Abstracts
Sex has been traditionally considered to be classified into two categories, male and female. However, numerous reports have shown examples of insects unfamiliar to this traditional binary sex view. Recently, the view of the sex spectrum has been proposed as a revised version. In this view, sex is recognised as a continuum from male to female (or female to male), and maleness or femaleness of any sexual traits is quantitatively interpreted as ‘a position on the continuum of sex’. This chapter discusses the molecular genetic mechanism defining a position on the continuum of sex based on the knowledge about the Japanese rhinoceros beetle Trypoxylus dichotomus.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Most insect species show sexual dimorphism, and their appearances are distinctively different between males and females. For example, in some beetles, males have magnificently developed mandibles, while females do not develop well. Based on the facts like this, sex has been traditionally considered to be classified into two categories, male and female. However, to date, numerous individuals cannot be classified as male or female by their appearances. For example, in damselflies, some females show male-like coloration though they produce typical female traits except for the coloration (Gossum and Sherratt 2008). Other examples are the sexually mosaic phenotypes (gynandromorph) and sexually intermediate phenotypes (intersex) accidentally produced by developmental abnormalities (Narita et al. 2010). These examples are not included in the traditionally binary view of sex, which needs to be revised (Nong et al. 2020).
The sex spectrum is a view of recognising sex as a continuum from male to female (or from female to male) (Nong et al. 2020; Preface). In this view, the gynandromorph and the intersex are interpreted to be placed between male and female in the sex spectrum. Furthermore, male-like female coloration in damselflies may be placed between midpoint and female. Thus, almost all differences among sexual traits in insects can be explained as differences in the location on the sex spectrum.
How is the location of the sex spectrum defined in insects? In the fruit fly Drosophila melanogaster, a major sex-determining gene doublesex (dsx) mutation yields the gynandromorph and the intersex in both females and males (Hildreth 1965). It is well known that dsx is essential for sexual differentiation not only in D. melanogaster but also in other holometabolous insects. These facts indicate that genetic mechanisms for sex determination have a considerable effect on determining insects’ location on the sex spectrum.
Here, we first overview the sex-determining mechanism in holometabolous insects. Then, based on an example of the Japanese rhinoceros beetle, we discuss a molecular mechanism to determine the location in the sex spectrum.
2 Sex-Determining Molecular Mechanism in Holometabolous Insects
This subsection first introduces a sex-determining molecular mechanism in the D. melanogaster, the most investigated model insect. Moreover, we also show the commonality of the sex-determining mechanism.
The primary signal for sex determination is the number of the X chromosome (Erickson and Quintero 2007). The sex of an individual with two X chromosomes, i.e., an XX individual, is finally determined as female; on the other hand, the sex of an individual with a single X chromosome (i.e., XY individual) is finally determined as male as a default state.
The initial signals from X chromosomes produce the sex-lethal (Sxl) protein only in XX early embryos (Cline 1978, 1986, 1988; Kramer et al. 1999; Sefton et al. 2000; Penalva and Sánchez 2003; Salz 2007). Sxl autoregulates and maintains the expression of another Sxl isoform (Cline 1984; Bell et al. 1988, 1991; Penalva and Sánchez 2003). The functional Sxl protein controls the RNA splicing of tra, and functional Tra protein is translated (Boggs et al. 1987; Bell et al. 1988; Penalva and Sánchez 2003). Then, Tra protein yields female-specific Dsx (DsxF) by regulating alternative splicing of dsx mRNA, which leads to female differentiation (Hoshijima et al. 1991).
On the other hand, in XY embryos, a non-functional Sxl protein is produced because of the lack of the initial signal from the X chromosome (Bell et al. 1988; Samuels et al. 1991; Keyes et al. 1992; Penalva and Sánchez 2003). Lack of functional Sxl leads to expression of non-functional Tra protein, and a male-specific Dsx (DsxM) is translated through lack of Tra-dependent splicing regulation, which leads to male differentiation (Bell et al. 1988).
Whether the sex-determining mechanism in Drosophila is conserved in other holometabolous insects has been recently studied by focusing on non-Drosophila insects. Sxl is a ‘master switch’ gene for sex determination in D. melanogaster. Sxl orthologues have been found in many holometabolous insects, including Diptera, Lepidoptera, Hymenoptera and Coleoptera (Traut et al. 2006). However, some dipteran species’ research revealed that Sxl orthologues are not responsible for sex determination (Meise et al. 1998; Saccone et al. 1998; Sievert et al. 2000). Furthermore, the Sxl orthologue in the silkworm Bombyx mori does not contribute to sex determination but regulates spermatogenesis (Niimi et al. 2006; Sakai et al. 2019). Therefore, Sxl is not a broadly conserved sex determination gene in holometabolous insects.
tra is an intermediate factor in the sex determination cascade of D. melanogaster. Tra orthologues are identified in holometabolous insects such as some species in Diptera, Coleoptera and Hymenoptera (O'Neil and Belote 1992; Pane et al. 2002; Kulathinal et al. 2003; Lagos et al. 2007; Ruiz et al. 2007; Hasselmann et al. 2008; Concha and Scott 2009; Schmieder et al. 2012; Shukla and Palli 2012; Geuverink and Beukeboom 2014; Morita et al. 2019). Furthermore, in some of these species, tra orthologues regulate female determination (Pane et al. 2002; Hasselmann et al. 2008; Concha and Scott 2009; Hediger et al. 2010; Shukla and Palli 2012; Morita et al. 2019). On the other hand, tra orthologues seem to have been lost in Lepidoptera and in some species in Strepsiptera and Diptera (Salvemini et al. 2013; Geuverink and Beukeboom 2014). These findings indicated that although tra orthologues are not found in some species, the sex-determining function of tra is conserved in a much more comprehensive range of holometabolous insect species than that of Sxl.
dsx is a bottom factor in the sex determination cascade of D. melanogaster, which directly regulates the transcription of a battery of genes responsible for sex differentiation. dsx orthologues are conserved in all of the insects investigated so far (Price et al. 2015). Furthermore, the dsx has sex-specific transcripts and contributes to sex determination in the various holometabolous insects such as Diptera, Lepidoptera, Hymenoptera and Coleoptera (Schütt and Nöthiger 2000; Ohbayashi et al. 2001; Cho et al. 2007; Chen et al. 2008; Oliveira et al. 2009; Shukla and Palli 2012; Ito et al. 2013; Mine et al. 2017; Taracena et al. 2019). Therefore, dsx is the conserved regulatory factor in the sex determination cascade among holometabolous insects.
The above comparative analysis focusing on the conserved genes associated with the insect sex determination pathway suggests that downstream genes tra and dsx are the core regulatory genes conserved among holometabolous insects. Therefore, to understand the determination mechanism of position in the sex spectrum in holometabolous insects, we focus on the role of tra and dsx in sexual traits. In the next section, we discuss the role of tra and dsx in the sex spectrum using the Japanese rhinoceros beetle, in which we can manipulate the location in the sex spectrum by controlling the expression level of sex-determining genes using RNA interference (RNAi) methods.
3 Sex Spectrum in T. dichotomus Horn Visualised by Manipulating the Sex Determination Pathway
The horn of the Japanese rhinoceros beetle Trypoxylus dichotomus (Coleoptera, Scarabaeoidea, Scarabaeidae) exhibits sexual dimorphism (Fig. 1.1a, EGFP). A male has an exaggerated long horn on the head and a short horn on the first thoracic segment (pronotum). On the other hand, a female has no horn neither on the head nor on the pronotum but has three small projections in the rostral region of the head (clypeolabral region).
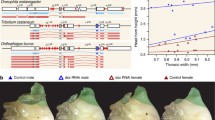
RNAi-mediated loss of function of sex-determining genes and morphological change of horn primordia. (a) Representative individuals in dsx and tra RNAi treatment in males and females. EGFP RNAi treatment (negative control) showed no morphological defects. The upper row, the dorsal views of adults. The second row, the lateral views of adults. Scale bars are 1 cm. (b) Sex-specific splicing of dsx in RNAi treatments targeting tra. RpL32 was used as an internal control for RT-PCR. Blue arrowheads, male-specific splicing patterns (dsxM). Magenta arrowheads, female-specific splicing patterns (dsxF). Black arrowheads, RpL32. (Adapted from Morita et al. PLOS Genet., 15: e1008063, 2019)
As described in the previous sections, sex-specific tra and dsx isoforms are essential genes to a sex-differentiating mechanism in holometabolous insects. In T. dichotomus, downregulation of dsx expression by RNAi results in short head horns in both males and females, while thoracic horns form in neither males nor females (Fig. 1.1a) (Ito et al. 2013). tra RNAi males showed no morphological changes, while females developed ectopic male-like horns on both the head and pronotum (Fig. 1.1a) (Morita et al. 2019). These facts indicated that in T. dichotomus, Tra and Dsx regulate sexual dimorphism in a horn (Fig. 1.1) (Morita et al. 2019).
Next, Morita et al. (2019) insufficiently suppressed tra expression levels in females. As a result, female-like and male-like traits coexist in a single tra RNAi female (Fig. 1.2) because tra regulates sex-specific splicing of dsx like other holometabolous insects (Fig. 1.1b) (Morita et al. 2019), and insufficient suppression of tra expression in tra RNAi females leads to incomplete switching from dsxF to dsxM.
tra RNAi phenotype induced by injection of small amounts of tra dsRNA. (a–c) Comparison of a wild-type female and ectopic intermediate sexual transformation of horns in females induced by tra RNAi treatments. (a) A wild-type female. (b, c) Small ectopic horn formation in tra RNAi females. Magenta arrowheads, three small protrusions formed in clypeolabrum. Green arrowheads, ectopic head horns formed in the region anterior to the three small protrusions in tra RNAi treatments. Scale bars are 5 mm. (d) Relationship between the head horn (left) and thoracic horn (right) length and body size in RNAi-treated individuals. The head and thoracic horn lengths and body sizes of control males (EGFP, blue dots), dsx RNAi-treated females (grey dots), and tra RNAi-treated females (green dots: b magenta diamond, c magenta hexagon) are plotted. HH, head horn; TH, thoracic horn. (Adapted from Morita et al. PLOS Genet., 15: e1008063, 2019)
Detailed observation of the tra RNAi phenotypes in Fig. 1.2 revealed that the degree of similarity to males is different between head and pronotum regions. This suggests that the location on the sex spectrum at the tissue level is different between head and pronotum regions. For example, in the head region, tra RNAi individuals show female-specific traits (three small projections) and male-specific traits (head horns) (Fig. 1.2b, c). However, in one individual (Fig. 1.2b), the ectopic head horn branched twice, forming a short stalk of head horns. On the other hand, the ectopic head horn branched only once in the other individual (Fig. 1.2c), and no stalk was formed. This suggests that the former phenotype was more similar to male-specific traits than the latter phenotype. Therefore, the locations on the sex spectrum of these tra RNAi phenotypes in the head region can be explained as shown in Fig. 1.3a.
Sex spectrum of T. dichotomus estimated from tra RNAi phenotypes. (a) The sex spectrum at the tissue level in the head region. (b) The sex spectrum at the tissue level in the pronotum region. The shorter head and thoracic horns in tra RNAi females than in control males may be due to differences in body size between males and females at the timing of injection. (c) The individual-level sex spectrum is understood from the sum of tissue-level sex spectrums. Blue dots, GFP RNAi male; magenta dots, GFP RNAi female; green diamond, tra RNAi female 1 (Fig. 1.2b); green square, tra RNAi female 2 (Fig. 1.2c). (Adapted from Morita et al. PLOS Genet., 15: e1008063, 2019)
Next, in the pronotum region, only the tra RNAi phenotype in Fig. 1.2b was that the ectopic thoracic horn was apparently like a male thoracic horn (Fig. 1.2d). The thoracic horn length is similar to that of a tra RNAi female injected with a sufficient amount of dsRNA (Fig. 1.2d). In contrast to the head horn in tra RNAi females, the incomplete formation of the thoracic horn has not been observed so far. This observation suggests that the thoracic horn phenotype is located closely at either endpoint on the sex spectrum (Fig. 1.3b).
These observations indicate that even within a single individual, the locations on the sex spectrums at the tissue level are tissue-dependent. Then, what mechanisms produce these differences? One possibility is a difference in the role of the sex-specific dsx isoforms among tissues. In the head region, male and female traits (the head horn and the three small projections, respectively) coexist in a single tra RNAi female (Fig. 1.2). In T. dichotomus, the dsx RNAi phenotype in both males and females showed the head horn was formed, albeit short (Fig. 1.1a). This head horn phenotype suggests that dsxM promotes the expression of the horn formation genes and dsxF represses (Ito et al. 2013). In Fig. 1.2, it is considered that dsxF and dsxM coexist in a single individual due to the insufficient suppression of tra expression. The antagonistic effects of dsxM and dsxF on the expression of horn formation genes might define the degree of similarity to males in the head region. In a region of the clypeolabrum where dsxF and dsxM coexist (Fig. 1.2b and c), dsxM functions to promote head horn formation, whereas dsxF functions to suppress head horn formation; therefore, this antagonistic effect would define the location on the sex spectrum at the tissue level in the head region. Conversely, male and female traits did not coexist in a tra RNAi female’s thoracic horn (Fig. 1.2). In the dsx RNAi phenotype, the thoracic horn was not formed in either males or females. These phenotypes indicate that dsxM promotes thoracic horn formation, while dsxF does not contribute to thoracic horn formation. This fact means that thoracic horn formation is regulated depending on the only dsxM. In other words, in tra RNAi females with insufficiently suppressed tra expression, when the expression level of dsxM exceeds a certain threshold, thoracic horn formation is promoted; otherwise, the horn is not formed. Therefore, the phenotype of the thoracic horn by tra RNAi (Fig. 1.2) is considered to be located at either endpoint on the sex spectrum (Fig. 1.3b).
This section described how sex is interpreted as a spectrum of continuous phenotypes in T. dichotomus, using the horn as an indicator of sex by manipulating the expression level of a sex-determining gene tra. In addition, the sex spectrum at the tissue level showed different locations in the head and pronotum regions. In the concept of sex spectrum, the sum of the sex spectrum at the tissue level can be understood as the sex spectrum at the individual level (Preface). Therefore, the location of the sex spectrum at the individual level for tra RNAi in T. dichotomus could be shown as in Fig. 1.3c. From the view of the sex spectrum, rather than the traditional binary sex view, it can be uniformly explained for individuals that cannot be classified by their appearance, as in Fig. 1.2.
References
Bell LR, Maine EM, Schedl P, Cline TW (1988) Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell 55:1037–1046
Bell LR, Horabin JI, Schedl P, Cline TW (1991) Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in drosophila. Cell 65:229–239
Boggs RT, Gregor P, Idriss S, Belote JM, McKeown M (1987) Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell 50:739–747
Chen SL, Dai SM, Lu KH, Chang C (2008) Female-specific doublesex dsRNA interrupts yolk protein gene expression and reproductive ability in oriental fruit fly, Bactrocera dorsalis (Hendel). Insect Biochem Mol Biol 38:155–165
Cho S, Huang ZY, Zhang J (2007) Sex-specific splicing of the honeybee doublesex gene reveals 300 million years of evolution at the bottom of the insect sex-determination pathway. Genetics 177:1733–1741
Cline TW (1978) Two closely linked mutations in Drosophila melanogaster that are lethal to opposite sexes and interact with daughterless. Genetics 90:683–697
Cline TW (1984) Autoregulatory functioning of a drosophila gene product that establishes and maintains the sexually determined state. Genetics 107:231–277
Cline TW (1986) A female-specific lethal lesion in an X-linked positive regulator of the Drosophila sex determination gene, sex-lethal. Genetics 113:641–663
Cline TW (1988) Evidence that sisterless-a and sisterless-b are two of several discrete "numerator elements" of the X/a sex determination signal in drosophila that switch Sxl between two alternative stable expression states. Genetics 119:829–862
Concha C, Scott MJ (2009) Sexual development in Lucilia cuprina (Diptera, Calliphoridae) is controlled by the transformer gene. Genetics 182:785–798
Erickson JW, Quintero JJ (2007) Indirect effects of ploidy suggest X chromosome dose, not the X: a ratio, signals sex in drosophila. PLoS Biol 5:e332
Geuverink E, Beukeboom LW (2014) Phylogenetic distribution and evolutionary dynamics of the sex determination genes doublesex and transformer in insects. Sex Dev 8:38–49
Gossum H, Sherratt TN (2008) A dynamic model of sexual harassment in damselflies and its implications for female-limited polymorphism. Ecol Model 210:212–220
Hasselmann M, Gempe T, Schiøtt M, Nunes-Silva CG, Otte M, Beye M (2008) Evidence for the evolutionary nascence of a novel sex determination pathway in honeybees. Nature 454:519–522
Hediger M, Henggeler C, Meier N, Perez R, Saccone G, Bopp D (2010) Molecular characterization of the key switch F provides a basis for understanding the rapid divergence of the sex-determining pathway in the housefly. Genetics 184:155–170
Hildreth PE (1965) Doublesex, recessive gene that transforms both males and females of drosophila into intersexes. Genetics 51:659–678
Hoshijima K, Inoue K, Higuchi I, Sakamoto H, Shimura Y (1991) Control of doublesex alternative splicing by transformer and transformer-2 in drosophila. Science 252:833–836
Ito Y, Harigai A, Nakata M, Hosoya T, Araya K, Oba Y, Ito A, Ohde T, Yaginuma T, Niimi T (2013) The role of doublesex in the evolution of exaggerated horns in the Japanese rhinoceros beetle. EMBO Rep 14:561–567
Keyes LN, Cline TW, Schedl P (1992) The primary sex determination signal of drosophila acts at the level of transcription. Cell 68:933–943
Kramer SG, Jinks TM, Schedl P, Gergen JP (1999) Direct activation of sex-lethal transcription by the drosophila runt protein. Development 126:191–200
Kulathinal RJ, Skwarek L, Morton RA, Singh RS (2003) Rapid evolution of the sex-determining gene, transformer: structural diversity and rate heterogeneity among sibling species of drosophila. Mol Biol Evol 20:441–452
Lagos D, Koukidou M, Savakis C, Komitopoulou K (2007) The transformer gene in Bactrocera oleae: the genetic switch that determines its sex fate. Insect Mol Biol 16:221–230
Meise M, Hilfiker-Kleiner D, Dubendorfer A, Brunner C, Nothiger R, Bopp D (1998) Sex-lethal, the master sex-determining gene in Drosophila, is not sex-specifically regulated in Musca domestica. Development 125:1487–1494
Mine S, Sumitani M, Aoki F, Hatakeyama M, Suzuki MG (2017) Identification and functional characterization of the sex-determining gene doublesex in the sawfly, Athalia rosae (Hymenoptera: Tenthredinidae). Appl Entomol Zool 52:497–509
Morita S, Ando T, Maeno A, Mizutani T, Mase M, Shigenobu S, Niimi T (2019) Precise staging of beetle horn formation in Trypoxylus dichotomus reveals the pleiotropic roles of doublesex depending on the spatiotemporal developmental contexts. PLoS Genet 15:e1008063
Narita S, Pereira RAS, Kjellberg F, Kageyama D (2010) Gynandromorphs and intersexes: potential to understand the mechanism of sex determination in arthropods. Terr Arthropod Rev 3:63–96
Niimi T, Sahara K, Oshima H, Yasukochi Y, Ikeo K, Traut W (2006) Molecular cloning and chromosomal localization of the bombyx sex-lethal gene. Genome 49:263–268
Nong QD, Matsuura T, Kato Y, Watanabe H (2020) Two Doublesex1 mutants revealed a tunable gene network underlying intersexuality in Daphnia magna. PLoS One 15:e0238256
Ohbayashi F, Suzuki MG, Mita K, Okano K, Shimada T (2001) A homologue of the drosophila doublesex gene is transcribed into sex-specific mRNA isoforms in the silkworm, Bombyx mori. Comp Biochem Physiol B Biochem Mol Biol 128:145–158
Oliveira DC, Werren JH, Verhulst EC, Giebel JD, Kamping A, Beukeboom LW, Van De Zande L (2009) Identification and characterization of the doublesex gene of Nasonia. Insect Mol Biol 18:315–324
O'Neil MT, Belote JM (1992) Interspecific comparison of the transformer gene of drosophila reveals an unusually high degree of evolutionary divergence. Genetics 131:113–128
Pane A, Salvemini M, Bovi PD, Polito C, Saccone G (2002) The transformer gene in Ceratitis capitata provides a genetic basis for selecting and remembering the sexual fate. Development 129:3715–3725
Penalva LO, Sánchez L (2003) RNA binding protein sex-lethal (Sxl) and control of Drosophila sex determination and dosage compensation. Microbiol Mol Biol Rev 67:343–359
Price DC, Egizi A, Fonseca DM (2015) The ubiquity and ancestry of insect doublesex. Sci Rep 5:1–9
Ruiz MF, Milano A, Salvemini M, Eirín-López JM, Perondini AL, Selivon D, Polito C, Saccone G, Sanchez L (2007) The gene transformer of Anastrepha fruit flies (Diptera, Tephritidae) and its evolution in insects. PLoS One 2:e1239
Saccone G, Peluso I, Artiaco D, Giordano E, Bopp D, Polito LC (1998) The Ceratitis capitata homologue of the Drosophila sex-determining gene sex-lethal is structurally conserved, but not sex-specifically regulated. Development 125:1495–1500
Sakai H, Oshima H, Yuri K, Gotoh H, Daimon T, Yaginuma T, Sahara K, Niimi T (2019) Dimorphic sperm formation by sex-lethal. Proc Natl Acad Sci U S A 116:10412–10417
Salvemini M, D'Amato R, Petrella V, Aceto S, Nimmo D, Neira M, Alphey L, Polito LC, Saccone G (2013) The orthologue of the fruitfly sex behaviour gene fruitless in the mosquito Aedes aegypti: evolution of genomic organisation and alternative splicing. PLoS One 8:e48554
Salz HK (2007) Male or female? The answer depends on when you ask. PLoS Biol 5:e335
Samuels ME, Schedl P, Cline TW (1991) The complex set of late transcripts from the Drosophila sex determination gene sex-lethal encodes multiple related polypeptides. Mol Cell Biol 11:3584–3602
Schmieder S, Colinet D, Poirié M (2012) Tracing back the nascence of a new sex-determination pathway to the ancestor of bees and ants. Nat Commun 3:1–7
Schütt C, Nöthiger R (2000) Structure, function and evolution of sex-determining systems in dipteran insects. Development 127:667–677
Sefton L, Timmer JR, Zhang Y, Beranger F, Cline TW (2000) An extracellular activator of the drosophila JAK/STAT pathway is a sex-determination signal element. Nature 405:970–973
Shukla JN, Palli SR (2012) Sex determination in beetles: production of all male progeny by parental RNAi knockdown of transformer. Sci Rep 2:1–9
Sievert V, Kuhn S, Paululat A, Traut W (2000) Sequence conservation and expression of the sex-lethal homologue in the fly Megaselia scalaris. Genome 43:382–390
Taracena ML, Hunt CM, Benedict MQ, Pennington PM, Dotson EM (2019) Downregulation of female doublesex expression by oral-mediated RNA interference reduces number and fitness of Anopheles gambiae adult females. Parasites Vectors 12:1–11
Traut W, Niimi T, Ikeo K, Sahara K (2006) Phylogeny of the sex-determining gene sex-lethal in insects. Genome 49:254–262
Acknowledgements
The authors would like to thank Minoru Tanaka for the invitation to submit this monograph. In addition, we thank Toshiya Ando, Taro Nakamura and Yasuhiko Chikami for the helpful discussion. This work was supported by MEXT KAKENHI Grant Numbers 20H05944, 20H04933, 18H04766 and 16H01452 (to T. N.) and JSPS KAKENHI Grant Numbers 19 K16181 and 21K15135 (to S. M.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Morita, S., Sakura, K., Niimi, T. (2022). Spectrum of Sex in a Horn of the Japanese Rhinoceros Beetle. In: Tanaka, M., Tachibana, M. (eds) Spectrum of Sex. Springer, Singapore. https://doi.org/10.1007/978-981-19-5359-0_1
Download citation
DOI: https://doi.org/10.1007/978-981-19-5359-0_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5358-3
Online ISBN: 978-981-19-5359-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)