Abstract
Autoimmune Addison’s disease (AAD) is an organ specific autoimmune disease which causes primary adrenocortical insufficiency. In AAD, autoantibodies and autoreactive T cells target cytochrome P450 21-hydroxylase enzyme, which is a major self-antigen. Similar to other autoimmune diseases, both genetic and environmental factors play an important role in AAD. Many researchers have proposed common pathways that link immunity and bacterial and viral infections in AAD. Moreover, studies have shown the association of viral infections with the initiation of AAD progression. The chapter summarizes the current aspects of microorganisms’ involvement in the pathogenesis and management of AAD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Autoimmune Addison’s disease (AAD)
- Autoimmunity
- Adrenal gland
- Adrenocortical insufficiency
- Cytochrome P450 21-hydroxylase enzyme
- Human immunodeficiency virus (HIV)
- Cytomegalovirus (CMV)
1 Introduction
Autoimmune Addison’s disease (AAD) is a rare autoimmune disease that occurs due to complex interaction of genetic, environmental and immunological factors and results in symptomatic adrenocorticol insufficiency and dependency on corticosteroid replacement therapy throughout life (Betterle et al. 2002). Dr. Thomas Addison from Guy’s Hospital, London reported the AAD’s first case in late mid-nineteenth century and described adrenocortical insufficiency and changes in adrenal glands as major symptoms (Addison 2009). The patients exhibited tumors or tuberculosis of adrenal glands, except the one case of idiopathic adrenal atrophy, now known as autoimmune Addison’s disease (AAD).
In most countries, the possible etiology behind primary adrenal insufficiency is represented as autoimmune adrenalitis (Neufeld et al. 1981; Ten et al. 2001). Malignancy and hemorrhage are considered as less frequently encountered cause of AAD, whereas if specifically considering cases in males, mostly rare X-linked condition and adrenoleukodystrophy are reported as a reason behind occurrence of AAD (Neufeld et al. 1981; Ten et al. 2001). In the past decades, tuberculous infiltration of the adrenal glands was considered as major cause of primary adrenal insufficiency globally and still is one of the persistent causes in several developing countries (Addison 2009; Soule 1999). Primary adrenal insufficiency is rare with an annual incidence of 4.7–6.2 per million in white populations with a prevalence of 93–140 per million (Laureti et al. 1999; Kong and Jeffcoate 1994; Mason et al. 1968). It is prevalent in women, irrespective of age; however, it is mostly reported between the 30 and 50 years of age groups (Kong and Jeffcoate 1994). In order to restore good health, AAD patients have to rely on supplementation therapy throughout their life, as the steroid hormone releasing cells of the adrenal cortex are attacked by immune cells which results into organ failure and lack of steroid hormone synthesis within adrenal gland of AAD patients (Bornstein 2009; Bratland and Husebye 2011). These patients suffer through higher rate of morbidity, mortality and reduced quality of life (Lovas et al. 2002; Erichsen et al. 2009; Bensing et al. 2008, 2016). Therefore, there is a need of improving insights regarding the unrevealed pathological mechanisms associated with AAD in order to form the grounds for rational design of molecular and cellular strategies which could be targeted for the treatment of the disease. Similar to most of the autoimmune diseases, AAD is also considered as a multi-factorial disease which involves several factors such as genetic, environmental, and failure to control autoreactive lymphocytes at different stages (Goodnow 2007). Various genetic risk factors are known to contribute to AAD, especially major histocompatibility complex (MHC) but hardly something is known about environmental factors (Erichsen et al. 2009; Skinningsrud et al. 2011). Suspected environmental factors which causes autoimmune diseases include infectious agents such as bacteria, viruses, and other pathogens (Ercolini and Miller 2009). Unlike other autoimmune diseases, no specific infectious agents have been identified for AAD (Christen and Hintermann 2018; Lassmann et al. 2011; Hyoty 2016). However, several pathogens are considered to infect the adrenal cortex and may also contribute to adrenal insufficiency (Paolo and Nosanchuk 2006). In this chapter, we highlight the role of infectious agents in the disease development and role of gut microbiota and probiotics in the management of AAD.
2 Role of Microorganisms in the Pathogenesis of Autoimmune Addison’s Disease
A variety of microorganisms have been reported to infect adrenal gland, adrenal cortex and cause adrenal insufficiency (Paolo and Nosanchuk 2006). These infectious agents cause opportunistic infections, primarily affecting immunocompromised individuals. The infectious agents involved in the AAD mainly include bacteria, viruses, fungi, and parasites. Among this infectious agents, bacteria and viruses are considered of major relevance with AAD just like any other autoimmune endocrinopathies. In fact other microbes like Paracoccidioides brasiliensis or M. tuberculosis with a tropism for the adrenals are not endemic in the Western world (Glaziou et al. 2014; Bocca et al. 2013). In the below sub-sections, we summarize the involvement of bacteria and viruses in pathogenesis of AAD.
2.1 Bacteria
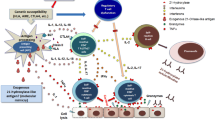
Bacterial infection influences the release of endotoxins or exotoxins resulting into dysregulation of the adrenal glands affecting the physiological host response (Fig. 7.1). The most commonly linked bacteria with adrenal damage is M. tuberculosis. M. tuberculosis can reside within adrenal gland without any clinical symptoms for up to 10 years (Kelestimur 2004). In a study conducted on 13,762 patients of tuberculosis, 6% of M. tuberculosis affected active patients exhibited adrenal insufficiency (Lam and Lo 2001). M. tuberculosis causes adrenal dysfunction through induction of degenerative cells within adrenal cortex (Kelestimur et al. 1994). The radiographic images of adrenal glands also suggests connection between length and tuberculosis activity in the adrenal gland (Kelestimur et al. 1994). Interestingly, reports revealed that treatment with anti-tuberculosis drugs does not ameliorate the condition of adrenal insufficiency (Bhatia et al. 1998). Another species of Mycobacterium, i.e., M. avium has been reported to cause infection in adrenals of AIDS patients (Glasgow et al. 1985). Although adrenal failure may result due to infiltrative disease; however, it may also occur without any clinical dysfunction (Guenthner et al. 1984). Unfortunately, the exact role of M. avium in adrenal destruction has not been explained completely, but its involvement in concomitant infection with cytomegalovirus virus (CMV) has been suggested. Similar to the above studies, bacterial sepsis is reported to produce bilateral adrenal hemorrhage in the case of Waterhouse-Friderichsen syndrome. One of the most frequently occurring bacterium linked with this syndrome is N. meningitidis (Bosworth 1979). However, it may also results during systemic infections caused by Haemophilus influenzae, Pneumococci, Streptococcus, Klebsiella oxytoca, Pasteurella multocida, Ewingella americana, and Capnocytophaga canimorsus (Doherty 2001; Hamilton et al. 2004; Karakousis et al. 2001; Tsokos 2003; Givner 1998; Hori et al. 1998; Ip et al. 1995; Gertner et al. 1992; McKinney and Agner 1989; Mirza et al. 2000; Morrison et al. 1985; Piccioli et al. 1994). Although these bacteria are considered to be linked with patients with adrenal insufficiency, but their direct involvement in AAD is still a mystery. Therefore, further studies are warranted to validate potential role of bacteria in pathogenesis of AAD.
Proposed mechanism for role of microorganisms in autoimmune Addison’s disease pathogenesis. Several environmental factors such as stress, bacteria, and viruses have been suggested to contribute in autoimmune Addison’s disease (AAD) pathogenesis. In AAD, autoantibodies and autoreactive T cells target cytochrome P450 21-hydroxylase enzyme, as autoantigen. IL-2 and IFN-γ cytokines activate autoreactive T cells (e.g., CD8+ T cells and CD4+ Th1 cells). In addition, IFN-γ activates macrophages and autoreactive B cells which then contribute to destruction of adrenal cortex with idiopathic primary adrenal insufficiency, and lead to autoimmune Addison’s disease
2.2 Viruses
Individuals with human immunodeficiency virus (HIV) infection are more susceptible towards several other infections including CMV infection, which results into adrenal dysfunction (Arlt and Allolio 2003; Nakamine et al. 1987; Glasgow et al. 1985; Huang et al. 2004; Angulo et al. 1994; Dore et al. 1995; Rodrigues et al. 2002). However, direct involvement of HIV in adrenal destruction is unusual (Sellmeyer and Grunfeld 1996). Autopsy studies have revealed that in HIV patients, adrenal gland is commonly affected endocrine organ (Welch et al. 1984; Hofbauer and Heufelder 1996). Autopsy study in 128 patients with AIDS showed pathologically compromised adrenal gland within 99.2% of the subjects (Rodrigues et al. 2002). Moreover, adrenal insufficiency was estimated in 5–8% AIDS patients, and it was significantly higher as compared to its incidence in the general population (Huang et al. 2004). Along with direct infection through HIV, other etiological reasons are also stated for adrenal malfunctions which include infections, viral-induced autoimmune deterioration and detrimental effects of chemotherapeutics (Huang et al. 2004). Furthermore, autopsy studies suggest that CMV plays substantial role in adrenal damage in AIDS patients. An adrenal pathology study in AIDS patients reported 21 cases in which adrenal gland was infected with CMV (Glasgow et al. 1985). Although, CMV has the highest involvement in adrenal infection of HIV patients, but antemortem diagnosis remains a rare occurrence (Dore et al. 1995; Eledrisi and Verghese 2001). Disturbance in function of adrenal gland can occur at any stage of HIV infection. Nonetheless, adrenocorticotrophin (ACTH) and cortisol levels occur early in HIV infection, and as HIV infection spreads, the insufficiency occurs with normal to low ACTH levels (Eledrisi and Verghese 2001). Advance HIV infection elevates the cortisol-binding globulin (CBG) within the serum in response to stimulation of adrenal cortex by IL-1β and IL-6 (Mayo et al. 2002). According to several studies, the progression to adrenal failure occurs in advanced HIV infection and the reason considered behind this is co-infection by opportunistic microbes, adrenal burnout, increased peripheral cortisol resistance, and anti-adrenal cell antibodies (Eledrisi and Verghese 2001; Mayo et al. 2002; Salim et al. 1988; Marik et al. 2002). A study conducted on 30 patients with AIDS depicted significant correlation between presence of CMV antigenemia and adrenal insufficiency (Hoshino et al. 1997). Controversy exists as there are no befitting diagnostic parameters for screening and delineating the probable basis for adrenal insufficiency, which majorly holds for some of the variance in number of cases reported (Eledrisi and Verghese 2001).
Moreover, some viruses also exhibit mutagenic potential, particularly Epstein–Barr virus (EBV), which was associated with adrenal gland lymphoma (Ohsawa et al. 1996; Suankratay et al. 2005; Ohshima et al. 1997; Jimenez-Heffernan et al. 1995; Prevot et al. 1994). Surprisingly, adrenal dysregulation of glucocorticoid levels, which is common in HIV patients may substantially promote latent EBV reactivation (Cacioppo et al. 2002). Acute EBV infection can potentially cause adrenalitis (Hertel et al. 1987).
There are also other frequent viruses that have a role in adrenal disease, such as newborn infections with echoviruses, which are linked to deadly disseminated intravascular coagulation, which causes severe damage due to multiple organ damage and adrenal hemorrhagic necrosis (Ventura et al. 2001; Speer and Yawn 1984; Mostoufizadeh et al. 1983; Reyes et al. 1983; Berry and Nagington 1982; Wreghitt et al. 1989). In neonates, Herpes simplex virus (HSV) can potentially cause injury to the adrenals (Nakamura et al. 1985). According to studies on mice, HSV-1 or HSV-2 causes fast infection of the CNS via adrenal gland (Irie et al. 1987; Hill et al. 1986; Aita et al. 2001). Adrenal glands contain the highest proportion of virus particles of any organ in the early stages of HSV-1 and HSV-2 infections (Potratz et al. 1986). In deadly infections with filoviruses, such as the Ebola virus, colossal apoptotic lysis of cells in numerous organs including liquefaction of the adrenals, has been documented (Mahanty and Bray 2004). Although the Marburg virus has low fatality rate, it can nevertheless harm the adrenal, particularly the cortical cells (Mahanty and Bray 2004; Geisbert and Jaax 1998). Similarly, an arenavirus, Lassa virus is also reported to infect the adrenals (Edington and White 1972). Influenza virus type A has also been suggested to potentially influence ACTH production and its release (Jefferies et al. 1998). The Influenza virus was found to be more fatal in adrenal insufficiency patients (Skanse and Miorner 1959). A study on avian influenza A virus suggests that H5N1 avian Influenza A virus has capacity to cause severe adrenal damage (Lee et al. 2005). The reports for involvement of viruses in adrenal infections are more in comparison with the bacterial involvement. Similar to the studies on bacteria, virus infected adrenal disease reports also fails to provide a direct association with adrenal insufficiency and AAD. Future studies on both animal and human subjects are needed to reach up to any concrete conclusion for the involvement of viruses in AAD.
2.2.1 SARS-CoV-2
In addition to the above reported virus infections, recently emerged causative agent of COVID-19 disease, i.e. severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) may also be involved in adrenal dysfunction (Ding et al. 2004). However, the cellular abnormalities found in the adrenals could be related to the virus’s direct cytopathic effects or systemic inflammatory reactions. Coronavirus can create peptides that are molecular mimics of ACTH, disrupts the host’s corticosteroid stress response (Wheatland 2004). Antibodies to viral peptides that bind to both the host’s ACTH and the viral protein impair the host’s capacity to release corticosteroids, resulting in adrenal insufficiency. Moreover, the use of glucocorticoids to treat coronavirus infection has been proposed as a way to prevent or modify the infection (Wheatland 2004). The COVID-19 was recently exposed to a 19-year-old female with a medical history of Raynaud’s phenomenon, and she tested positive for the disease (Bhattarai et al. 2021). The study reported primary adrenal insufficiency due to COVID-19 infection (Bhattarai et al. 2021). The COVID-19 illness and endocrine abnormalities have also been addressed by the European Society of Endocrinology (ESE) (Puig-Domingo et al. 2020). Moreover, a 32-year-old woman with autoimmune polyglandular syndrome type 1 (APS-1) was reported to develop COVID-19. The patient’s clinical, immunological and genetic patterns confirmed autoimmune polyendocrinopathy–candidiasis–ectodermaldystrophy (APECED), also known as APS-1 (Betterle et al. 2012; Beccuti et al. 2020). Similarly, a case study on 51-year-old man confirmed with COVID-19 infection exhibited probability of an underlying cortisol deficiency (Hashim et al. 2021). Furthermore, COVID-19 was found in 64-year-old lady with type 2 diabetes and hypothyroidism, who had nausea, vomiting, and abdominal pain since 1 week. The patient exhibited presence of 21-hydroxylase antibodies, high ACTH level and low cortisol level, indicative of Addison’s disease. After infection, the COVID-19 disease might have contributed to rapid, clinically meaningful disease progression. The study suggested that the development of AAD could be linked to a previous COVID-19 infection in the patient (Sánchez et al. 2022).
These case reports offer important insights into the COVID-19 related serious and rare medical conditions including adrenal insufficiency which can be unmasked by SARS-CoV-2 infection; thereby, rendering diagnostic and treatment processes complicate.
3 Role of Gut Microbiota in Autoimmune Addison’s Disease
The gut microbiota plays a crucial role in shaping activity of the hypothalamus-pituitary-adrenocortical (HPA) axis (Herman et al. 2016). Nevertheless, knowledge on microbiota’s effect on adrenals, pituitary, and hypothalamus is scarce (Sudo et al. 2004). The influence of microbiota on the acute restraint stress (ARS) response in the adrenal gland, pituitary, and gut (an organ of extra-adrenal glucocorticoid production) was investigated in a study on germ free (GF) mice (Vagnerová et al. 2019). A study using SPF and GF male BALB/c mice found that the GF mice’s plasma corticosterone reaction to ARS was higher than that of SPF mice (Vagnerová et al. 2019). ARS substantially activated the steroidogenic pathway in the adrenals at the levels of the steroidogenic transcriptional regulator Sf-1, cholesterol transporter Star, and Cyp11a1 (the first enzyme in the steroidogenic pathway). These findings show that the gut microbiota influences the adrenals’ and microbiota’s responses (Vagnerová et al. 2019). Although studies on the HPA axis and the neuroendocrine system have indicated the significance of gut microbiota in modulating adrenal glands, there is no clear indication whether it improves or worsens the adrenal function (Vagnerová et al. 2019; Farzi et al. 2018). So far, there is no study reported on the impact of gut microbiota on AAD. Upcoming research should be more focused on finding the role of intestinal microbiota in AAD and how they can be targeted for improving adrenal functions.
4 Role of Probiotics in Autoimmune Addison’s Disease
Probiotics have been found to have potential ameliorative benefits in the prevention and treatment of a wide range of systemic diseases in both animal and human investigations. Rheumatoid arthritis, ulcerative colitis, multiple sclerosis, and hepatic encephalopathy are some of the examples of inflammatory and autoimmune disorders in which probiotics have been found useful (Liu et al. 2018). The regulation of immune system function, which is typically reliant on the strain of probiotic bacteria, is one of the major benefits of probiotics. Some strains have been shown to stimulate the immune response, and thereby rendering it beneficial to patients with immune deficiencies (Ishizaki et al. 2017). Although, previous reports suggested the crucial role of probiotics in ameliorating the dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis induced by stress, but there is no report available which directly indicates role of probiotics in benefiting either adrenal complications or AAD (Eutamene and Bueno 2007; Smith et al. 2014). Therefore, future studies should be targeted for exploring the potential of probiotics in improving the adrenal functions in AAD.
5 Conclusion
Many organ specific autoimmune diseases including type 1 diabetes have been related to enteroviruses; however, the active involvement of any infectious agents in AAD has yet to be established. The immune system majorly target and coordinately attack on steroidogenic cells of the adrenal cortex by 21OH (one dominant self-antigen), and it is markedly similar to the immune system’s attack on viruses, CD4+ and CD8+ T cells, and antibodies specific for an intracellular antigen (Fig. 7.1). In addition, epidemiological research revealed that infections may have a role in the development of AAD. The protracted subclinical phase of AAD, on the other hand, makes it difficult to identify any probable viruses or bacteria that might play a role in the pathogenesis at early stages. Viruses such as CMV, EBV, and HSV-1 are involved in the AAD pathogenesis; however, more extensive research is needed to identify the exact pathomechanisms and other pathogens contributing to AAD. Furthermore, there are no studies that indicate the possible relevance of gut microbiota dysbiosis and probiotics in the treatment of AAD; therefore, animal model and clinical investigations are required. Certain studies, on the other hand, offer potential ways for taking use of AAD’s extraordinarily high heritability and gathering a large cohort of families with ADD aggregation (Skov et al. 2017; Mitchell and Pearce 2012). All family members could then be tracked prospectively and examined for evidence of functional adrenal impairment as well as exposure to infectious agents or other environmental factors.
References
Addison T (2009) On the constitutional and local effects of disease of the supra-renal capsules. BMJ Clin Res Ed 339:b4183
Aita K, Irie H, Koyama AH, Fukuda A, Yoshida T, Shiga J (2001) Acute adrenal infection by HSV-1: role of apoptosis in viral replication. Arch Virol 146:2009–2020
Angulo JC, Lopez JI, Flores N (1994) Lethal cytomegalovirus adrenalitis in a case of AIDS. Scand J Urol Nephrol 28:105–106
Arlt W, Allolio B (2003) Adrenal insufficiency. Lancet 361:1881–1893
Beccuti G, Ghizzoni L, Cambria V et al (2020) A COVID-19 pneumonia case report of autoimmune polyendocrine syndrome type 1 in Lombardy, Italy: letter to the editor. J Endocrinol Investig 43(8):1175–1177
Bensing S, Brandt L, Tabaroj F et al (2008) Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clin Endocrinol 69:697–704
Bensing S, Hulting AL, Husebye ES, Kampe O, Lovas K (2016) Management of endocrine disease: epidemiology, quality of life and complications of primary adrenal insufficiency: a review. Eur J Endocrinol 175:R107–R116
Berry PJ, Nagington J (1982) Fatal infection with echovirus 11. Arch Dis Child 57:22–29
Betterle C, Dal PC, Mantero F, Zanchetta R (2002) Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev 23:327–364
Betterle C, Ghizzoni L, Cassio A et al (2012) Autoimmune-polyendocrinopathy-candidiasis-ectodermal-dystrophy in Calabria: clinical, immunological and genetic patterns. J Endocrinol Investig 35:877–881
Bhatia EA, Jain SK, Gupta RK, Pandey R (1998) Tuberculous Addison’s disease: lack of normalization of adrenocortical function after anti-tuberculous chemotherapy. Clin Endocrinol 48:355–359
Bhattarai P, Allen H, Aggarwal A, Madden D, Dalton K (2021) Unmasking of Addison’s disease in COVID-19. SAGE Open Med Case Rep 9:2050313X211027758
Bocca AL, Amaral AC, Teixeira MM, Sato PK, Shikanai-Yasuda MA, Soares Felipe MS (2013) Paracoccidioidomycosis: ecoepidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol 8:1177–1191
Bornstein SR (2009) Predisposing factors for adrenal insufficiency. N Engl J Med 360:2328–2339
Bosworth D (1979) Reversible adrenocorticol insufficiency in fulminant meningococcemia. Arch Intern Med 139:823–824
Bratland E, Husebye ES (2011) Cellular immunity and immunopathology in autoimmune Addison’s disease. Mol Cell Endocrinol 336:180–190
Cacioppo JT, Kiecolt-Glaser JK, Malarkey WB et al (2002) Autonomic and glucocorticoid associations with the steady-state expression of latent Epstein–Barr virus. Horm Behav 42:32–41
Christen U, Hintermann E (2018) Autoantibodies in autoimmune hepatitis: can epitopes tell us about the etiology of the disease? Front Immunol 9:163
Ding Y, He L, Zhang Q et al (2004) Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol 203:622–630
Doherty S (2001) Fatal pneumococcal Waterhouse–Friderichsen syndrome. Emerg Med 13:237–239
Dore G, Marriott D, Duflou J (1995) Clinico-pathological study of cytomegalovirus (CMV) in AIDS autopsies: under-recognition of CMV pneumonitis and CMV adrenalitis. Aust NZ J Med 25:503–506
Edington GM, White HA (1972) The pathology of Lassa fever. Trans R Soc Trop Med Hyg 66:381–389
Eledrisi M, Verghese A (2001) Adrenal insufficiency in HIV infection; a review and recommendations. Am J Med Sci 321:137–144
Ercolini AM, Miller SD (2009) The role of infections in autoimmune disease. Clin Exp Immunol 155:1–15
Erichsen MM, Lovas K, Skinningsrud B et al (2009) Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: observations from a Norwegian registry. J Clin Endocrinol Metab 94:4882–4890
Eutamene H, Bueno L (2007) Role of probiotics in correcting abnormalities of colonic flora induced by stress. Gut 56(11):1495–1497
Farzi A, Fröhlich EE, Holzer P (2018) Gut microbiota and the neuroendocrine system. Neurotherapeutics 15(1):5–22
Geisbert TW, Jaax NK (1998) Marburg hemorrhagic fever: report of a case studied by immunohistochemistry and electron microscopy. Ultrastruct Pathol 22:3–17
Gertner M, Rodriguez L, Barnett SH, Shah K (1992) Group A betahemolytic Streptococcus and Waterhouse–Friderichsen syndrome. Pediatr Infect Dis J 11:595–596
Givner LB (1998) Invasive disease due to group A beta-hemolytic streptococci: continued occurrence in children in North Carolina. South Med J 91:333–337
Glasgow B, Steinsapie K, Anders K, Layfield L (1985) Adrenal pathology in the acquired immune deficiency syndrome. Am J Clin Pathol 84:594–597
Glaziou P, Sismanidis C, Floyd K, Raviglione M (2014) Global epidemiology of tuberculosis. Cold Spring Harb Perspect Med 5:a017798
Goodnow CC (2007) Multistep pathogenesis of autoimmune disease. Cell 130:25–35
Guenthner EE, Rabinowe SL, Van Niel A, Naftilan A, Dluhy RG (1984) Primary Addison’s disease in a patient with the acquired immunodeficiency syndrome. Ann Intern Med 100:847–848
Hamilton D, Harris MD, Foweraker J, Gresham GA (2004) Waterhouse–Friderichsen syndrome as a result of non-meningococcal infection. J Clin Pathol 57:208–209
Hashim M, Athar S, Gaba WH (2021) New onset adrenal insufficiency in a patient with COVID-19. BMJ Case Rep 14(1):e237690
Herman JP, McKlveen JM, Ghosal S et al (2016) Regulation of the hypothalamic–pituitary–adrenocortical stress response. Compr Physiol 6:603–621
Hertel NT, Jacobsen BB, Pedersen FK, Heilmann C (1987) Adrenocortical insufficiency associated with Epstein–Barr virus infection in a patient with the Wiskott–Aldrich syndrome. Eur J Pediatr 146:603–604
Hill TJ, Yirrell DL, Blyth WA (1986) Infection of the adrenal gland as a route to the central nervous system after viraemia with herpes simplex virus in the mouse. J Gen Virol 67(Pt 2):309–320
Hofbauer LC, Heufelder AE (1996) Endocrine implications of human immunodeficiency virus infection. Medicine (Baltimore) 75:262–278
Hori K, Yasoshima H, Yamada A, Sakurai K, Ohkubo E, Kubota A et al (1998) Adrenal hemorrhage associated with Klebsiella oxytocabacteremia. Intern Med 37:990–994
Hoshino Y, Nagata Y, Gatanaga H et al (1997) Cytomegalovirus (CMV) retinitis and CMV antigenemia as a clue to impaired adrenocortical function in patients with AIDS. AIDS 11:1719–1724
Huang Y-W, Chang C-C, Sun H-Y, Chen M-Y, Hung C-C, Chang S-C (2004) Primary adrenal insufficiency in patients with acquired immunodeficiency syndrome: report of four cases. J Microbiol Immunol Infect 37:250–253
Hyoty H (2016) Viruses in type 1 diabetes. Pediatr Diabetes 17(Suppl 22):56–64
Ip M, Teo JG, Cheng AF (1995) Waterhouse–Friderichsen syndrome complicating primary biliary sepsis due to Pasteurella multocida in a patient with cirrhosis. J Clin Pathol 48:775–777
Irie H, Harada Y, Kurokawa E et al (1987) Early adrenal infection by herpes simplex virus type-1 (Miyama + GC strain): special reference to inoculation dose and spread from the adrenal to the central nervous system. Virchows Arch B Cell Pathol Incl Mol Pathol 53:325–331
Ishizaki A, Bi X, Nguyen LV et al (2017) Effects of short-term probiotic ingestion on immune profiles and microbial translocation among HIV-1-infected vietnamese children. Int J Mol Sci 18:E2185
Jefferies WM, Turner JC, Lobo M, Gwaltney JM Jr (1998) Low plasma levels of adrenocorticotropic hormone in patients with acute influenza. Clin Infect Dis 26:708–710
Jimenez-Heffernan JA, Hardisson D, Palacios J, Garcia-Viera M, Gamallo C, Nistal M (1995) Adrenal gland leiomyoma in a child with acquired immunodeficiency syndrome. Pediatr Pathol Lab Med 15:923–929
Karakousis PC, Page KR, Varello MA, Howlett PJ, Stieritz DD (2001) Waterhouse–Friderichsen syndrome after infection with group A streptococcus. Mayo Clin Proc 76:1167–1170
Kelestimur F (2004) The endocrinology of adrenal tuberculosis: the effect of tuberculosis on the hypothalamic-pituitary–adrenal axis and adrenocortical function. J Endocrinol Investig 27:380–386
Kelestimur F, Unlu Y, Ozesmi M, Tolu I (1994) A hormonal and radiological evaluation of adrenal gland in patients with acute or chronic pulmonary tuberculosis. Clin Endocrinol 41:53–56
Kong MF, Jeffcoate W (1994) Eighty-six cases of Addison’s disease. Clin Endocrinol 2:757–761
Lam K, Lo C (2001) A critical examination of adrenal tuberculosis and a 28-year autopsy experience of active tuberculosis. Clin Endocrinol 54:633–639
Lassmann H, Niedobitek G, Aloisi F, Middeldorp JM, NeuroproMiSe (2011) EBV Working Group. Epstein–Barr virus in the multiple sclerosis brain: a controversial issue – report on a focused workshop held in the Centre for Brain Research of the Medical University of Vienna, Austria. Brain 134:2772–2786
Laureti S, Vecchi L, Santeusanio F, Falorni A (1999) Is the prevalence of Addison’s disease underestimated? J Clin Endocrinol Metab 84:1762
Lee CW, Suarez DL, Tumpey TM et al (2005) Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J Virol 79:3692–3702
Liu Y, Alookaran JJ, Rhoads JM (2018) Probiotics in autoimmune and inflammatory disorders. Nutrients 10(10):1537
Lovas K, Loge JH, Husebye ES (2002) Subjective health status in Norwegian patients with Addison’s disease. Clin Endocrinol 56:581–588
Mahanty S, Bray M (2004) Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis 4:487–498
Marik PE, Kiminyo K, Zaloga GP (2002) Adrenal insufficiency in critically ill patients with human immunodeficiency virus. Crit Care Med 30:1267–1273
Mason AS, Meade TW, Lee JA, Morris JN (1968) Epidemiological and clinical picture of Addison’s disease. Lancet 2:744–747
Mayo J, Collazos J, Martinez E, Ibarra S (2002) Adrenal function in the human immunodeficiency virus-infected patient. Arch Intern Med 162:1095–1098
McKinney WP, Agner RC (1989) Waterhouse–Friderichsen syndrome caused by Haemophilus influenzae type b in an immunocompetent young adult. South Med J 82:1571–1573
Mirza I, Wolk J, Toth L, Rostenberg P, Kranwinkel R, Sieber SC (2000) Waterhouse–Friderichsen syndrome secondary to Capnocytophaga canimorsus septicemia and demonstration of bacteremia by peripheral blood smear. Arch Pathol Lab Med 124:859–863
Mitchell AL, Pearce SH (2012) Autoimmune Addison disease: pathophysiology and genetic complexity. Nat Rev Endocrinol 8:306–316
Morrison U, Taylor M, Sheahan DG, Keane CT (1985) Waterhouse–Friderichsen syndrome without purpura due to Haemophilus influenzae group B. Postgrad Med J 61:67–68
Mostoufizadeh M, Lack EE, Gang DL, Perez-Atayde AR, Driscoll SG (1983) Postmortem manifestations of echovirus 11 sepsis in five newborn infants. Hum Pathol 14:818–823
Nakamine H, Shimizu E, Nishino E, Takenaka T, Maeda J, Hanaoka M (1987) Autopsy findings in a Japanese patient with acquired immunodeficiency syndrome. Acta Pathol Jpn 37:1797–1809
Nakamura Y, Yamamoto S, Tanaka S et al (1985) Herpes simplex viral infection in human neonates: an immunohistochemical and electron microscopic study. Hum Pathol 16:1091–1097
Neufeld M, Maclaren NK, Blizzard RM (1981) Two types of autoimmune Addison’s disease associated with different polyglandular autoimmune (PGA) syndromes. Medicine 60:355–362
Ohsawa M, Tomita Y, Hashimoto M, Yasunaga Y, Kanno H, Aozasa K (1996) Malignant lymphoma of the adrenal gland: its possible correlation with the Epstein–Barr virus. Mod Pathol 9:534–543
Ohshima K, Kobari S, Kuroiwa S et al (1997) Heterogeneity of systemic extra-nodal Epstein–Barr virus-associated lympho-histiocytic tumor—ten autopsy cases of human immunodeficiency virus-negative Japanese. Pathol Res Pract 193:257–265
Paolo WF Jr, Nosanchuk JD (2006) Adrenal infections. Int J Infect Dis 10:343–353
Piccioli A, Chini G, Mannelli M, Serio M (1994) Bilateral massive adrenal hemorrhage due to sepsis: report of two cases. J Endocrinol Investig 17:821–824
Potratz D, Brake B, Dienes HP et al (1986) Herpes simplex virus type 1 and 2 in the adrenal glands: replication and histopathology. Arch Virol 90:207–222
Prevot S, Neris J, de Saint Maur PP (1994) Detection of Epstein–Barr virus in an hepatic leiomyomatous neoplasm in an adult human immunodeficiency virus 1-infected patient. Virchows Arch 425:321–325
Puig-Domingo M, Marazuela M, Giustina A (2020) COVID-19 and endocrine diseases a statement from the European Society of Endocrinology. Endocrine 68:2–5
Reyes MP, Ostrea EM Jr, Roskamp J, Lerner AM (1983) Disseminated neonatal echovirus 11 disease following antenatal maternal infection with a virus-positive cervix and virus-negative gastrointestinal tract. J Med Virol 12:155–159
Rodrigues D, Reis M, Teixeira V, Silva-Vergara M, Filho DC, Adad S et al (2002) Pathologic findings in the adrenal glands of autopsied patients with acquired immunodeficiency syndrome. Pathol Res Pract 198:25–30
Salim YS, Faber V, Wiik A, Andersen PL, Hoier-Madsen M, Mouritsen S (1988) Anti-corticosteroid antibodies in AIDS patients. APMIS 96:889–894
Sánchez J, Cohen M, Zapater JL, Eisenberg Y (2022) Primary adrenal insufficiency after COVID-19 infection. AACE Clin Case Rep 8(2):51–53
Sellmeyer DE, Grunfeld C (1996) Endocrine and metabolic disturbances in human immunodeficiency virus infection and the acquired immune deficiency syndrome. Endocr Rev 17:518–532
Skanse B, Miorner G (1959) Asian influenza with adrenocortical insufficiency. Lancet 1959(1):1121–1122
Skinningsrud B, Lie BA, Lavant E et al (2011) Multiple loci in the HLA complex are associated with Addison’s disease. J Clin Endocrinol Metab 96:E1703–E1708
Skov J, Hoijer J, Magnusson PKE, Ludvigsson JF, Kampe O, Bensing S (2017) Heritability of Addison’s disease and prevalence of associated autoimmunity in a cohort of 112,100 Swedish twins. Endocrine 58:521–527
Smith CJ, Emge JR, Berzins K et al (2014) Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am J Physiol Gastrointest Liver Physiol 307(8):G793–G802
Soule S (1999) Addison’s disease in Africa – a teaching hospital experience. Clin Endocrinol 50:115–120
Speer ME, Yawn DH (1984) Fatal hepatoadrenal necrosis in the neonate associated with echovirus types 11 and 12 presenting as a surgical emergency. J Pediatr Surg 19:591–593
Suankratay C, Shuangshoti S, Mutirangura A et al (2005) Epstein–Barr virus infection associated smooth-muscle tumors in patients with AIDS. Clin Infect Dis 40:1521–1528
Sudo N, Chida Y, Aiba Y et al (2004) Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol 558:263–275
Ten S, New M, Maclaren N (2001) Clinical review 130: Addison’s disease 2001. J Clin Endocrinol Metab 86:2909–2922
Tsokos M (2003) Fatal Waterhouse–Friderichsen syndrome due to Ewingella americana infection. Am J Forensic Med Pathol 24:41–44
Vagnerová K, Vodička M, Hermanová P et al (2019) Interactions between gut microbiota and acute restraint stress in peripheral structures of the hypothalamic–pituitary–adrenal axis and the intestine of male mice. Front Immunol 10:2655
Ventura KC, Hawkins H, Smith MB, Walker DH (2001) Fatal neonatal echovirus 6 infection: autopsy case report and review of the literature. Mod Pathol 14:85–90
Welch K, Finkbeiner W, Alpers CE, Blumenfeld W, Davis RL, Smuckler EA et al (1984) Autopsy findings in the acquired immune deficiency syndrome. JAMA 252:1152–1159
Wheatland R (2004) Molecular mimicry of ACTH in SARS — implications for corticosteroid treatment and prophylaxis. Med Hypotheses 63:855–862
Wreghitt TG, Sutehall GM, King A, Gandy GM (1989) Fatal echovirus 7 infection during an outbreak in a special care baby unit. J Infect 19:229–236
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Shah, F., Dwivedi, M.K. (2022). Microorganisms in Pathogenesis and Management of Autoimmune Addison’s Disease (AAD). In: Dwivedi, M.K., Sankaranarayanan, A., Kemp, E.H., Shoenfeld, Y. (eds) Role of Microorganisms in Pathogenesis and Management of Autoimmune Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-19-4800-8_7
Download citation
DOI: https://doi.org/10.1007/978-981-19-4800-8_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4799-5
Online ISBN: 978-981-19-4800-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)