Abstract
During infancy sleep is at a lifetime maximum and the maturation of sleep is one of the most important physiological processes occurring during the first year of life, particularly the first 6 months. Sleep has a marked effect on cardiorespiratory control which is also rapidly maturing during infancy. Immaturity of cardiorespiratory control frequently leads to respiratory instability and prolonged pauses in breathing that manifest in apnoea of prematurity and periodic breathing. During infancy central apnoeas are common and obstructive apnoea rare. Although apnoea of prematurity is actively treated whilst preterm infants are in the neonatal intensive care unit (NICU), shorter apnoeas and periodic breathing go largely undetected and untreated. The hypoxia associated with apnoea of prematurity has been associated with adverse developmental outcomes, shorter central apnoeas are currently believed to be benign during this early period of development. However, there is growing evidence that these short central apnoeas may be associated with developmental deficits in neurocognition. Further research is required to optimise treatment of apnoea of prematurity and to identify if shorter central apnoeas also require treatment to improve developmental outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Respiratory instability during sleep is common in infancy, especially in those infants born preterm. This respiratory instability, which manifests as periods of apnoea, is thought to be due to immaturity of the central and peripheral mechanisms that control breathing [1, 2]. During an apnoea there is a fall in heart rate and blood pressure and a concomitant surge in these when breathing is resumed. The effects of apnoea are more marked in infants born preterm as these infants also have prolonged immaturity of cardiovascular control, manifest as lower blood pressure, delayed blood pressure recovery following a cardiovascular challenge and impaired control of blood pressure, heart rate and cerebral oxygenation cross the first 6 months after term corrected age, when compared with age matched term infants [3,4,5,6,7,8,9,10].
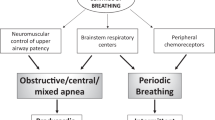
Apnoeas are characterised as central, obstructive or mixed. Central apnoeas are defined as a cessation of nasal and oral airflow in conjunction with an absence of respiratory effort. Obstructive apnoeas are defined as the cessation of nasal and oral airflow in the presence of continued respiratory effort against airway obstruction. Central apnoeas (defined as pauses in breathing ≥10 s in duration) are common in infancy and can occur spontaneously, but occur more frequently after a movement [11, 12]. Traditionally, these central apnoeas have been considered benign as they are not associated with significant desaturation and occur in healthy infants [12]. Currently, the American Academy of Sleep Medicine (AASM) recommends a central apnoea be scored if the event is and at least one of the following is met: (1) The event lasts 20 s or longer. (2) The event lasts at least the duration of two breaths during baseline breathing and is associated with an arousal or ≥3% oxygen desaturation. (3) For infants younger than 1 year of age, the event lasts at least the duration of two breaths during baseline breathing and is associated with a decrease in heart rate to less than 50 beats/min for at least 5 s or less than 60 beats/min for 15 s [13, 14]. Using this definition, the frequency of central apnoeas declines with age, with the median number of events per hour declining from 5.5 (minimum 0.9; maximum 44.3) at 1 month of age to 4.1 (minimum 1.2; maximum 27.3) at 3 months [15]. The authors suggested these high rates of central apnoea may be simply due to the fact that the current definitions for central apnoeas used for older children are not appropriate for young infants.
Apnoeas can occur as isolated events or in a repetitive pattern termed periodic breathing.
2 Apnoea of Prematurity
2.1 Epidemiology
Apnoea of prematurity is one of the most common diagnoses in the neonatal intensive care unit (NICU) [16]. An apnoea of prematurity episode is usually defined as a cessation of breathing for 20 s or longer, or a shorter pause accompanied by bradycardia (<100 bpm), cyanosis, or pallor. In practice, many apnoeic events in preterm infants are shorter than 20 s, because briefer pauses in airflow may result in bradycardia or hypoxaemia [16].
Apnoea of prematurity is extremely common, occurring in more than 85% of infants born prior to 34 weeks of gestation. The incidence of apnoea of prematurity is inversely related to gestational age occurring in: 3–5% of term-born infants, 7% of infants born at 34–35 weeks of gestational age (GA), 15% of infants born at 32–33 weeks of GA, 54% of infants born at 30–31 weeks of GA and nearly 100% of infants born less than 29 weeks of GA [17, 18]. There are also marked changes in apnoea frequency with postnatal age, with few events in the first week of life, then a progressive increase in weeks 2–3 which plateau in weeks 4–6 and then decrease in weeks 6–8 [19].
2.2 Aetiology
Preterm infants have a reduced ventilatory response to CO2 compared to infants born at term, and respond to increased CO2 levels with an increase in tidal volume, but little or no increase in respiratory frequency [20]. Furthermore, infants who exhibit apnoea have a reduced response to CO2 compared to who do not exhibit apnoeic periods. In addition, baseline PaCO2 is only 1 to 1.5 mmHg above the apnoeic threshold and thus only very small changes in PaCO2 can predispose to apnoea. Furthermore, respiratory instability is more marked in active sleep compared to quiet sleep, a sleep state in which preterm infants spend more of their time [21]. Vulnerable respiratory control is exacerbated by a compliant chest wall and reduced lung mechanics which lead to reduced functional residual capacity (FRC) [22]. Reduced FRC is further reduced by the reduced muscle tone which occurs during sleep.
2.3 Clinical Significance
Apnoea of prematurity is accompanied by both bradycardia and desaturation. The hypoxia associated with apnoea has detrimental effects on developing tissues and organs and can have long-term or permanent impairments [23,24,25,26]. Treatment of apnoea of prematurity, such as respiratory support and caffeine, are only partially successful in reducing bradycardia and desaturation [27] and the effects of apnoea of prematurity [28, 29]. A number of studies that have examined changes in cerebral haemodynamics associated with apnoea of prematurity. These have shown that both cerebral blood volume (CBV) and cerebral blood flow velocity fall [30], and the falls are greater when the apnoea is also associated with bradycardia [30, 31]. Although central apnoeas are more common than obstructive apnoeas in infants, obstructive apnoeas have been reported to be more common in the first days of life [32], when intraventricular haemorrhage usually occurs [17]. Obstructive apnoeas have been associated with greater falls in CBV compared to central and mixed apnoeas [33]. The decreases in both cerebral haemoglobin oxygenation index and CBV were greater during mixed and central apnoeas when infants slept supine compared to prone [34] and apnoea duration is positively associated with the decrease in CBV [35]. In a study which classified events as isolated bradycardias (heart rate <80 bpm), isolated hypoxaemia (oxygen saturation (SpO2) < 75%), simultaneous (within 4 s) bradycardia and hypoxemia, bradycardia followed by hypoxaemia and hypoxaemia followed by bradycardia, isolated hypoxaemias were the most common events and isolated bradycardias the least common [36]. Falls in cerebral oxygenation were smallest for isolated bradycardias and largest for combined bradycardia and hypoxaemia events. However, the majority of infants were able to maintain their cerebral oxygenation >60% despite severe falls in SpO2 [36]. Examples of falls in heart rate, SpO2 and cerebral oxygenation following central apnoeas in preterm infants are illustrated in Figs. 9.1 and 9.2.
Cerebral oxygenation measurements are not routine in most NICUs. Studies have identified that in general falls in cerebral oxygenation index in association with apnoeas >4 s in duration were not well correlated with falls in arterial oxygen saturation (SpO2) when falls in SpO2 were small (<3%) [37]. However, when falls in SpO2 associated with apnoeas >20 s in duration were >85%, SpO2 and cerebral oxygenation were well correlated, and that falls in SpO2 >85% could be used as an indication of clinically significant falls in cerebral oxygenation [38]. A more recent study identified that even the most sensitive oximeter setting of a 2 s averaging time underestimated the frequency of bradycardias, missing around 10% of bradycardias and showing significant delays in detecting bradycardias [39]. The study also identified that the falls in cerebral oxygenation during bradycardias were greater in very preterm infants (born ≤31 weeks GA) compared to those in late preterm infants (born >32 weeks GA). Even mild bradycardias (heart rate dropped to 60–80% of baseline) were associated with falls in cerebral oxygenation. The authors suggested that routine NIRS monitoring of cerebral oxygenation in NICUs may increase staff awareness for interventions to reduce the repetitive falls in cerebral oxygenation in preterm infants [39].
It is important to note that the magnitude and frequency of the apnoeic events are frequently underestimated in the NICU due to the current clinical settings of pulse oximeter monitors, which often are set with long averaging times and to alarm apnoeas of at 8–20 s duration. In one study 1958 apnoeas with desaturation <80% were recorded using an averaging time of 3 s, compared to only 339 when using a more conventional averaging time of 16 s [40]. In a study which used a 2 s averaging time and which counted apnoeas where oxygen saturation fell to ≤80% for between 3–10s, between 50–100 events/day were recorded [23]. Definitions for infant apnoea vary greatly in the literature. In scoring of sleep studies for children over a year of age, respiratory events representing the loss of two respiratory cycles are counted as apnoea with the qualification of an associated oxygen desaturation required if the event is central [13, 14]. The length of two respiratory cycles varies between 3 and 4.8 s in preterm infants and 4.2 and 5.5 s in term infants at birth depending on sleep state and the method of calculation [41]. It has therefore been suggested that a 5-s cut-off for defining central apnoeas in an infant is not unreasonable [41].
3 Short Central Apnoeas
Central apnoeas are frequent in infants, particularly in preterm born infants and as highlighted above can be associated with clinically significant falls in oxygen saturation and cerebral oxygenation. There have been limited studies that have examined the effects of short apnoea in preterm born infants after hospital discharge. A study investigating the longitudinal effects of persistent short apnoeas, studied 24 infants born between 27–36 weeks GA who underwent daytime polysomnographic studies at 2–4 weeks corrected age (CA), 2–3 months CA and 5–6 months CA [42]. Changes in heart rate, oxygen saturation and cerebral tissue oxygenation index were assessed for all apnoeas lasting ≥3 s. The study found that although overall apnoea frequency declined with age, apnoeas occurred in all infants at 2–4 weeks CA, 2–3 months CA and 5–6 months CA. Furthermore, there were no effects of GA at birth on the frequency or duration of apnoeas at any age studied. Interestingly, the effects of apnoeas on the falls in heart rate and cerebral oxygenation were more marked at the older ages than at 2–4 weeks CA. In contrast, apnoea duration had more marked effects on these variables at the younger ages [42]. The study showed that apnoea persists in infants born preterm after discharge home and similar to studies before term-equivalent age, apnoeas are associated with falls in cerebral oxygenation. When the data were compared to age-matched term-born infants, apnoea duration was not different between preterm-born and term-born groups, however the decline in apnoea index with postnatal age observed in the term-born infants was not seen in the preterm-born infants. Importantly, when compared to term infants, falls in cerebral oxygenation associated with apnoeas were greater in the preterm-born infants at all three ages studied [43].
3.1 Treatment
There is no consensus as to when to initiate therapy for apnoea of prematurity, however the first line of treatment is usually a methylxanthine [44] Methylxanthines, such as caffeine, aminophylline and theophylline have been used since the 1970’s for the treatment of apnoea of prematurity and also to facilitate extubation and weaning off mechanical ventilation [45, 46]. Methylxanthines cross the blood-brain barrier [47] and their primary action is to antagonise the A1/A2a adenosine receptors in the CNS. Methylxanthines improve apnoea of prematurity by increasing minute ventilation and improving both hypercapnic and hypoxic ventilatory drive [48, 49].
Today caffeine is the most commonly used methylxanthine in neonatal units worldwide [50]. Caffeine’s universal acceptance followed the 2006 CAP (Caffeine for Apnoea of Prematurity) randomised control trial, which compared caffeine citrate (20 mg/kg loading dose of caffeine citrate followed by 5 mg/kg/day) with placebo in very low birth weight preterm infants. The study demonstrated both significant short-term benefits of reduced incidence of bronchopulmonary dysplasia, medically and surgically treated ductus arteriosus, and long-term benefits of improved rates of survival without neurodevelopmental delay and significantly reduced incidences of cerebral palsy at 18–21 months [29, 51]. Improved microstructural development of white matter has been demonstrated in a subsample of these children who underwent brain magnetic resonance imaging (MRI) at term equivalent age, a finding which may explain the improved neurodevelopmental outcomes [52]. However, when reassessed at 5 years of age there was no longer any difference in rate of survival without disability between children treated with caffeine and those that were not [53]. A recent study confirmed the safety of maintenance doses up to 10 mg/kg/day in extremely preterm infants for longer durations than recommended on the drug label [54]. Other studies have suggested that higher dose regimens (loading doses up to 80 mg/kg, maintenance doses of 10–20 mg/kg/day) have been shown to be more effective in reducing apnoea and preventing extubation failure compared to conventional doses [55]. Higher average daily doses of caffeine have also been associated with improved neurodevelopmental outcomes [56]. However, there have been some concerns about adverse effects of high-dose caffeine with one study reporting a higher incidence of cerebellar haemorrhage with early high-dose caffeine compared to standard dosing, but there was no difference in developmental outcomes at 2 years [57]. Further randomised controlled trials are necessary to determine the optimal dose of caffeine to treat apnoea of prematurity to optimise neonatal outcomes [44].
In the immediate postnatal period O2 can trigger apnoea as the peripheral chemoreceptors have not had time to adjust to the higher O2 levels of extrauterine life. After this initial period, O2 therapy can be used to reduce the hypoxia associated with apnoea. Nasal continuous positive airway pressure (CPAP) at 4 to 6 cm H2O improves FRC and oxygenation [21, 22]. Heated humidified high-flow nasal cannula (HHFNC) at >2 L/kg/min provides similar effects to CPAP, using both O2 and room air [22]. In preterm infants born at 30.0 ± 3.2 (standard deviation) weeks’ gestational age and studied at 38.1 ± 4.4 weeks’ postconceptional age, supplemental low flow O2 delivered via nasal cannula at 0.25 L/min increased the amount of quiet sleep and decreased the amount of active sleep, apnoea index and also the amount of periodic breathing [58].
3.2 Research Gaps
As highlighted above studies are still required to elucidate the optimum dose of caffeine to optimise developmental outcomes to treat preterm infants with apnoea of prematurity. Studies are also required to determine if HHFNC is more advantageous than CPAP and if nasal intermittent positive pressure ventilation (NIPPV) is effective in reducing apnoea of prematurity symptoms. Anaemia may exacerbate apnoea by reducing the oxygen-carrying capacity of the blood and thus decreasing oxygen delivery to the brain. There have been limited studies to confirm that blood transfusions reduce apnoea in the short-term and none which have examined the long term effects on reducing apnoea and improving developmental outcomes [44].
While apnoea of prematurity is treated vigilantly in the NICU, it is unknown whether recurrent short apnoeas of 3–10 s, with relatively brief bradycardia and mild hypoxaemia in preterm infants are harmful and if treatment is warranted for the short apnoeas. Limited data suggest that the total number of days with apnoea and resolution of episodes at more than 36 weeks of postmenstrual age (PMA) are associated with worse neurodevelopmental outcome in preterm infants [24, 25]. In addition, levels of cerebral oxygenation of <55% in infants born <32 weeks GA, when measured by adult NIRS probes and <65% when measured with neonatal/paediatric probes, have been associated with adverse cognitive outcomes at 2 years of age [59]. Studies are urgently needed to identify if the short apnoeas experienced while in the NICU and after hospital discharge contribute to the neurodevelopmental deficits which are commonly associated with preterm birth.
3.3 Summary
In summary, both apnoea of prematurity and short apnoeas are extremely common in infancy, particularly in infants born preterm. Apnoea of prematurity in the NICU is actively treated, however the majority of apnoeas are short and do not trigger alarms and so go undetected. Even short apnoeas are associated with bradycardia, peripheral desaturation and falls in cerebral oxygenation, however their contribution to developmental outcomes requires further research.
4 Periodic Breathing
4.1 Epidemiology
Periodic breathing episodes are defined as 3 or more sequential apnoeas lasting >3 s separated by no more than 20 s of normal breathing [13, 14, 60]. Periodic breathing is common in term-born infants in the first 2 weeks of life, however less than 1% of total sleep time is spent in periodic breathing and it usually resolves with increasing postnatal age [61]. In preterm infants after term-equivalent age, periodic breathing is more prevalent, with one early study reporting an increased incidence of periodic breathing at 52 weeks PMA (i.e. 3 months CA) but a similar incidence at 64 weeks PMA (i.e. 6 months CA) compared to term-born infants [62]. At 40 weeks the density of all apnoeas in total sleep time was 2.5 times higher in the preterm group 126/100 min vs. 49/100 min in the term infants [62]. A study of 24 preterm infants born between 27–36 weeks GA, examined the incidence and consequences of periodic breathing in infants studied at 2–4 weeks CA, 2–3 months corrected age (CA) and 5–6 months CA [63]. Although all preterm infants had been discharged home with no clinical concerns of respiratory instability, a total of 261 individual episodes of periodic breathing were detected: 164 at Study 1, 62 at Study 2 and 35 at Study 3; 22 of the 24 infants (92%) exhibited periodic breathing during at least one of the three studies: 19 infants (79%) at 2–4 weeks corrected age (CA); 12 (50%) at 2–3 months CA and 10 (42%) at 5–6 months CA. Seven infants (29%) exhibited epochs of periodic breathing at all three studies and 10 infants (42%) at Studies 1 and 2. In term born infants studied at matched ages a total of 95 individual episodes of periodic breathing were detected; 64 at Study 1 at 2–4 weeks (one infant had 35 individual episodes), 24 at Study 2 at 2–3 months and 7 at Study 3 at 5–6 months. Eleven of the 17 infants (59%) exhibited periodic breathing during at least one of the three studies: 10 infants (79%) at 2–4 weeks; 7 (41%) at 2–3 months and 5 (29%) at 5–6 months. Four infants (24%) exhibited epochs of periodic breathing in all three studies and seven infants (41%) at Studies 1 and 2 [43].
4.2 Aetiology
Periodic breathing in the preterm infant is likely the result of several interacting mechanisms including chemoreceptor hypersensitivity and impaired gas exchange characteristics of the immature lung [2]. A recent study in preterm infants followed longitudinally from 32–36 weeks postmenstrual age (PMA) to 6 months post-term CA assessed ventilatory instability, using measurements of loop gain. Loop gain represents the sensitivity of the negative feedback loop that controls ventilation and can be defined as the ratio of the ventilatory response to the disturbance that elicited the response. A high loop gain represents a hypersensitive ventilatory control system, where a small disturbance leads to a large corrective response, ultimately causing cyclical oscillations in breathing such as occur in periodic breathing. The study showed that loop gain fell with postnatal age and was correlated with the decline in periodic breathing. Furthermore, those infants who continued to periodic breath at 6 months CA age had higher loop gain at 32–36 weeks PMA [64].
4.3 Clinical Significance
Because of its high prevalence, and no strong associations with significant hypoxia or bradycardia, the traditional view of periodic breathing is that it is simply due to immaturity of respiratory control and is benign [2]. However, the small number of studies, which have assessed the impact of periodic breathing, have found that repetitive short apnoeas can be associated with falls in both SpO2 and cerebral oxygenation. A study of a single preterm infant born at 27 weeks GA and studied at 37 weeks PMA showed significant cyclical changes in cerebral blood volume during episodes of periodic breathing [65]. A study in 10 term born infants studied at 6–8 weeks postnatal age also demonstrated that periodic breathing episodes >1 min in duration were associated with cyclical variations in haemoglobin oxygenation index [66]. These cyclic variations represent changes in CBV, and these occurred in 42% of episodes and were correlated with changes in heart rate [66].
There has been limited investigation of the short- and long-term consequences of periodic breathing after preterm infants are discharged home. The study by Decima et al., found that periodic breathing was associated with repetitive falls in cerebral tissue oxygenation index, heart rate and SpO2 as illustrated in Fig. 9.3, and these were apparent for up to 6 months CA in some infants [63]. When the data were compared to age matched term-born infants, the time spent in periodic breathing decreased with increasing PNA in both groups, however the falls in cerebral oxygenation associated with periodic breathing were greater at 2–3 months and 5–6 months CA in the preterm-born group [43].
Polysomnographic example of the effects of periodic breathing in an infant studied at 2–4 weeks post-term corrected age after discharge home. The short central apnoeas, indicated by the red boxes, are associated with repetitive oxygen desaturation, falls in cerebral tissue oxygen index to 55% (as measured with Near Infrared Spectroscopy), and repetitive bradycardia which worsens over time. This infant spent 28% of his total sleep time in periodic breathing. Note: lag time of falls in oxygen saturation is due to physiological and signal processing factors
Whilst the clinical significance of the repetitive falls in cerebral oxygenation in these infants remains unknown, it has been shown that a 10% reduction in cerebral oxygenation is of clinical concern for the development of cerebral hypoxic injury in preterm infants in NICU [67]. Cerebral tissue oxygenation index values fell by ≥10% of the baseline values in preterm-born infants at 2–3 and 5–6 months CA, while term-born infants did not experience these large changes at any age studied [43]. These findings, coupled with the increased frequency of both periodic breathing and apnoea, in the preterm group across the first 6 months CA suggest that even clinically well preterm infants are exposed to significantly greater levels of cerebral hypoxia compared to those born at term. It is well reported that obstructive sleep apnoea in children and adults is associated with neurocognitive deficits and the repetitive hypoxic events associated with this condition have been proposed as the primary mechanism. It is also possible that postnatal intermittent hypoxia can affect cardiovascular control beyond the neonatal period with studies in both rodent models [68] and human infants [69] demonstrating this. Further studies with neurodevelopmental follow-up in this population are required to ascertain if these brief falls in cerebral oxygenation with periodic breathing are associated with the neurocognitive deficits that are more prevalent in infants born preterm.
In addition, population cohort studies show that obstructive sleep disordered breathing is 3 to 6 times more likely in children who were born preterm and whether periodic breathing in infancy is a precursor to sleep disordered breathing later in life is a question requiring further research [70, 71].
4.4 Treatment
Periodic breathing is not routinely treated in preterm infants whilst in the NICU, however treatments for apnoea of prematurity (caffeine, CPAP and high-flow O2) are also effective in reducing the incidence of periodic breathing [50].
4.5 Research Gaps
Unlike apnoea of prematurity there is currently little evidence to associate periodic breathing with adverse developmental outcomes and further research is urgently needed.
5 Periodic Breathing Clinical Vignette
Figure 9.4 shows a sleep study report of an infant born at 29 weeks of gestational age and studied at 3 months of age. The infant was referred for assessment of central sleep apnoea.
The parent reported a normal night of sleep with 7.7 h of sleep available for analysis, with a feed at about 1 am. The study showed good sleep efficiency and normal sleep architecture for age. All sleep was supine. The arousal index was appropriate for age at 14.8/h overall with 57% being spontaneous arousals and 43% due to respiratory events. Nasal airflow was demonstrated. There were quiet breath sounds, no snoring and no increased work of breathing. Heart rate remained within normal limits throughout the study.
There was 4.8 h of sleep in room air. During this time the arousal index was 12.7/h with 61% due to respiratory events. There were no obstructive events. There were very frequent central events and periodic breathing in all sleep states. The central apnoea hypopnoea index (CAHI) was elevated at 152.4/h with central apnoeas associated with mild to moderate desaturation and occasional arousal. Average duration of central apnoeas was 6 s with the longest recorded being 12 s, both of which are within normal limits. There was frequent periodic breathing, which comprised 41% of the diagnostic portion. SpO2 levels returned to normal between events with a mean SpO2 of 98% and 1% of the time being spent <90%. The CO2 was normal.
Supplemental oxygen at a rate of 0.1 L/min was initiated at 02:45 with 2.9 h of sleep on of oxygen. The arousal index was 18.1/h but with 21% now being due to respiratory events. There were no obstructive events. There was a significant reduction in the number of central events on oxygen with the central apnoea hypopnoea index (CAHI) decreasing to 8.0/h. Events mainly occurred in REM sleep, with NREM breathing pattern mostly normalising. The central events were associated with brief and mild desaturation with a nadir of 93%. Periodic breathing comprised 18% of the sleep time when on oxygen. The CO2 remained normal.
Treatment: The baby was sent home on 0.125 L/min of oxygen with saturation monitoring and follow up with oximetry to track the expected improving course of this condition with time.
6 A Note on Obstructive Apnoea in Infants
Obstructive apnoeas are reported to be rare in infancy [15, 72]. However, snoring is reported to be common, with prevalence rates ranging from 5.6% to 26% [73,74,75,76]. These wide ranges in prevalence may have been due confounders with some studies including infants with colds and others studying different ethnicities. In a study of healthy predominantly Caucasian children aged 0–3 months a prevalence of 9% has been reported [77]. A significantly greater proportion of 2–3 month old infants were reported to snore habitually than 0–1 month old infants [77]. Cognitive ability at 6 months of age was found to be lower in those infants who began snoring frequently (≥3 nights/week) within the first month of life [78].
7 Summary
In summary, periodic breathing is very common in infancy, particularly in infants born preterm. Periodic breathing is considered to be a normal manifestation of immature respiratory control which improves with age. Because the apnoeas are short periodic breathing has been considered benign and is not routinely treated. However, there is growing evidence that periodic breathing persists well past term equivalent age in some infants and is associated with clinically significant falls in cerebral oxygenation and may contribute to neurological deficits.
References
Di Fiore JM, Martin RJ, Gauda EB. Apnea of prematurity—perfect storm. Respir Physiol Neurobiol. 2013;189:213–22.
Edwards BA, Sands SA, Berger PJ. Postnatal maturation of breathing stability and loop gain: the role of carotid chemoreceptor development. Respir Physiol Neurobiol. 2013;185:144–55.
Fyfe K, Odoi A, Yiallourou SR, Wong F, Walker AM, Horne RS. Preterm infants exhibit greater variability in cerebrovascular control than term infants. Sleep. 2015;38(9):1411–21.
Fyfe KL, Yiallourou SR, Wong FY, Odoi A, Walker AM, Horne RS. Cerebral oxygenation in preterm infants. Pediatrics. 2014;134:435–45.
Fyfe KL, Yiallourou SR, Wong FY, Odoi A, Walker AM, Horne RS. The effect of gestational age at birth on post-term maturation of heart rate variability. Sleep. 2015;38(10):1635–44.
Fyfe KL, Yiallourou SR, Wong FY, Odoi A, Walker AM, Horne RS. Gestational age at birth affects maturation of baroreflex control. J Pediatr. 2015;166:559–65.
Witcombe NB, Yiallourou SR, Sands SA, Walker AM, Horne RS. Preterm birth alters the maturation of baroreflex sensitivity in sleeping infants. Pediatrics. 2012;129:E89–96.
Witcombe NB, Yiallourou SR, Walker AM, Horne RSC. Blood pressure and heart rate patterns during sleep are altered in preterm-born infants: implications for sudden infant death syndrome. Pediatrics. 2008;122:1242–8.
Witcombe NB, Yiallourou SR, Walker AM, Horne RSC. Delayed blood pressure recovery after head-up tilting during sleep in preterm infants. J Sleep Res. 2010;19:93–102.
Yiallourou SR, Witcombe NB, Sands SA, Walker AM, Horne RS. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum Dev. 2013;89:145–52.
Carskadon MA, Harvey K, Dement WC, Guilleminault C, Simmons FB, Anders TF. Respiration during sleep in children. West J Med. 1978;128:477–81.
Marcus CL, Omlin KJ, Basinki DJ, Bailey SL, Rachal AB, Von Pechmann WS, Keens TG, Ward SL. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146:1235–9.
Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, Troester MT, Vaughn BV. Aasm scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13:665–6.
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM, American Academy of Sleep, M. Rules for scoring respiratory events in sleep: update of the 2007 Aasm manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the american academy of sleep medicine. J Clin Sleep Med. 2012;8:597–619.
Brockmann PE, Poets A, Poets CF. Reference values for respiratory events in overnight polygraphy from infants aged 1 and 3 months. Sleep Med. 2013;14:1323–7.
Eichenwald EC, Committee On F, Newborn AAOP. Apnea of prematurity. Pediatrics. 2016;137:e20153757.
Henderson-Smart D. The effect of gestational age on the incidence and duration of recurrent apnoea in newborn babies. Aust Paediatr J. 1981;17:273–6.
Picone S, Bedetta M, Paolillo P. Caffeine citrate: when and for how long. A literature review. J Matern Fetal Neonatal Med. 2012;25(Suppl 3):11–4.
Martin RJ, Di Fiore JM, Macfarlane PM, Wilson CG. Physiologic basis for intermittent hypoxic episodes in preterm infants. Adv Exp Med Biol. 2012;758:351–8.
Abu-Shaweesh JM, Martin RJ. Neonatal apnea: what’s new? Pediatr Pulmonol. 2008;43:937–44.
Martin RJ, Wilson CG. Apnea of prematurity. Compr Physiol. 2012;2:2923–31.
Morton SU, Smith VC. Treatment options for apnoea of prematurity. Arch Dis Child Fetal Neonatal Ed. 2016;101:F352–6.
Di Fiore JM, Bloom JN, Orge F, Schutt A, Schluchter M, Cheruvu VK, Walsh M, Finer N, Martin RJ. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. 2010;157:69–73.
Janvier A, Khairy M, Kokkotis A, Cormier C, Messmer D, Barrington KJ. Apnea is associated with neurodevelopmental impairment in very low birth weight iinfants. J Perinatol. 2004;24:763.
Pillekamp F, Hermann C, Keller T, Von Gontard A, Kribs A, Roth B. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155–61.
Poets CF, Roberts RS, Schmidt B, Whyte RK, Asztalos EV, Bader D, Bairam A, Moddemann D, Peliowski A, Rabi Y, Solimano A, Nelson H, Canadian Oxygen Trial I. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA. 2015;314:595–603.
Fairchild K, Mohr M, Paget-Brown A, Tabacaru C, Lake D, Delos J, Moorman JR, Kattwinkel J. Clinical associations of immature breathing in preterm infants: Part 1-central apnea. Pediatr Res. 2016;80:21–7.
Gizzi C, Montecchia F, Panetta V, Castellano C, Mariani C, Campelli M, Papoff P, Moretti C, Agostino R. Is synchronised nippv more effective than nippv and ncpap in treating apnoea of prematurity (Aop)? A randomised cross-over trial. Arch Dis Child Fetal Neonatal Ed. 2015;100:F17–23.
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W. Caffeine therapy for apnea of prematurity. NEJM. 2006;354:2112–21.
Livera LN, Spencer SA, Thorniley MS, Wickramasinghe YA, Rolfe P. Effects of hypoxaemia and bradycardia on neonatal cerebral haemodynamics. Arch Dis Child. 1991;66:376–80.
Pichler G, Urlesberger B, Muller W. Impact of bradycardia on cerebral oxygenation and cerebral blood volume during apnoea in preterm infants. Physiol Meas. 2003;24:671–80.
Perlman JM, Volpe JJ. Are venous circulatory abnormalities important in the pathogenesis of hemorrhagic and/or ischemic cerebral injury? Pediatrics. 1987;80:705–11.
Jenni OG, Wolf M, Hengartner M, Siebenthal K, Keel M, Bucher HU. Impact of central, obstructive and mixed apnea on cerebral hemodynamics in preterm infants. Biol Neonate. 1996;70:91–100.
Pichler G, Schmolzer G, Muller W, Urlesberger B. Body position-dependent changes in cerebral hemodynamics during apnea in preterm infants. Brain Dev. 2001;23:395–400.
Urlesberger B, Kaspirek A, Pichler G, Muller W. Apnoea of prematurity and changes in cerebral oxygenation and cerebral blood volume. Neuropediatrics. 1999;30:29–33.
Schmid MB, Hopfner RJ, Lenhof S, Hummler HD, Fuchs H. Cerebral oxygenation during intermittent hypoxemia and bradycardia in preterm infants. Neonatology. 2015;107:137–46.
Watkin SL, Spencer SA, Dimmock PW, Wickramasinghe Y, Rolfe P. A comparison of pulse oximetry and near infrared spectroscopy (Nirs) in the detection of hypoxaemia occurring with pauses in nasal airflow in neonates. J Clin Monit Comput. 1999;15:441–7.
Yamamoto A, Yokoyama N, Yonetani M, Uetani Y, Nakamura H, Nakao H. Evaluation of change of cerebral circulation by SpO2 In preterm infants with apneic episodes using near infrared spectroscopy. Pediatr Int. 2003;45:661–4.
Walter LM, Ahmed B, Odoi A, Cooney H, Horne RSC, Wong FY. Bradycardias are associated with more severe effects on cerebral oxygenation in very preterm infants than in late preterm infants. Early Hum Dev. 2018;127:33–41.
Vagedes J, Poets CF, Dietz K. Averaging time, desaturation level, duration and extent. Arch Dis Child Fetal Neonatal Ed. 2013;98:F265–6.
Elder DE, Whale J, Galletly D, Campbell AJ. Respiratory events in preterm infants prior to discharge: with and without clinically concerning apnoea. Sleep Breath. 2011;15:867–73.
Horne RSC, Fung ACH, Ncneil S, Fyfe KL, Odoi A, Wong FY. The longitudinal effects of persistent apnea on cerebral oxygenation in infants born preterm. J Pediatr. 2017;182:79–84.
Horne RSC, Sun S, Yiallourou SR, Fyfe KL, Odoi A, Wong FY. Comparison of the longitudinal effects of persistent periodic breathing and apnoea on cerebral oxygenation in term- and preterm-born infants. J Physiol. 2018;596:6021–31.
Erickson G, Dobson NR, Hunt CE. Immature control of breathing and apnea of prematurity: the known and unknown. J Perinatol. 2021; https://doi.org/10.1038/s41372-021-01010-zs.
Al-Saif S, Alvaro R, Manfreda J, Kwiatkowski K, Cates D, Qurashi M, Rigatto H. A randomized controlled trial of theophylline versus CO2 inhalation for treating apnea of prematurity. J Pediatr. 2008;153:513–8.
Henderson-Smart DJ, Steer P. Methylxanthine treatment for apnea in preterm infants. Cochrane Database Syst Rev. 2001:Cd000140.
Mccall AL, Millington WR, Wurtman RJ. Blood-brain barrier transport of caffeine: dose-related restriction of adenine transport. Life Sci. 1982;31:2709–15.
Chardon K, Bach V, Telliez F, Cardot V, Tourneux P, Leke A, Libert JP. Effect of caffeine on peripheral chemoreceptor activity in premature neonates: interaction with sleep stages. J Appl Physiol. 2004;96:2161–6.
Montandon G, Kinkead R, Bairam A. Adenosinergic modulation of respiratory activity: developmental plasticity induced by perinatal caffeine administration. Respir Physiol Neurobiol. 2008;164:87–95.
Eichenwald EC. National and international guidelines for neonatal caffeine use: are they evidenced-based? Semin Fetal Neonatal Med. 2020;25:101177.
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W. Long-term effects of caffeine therapy for apnea of prematurity. NEJM. 2007;357:1893–902.
Doyle LW, Cheong J, Hunt RW, Lee KJ, Thompson DK, Davis PG, Rees S, Anderson PJ, Inder TE. Caffeine and brain development in very preterm infants. Ann Neurol. 2010;68:734–42.
Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV, Davis PG, Tin W, Moddemann D, Solimano A, Ohlsson A, Barrington KJ, Roberts RS. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307:275–82.
Puia-Dumitrescu M, Smith PB, Zhao J, Soriano A, Payne EH, Harper B, Bendel-Stenzel E, Moya F, Chhabra R, Ku L, Laughon M, Wade KC, Best Pharmaceuticals For Children Act-Pediatric Trials Network Steering, C. Dosing and safety of off-label use of caffeine citrate in premature infants. J Pediatr. 2019;211:27–32 E1.
Brattstrom P, Russo C, Ley D, Bruschettini M. High-versus low-dose caffeine in preterm infants: a systematic review and meta-analysis. Acta Paediatr. 2019;108:401–10.
Ravichandran S, Chouthai NS, Patel B, Sharma A, Gupte A, Ma MM, Mamilla D, Lulic-Botica M, Thomas R, Kamat D. Higher daily doses of caffeine lowered the incidence of moderate to severe neurodevelopmental disabilities in very low birth weight infants. Acta Paediatr. 2019;108:430–5.
Mcpherson C, Neil JJ, Tjoeng TH, Pineda R, Inder TE. A pilot randomized trial of high-dose caffeine therapy in preterm infants. Pediatr Res. 2015;78:198–204.
Simakajornboon N, Beckerman RC, Mack C, Sharon D, Gozal D. Effect of supplemental oxygen on sleep architecture and cardiorespiratory events in preterm infants. Pediatrics. 2002;110:884–8.
Alderliesten T, Van Bel F, Van Der Aa NE, Steendijk P, Van Haastert IC, De Vries LS, Groenendaal F, Lemmers P. Low cerebral oxygenation in preterm infants is associated with adverse neurodevelopmental outcome. J Pediatr. 2019;207:109–116 E2.
Kelly DH, Shannon DC. Periodic breathing in infants with near-miss sudden infant death syndrome. Pediatrics. 1979;63:355–60.
Kelly DH, Stellwagen LM, Kaitz E, Shannon DC. Apnea and periodic breathing in normal full-term infants during the first twelve months. Pediatr Pulmonol. 1985;1:215–9.
Albani M, Bentele KH, Budde C, Schulte FJ. Infant sleep apnea profile: preterm vs. term infants. Eur J Pediatr. 1985;143:261–8.
Decima PF, Fyfe KL, Odoi A, Wong FY, Horne RS. The longitudinal effects of persistent periodic breathing on cerebral oxygenation in preterm infants. Sleep Med. 2015;16:729–35.
Siriwardhana LS., Lee A, Mann DL, Dawadi S, Nixon GM, Wong FY, Edwards BA, Horne RSC. Longitudinal assessment of ventilatory control instability in preterm infants with periodic breathing. J Pediatr. Submitted; 2021.
Jenni OG, Bucher HU, Von Siebenthal K, Wolf M, Keel M, Duc G. Cyclical variations in cerebral blood volume during periodic breathing. Acta Paediatr. 1994;83:1095–6.
Urlesberger B, Pichler G, Gradnitzer E, Reiterer F, Zobel G, Muller W. Changes in cerebral blood volume and cerebral oxygenation during periodic breathing in term infants. Neuropediatrics. 2000;31:75–81.
Van Bel F, Lemmers P, Naulaers G. Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls. Neonatology. 2008;94:237–44.
Soukhova-O'hare GK, Cheng ZJ, Roberts AM, Gozal D. Postnatal intermittent hypoxia alters baroreflex function in adult rats. Am J Physiol Heart Circ Physiol. 2006;290:H1157–64.
Cohen G, Lagercrantz H, Katz-Salamon M. Abnormal circulatory stress responses of preterm graduates. Pediatr Res. 2007;61:329–34.
Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–52.
Rosen CL, Larkin EK, Kirchner HL, Emancipator JL, Bivins SF, Surovec SA, Martin RJ, Redline S. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9.
Kato I, Franco P, Groswasser J, Kelmanson I, Togari H, Kahn A. Frequency of obstructive and mixed sleep apneas in 1,023 infants. Sleep. 2000;23:487–92.
Gislason T, Benediktsdottir B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old. an epidemiologic study of lower limit of prevalence. Chest. 1995;107:963–6.
Kelmanson IA. Snoring, noisy breathing in sleep and daytime behaviour in 2-4-month-old infants. Eur J Pediatr. 2000;159:734–9.
Mitchell EA, Thompson JM. Snoring in the first year of life. Acta Paediatr. 2003;92:425–9.
Montgomery-Downs HE, Gozal D. Sleep habits and risk factors for sleep-disordered breathing in infants and young toddlers in Louisville, Kentucky. Sleep Med. 2006;7:211–9.
Piteo AM, Lushington K, Roberts RM, Van Den Heuvel CJ, Nettelbeck T, Kohler MJ, Martin AJ, Kennedy JD. Prevalence of snoring and associated factors in infancy. Sleep Med. 2011;12:787–92.
Piteo AM, Kennedy JD, Roberts RM, Martin AJ, Nettelbeck T, Kohler MJ, Lushington K. Snoring and cognitive development in infancy. Sleep Med. 2011;12:981–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Horne, R.S.C., Wong, F.Y. (2022). Central Sleep Apnoea Syndromes in Infants. In: Li, A.M., Chan, K.Cc. (eds) Paediatric Sleep Disorders. Springer, Singapore. https://doi.org/10.1007/978-981-19-5791-8_9
Download citation
DOI: https://doi.org/10.1007/978-981-19-5791-8_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5790-1
Online ISBN: 978-981-19-5791-8
eBook Packages: MedicineMedicine (R0)