10.
Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149–62.
Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61(2):396–407.
Artigas A, Wernerman J, Arroyo V, Vincent JL, Levy M. Role of albumin in diseases associated with severe systemic inflammation: pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care. 2016;33:62–70.
Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417–29. [published correction appears in Lancet. 2018 Aug 4;392(10145):386].
Arora V, Vijayaraghavan R, Maiwall R, et al. Paracentesis-induced circulatory dysfunction with modest-volume paracentesis is partly ameliorated by albumin infusion in acute-on-chronic liver failure. Hepatology. 2019;72(3):1043–55. [published online ahead of print, 2019 Dec 17]. https://doi.org/10.1002/hep.31071.
Jindal A, Bhadoria AS, Maiwall R, Sarin SK. Evaluation of acute kidney injury and its response to terlipressin in patients with acute-on-chronic liver failure. Liver Int. 2016;36(1):59–67.
Arora V, Maiwall R, Rajan V, et al. Terlipressin is superior to noradrenaline in the management of acute kidney Injury in acute on chronic liver failure. Hepatology. 2020;71(2):600–10.
Larsen FS. Artificial liver support in acute and acute-on-chronic liver failure. Curr Opin Crit Care. 2019;25(2):187–91.
Maiwall R, Maras JS, Nayak SL, Sarin SK. Liver dialysis in acute-on-chronic liver failure: current and future perspectives. Hepatol Int. 2014;8(Suppl 2):505–13.
Bañares R, Nevens F, Larsen FS, et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57(3):1153–62.
Kribben A, Gerken G, Haag S, et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012;142(4):782–789.e3.
Tan EX, Wang MX, Pang J, Lee GH. Plasma exchange in patients with acute and acute-on-chronic liver failure: a systematic review. World J Gastroenterol. 2020;26(2):219–45.
Ma Y, Chen F, Xu Y, et al. Safety and efficacy of regional citrate anticoagulation during plasma adsorption plus plasma exchange therapy for patients with acute-on-chronic liver failure: a pilot study. Blood Purif. 2019;48(3):223–32.
Stahl K, Busch M, Fuge J, et al. Therapeutic plasma exchange in acute on chronic liver failure. J Clin Apher. 2020;35(4):316–27.
Maiwall R, Moreau R. Plasma exchange for acute on chronic liver failure: is there a light at the end of the tunnel? Hepatol Int. 2016;10(3):387–9.
Larsen FS, Schmidt LE, Bernsmeier C, et al. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol. 2016;64(1):69–78.
Garg V, Garg H, Khan A, et al. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142(3):505–512.e1.
Duan XZ, Liu FF, Tong JJ, et al. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol. 2013;19(7):1104–10.
Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol. 2014;109(9):1417–23.
Singh V, Keisham A, Bhalla A, et al. Efficacy of granulocyte colony-stimulating factor and N-acetylcysteine therapies in patients with severe alcoholic hepatitis. Clin Gastroenterol Hepatol. 2018;16(10):1650–1656.e2.
Shasthry SM, Sharma MK, Shasthry V, Pande A, Sarin SK. Efficacy of granulocyte colony-stimulating factor in the management of steroid-nonresponsive severe alcoholic hepatitis: a double-blind randomized controlled trial. Hepatology. 2019;70(3):802–11.
Cristani M, Speciale A, Saija A, Gangemi S, Minciullo PL, Cimino F. Circulating advanced oxidation protein products as oxidative stress biomarkers and progression mediators in pathological conditions related to inflammation and immune dysregulation. Curr Med Chem. 2016;23(34):3862–82. https://doi.org/10.2174/0929867323666160902154748.
Nabi T, Nabi S, Rafiq N, Shah A. Role of N-acetylcysteine treatment in non-acetaminophen-induced acute liver failure: a prospective study. Saudi J Gastroenterol. 2017;23(3):169–75. PMID: 28611340; PMCID: PMC5470376. https://doi.org/10.4103/1319-3767.207711.
Mumtaz K, Azam Z, Hamid S, Abid S, Memon S, Ali Shah H, Jafri W. Role of N-acetylcysteine in adults with non-acetaminophen-induced acute liver failure in a center without the facility of liver transplantation. Hepatol Int. 2009;3(4):563–70. Epub 2009 Aug 29. PMID: 19727985; PMCID: PMC2790590. https://doi.org/10.1007/s12072-009-9151-0.
Baniasadi S, Eftekhari P, Tabarsi P, Fahimi F, Raoufy MR, Masjedi MR, Velayati AA. Protective effect of N-acetylcysteine on antituberculosis drug-induced hepatotoxicity. Eur J Gastroenterol Hepatol. 2010;22(10):1235–8. PMID: 20461008. https://doi.org/10.1097/MEG.0b013e32833aa11b.
Hu J, Zhang Q, Ren X, Sun Z, Quan Q. Efficacy and safety of acetylcysteine in “non-acetaminophen” acute liver failure: a meta-analysis of prospective clinical trials. Clin Res Hepatol Gastroenterol. 2015;39(5):594–9. Epub 2015 Feb 26. https://doi.org/10.1016/j.clinre.2015.01.003.
Weiler N, Schlotmann A, Schnitzbauer AA, Zeuzem S, Welker MW. The epidemiology of acute liver failure. Dtsch Arztebl Int. 2020;117(4):43–50. PMID: 32036852; PMCID: PMC7036472. https://doi.org/10.3238/arztebl.2020.0043.
Artzner T, Michard B, Besch C, Levesque E, Faitot F. Liver transplantation for critically ill cirrhotic patients: overview and pragmatic proposals. World J Gastroenterol. 2018;24(46):5203–14. PMID: 30581269; PMCID: PMC6295835. https://doi.org/10.3748/wjg.v24.i46.5203.
Dong V, Karvellas CJ. Acute-on-chronic liver failure: objective admission and support criteria in the intensive care unit. JHEP Rep. 2019;1(1):44–52. PMID: 32039351; PMCID: PMC7001553. https://doi.org/10.1016/j.jhepr.2019.02.005.
Bhatti ABH, Dar FS, Butt MO, Sahaab E, Salih M, Shah NH, Khan NY, Zia HH, Khan EU, Khan NA. Living donor liver transplantation for acute on chronic liver failure based on EASL-CLIF diagnostic criteria. J Clin Exp Hepatol. 2018;8(2):136–43. Epub 2017 Nov 24. PMID: 29892176; PMCID: PMC5992305. https://doi.org/10.1016/j.jceh.2017.11.007.
Pan HC, Jenq CC, Lee WC, Tsai MH, Fan PC, Chang CH, Chang MY, Tian YC, Hung CC, Fang JT, Yang CW, Chen YC. Scoring systems for predicting mortality after liver transplantation. PLoS One. 2014;9(9):e107138. PMID: 25216239; PMCID: PMC4162558. https://doi.org/10.1371/journal.pone.0107138.
Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D, Soriano G, Hoefs J, Navasa M, International Ascites Club. Sepsis in cirrhosis: report on the 7th meeting of the international ascites Club. Gut. 2005;54(5):718–25. PMID: 15831923; PMCID: PMC1774473. https://doi.org/10.1136/gut.2004.038679.
Arroyo V. Acute-on-chronic liver failure in cirrhosis requires expedited decisions for liver transplantation. Gastroenterology. 2019;156(5):1248–9. Epub 2019 Mar 5. https://doi.org/10.1053/j.gastro.2019.03.004.
O'Grady JG. Acute liver failure. Postgrad Med J. 2005;81(953):148–54. PMID: 15749789; PMCID: PMC1743234. https://doi.org/10.1136/pgmj.2004.026005.
Mathurin P, O'Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, Ramond MJ, Naveau S, Maddrey WC, Morgan TR. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60(2):255–60. Epub 2010 Oct 12. https://doi.org/10.1136/gut.2010.224097.
Mathurin P, Moreno C, Samuel D, Pruvot FR, Valle’e JC. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790–800.
Anand L, Choudhury A, Bihari C, et al. Flare of autoimmune hepatitis causing acute on chronic liver failure: diagnosis and response to corticosteroid therapy. Hepatology. 2019;70(2):587–96.
Yeoman AD, Westbrook RH, Portmann C, O’Grady OJ, Harrison PM, Heneghan MA. Early predictors of corticosteroid treatment failure in icteric presentations of autoimmune hepatitis. Hepatology. 2011;53:926–34.
Choudhury A, Jindal A, Maiwall R, et al. Liver failure determines the outcome in patients of acute-on-chronic liver failure (ACLF): comparison of APASL ACLF research consortium (AARC) and CLIF-SOFA models. Hepatol Int. 2017;11(5):461–71.
Engelmann C, Thomsen KL, Zakeri N, et al. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care. 2018;22(1):254.
Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61(5):1038–47.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Ethics declarations
None.
Conflicts of Interest
None to declare.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gupta, M., Maiwall, R. (2023). Acute on Chronic Liver Failure: An Update. In: Vohra, V., Gupta, N., Jolly, A.S., Bhalotra, S. (eds) Peri-operative Anesthetic Management in Liver Transplantation. Springer, Singapore. https://doi.org/10.1007/978-981-19-6045-1_43
Download citation
DOI: https://doi.org/10.1007/978-981-19-6045-1_43
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-6044-4
Online ISBN: 978-981-19-6045-1
eBook Packages: MedicineMedicine (R0)