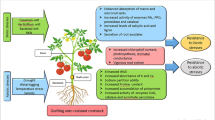
Abstract
Climate change seems to have an impact on agricultural and horticultural production systems through biotic stresses such as new disease races and insect pests, as well as abiotic stresses such as drought, flood, salinity, and heavy metal stress. Vegetable grafting is one of the most important procedures for improving vegetable production under a variety of environmental situations, as well as increasing yield and product nutritional quality. Many crops, including watermelon, tomato, eggplant, pepper, and cucumber, are currently grafted on a commercial basis. This method is viewed as a quick alternative to the somewhat laborious process of breeding fruits and vegetables to raise their environmental stress tolerance. Despite the fact that this is used in the majority of vegetables, the genetics and genomic foundation of gene transfer, interaction, and epigenetics mechanisms are unknown. Recent advances in molecular breeding and biotechnology techniques including as marker-assisted selection, genomic selection, and next-generation sequencing will help researchers to better understand the biological basis of root stock–scion interaction. In grafted scions, significant alterations in DNA methylation are seen, suggesting that these epigenetic pathways may be involved in grafting effects. Multiple resistant genes have a key role in the fight against different stresses when resistant rootstocks are used, whether intraspecific or interspecific. Genetic information is horizontally conveyed between the two grafted partners, either as DNA bits or plastids, according to transgenic lines with antibiotic markers in tobacco. Furthermore, the proteomics and transcriptomics research will reveal biochemical alterations in the products produced by the grafted plants. Mapping epigenetic markers and QTLs/genes in grafted crops can reveal fresh information about how to improve the crop. Vegetable grafting, thus, has a huge potential to improve the efficiency of modern and intelligent vegetable cultivation by increasing adaptability and resilience to various stress situations while also increasing yield.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Abiotic stress
- Biotic stress
- Climate change
- Genetics
- Genomics
- Marker-assisted selection
- Molecular breeding
- Proteomics
- QTLs
- Transcriptomics
- Vegetable grafting
- Yield stability
4.1 Introduction
Horticultural diversification is seen as critical to meeting the increasing demand for food and nourishment for an ever-increasing global population, and vegetables play a vital role in this direction (Jena et al. 2018). Vegetable production is hindered by biotic (pest and disease) and abiotic (environmental and soil stresses) factors (Pandey et al. 2017). These limits have been overcome by the development of new varieties or hybrids and the standardization of crop management procedures. Grafting has gain its popularity as a farming technique for fast improving modern vegetable cultivars flexibility or resistance to various conditions by grafting them onto stress-resistant rootstocks (Colla et al. 2013; Kumar et al. 2018). An important weapon in the fight against stresses appears to be the use of resistant rootstocks that are intraspecific (within the same species) selections with resistance genes or with resistance mechanisms that are not based on the host and are inter-specific (different species) and inter-generic (different genera) (Louws et al. 2010). Cucurbitaceous (cucumber, melon, and watermelon) and Solanaceous crops (eggplant, tomato, and pepper) employ grafting extensively (Kyriacou et al. 2016; Colla et al. 2008). It is possible to use natural genetic variation for specific root properties to alter the phenotype of the shoot by grafting (Kyriacou et al. 2017). Rootstock selection is an important step in grafting because it can influence the morphology and physiology of the scion, as well as the ability to manage environmental stresses, such as soil and foliar pathogens, arthropods and viral diseases, weeds and nematodes, and abiotic stresses such as thermal stress, drought, and salinity, as well as adverse soil pH (alkalinity and acidity) (Kumar et al. 2017). Grafting has been practiced for millennia, yet there are still many unanswered questions about the process. To better understand grafting and screening from genetics and genomics perspective, as well as the epigenetics and problems that come along with grafting, we look at a variety of grafting methods and screening techniques in this chapter. In addition, it expresses the potential of vegetable grafting for further study.
4.2 Grafting, Its Purpose, Historical Background to Current Status
This is crucial to consider grafting as a way to combat biotic and abiotic stresses, as well as organic vegetable cultivation. When two plants of different genetic backgrounds are joined together, a new plant is formed. One gives a stem, or scion, and one supplies a root system (rootstock). Since the 1970s, vegetable grafting has been used for commercial purposes around the world. For fruit and vegetable crops, the major goal of grafting is to boost yield and quality in the face of high soil-borne disease and nematode density and unfavorable circumstances. Increased yield and production efficiency, as well as improved economic viability, can be achieved through the use of grafting to reduce pesticide use in sustainable vegetable production through organic agriculture (Lee et al. 2010). For breeding, grafting can be used to create new genetic combinations (e.g., pomato). Grafted plants are more able to withstand both biotic and abiotic stresses, which can result in higher yields. Watermelon yields increased by almost 106% in Australia after grafting a specific rootstock (Yetisir and Sari 2003). To combat soil-borne diseases, such as Fusarium wilt, Verticillium wilt, Ralstonia wilt, Pyrenochaeta and Phomopsis rots, and root-knot nematodes, grafting has become increasingly common in the growth of fruit crops in many countries (Collonier et al. 2001). By employing different grafting tools, plant vigor is improved, harvesting time is extended, yield and fruit quality are improved as well as shelf life is extended, and nutrient uptake is increased. Low- and high-temperature tolerance, salinity and heavy metal stress tolerance, drought, and waterlogging tolerance are all made possible through the use of this substance. Cucumber (Cucumis sativus L.) grafting began in the late 1920s, but little success was reached until the 1960s (Sakata et al. 2008). In the 1950s, eggplant (Solanum melongena L.) was grafted into scarlet eggplant (Solanum integrifolium Poir.), and tomato (Lycopersicon esculentum Mill.) was introduced commercially (Lee and Oda 2003). In Japan and Korea, the use of grafting in S
Solanaceous (eggplant, tomato) and Cucurbitaceous (cucumber, melon) crops had increased by 59% and 81%, respectively (Lee 1994). On the commercial scale, grafted vegetables have gained appeal across the globe and are most commonly grown in greenhouses in nations such as the United States and China. In Asia and Europe, the market for grafted vegetable plants has already begun to grow outside Asia and now includes North America (Kubota et al. 2008). Watermelon grafting is a common practice around the world (Bekhradi et al. 2011). There were 40 million grafted seedlings utilized in greenhouse hydroponic tomato farming in North America, according to a study (Kubota et al. 2008). Watermelon, cucumber, eggplant, and 58% of the tomato plants grown in Japan were all grafted in the country (NARO 2011). Over the course of the last two decades, the number of plants produced by 16 Italian nurseries has climbed from ten million grafted plants to more than 60 million plants (Leonardi 2016). A timeline of vegetable grafting has been shown in Fig. 4.1. The use of desired rootstocks in vegetable grafting helps increase the plant’s resistance to abiotic stressors. Among other things, it increases vigor and precocity; improves production and quality and reduces soil-borne pathogen infection (Gaion et al. 2018). To feed the world’s ever-increasing population, grafting cucurbits, tomatoes, eggplants, and peppers onto hardy, disease-resistant rootstocks has become widely popular (Röös et al. 2017).
4.3 Genetic Basis of Vegetable Grafting
Numerous phenotypic polymorphisms in peppers have been described as a result of graft-induced changes in several plant traits (Tsaballa et al. 2011). In light of these findings, it is evident that genetic information is being traded between the grafted partners (Fig. 4.2). One of the most important issues in recent literature has been the movement of genetic information in the form of short RNAs. Plants are known to transport messenger RNA (mRNA) molecules via the phloem for decades, and grafting has been employed in numerous related research to confirm just that (Spiegelman et al. 2013; Turnbull and Lopez-Cobollo 2013). Using micro-grafting experiments, researchers found the miR399, a phosphorus deficiency-induced miRNA, for the first time in the phloem sap of rapeseed and pumpkin (Pant et al. 2008). A broader range of miRNAs, including those found in the scion but not in the rootstock, have been proposed for rootstock-to-scion transfer (Bhogale et al. 2014). A new understanding of the genetic information traveling through grafted plants as siRNAs has recently been made possible thanks to basic studies in Arabidopsis. De novo methylation of transposable elements (TEs) and repetitive DNA has been reported using 24-nt heterochromatic siRNAs, which leads to transcriptional gene silencing (TGS). A Dicer-like protein, DCL3, generates 24-nt siRNAs that can be transferred from the shoot to the root of grafted plants, according to Molnar et al. (2010), who employed wild-type (WT) Arabidopsis plants and mutants that were grafted onto each other. 24-nt siRNAs have been related to DNA methylation of three TEs in the roots, despite the fact that 22-nt and 23-nt siRNAs are also mobile (Molnar et al. 2010). The phloem is responsible for transporting siRNA. Only the mobile 24-nt siRNAs directed DNA methylation at recipient meristematic tissues and transgene promoter TGS in tests with Arabidopsis transgenic lines later on (Melnyk et al. 2011). Another finding was the ability of the non-exclusive 24-nt class of mobile siRNAs to go from shoot to root and directly modify hundreds of genomic locations related once more with TEs using mobile 24-nt siRNAs (Lewsey et al. 2016). For example, sRNAs that originate in the scion of a plant and travel toward the rootstock are more efficient at moving through the phloem and plasmodesmata than those that originate in the rootstock and travel toward the scion (Molnar et al. 2010; Melnyk et al. 2011). SiRNAs made in rootstock phloem partner cells have been shown to reach the WT scion and reduce viroid infection, even after lateral leaves and buds are removed (Kasai et al. 2013). In tomato ‘LeFAD7’ transgenic rootstocks, non-transgenic scions were used for grafting purposes. This study found that the LeFAD7 gene was under-expressed in grafted plants, indicating that the GM rootstock had transferred the gene to the scion. Before grafting, the scions in this project had their leaves removed as well (Nakamura et al. 2015). sRNA mobility in grafted plants could have significant practical ramifications, as can be seen from the evidence. Rootstock-to-scion transmission of resistance to viruses has been demonstrated in tomato. It has been shown that by grafting a tomato variety that is resistant to the TSWV virus onto another tomato variety that has a stronger RNA interference (RNAi) response to the viral infection, it is possible to create scions resistant to the virus. Roots of resistant grafted plants showed an increase in the expression of key RNAi genes, such as Argonaute (AGO) and RNA-Dependent RNA polymerase (RDR) genes. Researchers discovered that RNA silencing was significantly stronger in self-grafted plants, indicating that the mechanism might be activated by the act of itself (Spanò et al. 2015).
4.4 Crop Improvement via Vegetable Grafting
Plant breeding has largely focused on improving harvest and disease resistance, mechanical injury resilience, total postharvest performance, and quality attributes. It may take longer to create a high-yielding variety, and it may also necessitate sacrificing a desirable attribute in the name of productivity. Ethylene-dependent biosynthetic pathways have been connected to volatile fragrance components, as well as shelf-life performance (Pech et al. 2008). As a result, shelf-life breeding may have unintended side effects on sensory qualities that are otherwise beneficial (Causse et al. 2002). During the process of selecting for desirable features, undesirable impacts might impede breeding attempts. In some cases, independent selection of scion and rootstock traits may be possible by grafting, if the graft combination is compatible enough. It is also possible to boost yields through the use of marketable rootstocks and safe agriculture (Colla et al. 2011). Solanaceae and Cucurbitaceae grafting has been facilitated by using wild genetic resources to develop root physiological traits that are more tolerant to stress than scion characteristics under marginal conditions of salinity, nutrient stress, water stress, organic pollutants, and alkalinity (Schwarz et al. 2010; Borgognone et al. 2013). A new variety of vegetables can be developed more easily by grafting appropriate rootstock and scions. An autonomous rootstock and scion breeding program can do trait stacking. To better understand root-to-shoot signaling, scientists have used reverse genetics, which involves grafting genetically separate rootstock and scion. In contrast, the epigenetic and molecular components of vegetable grafting are still mostly unsearched. Epigenetics is the study of changes in gene expression that are not produced by changes in the primary DNA sequence, but rather by changes in how the DNA is packed (Bender 2002). Many plant characteristics and interactions with the environment have been connected to epigenetics. Crop breeders may now make better crop varieties that are more resilient to climate change by increasing and utilizing genomic diversity through genome-wide mapping of markers for epigenetic markings and epigenetic target identification. If heritable epigenetic modifications are caused by plant grafting and have a considerable impact on gene expression variability, it is evident that this should be investigated.
4.5 Epigenetics Basis of Vegetable Grafting
A plant’s genes can be activated or silenced by any one of three epigenetic mechanisms: DNA methylation/demethylation, histone changes, and non-coding RNA-mediated activity (Kapazoglou et al. 2018). There are three types of plant DNA methylation: the addition of the CH3 methyl group (CH3) to the nucleotides of DNA cytosines, which results in a 5-methylcytosine (He et al. 2011). To cleave or repress/inhibit the translation of homologous gene transcripts, non-coding RNA and specifically small non-coding RNAs (sRNAs) of 21–24 nucleotides (nt) (DCL) proteins target them. Four types of small RNAs exist in plants: micro-RNAs (miRNAs), which are post-transcriptional regulators; siRNAs, which are also engaged in transcriptional gene silencing; and snoRNAs, which are both. RNA-directed DNA methylation processes use siRNAs with a length of 24 nt to silence transposons (Chen 2009). DNA methylation and non-coding RNAs have been linked to epigenetic changes in grafting. When plants are grafted, they have been found to have different DNA methylation levels. Despite the fact that variations in DNA methylation have previously been connected to wound stress (Cao et al. 2016), it appears that the association between plant grafting and DNA methylation goes beyond wound stress. Using grafted tomato and eggplant plants, as well as pepper plants utilised exclusively as a rootstock for the tomato plants, Wu et al. (2013) reported a study indicating alterations in DNA methylation in these grafted Solanaceae plants.
Using MSAP analysis, no alterations in the global methylation of the grafted plants were seen. Only alterations in local methylation were found in the scions and pepper rootstocks, in a locus-specific manner. The self-pollinated grafted progeny carried a high proportion of the DNA methylation alterations from the scion. Bisulfite sequencing (BS) of specific loci indicated that while self-grafting can result in minor DNA methylation modifications, interspecies grafting in Solanaceae is accompanied by considerable heritable methylation changes. Genes all connected to DNA methylation, such as Methyl Transferase (MET) 1, displayed dramatically altered expression profiles in tomato-to-eggplant grafted plants compared to their seed non-grafted controls, but these profiles were reversed in the progenies (Wu et al. 2013). In this study, epigenetics and DNA methylation were closely linked to grafting, particularly in scions. Other plant groups that often employ interspecies propagation, such as the Cucurbitaceae, may similarly rely on DNA methylation for grafting effects. On pumpkins, we have found that MSAP markers may be used to detect a large increase in DNA methylation in cucumber scions, but not watermelon scions, when grafted onto pumpkins (Avramidou et al. 2015). That epigenetic changes in grafting scions are specific to the interaction between the rootstock and the scion could be suggested by this finding. Cucurbita pepo scion fruit quality was studied via intraspecies/inter-cultivar grafting, and we conducted methylation and miRNA studies to monitor the fruit phenotypic changes after grafting in order to understand the effects of grafting. Results show Cucurbita grafting modifies DNA methylation patterns and the expression of particular miRNAs, as demonstrated by MSAP and qRT-PCR (Xanthopoulou et al. 2019). Using both homo- and reciprocal grafting techniques, Li et al. (2014) grew cucumber and pumpkin on one another. Heterogeneous and homogeneous grafts were compared for miRNA levels by analyzing RNA from their leaves and root tips. Most of the miRNAs were observed to be altered in the hetero-grafted compared to the homo-grafted (Li et al. 2014). However, stress cannot be ignored when it comes to the expression of miRNAs in grafted plants. During salt stress, cucumbers grafted onto pumpkin rootstocks showed different miRNA expression patterns, suggesting that salt stress adaptation may play a role in the control of miRNA expression (Li et al. 2016). The role of diverse rootstock and scion combinations in stress adaptations and the role of miRNAs in these adaptations are still unanswered. However, greater investigation is needed into the origin, transport, and participation of these sRNAs in epigenetic modifications. To our knowledge, there has been no research tying vegetable grafting to histone alterations to this date.
4.6 Methods of Vegetable Grafting
Crop type, grower expertise, and the availability of grafting facilities all influence the grafting method used. There are a number of ways to graft vegetables, and examples of each are provided here. A schematic representation of vegetable grafting technique has been shown in Fig. 4.3, and the list of different grafting methods and the rootstock used are listed in Table 4.1. The scion meristematic tissues are in direct touch with the rootstock meristematic tissues. Callus tissue is formed when cambium cells from the rootstock and the scion fuse together to form a callus tissue when they are in close proximity (Acosta Muñoz 2005). Incompatible grafts as well as compatible grafts experience this first phase of cohesion, which is analogous to wound healing and does not need communication between the rootstock and scion. When a graft is appropriate, the callus shows a differentiation of some phloem vessels and sieve tube parts that are not generated from the cambium and form the first bridging and uninterrupted union between the rootstock and the scion. During the last stages of the grafting process, new vascular tissue is generated by the cambium layer that has grown in the bridge of the callus. For the first time, it is possible to establish an intercellular contact between rootstock and seedling (Pina and Errea 2005).
4.6.1 Cleft Grafting
Cucurbit cleft grafting was once common in several places, but these days, it is more common in solanaceous crops like tomatoes and brinjal. Rootstock seeds are sown 5–7 days before the scion’s seeds. They are cut at right angles 2–3 inches deep, with 2–3 leaves left on the stem of the seedlings that are selected for rootstock. One-quarter inch in diameter is the ideal diameter for the scion. To fit inside the vertical incision on the rootstock, the scion has two angled cuts on either side of the bottom end. Over the rootstock’s top, a grafting wax or clip is applied to seal the wounds and stabilize the graft Cleft grafting is a basic technique that works well with rootstocks that have hypocotyls that are rather wide (Gaion et al. 2018).
4.6.2 Tongue Approach/Approach Grafting
Farmers and small nurseries utilize this method the most but novice farmers and those without a greenhouse with a robust microclimate management system tend to prefer it. If you are just starting out, you can get started with this strategy thanks to the excellent seedling survival rate. Rootstocks with hollow hypocotyls should not be utilized on members of the cucurbitaceous vegetable family. To achieve a diameter of less than 1 inch, it is best if the rootstock and scion are grown at least 3 days apart. In order to prevent any further growth, the rootstock’s shoot apex is cut off. Hypocotyls are cut at 45° angles on both the scion and rootstock, resulting in a 45° hypocotyl. When the scion is interlocked with the rootstock via interlocking, a second, vertical incision is made to form notches or tabs. In order to allow the hypocotyl of the scion to fully heal, it is cut off from its roots and partially trimmed below the graft for 3–4 days. Finally, grafting tape is wrapped around the graft to keep it in place as it grows (Thakur 2020).
4.6.3 Hole Insertion/Top Insertion Grafting
Cucurbits scion and rootstock with hollow hypocotyls are commonly grown this way. The scion should be sown 3–8 days after the rootstock, in order to acquire the same diameter as the rootstock. The chasm widened or narrowed according on the rootstock type (Lee et al. 2010). Using a bamboo or plastic gimlet, cut a hole at a slant angle is cut to the longitudinal direction of the rootstock and the genuine leaf and growing tip are removed. By slant cutting the hypocotyl of the scion, it has a thin end that makes it easier to implant into the plant. It is incredibly cost effective for small farmers to produce up to 1500 grafts each day from one individual. High success rates can be achieved with 95% relative humidity and a temperature range of 21–36 °C from healing to transplantation. Hole insertion hypocotyl grafting is preferred by many farmers across the country because to the lower seedling size of watermelons compared to their rootstock (often Squash or Bottle gourd) (Lee et al. 2010). In comparison to tongue grafting, this procedure is quite prevalent in China since it produces a stronger union and vascular connection.
4.6.4 One Cotyledon/Slant/Splice Grafting
Expert growers and commercial nurseries are well versed in splice grafting. Vegetables of all kinds can be treated with this method, which can be done by hand, machine, or robot. Rootstock should be sown 7–10 days prior to scion sowing in order to guarantee equal hypocotyl diameter and hold the scion in place on the rootstock properly. Intact or excised, i.e., root-removed rootstock seedlings can be employed, depending on the preference of the growers and farmers. It is possible to do grafting by making slant incisions on both rootstock and scion while retaining only one cotyledon leaf on the cucurbit rootstock, which is also known as one cotyledon grafting (OC-SG). Grafting is typically done at the lower epicotyl of solanaceous plants and secured with simple clips. For 3 days, grafted plants should be kept at 25 °C and 100% humidity to ensure a successful graft union. Gluing tubes together A tube connect the rootstock and scion, similar to a slant, but instead of using clips, this approach uses an elastic tube (Kubota et al. 2008). A 45° angle under the cotyledons and a 5- to 10-mm-deep cut in the scion are also necessary. At the cut end of the rootstock hypocotyl, one tube about midway is placed down the stem. It is aimed for a flawless fit between scion and rootstock while inserting it into the grafting tube. Depending on the materials, the tube can be repurposed numerous times. Healing takes around 7 days. Tomatoes and brinjal are the most prevalent vegetables to contain it.
4.6.5 Pin Grafting
Splicing or slant grafting is the same procedure. Instead of grafting clips, specific pins are employed to keep the graft in place. Approximately 15 mm long and 0.5 mm wide, the ceramic pin has a hexagonal cross section. This pin is made of natural ceramic and can be left on a plant without any issues. Because ceramic pins are expensive, bamboo pins with rectangular cross-sectional shapes might easily replace them at a considerably reduced cost. Watermelons and other solanaceous plants may benefit from its application (Lee et al. 2010).
4.7 Diverse Applications of Vegetable Grafting
The leading objective of vegetable crop grafting is to develop resistant crops against serious diseases and insect pests as well as improve the fruit quality by using the desirable resistant root stock. Several advantages offered by vegetable grafting have been represented in Fig. 4.4, and stress resistance mechanism of grafted plants is schematically exemplified in Fig. 4.6.
4.7.1 Grafting Improves Biotic Stresses
Seedlings that are healthy and well established are essential to a successful vegetable farm’s profitability. By limiting plant death and the transmission of disease to new areas, these robust seedlings increase vegetable yields while lowering production costs (Ventura et al. 2019). However, due to a dearth of cultivable land, vegetables like cucurbits and solanaceous crops are commonly cultivated in disease polluted soil and environmental circumstances because of their high demand and market price (Schwarz et al. 2010). Diseases can stunt the growth of vegetable seedlings, resulting in a decrease in output and a decrease in the quality of the fruit. Planting disease-resistant cultivars is the most efficient method for preventing vegetable illnesses (Ventura et al. 2019). Sources of resistance have yet to be discovered in many plants, yet resistance may be reduced or lost as new pathogens, strains, or races are introduced. The grafting of vegetables onto rootstocks that can restrict or avoid the detrimental impact of external biotic stress on the plant is one technique to decrease production losses caused by soil-borne illnesses (Colla et al. 2013). The use of disease-resistant rootstocks in grafting methods has been shown to protect vegetable crops against a wide range of soil-borne illnesses in varied locales and situations (Rivard and Louws 2011). A list of crop plants is mentioned in Table 4.2 which is used for grafting to eradicate the fungal, bacterial, viral, and nematode pathogens. The use of grafting to combat Verticillium wilt, Fusarium wilt, corky root rot, and bacteria wilt diseases has proved successful in a number of nations. Grafting technology has evolved into a unique component for the improvement of pest management and crop productivity techniques in the production of several solanaceous (tomato and brinjal) and cucurbitaceous vegetables. Here, we want to highlight one case study about vegetable grafting in eggplant (Solanum melongena) to fight against fruit and shoot borer (Leucinodes orbonalis) at Regional Research and Technology Transfer Station, Odisha University of Agriculture and Technology, Keonjhar district, Odisha. The parent variety is highly susceptible to the fruit and shoot borer. Thus, looking to the severity, we planned to graft the scion on the rootstock of Solanum torvum (Turkey berry, wild eggplant relatives). The Solanum torvum plant is a perennial evergreen shrub or small tree with plant height of 4–5 m, and it produces small berry fruits. The observations are recorded that the grafted plant is completely resistant to fruit and shoot borer and the fruit size. As the life span of S. torvum is more, the plant is able to uptake more nutrient and water from the deeper soil layer for a longer period and the yield also increased as compared to the non-grafted plant (Figs. 4.5 and 4.6). The average fruit weight of grafted eggplant is 200–210 g, whereas in non-grafted, this is observed 90–95 g. Worldwide, grafted seedlings are becoming more popular due to the availability of disease-resistant rootstocks and the development of grafting technology. A better understanding of grafting-induced safeguards from inherent resistance to mediate systemic resistance in rootstocks was also provided by the research studies (Guan et al. 2012).
4.7.1.1 Vegetable Grafting to Induce Resistance Against Fungal Pathogens
In vegetable crops, soil-borne Fusarium and Verticillium wilt infections have been successfully avoided through the use of vegetable grafting (Louws et al. 2010). F. oxysporum formae speciales have not been found in many of the cucurbit rootstocks used for this purpose. This is why fusarium wilt disease in cucurbits was successfully managed by grafting (Louws et al. 2010). When Fusarium wilt first appeared in Japan in the 1920s, watermelon (Citrullus lanatus) was grafted onto bottle gourd (Lagenaria siceraria). The grafting method has since expanded to countries across the world. Watermelon grafts are used in nearly all of Japan and Korea’s vegetable farms (Lee et al. 2010). Squash (Cucurbita moschata), bottle gourd, and interspecific hybrid squash (C. maxima × C. moschat) have all been grafted onto it, and it has shown a great affinity for connected rootstocks (Gaion et al. 2018). Squash and interspecific hybrid squash have a superior root system and are more resistant to Fusarium wilt than the others (Keinath and Hassell 2014). Interspecific hybrid squash ‘Shintoza’ or ‘Super Shintoza’ provided watermelon plants with resistance to Fusarium wilt when grown in the presence of polluted soils. The rootstocks increased fruit size and yield compared to non-grafted plants. V. dahliae wilts Solanaceae and Cucurbitaceae plants by damaging the vascular system (Paplomatas et al. 2000). Vegetables infected with the V. dahliae bacterium can be grafted onto agricultural rootstocks and a scion infected with V. dahliae to create disease resistance in melons, watermelons, cucumbers, and tomatoes (Solanum lycopersicum). Resistance to Verticillium wilt was demonstrated by the ‘Super Shintoza’ rootstock, which decreased Verticillium microsclerotia incidence (Gaion et al. 2018). Watermelon and melon plants can be affected by Monosporascus cannonballus, a soil-borne disease that causes watermelon and melon plants to suddenly wilt (Edelstein et al. 1999). Melon resistance to M. cannonballus was improved by grafting sensitive types onto C. maxima and interspecific hybrid squash rootstocks (Cohen et al. 2005). However, it was discovered that the increased tolerance and increased production of grafted plants were not constant. Variations in the rootstock and scion combinations, as well as the surrounding environment, could account for the inconsistent results. In cucurbit production, the Phytophthora capsici pathogen is considered one of the most devastating. Grafting P. capsici onto bottle gourd, C. moschata, and wax gourd (Benincasa hispida) rootstocks increased yields and vegetative development in areas afflicted with P. capsici (Nemati and Banihashemi 2015). Scions of watermelons grafted onto Lagenaria siceraria rootstocks also demonstrated resistance to P. capsici (Kousik and Thies 2010). When grafted onto ‘Beaufort’ (S. lycopersicum, S. habrochaites) rootstocks, grafted tomatoes, and eggplants (S. melongena) had lower incidences of corky root disease, better yields, and larger fruits (Hasna et al. 2009). Rootstocks derived from watermelon, bottle gourd, pumpkin, and squash have been successfully grafted onto melon, and interspecific hybrids of C. melo, cucumber, and wax gourd have been developed, providing resistance to soil-borne diseases caused by Monosporascus cannonballus, F. oxysporum, and Stagonosporopsis spp (King et al. 2010; Lee et al. 2010; Zhou et al. 2014). Root and stem rot, caused by Fom, M. cannonballus, Macrophomina phaseolina, and Stagonosporopsis spp., are the primary opponents to rootstock generation (King et al. 2010). It has been difficult to establish melon cultivars that are completely resistant to all forms of Fom (Dhall 2015). Due to the discovery of rootstocks that are tolerant of all races, farmers in Fom-infested areas can utilize them to boost growth and development (Oumouloud et al. 2010). They can be used as rootstocks for melon because they are practically resistant to the Fusarium wilt disease. A variety of Fom races have been tolerated by interspecific hybrid rootstocks (SYTZ and NZ1), and their use has increased yields compared to non-grafted melon cv. Liyu’s (Zhou et al. 2014). Squash, interspecific hybrid squash, and pumpkin are commonly grafted onto cucumbers to protect them against Fusarium Wilt (C. ficifolia) (Dhall 2015). A frequent method is to graft tomato onto tomato genotypes, as well as tomato interspecific hybrids, to protect them from soil-borne fungus, such as Verticillium spp. (Polizzi et al. 2015). Traditionally, natural species like S. integrifolium or hybrid tomato rootstocks have been used to graft eggplants. Fusarium wilt-resistant rootstocks from hybrid tomatoes are more commonly used by farmers who use them for grafting tomatoes (King et al. 2010). Traditional rootstocks have been shown to lose their resistance to grafting or suffer detrimental effects as a result (Kawaguchi et al. 2008), which necessitated the development of new rootstocks, such as interspecific hybrids and those that are more closely related to wild species (King et al. 2010). S. sisymbriifolium or S. sisymbriifolium for eggplant grafting, which has shown strong resistance to Fusarium or Verticillium wilt, has proved its promise. Vegetable cultivars that were grafted onto S. sisymbriifolium roots and grown in either infested or uninfested soil saw increased yields as well as increased resistance to Verticillium wilt (Bletsos et al. 2003). Eggplant types grafted onto the ‘Beaufort’ F1 demonstrated enhanced yield and fruit output as a result of the increased vigor of the grafted plants A resistance to Verticillium wilt has been found in the grafted Epic eggplant cv. Beaufort F1 rootstock (Johnson et al. 2014; Miles et al. 2015). On cucumbers, tomatoes, and eggplant, diseases like the black root rot of cucumbers, the target leaf disease of cucumbers, and the Southern Blight disease of tomatoes have all been successfully prevented by the use of grafting (Louws et al. 2010). While using specific rootstocks, grafting has been shown to boost crop resistance to foliar diseases such as powdery mildew and downey mildew on cucurbits.
4.7.1.2 Vegetable Grafting to Induce Resistance Against Bacteria Pathogens
Tomatoes are susceptible to bacterial wilt, which is caused by the bacterium Ralstonia solanacearum. Tobacco plant resistance to this wilt disease is a measurable trait that is closely tied to the size of the fruits itself (Louws et al. 2010). Tomato varieties that are resistant to wilt are scarcely commercially stable (King et al. 2010). As produced on rootstocks that are resistant to the bacteria that cause tomato wilt, susceptible tomato cultivars (e.g., BHN 602 tomato line) were successfully grown to prevent the disease in tomato plants (Rivard et al. 2012). When grafted plants are more resistant to bacterial infections, it may be because the lower stems are less likely to become infected (Nakaho et al. 2000). To fight soil-borne bacterial wilt disease, the eggplant was grafted onto a wild scarlet eggplant rootstock (S. integrifolium) (King et al. 2010). Bacterial wilt resistance in wild relatives, such as S. torvum or S. sisymbriifolium, has been found to be higher (Gousset et al. 2005).
4.7.1.3 Vegetable Grafting to Induce Résistance Against Nematode
Root galling, which reduces nutrient and water intake, is a common sign of root-knot nematode (RKN) infection in vulnerable plants. M. incognita resistance was found in Cucumis metuliferus, Cucumis ficifolius, and bur cucumber (Sicyos angulatus) (Gu and Zhang 2006). Reduced root gall number and nematode infection were achieved by grafting C. metuliferus as rootstock on to RKN-susceptible melon cultivars (Sigüenza et al. 2005). In addition, a variety of melon cultivars have successfully been grafted onto C. metuliferus (Nisini et al. 2002). Using the bur cucumber as a rootstock, researchers found that it was more resistant to RKN (Zhang et al. 2006). The development of M. incognita-resistant rootstocks for wild watermelon has also shown promising results (Citrullus lanatus). RKN-resistant cucurbit rootstocks, on the other hand, are not widely available (Thies et al. 2010). The Mi gene was inserted into tomato rootstocks and cultivars, allowing for successful RKN control in tomatoes (Louws et al. 2010). It was possible to graft RKN-resistant rootstocks onto tomato cultivars because of the reduced RKN infestation in the field soils. However, it is possible that the temperature sensitivity of the Mi gene’s resistance to RKN is not always constant (Cortada et al. 2009). The rootstocks of Capsicum annuum (Capsicum annuum) with the N gene have proven beneficial in controlling RKNs (M. incognita, M. arenaria, and M. javanica) in pepper (Oka et al. 2004). Root-knot nematodes are resistant to grafted melon seedlings (Zhou et al. 2014). Resistance to nematodes (Meloidogyne incognita and M. javanica) is induced by wax gourds and squash, which have been employed as rootstocks (Galatti et al. 2013). Resistance to RKN in wild species like S. torvum and S. sisymbriifolium suggests that they could be used as rootstocks for eggplant. Eggplant can be grown on interspecific rootstocks that have good compatibility, strong plant vigor, enhanced yield, and mild root-knot nematode tolerance without affecting the quality of the fruit. Root-knot nematode in sweet pepper on Capsicum annuum rootstocks is the primary cause of disease, and successful grafting may be a potential strategy for managing this (Oka et al. 2004). The grafted cv. Celica, which is resistant to RKNs (M. incognita and M. javanica), performed much better than non-grafted plants cultivated in infested soils (Oka et al. 2004).
4.7.1.4 Vegetable Grafting to Induce Resistance Against Virus
Due to a paucity of thorough studies in this field, research into vegetable grafting-based resistant to viral illnesses (Spanò et al. 2020) has yielded mixed findings. Wang et al. (2002) found that grafted seedless watermelon plants with an anti-virus function performed better than seeded watermelon plants. Rootstocks that are resistant to melon necrotic spot virus in cucurbits were successfully used in Israel instead of soil fumigation with methyl bromide to control a soil-borne virus in cucurbits (Cohen et al. 2007). Tomato yellow leaf curl virus, tomato-spotted wilt virus, and the pepino mosaic virus can all be managed via grafting (Louws et al. 2010). There have been numerous reports that grafted plants are far more susceptible to viruses, probably due to graft incongruity that harms the scion’s health (Davis et al. 2008).
4.7.2 Grafting Improves Abiotic Stresses
In addition to salt, heat, soil alkalinity, heavy metals, and excess trace elements (Colla et al. 2013; Zhang et al. 2020; Huang et al. 2016a, b; Martínez-Andújar et al. 2017), vegetable crops are also subjected to numerous abiotic stresses, which have a substantial impact on crop growth and productivity. Grafting vegetables onto rootstocks can help lessen the effects of environmental stress on the shoots and help farmers to prevent or reduce production losses in times of bad weather (Schwarz et al. 2010). It is possible for both the scion and the rootstock to have a detrimental effect on the resistance of grafted plants to poor environmental conditions (Colla et al. 2010). When grown in difficult conditions, grafted plants were able to grow more quickly than un-grafted or self-grafted plants. They also had a greater photosynthetic rate and a lower concentration of heavy metals and a considerable number of trace necessary elements in their shoots (Siamak and Paolo 2019). Table 4.3 lists the abiotic stresses that have been reported to be alleviated by vegetable grafting.
4.7.3 Grafting Improving Yield Stability
Many fruit vegetables benefit significantly from grafting, even if they are infected with soil-borne illnesses. An increase in fresh fruit weight of 25–55% in oriental melons over own-rooted plants has been found. In addition to disease resistance, these yield increases were directly linked to high plant health throughout the growing season. Fusarium-infected plants produced virtually no commercial products. With tomato, the same results were achieved. Rootstocks ‘Kagemusia’ and ‘Helper’ increased tomato marketable output by up to 54% and 51%, respectively (Chung and Lee 2007). In comparison to the own-rooted ‘Seokwang’ tomato, plants grafted to most rootstocks had much less aberrant fruits. Watermelon, cucumber (Lee and Oda 2003), melon, pepper, and eggplant have all seen similar increases in output.
4.7.4 Improving the Fruit Quality
Several studies (Proietti et al. 2008; Flores et al. 2010) disagree over whether grafting improves fruit quality or has the opposite impact. A variety of factors, including the type of rootstock/scion combination utilized and harvest timing, could explain the discrepancies in results reported. When compared to watermelons from intact plants, the fruit size of grafted watermelons with strong root systems is sometimes greatly increased. This is why many growers employ grafting. Fruit shape and skin color; rind thickness; and soluble solid concentration are all influenced by the rootstock used in the cultivar. When it comes to exporting cucumbers, color and bloom development are critical. Despite the fact that these traits are typically viewed as cultivar specific, the rootstock can have a significant impact. As a result, rootstocks can negatively affect various aspects of cucumber fruit quality, including lower soluble solids and thicker peel as well as the flavor of rootstocks, as well as undesirable internal breakdown in mature fruit. Most new advice for producing grapes are geared at mitigating rootstock’s impact on fruit quality. Table 4.3 lists studies that show grafting improves fruit quality in vegetable crops.
4.8 Problems Associated with Vegetable Grafting
A wide range of issues arise while dealing with grafted grafts. The procedure is time-consuming and requires the expertise of qualified professionals. For graft healing, a regulated environment, and efficient grafting equipment and robots are all necessary. Time management for sowing rootstock and scion seeds is also necessary (Fig. 4.7). Scion fruit quality and output can be dramatically impacted if transplants develop too quickly in the field (Huang et al. 2015). Rootstock–scion incompatibility may be found in the early stages or during field transplantation. Depending on the soil and environmental circumstances, it is necessary to select rootstock and scion combinations with care. Seeds for both the rootstock and the scion must be purchased, and the cost of hybrid and special seed might be prohibitive. Suckers and offshoots of the rootstock that form during the healing process or in the field (following transplantation) must be removed. The spread of pathogens, especially seedborne ones (e.g., Clavibacter michiganensis subsp. michiganensis in tomato, bacterial fruit blotch caused by Acidovorax citrulli in watermelon and melon, charcoal rot caused by Macrophomina phaseolina in melon and bottle gourd, and tomato mosaic virus and pepino mosaic virus infections in tomato) can be increased by grafting, particularly in the nursery. This is because a grafted plant is made from two seeds and is grafted with cutting devices. In order to prevent the spread of pathogens in the nursery, it is necessary to use seeds that have been certified free of pathogens, disinfect cutting instruments, use clean clothing and sterilized hands by grafting workers, disinfect grafting areas and plant growing environments, and constantly monitor the phytosanitary status of seedlings. Vegetable grafting may provide several career opportunities, but researchers have discovered a number of hazards that may endanger the health of those who work in nurseries. During the months of April–June, September, and October, employees in greenhouses and growth chambers experience heat stress and pain while grafting plants (Lee et al. 2010; Marucci et al. 2012). However, workers’ health and safety can be improved by the use of cooling pads, blowers, and covering sheets, but better facilities (such as air-conditioned settings) are still needed. However, careful management approaches can greatly lessen the severity of these issues.
4.9 Conclusion and Future Prospects
Some soil-borne illnesses can have a dramatic impact on the production of vegetables, particularly tomatoes, in rural areas. There are many diseases that can damage tropical tomato plants, but one of the most common and most devastating is bacterial wilt. In the case of cucurbits, such as eggplant, this has also been the case. Soil-borne illnesses and nematodes can affect yields, but grafting sensitive cultivars onto wild rootstock has been shown to reduce such risks. It is necessary to conduct location-specific research in order to analyze and identify the most compatible rootstocks. As a vegetative growth technique, grafting can be used to overcome the incompatibility between two different species. Another major application of this approach is to offset yield losses due to abiotic stresses. Identification of appropriate disease-resistant rootstocks with tolerance to biotic and abiotic stressors is vital for the sustained success of grafting. As the molecular mechanism of gene flow between rootstock and scion is still not clearly understood, it needs further advanced study by using molecular markers and genome sequence study. The proteomics and metabolomics study can further clarify the fruit nutritional quality status in the grafted hybrid. The level of beneficial and anti-nutritional factors should need to analyze as most of the rootstocks are used from wild sources. In coming future, the technique will explore with more precision and innovation. The key to widespread adoption is the availability of reasonably priced, healthy grafted seedlings. It takes a lot of time and effort to grow and manage grafted plants in the nursery. Unemployed individuals may be able to find work as a result of this initiative. Preparation of the bed soil and planting for the creation of grafted nursery plants are just a few of the many stages involved. Farmers should use low-cost grafting techniques to ensure the long-term viability of grafted seedlings. Improvements in grafting technique and the healing environment are required before commercial use. Farmers need to be made more aware of grafted vegetable processes and advantages. Fruit and vegetable grafting can help boost agricultural output. A growing number of organic farmers are using vegetable grafting as a way to expand their harvests. To feed the predicted 10–11 billion people on the planet by 2050, agricultural production must rise by 60%. Sustainable use of natural resources can attain this goal. An growing number of biotic (soil-borne disease and nematode problems) and abiotic (salinity, drought, heat, waterlogging) challenges are having an effect on vegetable output. Grafting techniques and nursery management practices should be improved in future study to ensure organic farm growers receive high-quality grafted transplants. Grafting can be used in both breeding and research to advance sustainable agricultural production, as can many other methods.
References
Acosta Muñoz A (2005) La Técnica del Injerto en Plantas Hortícolas. Horticom (Extra Viveros I), Extra. pp. 62–65. http://bit.do/eAgQc. Accessed 15 Sept 2018
Ajuru MG, Okoli BE (2013) The morphological characterization of the melon species in the family Cucurbitaceae Juss., and their utilization in Nigeria. Int J Modern Botany 3(2):15–19
Al-Chaabi S, Koutifani O, Safeih MH, Sedawi A, Asmar J (2009) Management of root-knot nematodes and corky root disease of pepper plants by grafting technique onto resistant rootstocks under plastic house. Arab Gulf J Sci Res 27(3):178–186
Al-Debei HS, Makhadmeh I, Abu-Al Ruz I, Al-Abdallat AM, Ayad JY, Al Amin N (2012) Influence of different rootstocks on growth and yield of cucumber (Cucumissativus L.) under the impact of soil-borne pathogens in Jordan. J Food Agric Environ 10(2):343–349
Allevato E, Mauro RP, Stazi SR, Marabottini R, Leonardi C, Ierna A, Giuffrida F (2019) Arsenic accumulation in grafted melon plants: role of rootstock in modulating root-to-shoot translocation and physiological response. Agronomy 9(12):828
Álvarez-Hernández JC, Castellanos-Ramos JZ, Aguirre-Mancilla CL, Huitrón-Ramírez MV, Camacho-Ferre F (2015) Influence of rootstocks on fusarium wilt, nematode infestation, yield and fruit quality in watermelon production. Ciênc Agrotec 39(4):323–330. https://doi.org/10.1590/S1413-70542015000400002
Arao T, Takeda H, Nishihara E (2008) Reduction of cadmium translocation from roots to shoots in eggplant (Solanummelongena) by grafting onto Solanumtorvum rootstock. Soil Sci Plant Nutr 54(4):555–559
Ashok Kumar B, Sanket K (2017) Grafting of vegetable crops as a tool to improve yield and tolerance against diseases—a review. Int J Agri Sci:0975–3710
Asins MJ, Bolarín MC, Pérez-Alfocea F, Estañ MT, Martínez-Andújar C, Albacete A, Carbonell EA (2010) Genetic analysis of physiological components of salt tolerance conferred by Solanum rootstocks. What is the rootstock doing for the scion? Theor Appl Genet 121(1):105–115
Avramidou E, Kapazoglou A, Aravanopoulos FA, Xanthopoulou A, Ganopoulos I, Tsaballa A et al (2015) Global DNA methylation changes in Cucurbitaceae inter-species grafting. Crop Breed Appl Biotechnol 15:112–116. https://doi.org/10.1590/1984-70332015v15n2n20
Baron D, Saraiva GFR, Amador TS, Rodrigues JD, Goto R, Ono EO (2018) Anatomical and physiological aspects of cucumber graft. Comun Sci 9(2):282–286
Bekhradi F, Kashi A, Delshad M (2011) Effect of three cucurbits rootstocks on vegetative and yield of ‘Charleston Gray’ watermelon. Int J Plant Product 5(2):105–110
Bender J (2002) Plant epigenetics. Curr Biol 12:R412–R414. https://doi.org/10.1016/s0960-9822(02)00910-7
Bhogale S, Mahajan AS, Natarajan B, Rajabhoj M, Thulasiram HV, Banerjee AK (2014) MicroRNA156: a potential graft-transmissible microRNA that modulates plant architecture and tuberization in Solanumtuberosum ssp. andigena. Plant Physiol 164:1011–1027. https://doi.org/10.1104/pp.113.230714
Bletsos F, Thanassoulopoulos C, Roupakias D (2003) Effect of grafting on growth, yield, and Verticillium wilt of eggplant. Hortic Sci 38(2):183–186. https://doi.org/10.21273/HORTSCI.38.2.183
Borgognone D, Colla G, Rouphael Y, Cardarelli M, Rea E, Schwarz D (2013) Effect of nitrogen form and nutrient solution pH on growth and mineral composition of self-grafted and grafted tomatoes. Sci Hortic 149:61–69
Cao L, Yu N, Li J, Qi Z, Wang D, Chen L (2016) Heritability and reversibility of DNA methylation induced by in vitro grafting between Brassica juncea and B. oleracea. Sci Rep 6:27233. https://doi.org/10.1038/srep27233
Causse M, Saliba-Colombani V, Lecomte L, Duffe P, Rousselle P, Buret M (2002) QTL analysis of fruit quality in fresh market tomato: a few chromosome regions control the variation of sensory and instrumental traits. J Exp Bot 53(377):2089–2098
Chen X (2009) Small RNAs and their roles in plant development. Annual Review of Cell and Developmental Biolog 25:21–44. https://doi.org/10.1146/annurev.cellbio.042308.113417
Chung HD, Lee JM (2007) Rootstocks for grafting. In: Horticulture in Korea. Korean Society for Horticultural Science, pp 162–167
Cohen R, Burger Y, Horev C, Koren A (2007) Introducing grafted cucurbits to modern agriculture: the Israeli experience. Plant Dis 91(8):916–923. https://doi.org/10.1094/PDIS-91-8-0916
Cohen R, Burger Y, Horev C, Porat A, Edelstein M (2005) Performance of Galia-type melons grafted on to Cucurbita rootstock in Monosporascus cannonballus-infested and non-infested soils. Ann Appl Biol 146(3):381–387. https://doi.org/10.1111/j.1744-7348.2005.040010.x
Colla G, Fiorillo A, Cardarelli M, Rouphael Y (2013) Grafting to improve abiotic stress tolerance of fruit vegetables. Acta Hortic 1041:119–125. https://doi.org/10.17660/ActaHortic.2014.1041.12
Colla G, Rouphael Y, Cardarelli M, Temperini O, Rea E, Salerno A, Pierandrei F (2008) Influence of graftingon yield and fruit quality of pepper (Capsicum annuum L.) grown under greenhouse conditions. Acta Hortic 782:359–363
Colla G, Rouphael Y, Leonardi C, Bie Z (2010) Role of grafting in vegetable crops grown under saline conditions. Sci Hortic 127(2):147–155. https://doi.org/10.1016/j.scienta.2010.08.004
Colla G, Rouphael Y, Mirabelli C, Cardarelli M (2011) Nitrogen-use efficiency traits of mini watermelon in response to grafting and nitrogen-fertilization doses. J Plant Nutr Soil Sci 174:933–994. https://doi.org/10.1002/jpln.201000325
Collonier C, Fock I, Kashyap V, Rotino GL, Daunay MC, Lian N, Mariska LK, Rajam MV, Seraes A, Ducreux G, Sihachakr D (2001) Applications of biotechnology in eggplant. Plant Cell Issue Organ Culture 65:91–101. https://doi.org/10.1023/A:1010674425536
Cortada L, Sorribas FJ, Ornat C, Andrés MF, Verdejo-Lucas S (2009) Response of tomato rootstocks carrying the Mi-resistance gene to populations of Meloidogyne arenaria, M. incognita and M. javanica. Eur J Plant Pathol 124(2):337–343. https://doi.org/10.1007/s10658-008-9413-z
Davis AR, Perkins-Veazie P, Sakata Y, Lopez-Galarza S, Maroto JV, Lee SG, Huh YC, Sun Z, Miguel A, King SR, Cohen R (2008) Cucurbit grafting. Crit Rev Plant Sci 27(1):50–74. https://doi.org/10.1080/07352680802053940
Dhall RK (2015) Breeding for biotic stresses resistance in vegetable crops: a review. J Crop Sci Technol 4:13–27
Edelstein M, Cohen R, Burger Y, Shriber S, Pivonia S, Shtienberg D (1999) Integrated management of sudden wilt in melons, caused by Monosporascus cannonballus, using grafting and reduced rates of methyl bromide. Plant Dis 83(12):1142–1145
El-Eslamboly AASA, Deabes AAA (2014) Grafting cucumber onto some rootstocks for controlling root-knot nematodes. Minufiya J Agri Res 39:1109–1129
Fita A, Pico B, Roig C, Nuez F (2007) Performance of Cucumis melo ssp. agrestis as a rootstock for melon. J Hortic Sci Biotechnol 82(2):184–190
Flores FB, Sanchez-Bel P, Estan MT, Martinez-Rodriguez MM, Moyano E, Morales B, Campos JF, Garcia-Abellán JO, Egea MI, Fernández-Garcia N, Romojaro F, Bolarín MC (2010) The effectiveness of grafting to improve tomato fruit quality. Sci Hortic 125:211–217
Gaion LA, Braz LT, Carvalho RF (2018) Grafting in vegetable crops: a great technique for agriculture. Int J Veg Sci 24(1):85–102. https://doi.org/10.1080/19315260.2017.1357062
Galatti FDS, Franco AJ, Ito LA, Charlo HDO, Gaion LA, Braz LT (2013) Rootstocks resistant to Meloidogyne incognita and compatibility of grafting in net melon. Revista Ceres 60(3):432–436. https://doi.org/10.1590/S0034-737X2013000300018
Garibaldi A, Baudino M, Minuto A, Gullino ML (2008) Effectiveness of fumigants and grafting against tomato brown root rot caused by Colletotrichum coccodes. Phytoparasitica 36(5):483. https://doi.org/10.1007/BF03020294
Gilardi G, Gullino ML, Garibaldi A (2010) Reaction of tomato rootstocks to selected soil-borne pathogens under artificial inoculation conditions. Acta Hortic 914:345–348. https://doi.org/10.17660/ActaHortic.2011.914.63
Gousset C, Collonnier C, Mulya K, Mariska I, Rotino GL, Besse P, Servaes A, Sihachakr D (2005) Solanum torvum, as a useful source of resistance against bacterial and fungal diseases for improvement of eggplant (S. melongena L.). Plant Sci 168(2):319–327
Gu X, Zhang S (2006) The screening of cucumber rootstocks resistant to southern root-knot nematode. China Vegetables 2:4–8
Guan W, Zhao X, Hassell R, Thies J (2012) Defense mechanisms involved in disease resistance of grafted vegetables. Hortic Sci 47(2):164–170. https://doi.org/10.21273/HORTSCI.47.2.164
Hasama W, Morita S, Kato T (1993) Reduction of resistance to Corynespora target leaf spot in cucumber grafted on a bloomless rootstock. Jpn J Phytopathol 59(3):243–248. https://doi.org/10.3186/jjphytopath.59.243
Hasna MK, Ögren E, Persson P, Mårtensson A, Rämert B (2009) Management of corky root disease of tomato in participation with organic tomato growers. Crop Prot 28(2):155–161. https://doi.org/10.1016/j.cropro.2008.09.011
He XJ, Chen T, Zhu JK (2011) Regulation and function of DNA methylation in plants and animals. Cell Res 21:442–465. https://doi.org/10.1038/cr.2011.23
Huang Y, Jiao Y, Nawaz MA, Chen C, Liu L, Lu Z (2016a) Improving magnesium uptake, photosynthesis and antioxidant enzyme activities of watermelon by grafting onto pumpkin rootstock under low magnesium. Plant Soil 409:229–246. https://doi.org/10.1007/s11104-016-2965-3
Huang Y, Kong QS, Chen F, Bie Z (2015) The history, current status and future prospects of the vegetable grafting in China. Acta Hortic 1086:31–39
Huang Y, Zhao L, Kong Q, Cheng F, Niu M, Xie J, Bie Z (2016b) Comprehensive mineral nutrition analysis of watermelon grafted onto two different rootstocks. Hortic Plant J 2(2):105–113
Jacob A, Malpathak N (2004) Green hairy root cultures of Solanum khasianum Clarke—a new route to in vitro solasodine production. Curr Sci:1442–1447
Jang Y, Yang E, Cho M, Um Y, Ko K, Chun C (2012) Effect of grafting on growth and incidence of Phytophthora blight and bacterial wilt of pepper (Capsicum annuum L.). Hortic Environ Biotechnol 53(1):9–19. https://doi.org/10.1007/s13580-012-0074-7
Jena AK, Deuri R, Sharma P, Singh SP (2018) Underutilized vegetable crops and their importance. J Pharmacog Phytochem 7(5):402–407
Johnson S, Inglis D, Miles C (2014) Grafting effects on eggplant growth, yield, and verticillium wilt incidence. Int J Veg Sci 20(1):3–20. https://doi.org/10.1080/19315260.2012.751473
Kapazoglou A, Ganopoulos I, Tani E, Tsaftaris A (2018) Epigenetics, epigenomics and crop improvement. In: Kuntz M (ed) Advances in botanical research, vol 86. Academic Press, pp 287–324
Kasai A, Sano T, Harada T (2013) Scion on a stock producing siRNAs of potato spindle tuber viroid (PSTVd) attenuates accumulation of the viroid. PLoS One 8:e57736. https://doi.org/10.1371/journal.pone.0057736
Kawaguchi M, Taji A, Backhouse D, Oda M (2008) Anatomy and physiology of graft incompatibility in solanaceous plants. J Hortic Sci Biotechnol 83(5):581–588. https://doi.org/10.1080/14620316.2008.11512427
Keinath AP, Hassell RL (2014) Control of Fusarium wilt of watermelon by grafting onto bottlegourd or interspecific hybrid squash despite colonization of rootstocks by Fusarium. Plant Dis 98(2):255–266. https://doi.org/10.1094/PDIS-01-13-0100-RE
Keinath AP (2013) Susceptibility of cucurbit rootstocks to Didymella bryoniae and control of gummy stem blight on grafted watermelon seedlings with fungicides. Plant Dis 97(8):1018–1024. https://doi.org/10.1094/PDIS-12-12-1133-RE
Khah EM, Kakava E, Mavromatis A, Chachalis D, Goulas C (2006) Effect of grafting on growth and yield of tomato (Lycopersicon esculentum Mill.) in greenhouse and open-field. J Appl Hortic 8(1):3–7
King SR, Davis AR, Zhang X, Crosby K (2010) Genetics, breeding and selection of rootstocks for Solanaceae and Cucurbitaceae. Sci Hortic 127(2):106–111. https://doi.org/10.1016/j.scienta.2010.08.001
Kousik CS, Mandal M, Hassell R (2018) Powdery mildew resistant rootstocks that impart tolerance to grafted susceptible watermelon scion seedlings. Plant Dis 102(7):1290–1298. https://doi.org/10.1094/PDIS-09-17-1384-RE
Kousik CS, Thies JA (2010) Response of US bottle gourd (Lagenaria siceraria) plant introductions (PI) to crown rot caused by Phytophthora capsici. Phytopathology 100:65
Kubota C, Mcclure MA, Kokalis-Burelle N, Bausher MG, Rosskopf EN (2008) Vegetable grafting: history, use, and current technology status in North America. Hortic Sci 43(6):1664–1669. https://doi.org/10.21273/HORTSCI.43.6.1664
Kumar P, Rouphael Y, Cardarelli M, Colla G (2017) Vegetable grafting as a tool to improve drought resistance and water use efficiency. Front Plant Sci 8:1130
Kumar RV, Sagar RL, Ahuja AN, Karthick SK, Mehta A, Rajesh Saini R (2018) Vegetable grafting: a recent advance in olericulture: a review. Int J Curr Microbiol App Sci 7(09):1877–1882
Kyriacou MC, Rouphael Y, Colla G, Zrenner RM, Schwarz D (2017) Vegetable grafting: the implications of agrowing agronomic imperative for vegetable fruit quality and nutritive value. Front Plant Sci 8:741
Kyriacou MC, Soteriou GA, Rouphael Y, Siomos AS, Gerasopoulos D (2016) Configuration of watermelon fruit quality in response to rootstock-mediated harvest maturity and postharvest storage. J Sci Food Agric 96:2400–2409
Lee JM (1994) Cultivation of Grafted Vegetables I. Current status, grafting methods, and benefits. Horticulture. Science 29(4):235–239. https://doi.org/10.21273/HORTSCI.29.4.235
Lee JM, Oda M (2003) Grafting of herbaceous vegetable and ornamental crops. In: Janick J (ed) Horticultural reviews, vol 28. Wiley, New York, NY, pp 61–124. https://doi.org/10.1002/9780470650851.ch2
Lee JM, Kubota C, Tsao SJ, Bie Z, Echevarria PH, Morra L, Oda M (2010) Current status of vegetable grafting: diffusion, grafting techniques, automation. Sci Hortic 127:93–105. https://doi.org/10.1016/j.scienta.2010.08.003
Leonardi C (2016) Vegetable grafting tour introduction. University of Catania, Sicily, Italy, p 23
Lewsey MG, Hardcastle TJ, Melnyk CW, Molnar A, Valli A, Urich MA (2016) Mobile small RNAs regulate genome-wide DNA methylation. Proc Natl Acad Sci U S A 113:E801–E810. https://doi.org/10.1073/pnas.1515072113
Li C, Li Y, Bai L, Zhang T, He C, Yan Y (2014) Grafting-responsive miRNAs in cucumber and pumpkin seedlings identified by high-throughput sequencing at whole genome level. Phys Plant 151:406–422. https://doi.org/10.1111/ppl.12122
Li Y, Li C, Bai L, He C, Yu X (2016) MicroRNA and target gene responses to salt stress in grafted cucumber seedlings. Acta Phys Plant 38:42. https://doi.org/10.1007/s11738-016-2070-5
Liu S, Li H, Lv X, Ahammed GJ, Xia X, Zhou J, Shi K, Asami T, Yu J, Zhou Y (2016) Grafting cucumber onto luffa improves drought tolerance by increasing ABA biosynthesis and sensitivity. Sci Rep 6:202–212. https://doi.org/10.1038/srep20212
Louws FJ, Rivard CL, Kubota C (2010) Grafting fruiting vegetables to manage soilborne pathogens, foliar pathogens, arthropods and weeds. Sci Hortic 127:127–146. https://doi.org/10.1016/j.scienta.2010.09.023
Martínez-Andújar C, Ruiz-Lozano JM, Dodd IC, Albacete A, Pérez-Alfocea F (2017) Hormonal and nutritional features in contrasting rootstock-mediated tomato growth under low-phosphorus nutrition. Front Plant Sci 8:533. https://doi.org/10.3389/fpls.2017.00533
Marucci A, Pagniello B, Monarca D, Colantoni A, Biondi P, Cecchini M (2012) The heat stress for workers during vegetable grafting in greenhouses. In: International Conference RAGUSA SHWA. Ragusa, Italy. pp. 321–328
Maurya D, Pandey AK, Kumar V, Dubey S, Prakash V (2019) Grafting techniques in vegetable crops: a review. Int J Chem Stud 7(2):1664–1672
Melnyk CW, Molnar A, Bassett A, Baulcombe DC (2011) Mobile 24 nt small RNAs direct transcriptional gene silencing in the root meristems of Arabidopsis thaliana. Curr Biol 21:1678–1683. https://doi.org/10.1016/j.cub.2011.08.065
Miles C, Wimer J, Inglis D (2015) Grafting eggplant and tomato for Verticillium wilt resistance. Acta Hort 1086:113–118. https://doi.org/10.17660/ActaHortic.2015.1086.13
Miskovic A, Ilic O, Bacanovic J, Vujasinovic V, Kukic B (2016) Effect of eggplant rootstock on yield and quality parameters of grafted tomato. Acta Sci Pol Hortic 15:149–159
Mohamed FH, El-Hamed KEA, Elwan MWM, Hussien MNE (2014) Evaluation of different grafting methods and rootstocks in watermelon grown in Egypt. Sci Hortic 168:145–150. https://doi.org/10.1016/j.scienta.2014.01.029
Molnar A, Melnyk CW, Bassett A, Hardcastle TJ, Dunn R, Baulcombe DC (2010) Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 328:872–875. https://doi.org/10.1126/science.1187959
Mozafarian M, Ismail NSB, Kappel N (2020) Rootstock effects on yield and some consumer important fruit quality parameters of eggplant cv. ‘Madonna’ under protected cultivation. Agronomy 10(9):1442
Nakaho K, Hibino H, Miyagawa H (2000) Possible mechanisms limiting movement of Ralstonia solanacearum in resistant tomato tissues. J Phytopathol 148(3):181–190. https://doi.org/10.1046/j.1439-0434.2000.00476.x
Nakamura S, Hondo K, Kawara T, Okazaki Y, Saito K, Kobayashi K (2015) Conferring high-temperature tolerance to nontransgenic tomato scions using graft transmission of RNA silencing of the fatty acid desaturase gene. Plant Biotechnol J 14:783–790. https://doi.org/10.1111/pbi.12429
NARO (2011) Current status and issues of vegetable grafting. National Agricultural Research Organization, Research Institute of Vegetable and Tea, p 147
Nemati Z, Banihashemi Z (2015) Reaction of different Cucurbita species to Phytophthora capsici, P. melonis and P. drechsleri under greenhouse conditions. J Crop Protect 4(20):705–709
Nisini PT, Colla G, Granati E, Temperini O, Crino P, Saccardo F (2002) Rootstock resistance to fusarium wilt and effect on fruit yield and quality of two muskmelon cultivars. Sci Hortic 93(3–4):281–288. https://doi.org/10.1016/S0304-4238(01)00335-1
Noor RS, Wang Z, Umair M, Yaseen M, Ameen M, Rehman SU, Sun Y (2019) Interactive effects of grafting techniques and scion-rootstocks combinations on vegetative growth, yield and quality of cucumber (Cucumis sativus L.). Agronomy 9(6):288
Oda M (2006) Vegetable seedling grafting in Japan. In: XXVII international horticultural congress-IHC2006: global horticulture: diversity and harmony, an introduction to IHC2006 759 (pp. 175–180)
Oka Y, Offenbach R, Pivonia S (2004) Pepper rootstock graft compatibility and response to Meloidogyne javanica and M. incognita. J Nematol 36:137–141
Oumouloud A, Arnedo-Andrés MS, González-Torres R, Alvarez JM (2010) Inheritance of resistance to Fusarium oxysporum f. sp. melonis races 0 and 2 in melon accession Tortuga. Euphytica 176(2):183–189. https://doi.org/10.1007/s10681-010-0201-4
Owusu SB, Kwoseh CK, Starr JL, Davies FT (2016) Grafting for management of root-knot nematodes, Meloidogyne incognita, in tomato (Solanum lycopersicum L.). Nematropica 46(1):14–21
Pandey P, Irulappan V, Bagavathiannan MV, Senthil-Kumar M (2017) Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front Plant Sci 8:537
Pant BD, Buhtz A, Kehr J, Scheible W (2008) MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J 53:731–738. https://doi.org/10.1111/j.1365-313X.2007.03363.x
Paplomatas EJ, Elena K, Tsagkarakou A, Perdikaris A (2000) Control of Verticillium wilt of tomato and cucurbits through grafting of commercial varieties on resistant rootstocks. Acta Hortic 579:445–449. https://doi.org/10.17660/ActaHortic.2002.579.77
Park DK, Son SH, Kim S, Lee WM, Lee HJ, Choi HS, Yang EY, Chae WB, Ko HC, Huh YC (2013) Selection of melon genotypes with resistance to Fusarium wilt and Monosporascus root rot for rootstocks. Plant Breed Biotechnol 1(3):277–282. https://doi.org/10.9787/PBB.2013.1.3.277
Pech JC, Bouzayen M, Latché A (2008) Climacteric fruit ripening: ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Sci 175:114–120. https://doi.org/10.1016/j.plantsci.2008.01.003
Pina AY, Errea P (2005) A review of new advances in mechanism of graft compatibility–incompatibility. Sci Hortic 106:1–11
Polizzi G, Guarnaccia V, Vitale A, Marra M, Rocco M, Arena S, Scaloni A, Giuffrida F, Cassaniti C, Leonardi C (2015) Scion/rootstock interaction and tolerance expression of tomato to FORL. Acta Hortic 1086:189–194. https://doi.org/10.17660/ActaHortic.2015.1086.23
Poudyala D, Khatria L, Uptmoora R (2015) An introgression of Solanum habrochaites in the rootstock improves stomatal regulation and leaf area development of grafted tomatoes under drought and low root-zone-temperatures. Adv Crop Sci Technol 3(3):1–11
Proietti S, Rouphael Y, Colla G, Cardarelli M, De Agazio M, Zacchini M, Moscatello S, Battistelli A (2008) Fruit quality of mini-watermelon as affected by grafting and irrigation regimes. J Sci Food Agric 88:1107–1114
Rivard CL, Louws FJ (2011) Tomato grafting for disease resistance and increased productivity. Sustainable Agriculture Resserach and Education.(SARE) Factsheet, GS05–046
Rivard CL, O’connell S, Peet MM, Welker RM, Louws FJ (2012) Grafting tomato to manage bacterial wilt caused by Ralstonia solanacearum in the southeastern United States. Plant Dis 96(7):973–978. https://doi.org/10.1094/PDIS-12-10-0877
Röös E, Bajželj B, Smith P, Patel M, Little D, Garnett T (2017) Greedy or needy? Land use and climate impacts of food in 2050 under different livestock futures. Glob Environ Chang 47:1–12. https://doi.org/10.1016/j.gloenvcha.2017.09.001
Rouphael Y, Cardarelli M, Colla G, Rea E (2008) Yield, mineral composition, water relations, and water use efficiency of grafted mini-watermelon plants under deficit irrigation. Hortic Sci 43(3):730–736
Sabatino L, Iapichino G, Rotino GL, Palazzolo E, Mennella G, D’Anna F (2019) Solanum aethiopicum gr. gilo and its interspecific hybrid with S. melongena as alternative rootstocks for eggplant: effects on vigor, yield, and fruit physicochemical properties of cultivar ‘Scarlatti’. Agronomy 9(5):223
Sakata Y, Ohara T, Sugiyama M (2008) The history of melon and cucumber grafting in Japan. Acta Hortic 767:217–228. https://doi.org/10.17660/ActaHortic.2008.767.22
Sánchez-Rodríguez E, Leyva R, Constán-Aguilar C, Romero L, Ruiz JM (2012) Grafting under water stress in tomato cherry: improving the fruit yield and quality. Ann Appl Biol 161(3):302–312
Savvas D, Colla G, Rouphael Y, Schwarz D (2010) Amelioration of heavy metal and nutrient stress in fruit vegetables by grafting. Sci Hortic 127(2):156–161
Schwarz D, Rouphael Y, Colla G, Venema JH (2010) Grafting as a tool to improve tolerance of vegetables to abiotic stresses: thermal stress, water stress and organic pollutants. Sci Hortic 127:162–171. https://doi.org/10.1016/j.scienta.2010.09.016
Shishido M (2014) Black root rot caused by Diaporthe sclerotioides threatens cucurbit cultivation in Japan. Adv Hortic Sci:208–213
Siamak SB, Paolo S (2019) Responses of grafted watermelon onto Cucurbita pepo Tiana F1 hybrid to boron nutritional disorders. Hortic Plant J 5(5):213–220. https://doi.org/10.1016/j.hpj.2019.07.003
Sigüenza C, Schochow M, Turini T, Ploeg A (2005) Use of Cucumis metuliferus as a rootstock for melon to manage Meloidogyne incognita. J Nematol 37(3):276
Singh K (2021) Vegetable grafting: a tool for improving quality and yield of crop. Biotica Res Today 3(5):399–401
Spanò R, Ferrara M, Gallitelli D, Mascia T (2020) The role of grafting in the resistance of tomato to viruses. Plan Theory 9(8):1042
Spanò R, Mascia T, Kormelink R, Gallitelli D (2015) Grafting on a non-transgenic tolerant tomato variety confers resistance to the infection of a sw5-breaking strain of tomato spotted wilt virus via RNA silencing. PLoS One 10(10):e0141319. https://doi.org/10.1371/journal.pone.0141319
Spiegelman Z, Golan G, Wolf S (2013) Don’t kill the messenger: longdistance trafficking of mRNA molecules. Plant Sci 213:1–8. https://doi.org/10.1016/j.plantsci.2013.08.011
Sugiyama M, Sakata Y, Ohara T (2006) The history of melon and cucumber grafting in Japan. In: XXVII international horticultural congress-IHC2006: international symposium on sustainability through integrated and organic 767 (pp. 217–228)
Sun Z, Lin CC, Gu XF, Zhang MY (2009) Progress of cucurbit grafting in China. In: 4th International Cucurbitaceae Symposium, 21–26 September 2009, Changsha, Hunan, China
Thakur D (2020) Role of grafting in vegetable crops: a review. J Pharmacogn Phytochem 9(6):1170–1174
Thies JA, Ariss JJ, Hassell RL, Olson S, Kousik CS, Levi A (2010) Grafting for management of southern root-knot nematode, Meloidogyne incognita, in watermelon. Plant Dis 94(10):1195–1199. https://doi.org/10.1094/PDIS-09-09-0640
Tsaballa A, Pasentsis K, Darzentas N, Tsaftaris AS (2011) Multiple evidence for the role of an ovate-like gene in determining fruit shape in pepper. BMC Plant Biol 11:46. https://doi.org/10.1186/1471-2229-11-46
Turnbull CG, Lopez-Cobollo RM (2013) Heavy traffic in the fast lane: long-distance signalling by macromolecules. New Phytol 198:33–51. https://doi.org/10.1111/nph.12167
Ventura JA, Lima IDM, Martins MVV, Culik MP, Costa H (2019) Impact and management of diseases in the propagation of fruit plants. Rev Bras Frutic 41(4):647. https://doi.org/10.1590/0100-29452019647
Vitale A, Rocco M, Arena S, Giuffrida F, Cassaniti C, Scaloni A, Lomaglio T, Guarnaccia V, Polizzi G, Marra M, Leonardi C (2014) Tomato susceptibility to Fusarium crown and root rot: effect of grafting combination and proteomic analysis of tolerance expression in the rootstock. Plant Physiol Biochem 83:207–216. https://doi.org/10.1016/j.plaphy.2014.08.006
Wang J, Wang X, Lin L, Liao MA, Liu J, Tang Y, Jiang W (2021) Effects of different rootstocks on the growth and cadmium-accumulation characteristics of a post-grafting generation of Cyphomandra betacea seedlings. Int J Environ Anal Chem 101(3):370–378
Wang J, Zhang D, Fang Q (2002) Studies on antivirus disease mechanism of grafted seedless watermelon. J Anhui Agric College 29(4):336–339
Wehner TC, Shetty NV (1997) Downy mildew resistance of the cucumber germplasm collection in North Carolina field tests. Hortic Sci 32(3):450B. https://doi.org/10.21273/HORTSCI.32.3.450B
Wu R, Wang X, Lin Y, Ma Y, Liu G, Yu X (2013) Inter-species grafting caused extensive and heritable alterations of DNA methylation in solanaceae plants. PLoS One 8:e61995. https://doi.org/10.1371/journal.pone.0061995
Xanthopoulou A, Tsaballa A, Ganopoulos I, Kapazoglou A, Avramidou E, Aravanopoulos FA (2019) Ιntra-species grafting induces epigenetic and metabolic changes accompanied by alterations in fruit size and shape of Cucurbita pepo L. Plant Growth Regul 87:93–108. https://doi.org/10.1007/s10725-018-0456-7
Yetisir H, Erhan A (2013) Rootstocks effect on plant nutrition concentration in different organ of grafted watermelon. Agric Sci 5:4
Yetisir H, Sari N (2003) Effect of different rootstock on plant growth, yield and quality of watermelon. Aust J Exp Agric 43:1269–1274. https://doi.org/10.1071/EA02095
Yetisri H, Sari N (2004) Effect of hypocotyl morphology on survival rate and growth of watermelon seedlings grafted on rootstocks with different emergence performance at various temperatures. Turk J Agric For 28(4):231–237
Zhang S, Gu X, Wang Y (2006) Effect of bur cucumber (Sicyos angulatus L.) as rootstock on growth physiology and stress resistance of cucumber plants. Acta Hortic Sinica 33(6):1231–1236
Zhang ZK, Liu SQ, Hao SQ, Liu SH (2010) Grafting increases the copper tolerance of cucumber seedlings by improvement of polyamine contents and enhancement of antioxidant enzymes activity. Agric Sci China 9(7):985–994
Zhang Z, Liu Y, Cao B, Chen Z, Xu K (2020) The effectiveness of grafting to improve drought tolerance in tomato. Plant Growth Regul 91:157–167. https://doi.org/10.1007/s10725-020-00596-2
Zhou X, Wu Y, Chen S, Chen Y, Zhang W, Sun X, Zhao Y (2014) Using Cucurbita rootstocks to reduce fusarium wilt incidence and increase fruit yield and carotenoid content in oriental melons. Hortic Sci 49(11):1365–1369. https://doi.org/10.21273/HORTSCI.49.11.1365
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Majhi, P.K. et al. (2023). Understanding the Genetics and Genomics of Vegetable Grafting to Ensure Yield Stability. In: Singh, S., Sharma, D., Sharma, S.K., Singh, R. (eds) Smart Plant Breeding for Vegetable Crops in Post-genomics Era . Springer, Singapore. https://doi.org/10.1007/978-981-19-5367-5_4
Download citation
DOI: https://doi.org/10.1007/978-981-19-5367-5_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5366-8
Online ISBN: 978-981-19-5367-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)