Abstract
Cancer, a debilitating disease by uncontrolled cell differentiation in the body, has been described to have over 200 different characteristic manifestations and clinical types. Conventional treatment strategies like chemotherapy, surgery, and radiotherapy (applying radiations) have been employed to treat the majority of the malignancies but acute side effects like hair loss, anaemia, oedema, bruising, fatigue, etc., have compelled scientists all around the world to look for alternate treatment regimes. Recent developments in nanoscience have revealed it to be highly effective in the detection and cure of cancers. One such class of nanomaterials that possesses a lot of potential in the biomedical domain is nanocomposites which can be roughly defined as a combination of nanoscale substances having no less than one dimension in the nanoscale range that are arranged in terms of a polymeric matrix, with the materials being in various combinations of organic and inorganic origin. Their rise in demand and research is because of their unusual properties and flexible nature that is relevant in the biomedical landscape. When associated with other biomaterials, they become even more functionally advantageous and the most promising one in cancer diagnostics, and treatment is chitosan. Chitosan, being a biopolymer, is produced by deacetylating chitin, a widely found polymeric form of N-acetylglucosamine which contains active functional groups that are highly susceptible to chemical reactions. This results in many unique properties like biocompatibility, biodegradability, non-toxicity, antimicrobial activity, etc. Thus, this chapter focusses on the up-gradation of nanocomposite properties when introduced with chitosan along with highlighting the multifarious uses of these chitosan-based nanocomposites in the domain of biomedicine, with special emphasis on cancer diagnostics and treatment. The chapter also aims to put into perspective, the recent developments in these biomaterials and discusses their functionalities and attributes whilst describing their applications in cancer healthcare concerning future advancements.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Currently, cancer is one of the most debilitating diseases and is amongst the leading causes of death across the globe, after the spectrum of diseases like cardiovascular problems. According to the well-researched data by the International Agency for Research on Cancer (IARC), approximately 13.1, a million casualties associated with cancer are estimated by 2030. Some reasons behind the occurrence of metastasis may be external, such as urbanization, lifestyle, and overgrowing population, or internal, such as hormonal, poor immune system, or even genetic [1]. Evidence suggests that carcinogenesis is primarily caused due to DNA damages that lead to genomic instability, a phenomenon in which chronic mutations are induced in the genetic material and can be initiated by agents like ionizing radiation, heavy metals, etc. This instability can be passed on to the next generation and is responsible for increasing the risk of carcinogenesis and may even lead to secondary cancers in patients undergoing chemotherapy or radiotherapy. Traditional methods of treating cancer include surgery, chemotherapy, and radiotherapy, with chemotherapy being the one that is used most widely. But it has its detrimental effects such as sub-par bioavailability, multi-drug resistance, deleterious physiological manifestations in the form of hair loss, anaemia, etc., due to poor targeting and low-cost efficacy. In the process of finding novel methods of treating cancer, scientists have started exploring nanotechnology for overcoming the limitations of conventional treatment procedures.
Ever since Richard Feynman gave the definition of nanoparticles, science has progressed a long way in the arena of nanotechnology and is making continuous strides in increasing the applications of this field. In a myriad of fields, nanoformulations are being applied because of their distinctive properties which are exemplified by their high-surface area, remarkable mobility in their free states, reactivity, stable carrier structures, quantum effects, etc. Such attributes are particularly useful in the field of biomedicine where these are put to use in a multitude of ways, ranging from diagnostics to therapy. There are a large variety of nanoscale formulations possible like nanoparticles of numerous origins (metallic, organic, inorganic, etc.), nanotubes, dendrimers, liposomes, polymeric nanoparticles, nanocomposites, and many more. Out of these, one class that has garnered large-scale attention is nanocomposites especially the ones of polymeric origin.
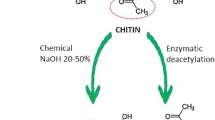
To recapitulate, nanocomposites are combinations of nanoscale substances with at least one dimension in the nanorange that are arranged in terms of a polymeric matrix, with the materials being a combination of organic/organic, organic/inorganic, or even inorganic/inorganic [2]. The textbook definition of nanocomposites has experienced many changes with the ongoing research and presently, is referred to as those systems with combinations of varied dimensional materials, mixed with amorphous materials at the nanoscale [3]. They showcase a multitude of beneficial properties that are applicable in industries like food packaging, healthcare, purification, etc., but they still possess the problem of biodegradability which is solved by another sub-class of these nanomaterials called “bionanocomposites” [4]. These are hybrid substances that comprise inorganic solids and biopolymers with the most important advantage being their biodegradability, that is, these green and eco-friendly formulations are subject to degradation by the action of living organisms in the biosphere, making them safe for the environment. They are also abundant and renewable, making them an ideal option over the other existing alternatives. Amidst the magnitude of biopolymers available, one of the first, most researched, and widely applicable biopolymer is “chitosan”, obtained from the second most abundant polysaccharide. This compound is derived after limited or incomplete deacetylation of chitin (Fig. 1), and some of its multifarious properties are excellent biodegradability, biocompatibility, non-toxicity, biodistribution, along with significant anti-tumour activity, and such unique and highly advantageous attributes have a huge scope in oncotherapy, which is explored in its depth throughout the course of this chapter.
2 Emerging Role of Nanotechnology in Oncotherapy
Throughout the history of scientific research, experts have been looking for the correct answer to treat one of the deadliest diseases to ever plague mankind, which is cancer. Extensive research has been going on for years, in search of newer approaches for cancer therapy that do not have the disadvantages of conventional methods and are safer for public use. A promising answer has been discovered in the form of nanotechnology. Nanoformulations are extremely short in size, their dimensions ranging between 10 and 1000 nm. Its most utilized form is a “nanoparticle”, which can be organic or inorganic and can be created from a variety of materials that provides it with specific and high-yielding properties, that are utilized in cancer therapy. Nanotechnology can rightly be referred to as the “Science of today”, owing to its tremendous applicability in every field with far better results than the conventional solutions, and consequently, the area of cancer detection and treatment has seen vast development in the landscape of nanoscience. Cancer nanotechnology provides a distinguished approach where it provides a holistic solution and covers areas of prevention, timely diagnosis, effective therapy, and even precision medicine.
2.1 Nanoparticles: What Are They?
Discovered almost 50 years before by scientist Richard Feynman, who is “The Father of Nanotechnology”, nanoparticles are reported to be sub-microscopic, ultrafine units that have the ability to be measured in dimensions of nanometres (nm; 1 nm = 10–9 m). Its definition was initially proposed in 2008 by the union, International Organization for Standardization (ISO) as an individual nano-object having all the 3 Cartesian dimensions to not be more than 100 nm. Following this, in the year 2011, the Commission of the European Union gave its approval to a more-technical and all-encompassing definition: a natural, incidental, or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where for 50% or more of the particles in the number size distribution, one or more external dimensions are in the size range 1 nm–100 nm [5]. Based on this expression, a nanoscale object is required to have a minimum of at least one dimension in the range 1–100 nm so that it can be classified as a nanostructure, even if its remaining two dimensions are not incorporated in the range specified above.
Nanoparticles have been segregated into numerous types based on a multitude of reasons like- size, shape, properties of a material, source, etc. A few classifications bifurcate nanoparticles into nanoparticles of organic and inorganic origin, the former includes metallic oxide nanoparticles, metallic nanoparticles (gold, silver, silicon, zinc, copper, etc.), quantum dots, etc., and the latter accounts for nanoparticles made of polymers like carbon and carbon-based, fullerenes, ceramics, liposomes, micelles, quantum dots, chitosan, dendrimers, etc. Nanoparticles are able to depict several distinctive effects and unique properties in different compositions due to their three major attributes:
-
1.
Possess a high level of mobility when present in the free state.
-
2.
Own exceptionally massive specific areas.
-
3.
Showcase unique quantum effects in a variety of compositions and conditions.
The catalytic, mechanical, and thermal properties of nanoparticles have the tendency to become altered either by increasing or by decreasing the surface area/volume ratio, and the new as well as individual properties generated by such changes allow applications of nanoformulations in a variety of biological fields like cancer therapy, drug administration, wound healing, diagnostic tests, biosensors, etc. The nanoparticles of chemically manufactured structures like polysaccharides, metals, and other polymers incorporated with active drugs and anti-cancer pharmaceuticals like Abraxane, Doxil, DaunoXome, Onyvide, etc., pose a highly efficient, safe, and targeted treatment for cancer [6, 7].
3 Advantages of Nanoformulations in Cancer Therapy
Nanotechnology is an extremely rapidly emerging field of science with ground-breaking applications in various areas, especially in medicine. Its most prominent application is found to be the treatment of cancer mainly because of the following characteristics of nanoformulations,
-
(i)
Extremely minute size enables them to cross physiological barriers easily and reach the target size properly,
-
(ii)
Enhanced stability inside anatomic sites of action which in turn increases the time of action of therapeutic agents adsorbed on the surface of these nanoformulations,
-
(iii)
Safer as compared to other alternatives as they can reduce the toxicity of chemotherapeutic drugs when combined with active pharmaceutical ingredients (APIs)
-
(iv)
Enables targeted mode of action and precision in working due to its bioavailability, etc. [8],
-
(v)
Interaction of nanoparticles does not cause any harm or necrosis to the physiological constituents of protein and cellulose,
-
(vi)
The presence of targeting ligands on the surface prevents the scattering of the therapeutic agent in other premises of the body and payloads it on the required site,
-
(vii)
Biodegradable composition of nanoformulations can be achieved so that complete removal can be achieved after its action is accomplished
These advantages of nanoformulations can be summarized in the following Fig. 2.
4 What Are Nanocomposites?
A nanocomposite is a multiphase solid substance with one, two, or three proportions of less than 100 nm in one of the phases, or even structures with nanoscale distances between the distinct phases that make up the material, nanocomposites are utilized as building blocks to construct materials with exceptional flexibility and enhancement in physical properties. In a broad sense, this term can refer to porous media, colloids, gels, and copolymers, although it is most usually associated with the solid combination of a bulk matrix and a nano-dimensional phase (s) with properties that differ due to structural and chemical similarities [9].
Abalone shells and bone structures are examples of nanocomposites found in natural environments [10]. The application of nanoparticle-rich materials predates our understanding of their physicochemical properties. Jose-Yacaman et al. looked into the origins of Maya blue paint’s colour depth and resilience to acids and bio-corrosion, putting it down to a nanoparticle mechanism [11]. Nanoscale organo-clays have been employed to control the flow of polymer solutions (e.g. as paint viscosifiers) and the compositions of gels since the mid-1950s (e.g. as a thickening substance in cosmetics, keeping the preparations in homogeneous form). Ceramic matrix nanocomposites, metal matrix nanocomposites, polymer matrix nanocomposites, and magnetic matrix nanocomposites are examples of nanocomposites (Fig. 3).
Because of the extremely high-surface-to-volume ratio of the reinforcing phase, nanocomposites differ from traditional composite materials in terms of mechanical structure. Particles (e.g. minerals), sheets (e.g. exfoliated clay stacks), or fibres can be used as reinforcing materials (e.g. carbon nanotubes or electrospun fibres). In comparison with conventional composite materials, the area of the interphase between the matrix and reinforcement phase(s) is often an order of magnitude larger. In the area of reinforcement, the matrix material’s characteristics are considerably influenced [12]. Due to the vast amount of surface area available for reinforcement, even a little amount of nanoscale reinforcement can have a significant impact on the macroscale properties of the composite. Carbon nanotubes, for example, improve both electrical and thermal conductivity. Other types of nanoparticulate can improve optical properties, dielectric properties, heat resistance, or mechanical properties like stiffness, strength, and wear and damage resistance. During processing, the reinforcement is generally dispersed into matrices. Because of the low-filler percolation threshold, the mass fraction of nanoparticles introduced can remain very low (about 0.5–5%), especially for the most commonly used non-spherical, high-aspect ratio filler (e.g. nanometre-thin platelets, like clays, or nanometre-diameter cylinders, like carbon nanotubes). The effectiveness of thermal conductivity of nanocomposites is highly influenced by the orientation and arrangement of asymmetrical nanoparticles, thermal characteristics that do not match at the interface, interface density per unit volume at the nanocomposite, and nanoparticle polydispersity.
5 Chitosan: A Versatile Super Polymer in Treating Cancer
Chitosan, being a linear polysaccharide, is polycationic in nature (having a positive charge at multiple sites) with a composition of β-(1-4)-linked-D-glucosamine in association with N-acetyl-D-glucosamine, and additionally is a derivative of “chitin” [13]. This biopolymer is extremely beneficial in the biomedical domain owing to its remarkable properties which arise as a result of its amino groups, conferring it anti-cancerous attributes, namely efficient cell permeability, biocompatibility, anti-angiogenesis, immune enhancement, anti-oxidation, and even apoptotic tendencies [14]. In general, chitosan exhibits a myriad of unique characteristics that make them ideal in numerous aspects like hydrophilicity, tunable molecular weight, antimicrobial nature, wound healing, anti-virulence, analgesic, haemostatic, and mucoadhesive behaviour that render them superior to other similar compounds [15,16,17,18]. Chitosan can induce apoptosis in malignant outgrowths by activating specific pathways of caspase-3 and caspase-8 which results in cell cycle dysfunction and inhibition of transcription along with a translation, including disruption of hormonal cascades [19]. This multifunctional biopolymer also has reported improvement in immunity by arresting the dividing cells in G1/S phases that in turn downregulate the expression of caspase-3, resulting in apoptosis (controlled cell death). As mentioned previously, the amine group of chitosan plays a highly essential role in exhibiting anti-tumour activity as it expresses cytotoxicity amongst varying malignancies by interacting directly, for example, owing to its low-molecular weight and high degrees of electrostatic interactions, chitosan adheres to the membranes of cancer cells to aid in their endocytosis and therefore, stands to be a remarkable delivery vehicle for anti-cancer drug formulations [20]. The advantageous traits of chitosan, with respect to cancer therapy, are showcased in Fig. 4.
The functional groups present on chitosan, which are the amine and hydroxyl groups have the ability to become functionalized and with a variety of chemicals and drugs that lead to chemical alterations resulting in increased solubility of chitosan-based carriers and even enhanced drug loading capacity. Its unique properties allow improved binding capacity towards mucosal membranes, resulting in successful transmucosal delivery, intrapulmonary, and intranasal delivery of therapeutic drugs against metastasis, and sometimes even acting as an immunoadjuvant for immunotherapy against cancer [21].
6 Synthesis and Attributes of Chitosan-Based Bionanocomposites
Because of the poor solubility of chitosan, a solution of 2 mg/ml in 1% acetic acid was prepared first. The mixture was gyrated to completely dissolve and kept overnight before being filtered through 0.22 m Millipore syringe filters to remove any impurities. Metal–chitosan nanocomposites are easy to make; in general, metal nanoparticles are obtained via chemical reduction of metal salts to yield zero-valent metal nanoparticles with NaBH4. The concentration of NaBH4 has to be 10 times higher than that of the metal salt to enable complete reduction. A 50 l, 20 mM HAuCl4, AgNO3, H2PtCl6, or Na2PdCl4 aqueous solution were mixed with 3 ml, 2.0 mg/ml chitosan and stirred for 30 min, after which freshly made aqueous solutions of NaBH4 (50 l, 0.2 M) were added to the mixture and stirred for another 90 min until the entire metal salts were reduced [22].
Chitosan-based nanoparticles have been made using solvent evaporation, emulsion, diffusion, ionic gelation, coacervation or precipitation, spray dyeing, self-assembly, and cross-linking. Chitosan/nanometric cellulose composites combine the properties of chitosan (e.g. biodegradability, antibacterial and antimicrobial activity, transparency, etc.) with those of nanometric cellulose (e.g. high-surface area, very good barrier as mechanical properties) to produce composite materials that can be used in the packaging industry (e.g. food film, paper coatings), the chemical industry (e.g. catalysts, adsorbents) (e.g. carrier of active substances, filaments) [23, 24].
7 Biomedical Relevance of Chitosan-Based Nanoformulations
Pharmaceutical formulation and drug delivery systems such as antibiotics, anti-inflammatory drugs, vaccines, proteins, and peptides are amongst the possible biomedical applications of chitosan. Antimicrobial applications, gene delivery, gene therapy, wound healing and burns, regenerative medicine, tissue engineering on bone, ligament, cartilage, tendon, liver, neural and skin regeneration, cancer applications (treatment, therapy, diagnostic strategy), dermatology, ophthalmology, dentistry, biosensors, and many other applications including bioimaging (for example, magnetic resonance imaging), supporting immobilized enzymes, and veterinary medicine.
7.1 Antimicrobial Outcomes
Despite brilliant progress in the development of antimicrobial mediums, many infectious diseases are still difficult to treat, due to a lot of reasons such as the rise and spread of resistant clones, the lack of the antimicrobial bodies, and inadequate pharmaceutical properties of existent antimicrobial bodies, that sometimes are difficult to reach active concentrations inside bacterial strains or in some body areas. Chitosan is a promising biocompatible and biodegradable biopolymer that shows potential antimicrobial activity [25]. Unlike chitosan, the nanocomposites of chitosan have been found to exhibit a broad spectrum of antimicrobial activity against various pathogens (both gram-positive and gram-negative bacteria) [15].
Lavorgna et al. [16] created silver montmorillonite antibacterial nanocomposites with a chitosan matrix by substituting the natural montmorillonite’s (MMT) Na+ ions with silver ions. They were able to improve mechanical performance, but more importantly, they demonstrated that after 24 h, pseudomonas aeruginosa acquired a considerable delay [26]. Lim et al. also looked into how rGO and CHI nanocomposites affected pseudomonas aeruginosa development. The results demonstrated that bacterial growth was not reliant on rGO concentration or size, but that a modest concentration of rGO in the chitosan solution may totally suppress bacterial growth, resulting in a 100% viability loss [27].
7.2 Anti-Cancer Drugs
Despite incredible advances in medical research, cancer remains one of the leading causes of mortality worldwide. The foundation of successful cancer management is accurate diagnosis and tailored treatment strategies. Chitosan and its derivatives are ideal for cancer diagnosis because their chemical features make them simple to produce into gels, sponges, membranes, beads, and scaffolds.
The chitosan oligosaccharide-arachidonic acid conjugate has been successfully produced and used to develop self-assembled nanoparticles for the administration of doxorubicin [28]. Grafting the targeting function via the thiolation procedure, for example, RGD (arginine-glycine-aspartic acid) improves selective intratumoural transport of siRNA packaged in PGD-chitosan nanoparticles (RGD-CH-NPS) and measures antitumoural activity [29].
A biodegradable polymer-drug combination of doxorubicin conjugated with stearic-acid-grafted chitosan nanosized oligosaccharide recently demonstrated excellent effectiveness for cellular absorption and tumour growth.
7.3 Drug Delivery Frameworks
Because of non-specific cell targeting and tissue biodispersion, as well as their quick metabolism and excretion, standard medications are becoming increasingly limited, resulting in a high need for high-performance solutions [30, 31]. Nanocarriers have emerged as one of the most promising drug delivery methods due to their ability to interface with the cell membranes and enter via endocytosis, departing for the endosomal compartment and releasing medication in cytosolic compartments [32]. Biodegradability and biocompatibility, which are often offered by natural polymers such as CHI, are the most important prerequisites for creating these systems.
It is less expensive to apply novel distribution methods than it is to develop new medications. Chitosan nanoparticles were found to be well adjusted towards nanocarriers used as an antimalarial drug delivery system [33], as a promising drug delivery system to improve antiviral drugs for the treatment of HIV infection [34], transformed by folic acid towards targeted drug delivery [35], developed responsive hybrid nanogels by poly(methacrylic acid) for pH-responsive drug release, and magnetic chitosan nanoparticles used as multifunctional nanocarriers were loaded with bleomycin to function as a nanocarrier proved to be effective towards targeting system [36].
7.4 Tissue Engineering Applications
Tissue engineering is the study of how to use structural and functional relationships in normal and infected tissues to generate biological substitutes that restore and improve biofunction. Different forms of bone grafts, such as autografts, allografts, and synthetic bone grafts, were employed for fracture repair in bone tissue engineering [37]. Autografting is the gold standard for bone repair, but it has several drawbacks, including limited availability, donor site morbidity, and danger of disease transmission from donor to recipient [38]. Allografts have some drawbacks, such as a lack of osteoinduction, the risk of disease transmission, and poor mechanical qualities. As a result, the development of synthetic bone grafts that overcome these disadvantages represents a huge demand for bone regeneration, especially through biomimetic devices with osteoconductive properties.
Biocompatible polysaccharides, such as chitosan-based materials, increase cell adhesion, proliferation, and differentiation and have been utilized extensively in orthopaedic tissue engineering [39]. Bone tissue engineering materials [40], cartilage regeneration [41], and liver and nerve tissue engineering [42] have all been described using chitosan-based hybrid nanocomposites.
7.5 Wound Healing Applications
Wound healing is a complex series of biologically regulated processes linked to tissue growth and regeneration. It goes through a variety of stages in which numerous cellular and matrix components work together to repair the damage and restore the tissues that have been lost [43]. Chitosan-based nanomaterials have been employed in a variety of wound healing applications, including composite scaffolds, chitosan-based sponges, immobilized scaffolds, and drug-loaded scaffolds [44].
Sulfated chitosan has the ability to disrupt the coagulation process. There are numerous medical applications for this. This chitosan derivative has been proven to have high-anticoagulant action when compared to heparin. Sulfated chitosan, unlike heparin, is not known to have antiplatelet activity, which can lead to excessive bleeding in individuals. Pure CHI-based hydrogels with high toughness may be obtained using, for example, double-network strategies; however, using nanocomposites based on CHI matrices is much more general. Considering these features, Lu et al. reported the use of CHI-PVA/graphene nanofibers, produced by electrospinning, for wound healing applications [45]. The potential of these membranes was tested on mouse and rabbit skin wounds and found that after five days, the wound area significantly decreased, and at the end of 10 days, the skin was completely recovered, whereas, for membranes lacking graphene, these wound areas still existed. Aguzzi and co-workers explored the use of CHI/MMT nanocomposites combined with silver sulfadiazine for the same purpose. They demonstrated a successful loading of the silver sulfadiazine in the nanocomposite structure, as XRD tests have shown no free drug in the composite matrix, which revealed that the intercalated nanocomposite was made by insertion of drug and/or polymer molecules, having a homogeneous dispersion in the nanocomposite structures [46].
7.6 Gene Therapy and Bioimaging Applications
The transfer of nucleic acids by the cell is at the heart of gene therapy, which offers the potential to heal a wide range of currently incurable diseases. Chitosan possesses a number of characteristics that make it an ideal gene delivery system. Chitosan formulation is simple, as the positive amine group reacts with negatively charged phosphate groups on the DNA, resulting in increased stability and improved gene transfer capabilities [47]. Because of its biocompatibility, bioimaging applications of chitosan nanoparticles are also gaining traction. Imaging agents, such as Fe3O4 for MRI, were incorporated into self-assembled nanoparticles to target tumour imaging [48]. Nanoparticles containing imaging agents were investigated for radio-pharmacological and MRI applications [49, 50].
7.7 Biosensing
Biosensing is the detection of target molecules using principles similar to those utilized by a living system such as the immune system. The main factors to consider when performing biosensing are detection specificity and sensitivity [51]. As shown by Singh and co-workers, CHI/GO nanocomposites have shown the ability to detect DNA for rapid and sensitive detection of typhoid, using a Salmonella typhi specific 5′-amine labelled single strand (ss) DNA (5′NH2 -ssDNA), covalently bound through CHI/GO by glutaraldehyde. The developed bioelectrode was able to distinguish between complementary and non-complementary sequences, which could be owing in part to the basic properties listed above, but to CHI compatibility, which enhances the DNA immobility and facilitates electron transfer between DNA and electrode surface [52].
Song et al. developed a glucose biosensor with cytochrome c and glucose oxidase entrapped on Au NPs and CHI over a glassy carbon electrode, demonstrating that the deposition of CHI/Au NPs increased its roughness to 9.5 0.1 nm, which was found to be critical for achieving a high-surface-to-volume ratio. Furthermore, higher glucose sensitivity and a lower detection limit were found [53].
Zhang et al. announced the development of a haemoglobin/Au NPs/CHI/graphene biosensor for hydrogen peroxide detection based on a glassy carbon electrode. The electron transport parameters of the biosensor were investigated using electrochemical impedance spectroscopy. The data showed that using haemoglobin, Au NPs, and graphene improves the electron transfer, reducing the transfer resistance supplied by CHI. In addition, a low-detection limit (0.35 µM), good stability (94%) for over one month, and a high sensitivity (347.1 mA/cm2 M) were found for these biosensors [54].
8 Chitosan-Based Nanocomposites: An Upcoming Domain in Cancer Diagnosis and Therapy
Bionanocomposites are distinctive, hybrid nanoformulations that possess inherent properties like biodegradability, biocompatibility, non-toxicity, etc., accompanied by special structural and functional qualities, owing to their unique composition of inorganic solids and natural polymers, including the cumulative impact of green/natural nanofillers that leads to increased sustainability, making them a green choice. Amongst these, chitosan-based nanocomposites have garnered significant attention in the arena of biomedical applications because of enhanced chemical, biological, and physical characteristics in a cost-efficient and eco-friendly manner. As discussed previously, chitosan has promising functional groups that possess the ability to interact with a variety of compounds, namely gold, silver, zinc oxide, copper, zinc oxide, etc., and therefore are attainable as films, hydrogels, beads, membranes, pervious frameworks, fibrous meshes, and even powdered form. Such variable chitosan nanocomposites hold a great value in the domain of cancer treatment and diagnosis owing to their intensified surface area-to-volume ratio, bioavailability, solubility, active, and specific targeting, reduced systemic damages, a high index of therapy, and even a greater circulation time within the biological setting [55]. Consequently, chitosan-based bionanocomposites are employed in oncotherapy in multiple formats like drug delivery systems, induction of apoptosis as well as necrosis, tumour imaging, biosensing of malignant tissues, photodynamic therapy, etc.
Some of the required abilities of efficient nanocarriers in promising drug delivery systems are susceptibility towards the light, pH, enzymes, temperature, and even magnetism that are portrayed by chitosan bionanocomposites after conjugation with a drug-induced pH-sensitive linker, allowing malignancy disruption. Resultantly, such a difference between the pH of malignant and typical cells allows the nanocarriers to become even more profitable as compared to other conventional drug delivery systems [21]. In the subsequent sections, the applications of chitosan-based bionanocomposites are described in various models for cancer theranostics.
-
1.
Chitosan-based Gold Nanocomposites
These bionanocomposites can be developed for utilization in photothermal therapy which was created by Zhang et al. [56]. After amplified laser ablation efficiency, such nanocomposites displayed a higher degree of accumulation on the surface of cancerous growths, when compared to normal healthy cells. Additionally, these nanocomposites possessed an increased tendency of selectivity towards malignancies than the normal cells, during photothermal ablation, which, in this case, involved cell lines of human dermal fibroblast cells/HDF as well as hepatocellular carcinoma/HepG2. After adherence to the cancer cells, these components generate a significant amount of heat that is able to destroy cancerous cells but not healthy ones; therefore, this study proved chitosan-based gold nanocomposites to have advantages like faster ablation with low-power near-infrared radiation, accompanied with low dosage.
Apart from this, scientists recently developed gold-chitosan nanoparticle-based films for rapidly and accurately detecting prostate cancer with the help of a special prostate-specific antigen, known as a “bio-marker”. This advancement of an electrochemical immunosensor that resembles a sandwich model reportedly showcases increased stability, promising biocompatibility, more current response tendency along with higher electrocatalytic activity [57].
Another example is the development of a localized combinatorial therapy for delivering anti-cancer drugs with the help of injectable chitosan-hydrogel incorporated gold nanocomposites or porous silica nanocomposites, which exhibited the advantage of a sustained release capacity for doxorubicin hydrochloride under acidic environment, near the malignant area [58].
-
2.
Chitosan-based Copper Nanocomposites
The ability to respond to biomarkers is a promising ability with respect to precisely diagnosing cancer, and this can be done in nanocomposites via functionalization with special molecules that are sensitive to these biomarkers, like copper. For instance, the diagnosis of prostate cancer is tedious as it requires the detection of sarcosine biomarkers which is expensive, complex, and time-taking. To combat this, chitosan-copper nanoparticles conjugated with carbon nanotubes were probed and characterized to become immobilized by the help of sarcosine oxidase and their analysis suggested that such biosensors portray a superior analytical performance with the ability to examine them in real-time [59]. A similar bioinspired fabrication was applied to chitosan-copper oxide bionanocomposites that were reported with bioflavonoid rutin. In this case, the in vitro examinations propounded the concentration-dependent activity regarding anti-proliferation along with apoptotic induction in the human cancerous cell line of A549 cells [60].
-
3.
Chitosan-based Silver Nanocomposites
A vital example of this category is chitosan conjugated with silver and phycoerythrin nanocomposites, abbreviated as “CS-Ag-PE-NCs” which have given a promising result of significant apoptotic activity induction in breast cancer cells of triple-negative nature. Owing to their internalization by cancerous cells due to an amplification in the reactive oxygen species (ROS), subsequent activation of caspase cascades was observed, resulting in the subsequent release of apoptotic factors that caused mitochondrial-moderated apoptosis in malignancies [61].
Another instance of chitosan stabilized by Ag nanoparticles was studied to give vital evidence in inducing apoptosis in cancer cells of HT-29 cell line, along with increased production of ROS intracellularly, which stimulated the required caspase signalling cascades needed for apoptotic induction [62].
Comparably, their usage against A549 lung carcinoma cell line and Salmonella sp. also shows similar action of ROS production to induce apoptosis with additional cytotoxic activity against A549 cells as a result of green synthesis along with inhibition of associated pathogen, Mycobacterium tuberculosis [63]. After morphogenic analysis of A549 carcinomic cells, following treatment with this nanocomposite indicated a concentration-dependent downfall in the capacity of adherence in tumour cells when compared with normal cells, accompanying a shape of round cells in carcinoma tissue in contrast with a normal polygonal occurrence in WI 38 cells.
-
4.
Chitosan-based Iron Oxide Nanocomposites
The anti-cancerous qualities of iron oxide chitosan bionanocomposites were originally examined in vitro against HepG2 cell line, as a result of enhanced cytotoxicity in tumour cells because of iron oxide incorporation [64]. Their incorporation also reported the activity of chitosan as a chelating agent towards metal ions causing a rise in the occurrence of hydroxyl and amino groups that were speculated to be involved in anti-cancerous outcomes due to free radical scavenging. Additionally, these iron oxide-based chitosan nanocomposites have given evidence of their activity against malignancies as well as multi-drug resistance in pathogens [65]. Another indication of apoptosis in nanocomposite treated A549 carcinoma cell line was observed to be fragmentation in nuclear material accompanied by nuclear condensation.
-
5.
Chitosan-based Graphene Oxide Nanocomposites
The nanocomposites functionalized with graphene oxide have reported a good yield of delivery of the anti-cancerous drug, doxorubicin hydrochloride, due to their internalization inside the MCF-7 cells causing the successive release of doxorubicin to kill tumorigenesis [66]. Moreover, the conjugation of iron oxide with chitosan nanocomposites also renders them more soluble in the biological environment and also reduces the binding of proteins of non-specific nature in the physiological setting.
The modelling of 5-fluorouracil-loaded chitosan-graphene oxide bionanocomposites along with curcumin was employed in treating HT-29 colon cancer cell lines [67]. Besides this, polyelectrolytic chitosan nanocomposites conjugated with graphene oxide were developed recently for achieving targeted drug delivery of doxorubicin and its proper loading in the polymeric matrix along with controlled release was examined in HeLa cells [68].
-
6.
Chitosan-based Zinc Oxide Nanocomposites
A potent anti-cancer and antimicrobial activity has been studied in polypyrrole-grafted ZnO/chitosan nanocomposites that were synthesized in situ. The created structure was observed to have the ability to induce apoptosis in the cell line of cervical cancer, studied in HeLa cells, and even breast cancer which was studied in MCF-7 cells [69].
-
7.
Chitosan-based Magnetic Nanocomposites
The synthesis of folic acid-based magnetic nanocomposites conjugated with chitosan has proven to show targeted tumour imaging [70]. Selective toxicity against tumorigenesis was exhibited by these drug-loaded nanocomposites which were coupled with controlled drug release and extremely sensitive selection of cancerous cells in acidic pH, making them suitable for breast cancer treatment. Besides this, magnetic resonance imaging studies done in vivo and in vitro displayed an enhanced negative signal in the malignancy, confirming the MRI contrast performance.
-
8.
Chitosan-based Carbon Nanocomposites
Multiwalled carbon nanotubes coated with chitosan/silver and enveloped with 5-fluorouracil were formulated and analyzed to exhibit cytotoxicity against the cancerous cell line of MCF-7 with reported sustained release of anti-cancerous drug formulations against the same cell line [71].
-
9.
Other Chitosan-based Nanocomposites
Photothermal ablation of tumorigenesis via photoacoustic imaging was seen in polypyrrole-based chitosan bionanocomposites with the results showing complete healing of mice tumour with dual treatment of nanocomposites and NIR 808 nm laser [72]. Moreover, the viability of breast carcinoma cells, namely MDA-MB-231, was lessened due to the photothermal effect of these nanocomposites. Apart from this, in vivo photodynamic therapy and targeted drug release done by involving near-infrared radiation achieved by graphene oxide nanocomposites have also been reported [73].
Colon cancer treatment has been reported in celecoxib-loaded chitosan nanocomposites based on hydroxyapatite, with their in vitro analysis showing a time-dependent take-up of nanocomposite in HT-29 and HCT-15 cancerous cell line succeeded by apoptosis and anti-proliferation, observed during in vivo studies in mice [74].
Some major applications of chitosan-based bionanocomposites are listed in Table 1.
9 Concluding Remarks and Future Perspective
Cancer is the second-largest contributor to the global disease burden and novel studies, and research on domains like nanocomposites and chitosan is the need of the hour so that this enfeebling malady can be cured of its core. The unique and outstanding properties of chitosan, namely biocompatibility, chemical receptivity, and anti-inflammatory and non-toxic nature, are especially beneficial in exhibiting anti-malignant behaviour. Chitosan-based nanocomposites derived from combining organic/inorganic nanoformulations with chitosan biopolymers have exhibited their enormous potential in numerous fields ranging from food packaging, material science, and water purification to mainstream biomedicine, especially oncotherapy.
The anti-tumour efficacy shown by chitosan-based nanocomposites shows precise diagnostic methods, preventive measures, treatment strategies, and personalized medicine through efficient drug delivery systems, biosensors, gene delivery systems, phototherapy agents, and other theragnostic instruments that have proven their remarkable potential in cancer diagnosis and treatment. The most promising outcome of chitosan bionanocomposites is their ability to provide target-specific drug deliveries that abstain from negatively influencing the usual healthy tissues around the target site.
However, there are still many challenges faced by developed bionanocomposites that have clinical applications in cancer with respect to toxicity-related issues, the specific underlying mechanism of treatment, and other complications. Thus, there is an urgent need to critically examine and study these revolutionary structures in the field of oncotherapy along with toxicological surveys, molecular medicine, clinical trials, and antibody generation so as to transform the clinical landscape in the future of cancer therapeutics as well as diagnostics.
Abbreviations
- A549:
-
Adenocarcinomic human alveolar basal epithelial cell line
- API:
-
Active pharmaceutical ingredient
- CHI:
-
Chitosan
- CHI/GO:
-
Chitosan conjugated graphene oxide
- HCT-15:
-
Human colorectal carcinoma cell line
- HeLa cells:
-
Henrietta lacks cell line
- HepG2:
-
Liver hepatocellular carcinoma
- HT-29:
-
Human colorectal adenocarcinoma cell line
- IARC:
-
International agency for research on cancer
- ISO:
-
International organization for standardization
- MCF-7:
-
Michigan cancer foundation
- NP:
-
Nanoparticle
- PLGA:
-
Poly(lactic-co-glycolic) acid
- ROS:
-
Reactive oxygen species
- XRD:
-
X-ray diffraction
References
Gulati S, Kumar S, Singh P, Diwan A, Mongia A (2020) Biocompatible Chitosan-coated gold nanoparticles: novel, efficient, and promising nanosystems for cancer treatment. In: Handbook of polymer and ceramic nanotechnology. Springer International Publishing, pp 1–29
Arora B, Bhatia R, Attri P (2018) In: Bionanocomposites: green materials for a sustainable future. Elsevier Inc.
Kamel S (2007) Nanotechnology and its applications in lignocellulosic composites, a mini review. Express Polym Lett 1(9):546–575. https://doi.org/10.3144/expresspolymlett.2007.78
Gulati S et al (2022) Starch based bio-nanocomposites: modern and benign materials in food packaging industry. In: Handbook of consumer nanoproducts, pp 881–909. https://doi.org/10.1007/978-981-16-8698-6_96
Wang EC, Wang AZ (2014) Nanoparticles and their applications in cell and molecular biology. Integr Biol (United Kingdom) 6(1):9–26. https://doi.org/10.1039/c3ib40165k
Kumar S, Mongia A, Gulati S, Singh P, Diwan A, Shukla S (2020) Emerging theranostic gold nanostructures to combat cancer: novel probes for combinatorial immunotherapy and photothermal therapy. Cancer Treat Res Commun 25:100258. https://doi.org/10.1016/J.CTARC.2020.100258
van der Meel R, Lammers T, Hennink WE (2017) Cancer nanomedicines: oversold or underappreciated? Expert Opinion Drug Delivery 14(1):1–5, 20 Jan 2017. Taylor and Francis Ltd. https://doi.org/10.1080/17425247.2017.1262346
Grodzinski P, Kircher M, Goldberg M, Gabizon A (2019) Integrating nanotechnology into cancer care. ACS Nano 13(7):7370–7376. https://doi.org/10.1021/acsnano.9b04266
Chikwendu Okpala C The benefits and applications of nanocomposites. Int J Adv Eng Technol
Yuan J, Zhang X, Qian H (2010) A novel approach to fabrication of superparamagnetite hollow silica/magnetic composite spheres. J Magn Magn Mater 15(322):2172–2176. https://doi.org/10.1016/J.JMMM.2010.02.004
José-Yacamán M, Rendón L, Arenas J, Serra Puche MC (1996) Maya blue paint: an ancient nanostructured material. Science (80) 273(5272):223–225. https://doi.org/10.1126/SCIENCE.273.5272.223
Tian Z, Hu H, Sun Y (2013) A molecular dynamics study of effective thermal conductivity in nanocomposites. Int J Heat Mass Transf 61(1):577–582. https://doi.org/10.1016/J.IJHEATMASSTRANSFER.2013.02.023
Cheung RCF, Ng TB, Wong JH, Chan WY (2015) Chitosan: an update on potential biomedical and pharmaceutical applications. Mar Drugs 13(8):5156. https://doi.org/10.3390/MD13085156
Adhikari HS, Yadav PN (2018) Anticancer activity of chitosan, chitosan derivatives, and their mechanism of action. Int J Biomater 2018. https://doi.org/10.1155/2018/2952085
Benhabiles MS, Salah R, Lounici H, Drouiche N, Goosen MFA, Mameri N (2012) Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll 29(1):48–56. https://doi.org/10.1016/J.FOODHYD.2012.02.013
Sandri G, Rossi S, Bonferoni MC, Ferrari F, Mori M, Caramella C (2012) The role of chitosan as a mucoadhesive agent in mucosal drug delivery. J Drug Deliv Sci Technol 22(4):275–284. https://doi.org/10.1016/S1773-2247(12)50046-8
Pogorielov MV, Sikora VZ (2015) Chitosan as a hemostatic agent: current state. Eur J Med Ser B 2(1):24–33. https://doi.org/10.13187/EJM.S.B.2015.2.24
Hu Z et al (2018) Investigation of the effects of molecular parameters on the hemostatic properties of chitosan. Molecules 23(12). https://doi.org/10.3390/MOLECULES23123147
Hasegawa M, Yagi K, Iwakawa S, Hirai M (2001) Chitosan induces apoptosis via caspase-3 activation in bladder tumor cells. Jpn J Cancer Res 92(4):459–466. https://doi.org/10.1111/J.1349-7006.2001.TB01116.X
Wang W et al (2020) Chitosan derivatives and their application in biomedicine. Int J Mol Sci 21(2). https://doi.org/10.3390/IJMS21020487
Babu A, Ramesh R (2017) Multifaceted applications of chitosan in cancer drug delivery and therapy. Mar Drugs 15(4). https://doi.org/10.3390/MD15040096
Huang H, Yuan Q, Yang X (2004) Preparation and characterization of metal–chitosan nanocomposites. Colloids Surf B Biointerfaces 39(1–2):31–37. https://doi.org/10.1016/J.COLSURFB.2004.08.014
Thakur VK, Thakur MK (2014) Recent advances in graft copolymerization and applications of chitosan: a review. ACS Sustain Chem Eng 2(12):2637–2652. https://doi.org/10.1021/SC500634P/ASSET/IMAGES/MEDIUM/SC-2014-00634P_0011.GIF
Fernandes SCM, Freire CSR, Silvestre AJD, Pascoal Neto C, Gandini A (2011) Novel materials based on chitosan and cellulose. Polym Int 60(6):875–882. https://doi.org/10.1002/PI.3024
Zheng LY, Zhu JF (2003) Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr Polym 4(54):527–530. https://doi.org/10.1016/J.CARBPOL.2003.07.009
Lavorgna M et al (2014) MMT-supported Ag nanoparticles for chitosan nanocomposites: structural properties and antibacterial activity. Carbohydr Polym 102(1):385–392. https://doi.org/10.1016/J.CARBPOL.2013.11.026
Lim HN, Huang NM, Loo CH (2012) Facile preparation of graphene-based chitosan films: enhanced thermal, mechanical and antibacterial properties. J Non Cryst Solids 3(358):525–530. https://doi.org/10.1016/J.JNONCRYSOL.2011.11.007
Termsarasab U et al (2013) Chitosan oligosaccharide–arachidic acid-based nanoparticles for anti-cancer drug delivery. Int J Pharm 441(1–2):373–380. https://doi.org/10.1016/J.IJPHARM.2012.11.018
Han HD et al (2010) Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin Cancer Res 16(15):3910–3922. https://doi.org/10.1158/1078-0432.CCR-10-0005
Mura S, Nicolas J, Couvreur P (2013) Stimuli-responsive nanocarriers for drug delivery. Nat Mater 2013 1211 12(11):991–1003. https://doi.org/10.1038/nmat3776
Lima AC, Sher P, Mano JF (2012) Production methodologies of polymeric and hydrogel particles for drug delivery applications. 9(2):231–248. https://doi.org/10.1517/17425247.2012.652614
Kavaz A et al (2010) Bleomycin loaded magnetic chitosan nanoparticles as multifunctional nanocarriers. https://doi.org/10.1177/0883911509360735
Tripathy S, Das S, Chakraborty SP, Sahu SK, Pramanik P, Roy S (2012) Synthesis, characterization of chitosan-tripolyphosphate conjugated chloroquine nanoparticle and its in vivo anti-malarial efficacy against rodent parasite: a dose and duration dependent approach. Int J Pharm 434(1–2):292–305. https://doi.org/10.1016/J.IJPHARM.2012.05.064
Wu D et al (2016) Zinc-stabilized chitosan-chondroitin sulfate nanocomplexes for HIV-1 infection inhibition application. Mol Pharm 13(9):3279–3291. https://doi.org/10.1021/ACS.MOLPHARMACEUT.6B00568/SUPPL_FILE/MP6B00568_SI_001.PDF
Yuan Q, Hein S, Misra RDK (2010) New generation of chitosan-encapsulated ZnO quantum dots loaded with drug: synthesis, characterization and in vitro drug delivery response. Acta Biomater 6(7):2732–2739. https://doi.org/10.1016/J.ACTBIO.2010.01.025
Wu W, Shen J, Banerjee P, Zhou S (2010) Chitosan-based responsive hybrid nanogels for integration of optical pH-sensing, tumor cell imaging and controlled drug delivery. Biomaterials 31(32):8371–8381. https://doi.org/10.1016/J.BIOMATERIALS.2010.07.061
James R et al (2011) Nanocomposites and bone regeneration. Front Mater Sci 5(4):342–357. https://doi.org/10.1007/S11706-011-0151-3
Duan B, Wang M, Zhou WY, Cheung WL, Li ZY, Lu WW (2010) Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater 6(12):4495–4505. https://doi.org/10.1016/j.actbio.2010.06.024
Di Martino A, Sittinger M, Risbud MV (2005) Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 26(30):5983–5990. https://doi.org/10.1016/J.BIOMATERIALS.2005.03.016
Bhowmick A et al (2016) Multifunctional zirconium oxide doped chitosan based hybrid nanocomposites as bone tissue engineering materials. Carbohydr Polym 151:879–888. https://doi.org/10.1016/J.CARBPOL.2016.06.034
In KS et al (2009) Chitosan nano-/microfibrous double-layered membrane with rolled-up three-dimensional structures for chondrocyte cultivation. J Biomed Mater Res A 90(2):595–602. https://doi.org/10.1002/JBM.A.32109
Mottaghitalab F, Farokhi M, Mottaghitalab V, Ziabari M, Divsalar A, Shokrgozar MA (2011) Enhancement of neural cell lines proliferation using nano-structured chitosan/poly(vinyl alcohol) scaffolds conjugated with nerve growth factor. Carbohydr Polym 86(2):526–535. https://doi.org/10.1016/J.CARBPOL.2011.04.066
Boateng JS, Matthews KH, Stevens HNE, Eccleston GM (2008) Wound healing dressings and drug delivery systems: a review. J Pharm Sci 97(8):2892–2923. https://doi.org/10.1002/JPS.21210
Ali A, Ahmed S (2018) A review on chitosan and its nanocomposites in drug delivery. Int J Biol Macromol 109:273–286. https://doi.org/10.1016/J.IJBIOMAC.2017.12.078
Lu B et al (2012) Graphene-based composite materials beneficial to wound healing. Nanoscale 4(9):2978–2982. https://doi.org/10.1039/C2NR11958G
Aguzzi C et al (2014) Solid state characterisation of silver sulfadiazine loaded on montmorillonite/chitosan nanocomposite for wound healing. Colloids Surf B Biointerfaces 113:152–157. https://doi.org/10.1016/J.COLSURFB.2013.08.043
Malmo J, Vårum KM, Strand SP (2011) Effect of chitosan chain architecture on gene delivery: comparison of self-branched and linear chitosans. Biomacromol 12(3):721–729. https://doi.org/10.1021/BM1013525
Lee CM et al (2011) Oleyl-chitosan nanoparticles based on a dual probe for optical/MR imaging in vivo. Bioconjug Chem 22(2):186–192. https://doi.org/10.1021/BC100241A
Kumar MNVR, Muzzarelli RAA, Muzzarelli C, Sashiwa H, Domb AJ (2004) Chitosan chemistry and pharmaceutical perspectives. Chem Rev 104(12):6017–6084. https://doi.org/10.1021/CR030441B/ASSET/CR030441B.FP.PNG_V03
Dash M, Chiellini F, Ottenbrite RM, Chiellini E (2011) Chitosan—a versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci 36:981–1014. https://doi.org/10.1016/j.progpolymsci.2011.02.001
Yasuga H, Shoji K, Koiwai K, Kawano R (2021) New sensing technologies: Microtas/NEMS/MEMS. Ref Modul Biomed Sci. https://doi.org/10.1016/B978-0-12-822548-6.00046-7
Singh A et al (2013) Graphene oxide-chitosan nanocomposite based electrochemical DNA biosensor for detection of typhoid. Sens Actuators, B Chem 185:675–684. https://doi.org/10.1016/J.SNB.2013.05.014
Qin FX, Jia SY, Wang FF, Wu SH, Song J, Liu Y (2013) Hemin@metal-organic framework with peroxidase-like activity and its application to glucose detection. Catal Sci Technol 3(10):2761–2768. https://doi.org/10.1039/c3cy00268c
Zhang L, Han G, Liu Y, Tang J, Tang W (2014) Immobilizing haemoglobin on gold/graphene–chitosan nanocomposite as efficient hydrogen peroxide biosensor. Sens Actuators B Chem (197):164–171. https://doi.org/10.1016/J.SNB.2014.02.077
Din FU et al (2017) Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed 12:7291. https://doi.org/10.2147/IJN.S146315
Zhang G, Sun X, Jasinski J, Patel D, Gobin AM (2012) Gold/chitosan nanocomposites with specific near infrared absorption for photothermal therapy applications. J Nanomater 2012. https://doi.org/10.1155/2012/853416
Suresh L, Brahman PK, Reddy KR, Bondili JS (2018) Development of an electrochemical immunosensor based on gold nanoparticles incorporated chitosan biopolymer nanocomposite film for the detection of prostate cancer using PSA as biomarker. Enzyme Microb Technol 112:43–51. https://doi.org/10.1016/J.ENZMICTEC.2017.10.009
Xia B, Zhang W, Tong H, Li J, Chen Z, Shi J (2019) Multifunctional Chitosan/Porous Silicon@Au nanocomposite hydrogels for long-term and repeatedly localized combinatorial therapy of cancer via a single injection. ACS Biomater Sci Eng 5(4):1857–1867. https://doi.org/10.1021/ACSBIOMATERIALS.8B01533/SUPPL_FILE/AB8B01533_SI_001.PDF
Narwal V, Kumar P, Joon P, Pundir CS (2018) Fabrication of an amperometric sarcosine biosensor based on sarcosine oxidase/chitosan/CuNPs/c-MWCNT/Au electrode for detection of prostate cancer. Enzyme Microb Technol 113:44–51. https://doi.org/10.1016/J.ENZMICTEC.2018.02.010
Bharathi D, Ranjithkumar R, Chandarshekar B, Bhuvaneshwari V (2019) Bio-inspired synthesis of chitosan/copper oxide nanocomposite using rutin and their anti-proliferative activity in human lung cancer cells. Int J Biol Macromol 141:476–483. https://doi.org/10.1016/J.IJBIOMAC.2019.08.235
Thangam R et al (2015) Theranostic potentials of multifunctional chitosan-silver-phycoerythrin nanocomposites against triple negative breast cancer cells. RSC Adv 5(16):12209–12223. https://doi.org/10.1039/C4RA14043E
Sanpui P, Chattopadhyay A, Ghosh SS (2011) Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl Mater Interfaces 3(2):218–228. https://doi.org/10.1021/AM100840C/SUPPL_FILE/AM100840C_SI_001.PDF
Arjunan N, Kumari HLJ, Singaravelu CM, Kandasamy R, Kandasamy J (2016) Physicochemical investigations of biogenic chitosan-silver nanocomposite as antimicrobial and anticancer agent. Int J Biol Macromol 92:77–87. https://doi.org/10.1016/J.IJBIOMAC.2016.07.003
Dawy Badry M, Ahmed Wahba M, Khaled R, Moawad Ali M, Ali Farghali A (2017) Synthesis, characterization, and in-vitro anticancer evaluation of iron oxide/chitosan nanocomposites. Inorganic Nano-Metal Chem 47(3):405–411. https://doi.org/10.1080/15533174.2016.1186064
Rabel AM, Namasivayam SKR, Prasanna M, Bharani RSA (2019) A green chemistry to produce iron oxide—chitosan nanocomposite (CS-IONC) for the upgraded bio-restorative and pharmacotherapeutic activities—supra molecular nanoformulation against drug-resistant pathogens and malignant growth. Int J Biol Macromol 138:1109–1129. https://doi.org/10.1016/J.IJBIOMAC.2019.07.158
Lei H, Xie M, Zhao Y, Zhang F, Xu Y, Xie J (2016) Chitosan/sodium alginate modificated graphene oxide-based nanocomposite as a carrier for drug delivery. Ceram Int 42(15):17798–17805. https://doi.org/10.1016/J.CERAMINT.2016.08.108
Dhanavel S et al (2020) 5-Fluorouracil and curcumin co-encapsulated chitosan/reduced graphene oxide nanocomposites against human colon cancer cell lines. Polym Bull 77(1):213–233. https://doi.org/10.1007/S00289-019-02734-X
Anirudhan TS, Chithra Sekhar V, Athira VS (2020) Graphene oxide based functionalized chitosan polyelectrolyte nanocomposite for targeted and pH responsive drug delivery. Int J Biol Macromol 150:468–479. https://doi.org/10.1016/J.IJBIOMAC.2020.02.053
Ahmad N, Sultana S, Faisal SM, Ahmed A, Sabir S, Khan MZ (2019) Zinc oxide-decorated polypyrrole/chitosan bionanocomposites with enhanced photocatalytic, antibacterial and anticancer performance. RSC Adv 9(70):41135–41150. https://doi.org/10.1039/C9RA06493A
Nejadshafiee V et al (2019) Magnetic bio-metal–organic framework nanocomposites decorated with folic acid conjugated chitosan as a promising biocompatible targeted theranostic system for cancer treatment. Mater Sci Eng C 99:805–815. https://doi.org/10.1016/J.MSEC.2019.02.017
Nivethaa EAK, Dhanavel S, Rebekah A, Narayanan V, Stephen A (2016) A comparative study of 5-Fluorouracil release from chitosan/silver and chitosan/silver/MWCNT nanocomposites and their cytotoxicity towards MCF-7. Mater Sci Eng C Mater Biol Appl 66:244–250. https://doi.org/10.1016/J.MSEC.2016.04.080
Manivasagan P et al (2017) Multifunctional biocompatible chitosan-polypyrrole nanocomposites as novel agents for photoacoustic imaging-guided photothermal ablation of cancer. Sci Rep 7. https://doi.org/10.1038/SREP43593
Sharma H, Mondal S (2020) Functionalized graphene oxide for chemotherapeutic drug delivery and cancer treatment: a promising material in nanomedicine. Int J Mol Sci 21(17):1–42. https://doi.org/10.3390/IJMS21176280
Venkatesan P et al (2011) The potential of celecoxib-loaded hydroxyapatite-chitosan nanocomposite for the treatment of colon cancer. Biomaterials 32(15):3794–3806. https://doi.org/10.1016/J.BIOMATERIALS.2011.01.027
Abdel-Aziz MM, Elella MHA, Mohamed RR (2020) Green synthesis of quaternized chitosan/silver nanocomposites for targeting mycobacterium tuberculosis and lung carcinoma cells (A-549). Int J Biol Macromol 142:244–253. https://doi.org/10.1016/J.IJBIOMAC.2019.09.096
Vivek R, Nipun Babu V, Thangam R, Subramanian KS, Kannan S (2013) PH-responsive drug delivery of chitosan nanoparticles as Tamoxifen carriers for effective anti-tumor activity in breast cancer cells. Colloids Surf B Biointerfaces 111:117–123. https://doi.org/10.1016/J.COLSURFB.2013.05.018
Arya G, Vandana M, Acharya S, Sahoo SK (2011) Enhanced antiproliferative activity of Herceptin (HER2)-conjugated gemcitabine-loaded chitosan nanoparticle in pancreatic cancer therapy. Nanomed Nanotechnol Biol Med 7(6):859–870. https://doi.org/10.1016/J.NANO.2011.03.009
Important Websites
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sharma, N., Gulati, S., Bhat, J. (2022). Emerging Applications of Chitosan-Based Nanocomposites in Multifarious Cancer Diagnosis and Therapeutics. In: Gulati, S. (eds) Chitosan-Based Nanocomposite Materials. Springer, Singapore. https://doi.org/10.1007/978-981-19-5338-5_7
Download citation
DOI: https://doi.org/10.1007/978-981-19-5338-5_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5337-8
Online ISBN: 978-981-19-5338-5
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)