Abstract
Autoimmune diseases are characterized by aberrant immune response against host own tissues. Studies have suggested that through molecular mimicry, bystander activation, and cross-reactivity, infections can trigger autoimmune diseases. However, paradoxically recent studies have highlighted the role of bacteria, viruses, and parasites in protection against autoimmune diseases. Epidemiological evidences and hygiene hypothesis also highlight the involvement of microbes in protection against autoimmune diseases. Interestingly, the data suggests increased incidence of the autoimmune diseases in developed countries. Microorganisms can protect against autoimmune diseases by antigenic competition, innate immune mechanisms, immune regulation; however, the detailed mechanisms underlying the involvement of microorganisms in protection of autoimmune diseases is unknown. The detailed understanding of mechanisms involved could lead to efficient therapeutics to treat autoimmune diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Autoimmune disease are chronic disorders characterized by loss of immune tolerance leading to aberrant immune response against hosts own tissues (Wang et al. 2015; Giri et al. 2022). The loss of immune tolerance leads to organ specific or systemic damage to the host (Janeway et al. 2001; Wang et al. 2015). Multiple factors like genetics, epigenetics, stress, environment, tobacco smoke, pharmaceutical agents, hormones, could trigger the development of autoimmune diseases (Costenbader et al. 2012). Moreover, recent studies suggest the role of infections in triggering autoimmune diseases (Arango et al. 2013). Conversely, studies have highlighted that infections can prevent or even suppress the development of autoimmune diseases (Arango et al. 2013).
According to the hygiene hypothesis, the decreased infections may lead to increase in the occurrence of allergies and autoimmune diseases (Sironi and Clerici 2010). The evidence for the hygiene hypothesis has been demonstrated worldwide (Bloomfield et al. 2006; Okada et al. 2010). Additionally, the animal model experiments have provided evidences for the hypothesis (Okada et al. 2010). Furthermore, studies suggest that the decreased rate of infections may be a likely explanation for increased incidence of autoimmune diseases in developed countries (Okada et al. 2010). Moreover, the prevalence of parasitic infections has been associated with increased risk of autoimmune disease (Strachan 2000; Arango et al. 2013). For example, the Schistosoma mansoni infection has been associated with protection of Type 1 diabetes mellitus (T1DM) (Cooke et al. 1999; Zaccone et al. 2003; Arango et al. 2013). Therefore, previous studies suggest that infections may be a potent immune system modulator (Arango et al. 2013).
However, the mechanism explaining the casual link between protective function of infection in development of autoimmunity is unclear. Various factors such as reduced regulatory T (Treg) cells activation, altered pro-inflammatory, and anti-inflammatory cytokine levels, changes in the microbiota may be linked with increased incidence of autoimmune diseases (Moudgil and Choubey 2011; Dwivedi et al. 2013a, 2015, 2017; Giri et al. 2020b, 2021a, 2022). Moreover, studies suggest that infections may suppress variety of autoimmune diseases by modulating immune response, non-specific to the particular pathogen (Sfriso et al. 2010). Given the role of infections in controlling aberrant immune response, this chapter focuses on the involvement of infections in protection of autoimmune diseases.
2 Autoimmune Diseases
Autoimmune diseases are characterized by loss of immune tolerance leading to destruction of bodies own tissues by self-reactive immune cells (Wang et al. 2015; Giri et al. 2022). The prevalence of autoimmune diseases is about 5% worldwide, and they represent a major concern of mortality and morbidity (Leslie and Hawa 1994; Wang et al. 2015). The autoimmune diseases are generally divided into two types organ specific autoimmunity, where the immune system mediated destruction is localized to a particular organ, the other type in systemic autoimmunity, where multiple organs are involved (Janeway et al. 2001). Despite enormous research in the field there is no cure for most of the autoimmune diseases, and the current therapeutics mostly focus on symptomatic relief (Chandrashekara 2012).
The exact triggering factor is unknown but multiple factors such as environment, genetics, epigenetics, tobacco smoke, infections may be responsible for the triggering the development of autoimmune response (Giri et al. 2022). The initial trigger generally activates the innate immune cells, which leads to activation of antigen presenting cells (APCs) and increased production of pro-inflammatory cytokines (Gandhi et al. 2010; Thanapati et al. 2017; Giri et al. 2022). The activated APCs stimulate the adaptive immune response by activating self-reactive CD4+ and CD8+ T cells (Skapenko et al. 2005; Giri et al. 2022). The self-reactive CD4+ T cells further aids in activating self-reactive CD8+ T cells and B cells, which exacerbates the autoimmune response (Skapenko et al. 2005; Giri et al. 2022). Additionally, the self-reactive CD4+ T cells mediate autoimmune response by FAS-FASL-mediated apoptosis (Tateyama et al. 2000; Giri et al. 2020b).
The self-reactive CD8+ T cells are the major culprits of the autoimmune response that mediate autoimmunity by production of the cytotoxic granules like granzyme B and perforin, resulting in apoptosis of target cells (Janeway et al. 2001). Additionally, they exacerbate the tissue damage by production of pro-inflammatory cytokines and FAS-FASL mediated apoptosis (Tateyama et al. 2000; Giri et al. 2020b). Apart from this, autoreactive B cells produce autoantibodies, which are the hallmark of various autoimmune diseases like RA, MS, SLE, and T1DMM (Hampe 2012). The autoantibodies after binding to the cellular receptors mediate cell lysis through complement activation and antibody-dependent cellular toxicity (Hampe 2012).
The subset of CD4+ T cells known as regulatory T cells (Tregs), maintains immune tolerance by suppressing such self-reactive T and B cells (Dwivedi et al. 2013a, 2015; Giri et al. 2020a, 2021c). However, studies suggest that the decreased expression of FOXP3 (the master regulator of Tregs), leads to quantitate and functional Tregs defects in various autoimmune diseases (Long and Buckner 2011; Dwivedi et al. 2013b; Giri et al. 2020a, b). Overall, the initial trigger of autoimmune response and failure of immunological tolerance leads to widespread activation of self-reactive T and B cells contributing to pathogenesis of autoimmune diseases (Giri et al. 2022).
3 Proposed Mechanisms for Protective Effect of Infections on Autoimmune Diseases
Compelling evidence suggest correlation between decreased incidence of infections and increase in occurrence of autoimmune diseases and allergies in developed north American and European countries (Gale 2002; Mayr et al. 2003; Joner et al. 2004; Zaccone et al. 2006; Okada et al. 2010). There is an increase in the development of T1DM and multiple sclerosis for the past decade in the Western countries (Bach 2009; Okada et al. 2010). Such trend is not observed in less developed countries. Moreover, such high incidence cannot be attributed solely to genetic factors since such increased autoimmune disease incidence have also been observed in immigrated families (Detels et al. 1972; Leibowitz et al. 1973; Bodansky et al. 1992; Symmons 1995; Staines et al. 1997; Hammond et al. 2000; Okada et al. 2010). The mechanism of protective effect mediated by infections on autoimmune diseases is multifactorial (Okada et al. 2010). Here, we discuss certain mechanisms like antigenic competition, innate immune mechanisms, immune regulation, mediated by infections which could lead to protection against autoimmune diseases.
3.1 Antigenic Competition
Antigenic competition is defined by diminished immune response to one antigen in the presence of another antigen (Pross and Eidinger 1974; Liacopoulos and Ben-Efraim 1975; Bach 2001). It occurs between closely related and unrelated antigens (Fujinami and Oldstone 1989; Oldstone 1998; Bach 2001). The phenomena are well studied in multicomponent vaccines such as diphtheria–pertussis–tetanus (DPT), and Haemophilus influenzae-tetanus vaccines (Table 4.1) (Halperin et al. 1999; Jatana and Nair 2007). Additionally, envelope component of human immunodeficiency virus (HIV) vaccine leads to reduced CD4+ T cells response against Gag/Pol antigens due to antigenic competition (Table 4.1) (Kallas et al. 2019). Moreover, similar phenomena have been observed in case of influenza virus, where the antigenic competition leads to increased immune response against hemagglutinin and decreased immune response against neuraminidase (Table 4.1) (Johansson 1988). This antigenic competition may be due to presence of multiple components or multiple antigens (Bach 2001). In some cases, the antigenic competition could lead to one antigen being dominated and other being suppressed or in other cases both the antigens can be mutually suppressed (Bach 2001).
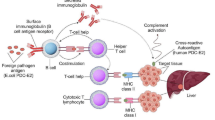
In the cases of autoimmune diseases, the infections can lead to increased competition with self-antigens, which could result in suppressed autoimmune response (Hara and Iwasa 2020). For example, administration of Streptococcal and Klebsiella extracts significantly reduces diabetes in non-obese diabetic (NOD) mice (Toyota et al. 1986; Saï and Rivereau 1996). Moreover, the immunization against bovine serum albumin (BSA) drastically reduces thyroiditis (McMaster and Kyriakos 1970). There may be multiple underlying mechanisms through which these infections can induce antigenic competition. The activation of adaptive immune response is triggered by antigen presenting cells (APCs). The APCs through the process of phagocytosis present the antigens on their surface (Janeway et al. 2001). These phagocytosis and subsequent antigen presentation process could be subject to saturation (Fig. 4.1) (Babbitt et al. 1986; Adorini et al. 1988; Bach 2001). Moreover, Fc receptors may also be saturated due to presence of antibodies against particular pathogens (Fig. 4.1) (Babbitt et al. 1986; Adorini et al. 1988; Bach 2001). Furthermore, evidences suggest the major histocompatibility complex (MHC) may be saturated by the presence of foreign antigens (Fig. 4.1) (Babbitt et al. 1986; Adorini et al. 1988; Bach 2001). Thus, the presence of infectious pathogen through antigenic competition could lead to reduced immune response against self-antigens contributing to decreased autoimmunity (Babbitt et al. 1986; Adorini et al. 1988; Bach 2001).
Proposed mechanism for the beneficial role of infections in protection towards autoimmune diseases. Increased consumption of IL-2 by pathogen specific T cells, can lead to reduced activation of self-reactive CD4+ T cells, which could contribute to reduced activation of self-reactive B and cytotoxic T cells leading to decreased autoimmune response. Additionally, pathogen specific antibodies can be more immunodominant compared to autoantibodies. Moreover, saturation of antigen presentation process and antibody production can lead to decreased self-reactive T and B cells response. Apart from this, the binding of fungi to TLR-2 promotes Th2 response. Additionally, soluble antigens of worm induce NKT cells which inhibit autoimmune response. Furthermore, helminths activate a subset of macrophage that produce anti-inflammatory cytokines like IL-10, IL-13, and TGF-β, which recruit Tregs. Overall pathogens through antigen competition, innate immune mechanisms and immune tolerance suppress autoimmune response
Furthermore, B cells also act as APCs, and the complex procedure of processing antigens, B cell differentiation, proliferation, and antibody production could lead to antigenic competition through various mechanisms (Bach 2001). The antibody directed towards the pathogen could be more immunodominant compared to self-antigens: as it may be present in a relatively higher numbers or it may have high affinity and avidity. Moreover, B cells precursors could be present in higher numbers for the particular antigen (Adorini 1998; Bach 2001, 2005; Gaisford and Cooke 2009). Thus, the antigenic competition mediated antibody against pathogen could lead to reduced autoimmune response.
Apart from this, restriction of the activated CD4+ T cells’ number could also influence B cells help. The presence of pre-existing pathogen specific T cells could interfere in activation of self-reactive T cells by consumption of IL-2 (Chatenoud et al. 1986; Bach 2001). Moreover, closely related antigens can act as T cells antagonist and can inhibit the activation of T cells specific to self-antigens (Fig. 4.1) (Chatenoud et al. 1986; Bach 2001). Furthermore, immune responses against pathogens could induce Treg cells which could suppress self-reactive T and B cells (Fig. 4.1) (Bach 2001). Thus, the decreased activation of self-reactive CD4+ T cells could contribute to reduced activation of self-reactive B and cytotoxic T cells leading to decreased autoimmune response.
3.2 Innate Immune Mechanisms
Autoimmune diseases are characterized by specific adaptive immune response against the host cells; however, the role of innate immune response cannot be denied in autoimmune diseases (Waldner 2009). Specifically, studies have suggested the involvement of toll like receptors (TLRs) in development of autoimmune response, in RIP-LCMV mice where, TLR3 binding and subsequent IFN-α production is crucial in development of autoimmunity (Bach 2005; Lincez et al. 2021). However, in vivo and in vitro studies have suggested that TLR-dependent production of IL-10 and TGF-β are crucial in regulation of autoimmunity (Table 4.1) (Bach 2005; Lang et al. 2005).
Microbial infections, and commensal bacteria can modulate the immune response by binding to various TLRs (Fig. 4.1) (Bach 2005). The binding of pathogens to the TLRs could lead to production of vast array of cytokines which could include regulatory cytokines (Bach 2005). For example, the binding of fungi to TLR2 could activate Th2 type response (Table 4.1, Fig. 4.1) (Bartemes and Kita 2018). Moreover, fungi can regulate the inflammatory response through activating Tregs and production of anti-inflammatory cytokines (Table 4.1) (Bartemes and Kita 2018). This could lead to suppression of self-reactive T and B cells leading to reduced autoimmune response. Moreover, they are known to induce Treg cells by binding to TLR2 on dendritic cells (DCs) (Fig. 4.1) (Van der Kleij et al. 2002; Oliveira-Nascimento et al. 2012). Additionally, soluble antigens of worm induce NKT cells which inhibit T1DM (Fig. 4.1) (Zaccone et al. 2003). Moreover, superantigens such as Staphylococcal enterotoxin B are known to suppress the pathogenesis of experimental allergic encephalomyelitis and collagen-induced arthritis by inhibiting Vβ T cell subsets (Prabhu Das et al. 1996; Bach 2001). Overall, these studies highlight the importance of innate immune mechanisms in suppression of autoimmunity.
3.3 Infections Induced Immunoregulation
The suppressive effect induced against a particular antigen could through bystander activation suppress the autoimmune response (Bach 2005). Therefore, the Treg cells activated in response to the particular antigen could in turn dampen the autoimmune response (Bach 2005). The mechanisms involved could be by enhancement of Th2 cells which could suppress the inflammatory response leading to protection against autoimmune diseases (Table 4.1).
Experimental evidence suggests that administration of gram-positive bacterial extract in NOD mice enhances the anti-inflammatory cytokine TGF-β production resulting in suppression of T1DM (Alyanakian et al. 2006). Furthermore, helminths activate a subset of macrophage that produce anti-inflammatory cytokines like IL-10, IL-13, and TGF-β, which recruit Tregs (Fig. 4.1) (McSorley and Maizels 2012). Furthermore, in MS, mycobacterial infections control trafficking of autoreactive T cells (Lee et al. 2008; Gaisford and Cooke 2009). Moreover, in T1DM the Salmonella typhimurium infection has been linked with inhibition of trafficking of autoreactive T cells trafficking to pancreas (Table 4.1) (Raine et al. 2006; Gaisford and Cooke 2009).
Several viruses have tropism towards immune cells and viral infections such as LCMV could infect the immune cells (Zinkernagel et al. 1999; Bach 2001). This could lead to reduction in autoreactive immune cells, resulting in suppression of autoimmune responses (Bach 2001). The most evident case of infection induced immunosuppression is of human immunodeficiency virus, which is known to infect CD4+ T cells, leading to depletion of the host immune response (Bach 2001). Moreover, viral infections could lead to increased production of IFN-β, an immunoregulatory cytokine (Bach 2001). Additionally, the IFN-β immunomodulatory properties have been used in the treatment of multiple sclerosis (Bach 2001). Interestingly a study has highlighted that lymphocytic choriomeningitis virus (LCMV) infection in diabetic NOD mice, delayed the onset of disease (Oldstone et al. 1990; Bach 2001). This could be due to suppression of CD8+ T cells by TGF-β producing Treg cells.
Overall, the studies suggest microorganisms and viruses could suppress the ongoing autoimmune diseases by suppressing pro-inflammatory Th1 response and promoting anti-inflammatory Th2 response (Fig. 4.1) (Bach 2001, 2005; Gaisford and Cooke 2009). However, detailed mechanistic studies assessing how microbes regulate the autoimmune response can lead novel therapeutics for autoimmune diseases. Additionally, studies will highlight the complex interactions between microbes and cellular signaling pathways involved in development of autoimmunity.
4 Epidemiological Evidence for the Protective Role of Infections in Human Autoimmune Diseases
Incidence of autoimmune diseases including MS, RA, T1DM have increased dramatically over the past few decades (Poser et al. 1989; Green and Patterson 2001; Myasoedova et al. 2010). Furthermore, this increased incidence has been observed prominently in the developed countries (Bach 2001). Epidemiological data suggests the increased incidence of MS, T1DM and Crohn’s diseases in North America and Europe (Bauer 1987; Bach 1994; Kurtzke 1995; Green and Patterson 2001). The increased incidence cannot be solely linked with genetics (Okada et al. 2010). Interestingly recent evidence has suggested changes in lifestyle in the developed countries could lead to increased occurrence of allergic and autoimmune diseases (Okada et al. 2010). Moreover, according to the hygiene hypothesis the decreasing incidence of infections in developed countries could lead to increased incidence of allergic and autoimmune diseases (Okada et al. 2010). The hygiene hypothesis is supported by the fact that the autoimmune diseases have increased in immigrants from low income countries to developed countries (Okada et al. 2010).
In developing countries, the incidence of asthma, allergic rhinitis and atopic dermatitis has increased by over 15% in United Kingdom, New Zealand, and Australia (Okada et al. 2010). Moreover, there is an increased prevalence of autoimmune diseases such T1DM in European countries such as Finland (Harjutsalo et al. 2008). Furthermore, the prevalence of inflammatory bowel diseases (IBD), such as Crohn’s disease or ulcerative colitis (Bach 2002) and primary biliary cirrhosis (Rautiainen et al. 2007), has increased. Interestingly, the incidence of T1DM and MS has also increased in the Asian and African immigrants in the US (Detels et al. 1972; Staines et al. 1997).
Multiple factors could explain the increased incidence of autoimmune diseases. Recent studies suggest the change in the microbiota and decreased exposure to infections in childhood could lead to autoimmune diseases, by multiple mechanisms like decreased immune regulation, antigenic completion and innate immune factors (Bach 2001, 2005; Gaisford and Cooke 2009). Moreover, studies suggest that increased exposure to farming and cowsheds in early life could prevent atopic diseases (Riedler et al. 2001; Ege et al. 2006). Moreover, exposure to endotoxin in the childhood protects against asthma and atopy (Braun-Fahrländer et al. 2002). Furthermore, Schistosoma infections have also been reported to protect against atopy (Flohr et al. 2006; Okada et al. 2010). Therefore, the detailed understanding of the role of microorganisms could lead to potent therapeutics for treatment of autoimmune diseases.
5 Animal Model Studies for Exploring the Protective Effects of Infections on Autoimmune Diseases
Studies have suggested a strong correlation between infections and incidence of autoimmunity (Okada et al. 2010). Here, we discuss few animal model studies which suggest the role of infections towards protection of autoimmune diseases.
5.1 Type 1 Diabetes Mellitus
Type 1 diabetes mellitus (T1DM) is an autoimmune disease characterized by loss of insulin producing β-cells (Hara et al. 2013). The exact etiology is unknown, but multiple factors like genetics, autoimmunity, and environmental factors can trigger T1DM. Insulitis and elevated autoantibodies produced against β-cells lead to β-cell death (Pihoker et al. 2005). The destruction of β-cell death leads to symptoms like urination, loss of appetite, fatigue, thirst, and hyperglycaemia (Pihoker et al. 2005).
Although microorganisms are thought to be triggering factors for development of autoimmune diseases, but animal model studies suggest that T1DM is associated with sanitary conditions of animal facilities (Bach 2002). The studies suggest that lower the burden of infection, the higher the incidence of diabetes (Like et al. 1991; Okada et al. 2010). Moreover, infection of NOD mice with bacteria, viruses, and parasites prevents NOD mice from diabetes (Table 4.2) (Bach 2002). Apart from this, probiotics and microbial components also protect NOD mice from diabetes (Petrovsky 2010; Kim et al. 2020). Additionally, exposure to components like soluble worm antigen and soluble egg antigen from Shistosoma mansoni, OM89 and OM85 from Escherichia coli and ES62 from Acanthocheilonema viteae can protect from autoimmune diseases like T1DM, SLE, and RA (Zaccone et al. 2003; Alyanakian et al. 2006; Harnett and Harnett 2006; Toussirot et al. 2006; Gaisford and Cooke 2009).
Additionally, administration with complete Freund’s adjuvant (CFA) in NOD mice has shown to protect from T1DM (Qin et al. 1993). Similarly, Mycobacterium bovis and Mycobacterium avium have shown to suppress autoimmune diabetes (Brás and Águas 1996; Inafuku et al. 2015). Interestingly, infection with viruses such as LCMV, murine hepatitis virus and LDV and Schistosoma mansoni parasites have shown to suppress T1DM in NOD mice (Oldstone et al. 1990; Wilberz et al. 1991; Takei et al. 1992; Bach 2002; Zaccone et al. 2009). Moreover, Pseudomonas aeruginosa signaling molecule, N-(3-oxododecanoyl)-l-homoserine lactone suppresses insulitis and controls T1DM in NOD mice (Pritchard et al. 2005). Additionally, Salmonella typhimurium infection generates immunomodulatory DCs which suppress T1DM in NOD mice (Raine et al. 2006). Moreover, gastrointestinal helminths such as Trichinella spiralis and Heligmosomoides polygyrus inhibit autoimmune diabetes in NOD mice (Saunders et al. 2007).
Although the data suggests the involvement of infections in protection against T1DM; however, the data is currently limited to animal model of diabetes (Bach 2001; Gaisford and Cooke 2009). Moreover, the underlying mechanisms how infections could suppress such autoimmune response is unknown. Infections and microbial components through antigenic competition, innate immune factors, immunoregulation could suppress the pro-inflammatory environment, promote anti-inflammatory cytokines and induce Treg cells, which could control the ongoing autoimmune response (Fig. 4.1) (Bach 2001, 2005; Gaisford and Cooke 2009). However, future animal model studies must explore the underlying mechanisms which could lead to development of potent therapeutics for treatment of T1DM.
5.2 Rheumatoid Arthritis
Rheumatoid arthritis (RA) is an autoimmune disease, characterized by chronic joint inflammation leading to bone and cartilage damage. RA is responsible for significant morbidity as it causes disability, discomfort, stiffness, and decreased life expectancy (Thanapati et al. 2017; Carbone et al. 2020; Giri et al. 2021b). Although the exact etilogy for RA is unknown, multiple factors such as genetics, autoimmunity, environment, diet alcohol, and smoking can trigger RA development (Giri et al. 2022). Although infections are considered to trigger autoimmune RA (Mahajna et al. 2015); however, recent evidence in animal model studies suggests that infections can protect from RA (Table 4.2) (Vischer 1993).
Evidence for pathogen induced RA protection are found in type II collagen-induced arthritis (CIA) arthritis model (Harnett et al. 2008). Studies suggests Syphacia obvelata parasite’s infection suppresses CFA arthritis in rats (Pearson and Taylor 1975). Moreover, S. mansoni infections reduces autoantibodies and pro-inflammatory cytokine production in CIA arthritis models (Osada et al. 2009). Additionally, tapeworm, Hymenolepis diminuta infections in rats induces IL-10 dependent CFA arthritis (Shi et al. 2011). Overall, animal model studies suggest parasite infections can alleviate arthritis pathology by suppressing pro-inflammatory cytokine production and inducing anti-inflammatory cytokine production (McSorley and Maizels 2012). Additionally, bacterial extract OM-89 showed inhibition of arthritis by inducing IL-10 production and suppressing pro-inflammatory cytokine production in RA patients (Toussirot et al. 2006). The above mentioned studies suggest that bacterial and parasitic infections can protect from ongoing autoimmunity in RA by promoting anti-inflammatory response and suppressing pro-inflammatory response. However, future in vitro and in vivo studies are warranted to understand the underlying mechanism for development of potent therapeutics for treatment of RA.
5.3 Multiple Sclerosis
Multiple sclerosis (MS) is a chronic autoimmune neurological disease, characterized by chronic inflammation and demyelination resulting in symptoms such as vision loss, cognitive defects, depression, and bowel defects (Filippi et al. 2018). The pathogenesis of MS is due to autoimmune reactions against myelin proteins and gangliosides (Prat and Martin 2002). Multiple factors such as vitamin D deficiency, intestinal dysbiosis, viral infections, a hypercaloric diet, genetics, and environmental factors can trigger MS development (Milo and Kahana 2010). Although infections, particularly viral infections are considered to trigger MS development, but animal model studies suggest certain bacterial and helminth infection can protect against autoimmune MS (Table 4.2) (Sewell et al. 2003; La Flamme et al. 2003).
Several bacterial and parasitic infections have shown disease protection in experimental autoimmune encephalomyelitis (EAE), the mouse model of MS (Lehmann and Ben-Nun 1992; Sewell et al. 2003; La Flamme et al. 2003; Gruden-Movsesijan et al. 2008). Studies suggest Mycobacteria infection can prevent mice from EAE (Lehmann and Ben-Nun 1992; Sewell et al. 2003). Mycobacterium bovis BCG diverts self-reactive T cells away from the central nervous system (CNS) which then suppresses EAE in mice (Sewell et al. 2003). Moreover, Mycobacterium tuberculosis exposure protects EAE-susceptible mice against induction of disease (Lehmann and Ben-Nun 1992). Moreover, Bordetella pertussis could also protect mice from EAE development (Lehmann and Ben-Nun 1992). Additionally, Escherichia coli, Shigella, and Staphylococcus aureus were found to be effective in suppressing EAE (Lehmann and Ben-Nun 1992). Moreover, S. mansoni parasite has shown to alleviate the EAE pathology (Cleenewerk et al. 2020). It converts the pro-inflammatory Th1/Th17 response to anti-inflammatory Th2 response (Cleenewerk et al. 2020). Interestingly, Heligmosomoides polygyrus infection suppressed the EAE in IL-4Rα-dependent manner (White et al. 2020). Fasciola hepatica infections has been shown to control EAE through TGF-β-Mediated suppression of Th17 and Th1 responses (Walsh et al. 2009). Additionally, Schistosomiasis (a parasitic infection) reduces the inflammation in the CNS, thereby alleviating the EAE pathology (La Flamme et al. 2003). Trichinella spiralis infection also showed to ameliorate the clinical course of EAE in dose dependent manner in Dark Agouti rats (Gruden-Movsesijan et al. 2008).
Overall, the above mentioned studies suggest that bacterial and parasitic infections can control EAE; however, studies in this field are scarce and only limited to animal models of MS. Therefore, future in vitro and in vivo studies must be carried to identify the exact underlying mechanism to explore the role of infections in protection of MS, which will aid in the development of potent therapeutics for treatment of MS.
5.4 Inflammatory Bowel Disease (IBD)
Crohn’s disease and ulcerative colitis are two main inflammatory bowel diseases (Seyedian et al. 2019). They are characterized by chronic inflammation in the digestive tract (Seyedian et al. 2019). The condition ulcerative colitis is characterized by chronic inflammation in the colon and rectum. Crohn’s disease involves chronic inflammation in the lining of digestive tract (Seyedian et al. 2019). IBD is generally characterized by diarrhea, rectal bleeding fatigue, weight loss, abdominal pain, and cramping (Seyedian et al. 2019).
In mouse model of IBD, helminths’ infections have been demonstrated to suppress disease pathology (Summers et al. 2005a, b; McSorley and Maizels 2012). For instance, S. mansoni infection suppressed the IBD by macrophage and IL-10 dependent mechanisms (Cleenewerk et al. 2020). Additionally, Ancylostoma hookworm products’ administration suppressed the colitis (Ruyssers et al. 2008). T. spiralis infections and antigens also suppressed the colitis pathology (Khan et al. 2002; Motomura et al. 2009). Moreover, Schistosome egg has been shown to protect colitis by suppressing the production of pro-inflammatory cytokines as well as by inducing anti-inflammatory cytokines such as IL-10 and TGF-β (Elliott et al. 2003; McSorley and Maizels 2012). Moreover, Heligmosomoides polygyrus was demonstrated to dampen the colitis in an IL-10 deficient manner (Elliott et al. 2004). The Hymenolepis diminuta infections were also able to suppress colitis pathology by suppressing macrophage activation (Johnston et al. 2010), increased IL-10 and Tregs’ production (Johnston et al. 2010; McSorley and Maizels 2012). Additionally, Trichuris suis improved the disease activity index of ulcerative colitis (Summers et al. 2005b). Furthermore, Trichuris suis was also shown to control Crohn’s disease (Summers et al. 2005a).
The suppressive effects of the infections on IBD are not characterized well. However, the findings suggest that the infections suppress the ongoing infections by promoting the production of anti-inflammatory cytokines like IL-10 and TGF-β (McSorley and Maizels 2012). Additionally it induces Tregs and suppresses Th1/Th17 associated cytokines after infection (McSorley and Maizels 2012). Thus, as shown in animal models of IBD the anti-inflammatory environment induced by infections can further lead to development of potent therapeutic strategies for IBD.
6 Conclusions
Infections are one of the major players which modulate the development of autoimmune diseases. Recently, compelling evidences have suggested the role of infections in protection of autoimmune diseases. However, the detailed underlying mechanisms how the infection could protect against autoimmune and allergic diseases are unclear. Therefore, in vitro and in vivo approaches studying the role of infections in suppression of autoimmune diseases are warranted. Moreover, considering ethical limitations for using infections in treatment of human autoimmune diseases, the therapeutic potentials of bacterial extracts in experimental models of autoimmune diseases must be investigated. Overall, a far better understanding for the underlying mechanisms for role of infections in protection of autoimmune diseases could pave a way to novel therapeutics for the treatment of autoimmune diseases.
References
Adorini L (1998) Immunodominance. Encycl Immunol 1290–1292. https://doi.org/10.1006/RWEI.1999.0331
Adorini L, Muller S, Cardinaux F, Lehmann PV, Falcioni F, Nagy ZA (1988) In vivo competition between self peptides and foreign antigens in T-cell activation. Nature 334:623–625. https://doi.org/10.1038/334623a0
Alyanakian M-A, Grela F, Aumeunier A et al (2006) Transforming growth factor-beta and natural killer T-cells are involved in the protective effect of a bacterial extract on type 1 diabetes. Diabetes 55:179–185
Arango M-T, Shoenfeld Y, Cervera R, Anaya J-M (2013) Infection and autoimmune diseases. El Rosario University Press, Bogota
Babbitt BP, Matsueda G, Haber E, Unanue ER, Allen PM (1986) Antigenic competition at the level of peptide-Ia binding. Proc Natl Acad Sci U S A 83:4509–4513. https://doi.org/10.1073/pnas.83.12.4509
Bach JF (1994) Predictive medicine in autoimmune diseases: from the identification of genetic predisposition and environmental influence to precocious immunotherapy. Clin Immunol Immunopathol 72:156–161. https://doi.org/10.1006/CLIN.1994.1122
Bach JF (2001) Protective role of infections and vaccinations on autoimmune diseases. J Autoimmun 16:347–353. https://doi.org/10.1006/JAUT.2000.0478
Bach J-F (2002) The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347:911–920. https://doi.org/10.1056/NEJMRA020100
Bach JF (2005) Infections and autoimmune diseases. J Autoimmun 25:74–80. https://doi.org/10.1016/J.JAUT.2005.09.024
Bach J-F (2009) The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347:911–920. https://doi.org/10.1056/NEJMRA020100
Bartemes KR, Kita H (2018) Innate and adaptive immune responses to fungi in the airway. J Allergy Clin Immunol 142:353. https://doi.org/10.1016/J.JACI.2018.06.015
Bauer HJ (1987) Multiple sclerosis in Europe. J Neurol 234:195–206. https://doi.org/10.1007/BF00618250
Bloomfield SF, Stanwell-Smith R, Crevel RWR, Pickup J (2006) Too clean, or not too clean: the hygiene hypothesis and home hygiene. Clin Exp Allergy 36:402–425. https://doi.org/10.1111/j.1365-2222.2006.02463.x
Bodansky HJ, Staines A, Stephenson C, Haigh D, Cartwrigth R (1992) Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. Br Med J 304:1020. https://doi.org/10.1136/BMJ.304.6833.1020
Brás A, Águas AP (1996) Diabetes-prone NOD mice are resistant to Mycobacterium avium and the infection prevents autoimmune disease. Immunology 89:20–25. https://doi.org/10.1046/J.1365-2567.1996.D01-717.X
Braun-Fahrländer C, Riedler J, Herz U et al (2002) Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med 347:869–877. https://doi.org/10.1056/NEJMOA020057
Carbone F, Bonaventura A, Liberale L et al (2020) Atherosclerosis in rheumatoid arthritis: promoters and opponents. Clin Rev Allergy Immunol 58:1–14
Chandrashekara S (2012) The treatment strategies of autoimmune disease may need a different approach from conventional protocol: a review. Indian J Pharm 44:665–671
Chatenoud L, Dugas B, Beaurain G et al (1986) Presence of preactivated T cells in hemodialyzed patients: their possible role in altered immunity. Proc Natl Acad Sci U S A 83:7457. https://doi.org/10.1073/PNAS.83.19.7457
Cleenewerk L, Garssen J, Hogenkamp A (2020) Clinical use of Schistosoma mansoni antigens as novel immunotherapies for autoimmune disorders. Front Immunol 11:1821. https://doi.org/10.3389/fimmu.2020.01821
Cooke A, Tonks P, Jones FM et al (1999) Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol 21:169–176. https://doi.org/10.1046/j.1365-3024.1999.00213.x
Costenbader KH, Gay S, Alarcón-Riquelme ME, Iaccarino L, Doria A (2012) Genes, epigenetic regulation and environmental factors: which is the most relevant in developing autoimmune diseases? Autoimmun Rev 11:604–609. https://doi.org/10.1016/j.autrev.2011.10.022
Detels R, Brody JA, Edgar AH (1972) Multiple sclerosis among American, Japanese and Chinese migrants to California and Washington. J Chronic Dis 25:3–10. https://doi.org/10.1016/0021-9681(72)90016-1
Dwivedi M, Laddha NC, Arora P, Marfatia YS, Begum R (2013a) Decreased regulatory T-cells and CD4+/CD8+ ratio correlate with disease onset and progression in patients with generalized vitiligo. Pigment Cell Melanoma Res 26:586–591. https://doi.org/10.1111/PCMR.12105
Dwivedi M, Laddha NC, Shah K, Shah BJ, Begum R (2013b) Involvement of interferon-gamma genetic variants and intercellular adhesion molecule-1 in onset and progression of generalized Vitiligo. J Interf Cytokine Res 33:646–659. https://doi.org/10.1089/jir.2012.0171
Dwivedi M, Kemp EH, Laddha NC, Mansuri MS, Weetman AP, Begum R (2015) Regulatory T cells in vitiligo: implications for pathogenesis and therapeutics. Autoimmun Rev 14:49–56. https://doi.org/10.1016/j.autrev.2014.10.002
Dwivedi M, Ansarullah, Radichev I, Kemp EH (2017) Alteration of immune-mechanisms by human microbiota and development and prevention of human diseases. J Immunol Res 2017:6985256. https://doi.org/10.1155/2017/6985256
Ege MJ, Bieli C, Frei R et al (2006) Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol 117:817–823. https://doi.org/10.1016/J.JACI.2005.12.1307
Elliott DE, Li J, Blum A et al (2003) Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol 284:G385–G391. https://doi.org/10.1152/ajpgi.00049.2002
Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF Jr, Weinstock JV (2004) Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol 34:2690–2698. https://doi.org/10.1002/eji.200324833
Filippi M, Bar-Or A, Piehl F et al (2018) Multiple sclerosis. Nat Rev Dis Prim 4(1):1–27. https://doi.org/10.1038/s41572-018-0041-4
Flohr C, Tuyen LN, Lewis S et al (2006) Poor sanitation and helminth infection protect against skin sensitization in Vietnamese children: a cross-sectional study. J Allergy Clin Immunol 118:1305–1311. https://doi.org/10.1016/J.JACI.2006.08.035
Fujinami RS, Oldstone MB (1989) Molecular mimicry as a mechanism for virus-induced autoimmunity. Immunol Res 8:3–15. https://doi.org/10.1007/BF02918552
Gaisford W, Cooke A (2009) Can infections protect against autoimmunity? Curr Opin Rheumatol 21:391–396. https://doi.org/10.1097/BOR.0B013E32832C2DEE
Gale EAM (2002) The rise of childhood Type 1 diabetes mellitusin the 20th century. Diabetes 51:3353–3361. https://doi.org/10.2337/DIABETES.51.12.3353
Gandhi R, Laroni A, Weiner HL (2010) Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol 221:7–14. https://doi.org/10.1016/j.jneuroim.2009.10.015
Giri PS, Dwivedi M, Laddha NC, Begum R, Bharti AH (2020a) Altered expression of nuclear factor of activated T cells, forkhead box P3, and immune-suppressive genes in regulatory T cells of generalized vitiligo patients. Pigment Cell Melanoma Res 33:566–578. https://doi.org/10.1111/PCMR.12862
Giri PS, Dwivedi M, Begum R (2020b) Decreased suppression of CD8+ and CD4+ T cells by peripheral regulatory T cells in generalized vitiligo due to reduced NFATC1 and FOXP3 proteins. Exp Dermatol 29:759–775. https://doi.org/10.1111/exd.14157
Giri P, Begum R, Dwivedi M (2021a) Meta-analysis for association of TNFA-308(G>A) polymorphism with vitiligo susceptibility. Gene 809:146027
Giri P, Shah F, Gupta B et al (2021b) Genetic association of interleukin-4 VNTR polymorphism with susceptibility to rheumatoid arthritis in South Gujarat population. Gene Rep 25:101322
Giri PS, Patel S, Begum R, Dwivedi M (2021c) Association of FOXP3 and GAGE10 promoter polymorphisms and decreased FOXP3 expression in regulatory T cells with susceptibility to generalized vitiligo in Gujarat population. Gene 768:145295. https://doi.org/10.1016/j.gene.2020.145295
Giri PS, Shah F, Dwivedi MK (2022) Probiotics and prebiotics in the suppression of autoimmune diseases. In: Probiotics in the prevention and management of human diseases, vol 161–186. Academic Press, London. https://doi.org/10.1016/B978-0-12-823733-5.00019-2
Green A, Patterson CC (2001) Trends in the incidence of childhood-onset diabetes in Europe 1989-1998. Diabetologia 44:B3. https://doi.org/10.1007/pl00002950
Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M, Stosic-Grujicic S, Milic M, Sofronic-Milosavljevic L (2008) Trichinella spiralis: modulation of experimental autoimmune encephalomyelitis in DA rats. Exp Parasitol 118:641–647. https://doi.org/10.1016/j.exppara.2007.12.003
Halperin SA, King J, Law B, Mills E, Willems P (1999) Safety and immunogenicity of Haemophilus influenzae-tetanus toxoid conjugate vaccine given separately or in combination with a three-component acellular pertussis vaccine combined with diphtheria and tetanus toxoids and inactivated poliovirus vaccine for the first four doses. Clin Infect Dis 28:995–1001. https://doi.org/10.1086/514741
Hammond SR, English DR, McLeod JG (2000) The age-range of risk of developing multiple sclerosis: evidence from a migrant population in Australia. Brain 123(Pt 5):968–974. https://doi.org/10.1093/BRAIN/123.5.968
Hampe CS (2012) B cells in autoimmune disease. Scientifica 2012:215308. https://doi.org/10.6064/2012/215308
Hara A, Iwasa Y (2020) Autoimmune diseases initiated by pathogen infection: mathematical modeling. J Theor Biol 498:110296. https://doi.org/10.1016/J.JTBI.2020.110296
Hara N, Alkanani AK, Ir D et al (2013) The role of the intestinal microbiota in type 1 diabetes. Clin Immunol 146:112–119
Harjutsalo V, Sjöberg L, Tuomilehto J (2008) Time trends in the incidence of Type 1 diabetes mellitusin Finnish children: a cohort study. Lancet 371:1777–1782. https://doi.org/10.1016/S0140-6736(08)60765-5
Harnett W, Harnett MM (2006) Filarial nematode secreted product ES-62 is an anti-inflammatory agent: therapeutic potential of small molecule derivatives and ES-62 peptide mimetics. Clin Exp Pharmacol Physiol 33:511–518. https://doi.org/10.1111/J.1440-1681.2006.04400.X
Harnett MM, Kean DE, Boitelle A et al (2008) The phosphorycholine moiety of the filarial nematode immunomodulator ES-62 is responsible for its anti-inflammatory action in arthritis. Ann Rheum Dis 67:518–523. https://doi.org/10.1136/ARD.2007.073502
Inafuku M, Matsuzaki G, Oku H (2015) Intravenous Mycobacterium Bovis Bacillus Calmette-Guérin Ameliorates nonalcoholic fatty liver disease in obese, diabetic ob/ob Mice. PLoS One 10:e0128676. https://doi.org/10.1371/JOURNAL.PONE.0128676
Janeway CA, Travers P, Walport M et al (2001) Immunobiology: the immune system in health and disease. In: Principles of innate and adaptive immunity, 5th edn. Garland Science, New York, NY
Jatana SK, Nair MNG (2007) Combination vaccines. Med J 63:167. https://doi.org/10.1016/S0377-1237(07)80067-0
Johansson BE (1988) Antigenic competition of influenza virus glycoproteins. Immunological and cellular mechanisms responsible for the influence of hemagglutinin priming on the immunogenicity of the viral neuraminidase. Dissertation. ProQuest
Johnston MJG, Wang A, Catarino MED et al (2010) Extracts of the rat tapeworm, Hymenolepis diminuta, suppress macrophage activation in vitro and alleviate chemically induced colitis in mice. Infect Immun 78:1364–1375. https://doi.org/10.1128/IAI.01349-08
Joner G, Stene LC, Søvik O (2004) Nationwide, prospective registration of Type 1 diabetes mellitusin children aged. Diabetes Care 27:1618–1622. https://doi.org/10.2337/DIACARE.27.7.1618
Kallas EG, Grunenberg NA, Yu C et al (2019) Antigenic competition in CD4 + T cell responses in a randomized, multicenter, double-blind clinical HIV vaccine trial. Sci Transl Med 11:eaaw1673. https://doi.org/10.1126/SCITRANSLMED.AAW1673
Khan WI, Blennerhasset PA, Varghese AK et al (2002) Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun 70:5931–5937. https://doi.org/10.1128/IAI.70.11.5931-5937.2002
Kim TK, Lee JC, Im SH, Lee MS (2020) Amelioration of autoimmune diabetes of NOD mice by immunomodulating probiotics. Front Immunol 11:1832. https://doi.org/10.3389/FIMMU.2020.01832/BIBTEX
Kurtzke JF (1995) MS epidemiology world wide. One view of current status. Acta Neurol Scand Suppl 161:23–33. https://doi.org/10.1111/J.1600-0404.1995.TB05853.X
La Flamme AC, Ruddenklau K, Bäckström BT (2003) Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect Immun 71:4996. https://doi.org/10.1128/IAI.71.9.4996-5004.2003
Lang KS, Recher M, Junt T et al (2005) Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med 11:138–145. https://doi.org/10.1038/NM1176
Lee J, Reinke EK, Zozulya AL, Sandor M, Fabry Z (2008) Mycobacterium bovis bacille Calmette-Guérin infection in the CNS suppresses experimental autoimmune encephalomyelitis and Th17 responses in an IFN-gamma-independent manner. J Immunol 181:6201–6212. https://doi.org/10.4049/jimmunol.181.9.6201
Lehmann D, Ben-Nun A (1992) Bacterial agents protect against autoimmune disease. I. Mice pre-exposed to Bordetella pertussis or Mycobacterium tuberculosis are highly refractory to induction of experimental autoimmune encephalomyelitis. J Autoimmun 5:675–690. https://doi.org/10.1016/0896-8411(92)90185-s
Leibowitz U, Kahana E, Alter M (1973) The changing frequency of multiple sclerosis in Israel. Arch Neurol 29:107–110. https://doi.org/10.1001/ARCHNEUR.1973.00490260051010
Leslie RD, Hawa M (1994) Twin studies in auto-immune disease. Acta Genet Med Gemellol 43:71–81. https://doi.org/10.1017/s000156600000297x
Liacopoulos P, Ben-Efraim S (1975) Antigenic competition. Prog Allergy 18:97–204. https://doi.org/10.1159/000395257
Like AA, Guberski DL, Butler L (1991) Influence of environmental viral agents on frequency and tempo of diabetes mellitus in BB/Wor rats. Diabetes 40:259–262. https://doi.org/10.2337/DIAB.40.2.259
Lincez PJ, Shanina I, Horwitz MS (2021) Changes in MDA5 and TLR3 sensing of the same diabetogenic virus result in different autoimmune disease outcomes. Front Immunol 12:4661. https://doi.org/10.3389/FIMMU.2021.751341/BIBTEX
Long SA, Buckner JH (2011) CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol 187:2061–2066. https://doi.org/10.4049/jimmunol.1003224
Mahajna H, Mahroum N, Amital H (2015) Rheumatoid arthritis and infections: more than an association? In: Infection and autoimmunity. Academic Press, London, pp 729–734. https://doi.org/10.1016/B978-0-444-63269-2.00065-9
Mayr WT, Pittock SJ, McClelland RL, Jorgensen NW, Noseworthy JH, Rodriguez M (2003) Incidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985-2000. Neurology 61:1373–1377. https://doi.org/10.1212/01.WNL.0000094316.90240.EB
McMaster PR, Kyriakos M (1970) The prevention of autoimmunity to the thyroid and allergic thyroiditis bantigenic competition. J Immunol 105:1201–1205
McSorley HJ, Maizels RM (2012) Helminth infections and host immune regulation. Clin Microbiol Rev 25:585. https://doi.org/10.1128/CMR.05040-11
Milo R, Kahana E (2010) Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev 9:A387
Motomura Y, Wang H, Deng Y, El-Sharkawy RT, Verdu EF, Khan WI (2009) Helminth antigen-based strategy to ameliorate inflammation in an experimental model of colitis. Clin Exp Immunol 155:88–95. https://doi.org/10.1111/j.1365-2249.2008.03805.x
Moudgil KD, Choubey D (2011) Cytokines in autoimmunity: role in induction, regulation, and treatment. J Interf Cytokine Res 31:695–703. https://doi.org/10.1089/jir.2011.0065
Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE (2010) Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheumatol 62:1576. https://doi.org/10.1002/ART.27425
Okada H, Kuhn C, Feillet H, Bach J-F (2010) The “hygiene hypothesis” for autoimmune and allergic diseases: an update. Clin Exp Immunol 160:1–9. https://doi.org/10.1111/j.1365-2249.2010.04139.x
Oldstone MB (1998) Molecular mimicry and immune-mediated diseases. FASEB J 12:1255–1265. https://doi.org/10.1096/fasebj.12.13.1255
Oldstone MBA, Ahmed R, Salvato M (1990) Viruses as therapeutic agents. II. Viral reassortants map prevention of insulin-dependent diabetes mellitus to the small RNA of lymphocytic choriomeningitis virus. J Exp Med 171:2091. https://doi.org/10.1084/JEM.171.6.2091
Oliveira-Nascimento L, Massari P, Wetzler LM (2012) The role of TLR2 in infection and immunity. Front Immunol 3:79. https://doi.org/10.3389/FIMMU.2012.00079/BIBTEX
Osada Y, Shimizu S, Kumagai T, Yamada S, Kanazawa T (2009) Schistosoma mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int J Parasitol 39:457–464. https://doi.org/10.1016/j.ijpara.2008.08.007
Pearson DJ, Taylor G (1975) The influence of the nematode Syphacia oblevata on adjuvant arthritis in the rat. Immunology 29:391–396
Petrovsky N (2010) Immunomodulation with microbial vaccines to prevent Type 1 diabetes mellitusmellitus. Nat Rev Endocrinol 6(3):131–138. https://doi.org/10.1038/nrendo.2009.273
Pihoker C, Gilliam LK, Hampe CS, Lernmark Å (2005) Autoantibodies in diabetes. Diabetes 54:S52–S61. https://doi.org/10.2337/diabetes.54.suppl_2.S52
Poser S, Stickel B, Krtsch U, Burckhard D, Nordman B (1989) Increasing incidence of multiple sclerosis in South Lower Saxony, Germany. Neuroepidemiology 8:207–213. https://doi.org/10.1159/000110184
Prabhu Das MR, Cohen A, Zamvil SS, Offner H, Kuchroo VK (1996) Prior exposure to superantigen can inhibit or exacerbate autoimmune encephalomyelitis: T-cell repertoire engaged by the autoantigen determines clinical outcome. J Neuroimmunol 71:3–10. https://doi.org/10.1016/S0165-5728(96)00107-5
Prat E, Martin R (2002) The immunopathogenesis of multiple sclerosis. J Rehabil Res Dev 39:187–200
Pritchard DI, Todd I, Brown A et al (2005) Alleviation of insulitis and moderation of diabetes in NOD mice following treatment with a synthetic Pseudomonas aeruginosa signal molecule, N-(3-oxododecanoyl)-L-homoserine lactone. Acta Diabetol 42:119–122. https://doi.org/10.1007/S00592-005-0190-2
Pross HF, Eidinger D (1974) Antigenic competition: a review of nonspecific antigen-induced suppression. Adv Immunol 18:133–168. https://doi.org/10.1016/S0065-2776(08)60309-0
Qin HY, Sadelain MW, Hitchon C, Lauzon J, Singh B (1993) Complete Freund’s adjuvant-induced T cells prevent the development and adoptive transfer of diabetes in nonobese diabetic mice. J Immunol 150:2072–2080
Raine T, Zaccone P, Mastroeni P, Cooke A (2006) Salmonella typhimurium infection in nonobese diabetic mice generates immunomodulatory dendritic cells able to prevent type 1 diabetes. J Immunol 177:2224–2233. https://doi.org/10.4049/JIMMUNOL.177.4.2224
Rautiainen H, Salomaa V, Niemelä S et al (2007) Prevalence and incidence of primary biliary cirrhosis are increasing in Finland. Scand J Gastroenterol 42:1347–1353. https://doi.org/10.1080/00365520701396034
Riedler J, Braun-Fahrländer C, Eder W et al (2001) Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 358:1129–1133. https://doi.org/10.1016/S0140-6736(01)06252-3
Ruyssers NE, De Winter BY, De Man JG et al (2008) Worms and the treatment of inflammatory bowel disease: are molecules the answer? Clin Dev Immunol 2008:567314. https://doi.org/10.1155/2008/567314
Saï P, Rivereau AS (1996) Prevention of diabetes in the nonobese diabetic mouse by oral immunological treatments. Comparative efficiency of human insulin and two bacterial antigens, lipopolysacharide from Escherichia coli and glycoprotein extract from Klebsiella pneumoniae. Diabetes Metab 22:341–348
Saunders KA, Raine T, Cooke A, Lawrence CE (2007) Inhibition of autoimmune Type 1 diabetes mellitus by gastrointestinal helminth infection. Infect Immun 75:397–407. https://doi.org/10.1128/IAI.00664-06
Sewell DL, Reinke EK, Co DO et al (2003) Infection with Mycobacterium bovis BCG diverts traffic of myelin oligodendroglial glycoprotein autoantigen-specific T cells away from the central nervous system and ameliorates experimental autoimmune encephalomyelitis. Clin Diagn Lab Immunol 10:564–572. https://doi.org/10.1128/CDLI.10.4.564-572.2003
Seyedian SS, Nokhostin F, Malamir MD (2019) A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life 12:113–122. https://doi.org/10.25122/jml-2018-0075
Sfriso P, Ghirardello A, Botsios C et al (2010) Infections and autoimmunity: the multifaceted relationship. J Leukoc Biol 87:385–395. https://doi.org/10.1189/JLB.0709517
Shi M, Wang A, Prescott D et al (2011) Infection with an intestinal helminth parasite reduces Freund’s complete adjuvant-induced monoarthritis in mice. Arthritis Rheumatol 63:434–444. https://doi.org/10.1002/art.30098
Sironi M, Clerici M (2010) The hygiene hypothesis: an evolutionary perspective. Microbes Infect 12:421–427. https://doi.org/10.1016/j.micinf.2010.02.002
Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H (2005) The role of the T cell in autoimmune inflammation. Arthritis Res Ther 7:S4. https://doi.org/10.1186/ar1703
Staines A, Hanif S, Ahmed S, McKinney PA, Shera S, Bodansky HJ (1997) Incidence of insulin dependent diabetes mellitus in Karachi, Pakistan. Arch Dis Child 76:121. https://doi.org/10.1136/ADC.76.2.121
Strachan DP (2000) Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax 55(Suppl 1):S2–S10. https://doi.org/10.1136/thorax.55.suppl_1.s2
Summers RW, Elliott DE, Urban JFJ, Thompson R, Weinstock JV (2005a) Trichuris suis therapy in Crohn’s disease. Gut 54:87–90. https://doi.org/10.1136/gut.2004.041749
Summers RW, Elliott DE, Urban JFJ, Thompson RA, Weinstock JV (2005b) Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 128:825–832. https://doi.org/10.1053/j.gastro.2005.01.005
Symmons DPM (1995) Frequency of lupus in people of African origin. Lupus 4:176–178. https://doi.org/10.1177/096120339500400303
Takei I, Asaba Y, Kasatani T et al (1992) Suppression of development of diabetes in NOD mice by lactate dehydrogenase virus infection. J Autoimmun 5:665–673. https://doi.org/10.1016/0896-8411(92)90184-R
Tateyama M, Oyaizu N, McCloskey TW, Than S, Pahwa S (2000) CD4 T lymphocytes are primed to express Fas ligand by CD4 cross-linking and to contribute to CD8 T-cell apoptosis via Fas/FasL death signaling pathway. Blood 96:195–202
Thanapati S, Ganu M, Giri P et al (2017) Impaired NK cell functionality and increased TNF-α production as biomarkers of chronic chikungunya arthritis and rheumatoid arthritis. Hum Immunol 78:370–374. https://doi.org/10.1016/j.humimm.2017.02.006
Toussirot É, Robinet É, Saas P et al (2006) Bacterial extract (OM-89) specific and non specific immunomodulation in rheumatoid arthritis patients. Autoimmunity 39:299–306. https://doi.org/10.1080/08916930600738425
Toyota T, Satoh J, Oya K, Shintani S, Okano T (1986) Streptococcal preparation (OK-432) inhibits development of type I diabetes in NOD mice. Diabetes 35:496–499. https://doi.org/10.2337/DIAB.35.4.496
Van der Kleij D, Latz E, Brouwers JFHM et al (2002) A novel host-parasite lipid cross-talk: schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization *. J Biol Chem 277:48122–48129. https://doi.org/10.1074/JBC.M206941200
Vischer TL (1993) Subreum (OM-8980): an anti-arthritic E. coli extract. Clin Exp Rheumatol 11(Suppl 8):S121–S123
Waldner H (2009) The role of innate immune responses in autoimmune disease development. Autoimmun Rev 8:400–404. https://doi.org/10.1016/J.AUTREV.2008.12.019
Walsh KP, Brady MT, Finlay CM, Boon L, Mills KHG (2009) Infection with a helminth parasite attenuates autoimmunity through TGF-β-mediated suppression of Th17 and Th1 responses. J Immunol 183:1577–1586. https://doi.org/10.4049/JIMMUNOL.0803803
Wang L, Wang FS, Gershwin ME (2015) Human autoimmune diseases: a comprehensive update. J Intern Med 278:369–395
White MPJ, Johnston CJC, Grainger JR et al (2020) The helminth parasite Heligmosomoides polygyrus attenuates EAE in an IL-4Rα-dependent manner. Front Immunol 11:1830. https://doi.org/10.3389/FIMMU.2020.01830/BIBTEX
Wilberz S, Partke HJ, Dagnaes-Hansen F, Herberg L (1991) Persistent MHV (mouse hepatitis virus) infection reduces the incidence of diabetes mellitus in non-obese diabetic mice. Diabetologia 34:2–5. https://doi.org/10.1007/BF00404016
Zaccone P, Fehérvári Z, Jones FM et al (2003) Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol 33:1439–1449. https://doi.org/10.1002/eji.200323910
Zaccone P, Fehervari Z, Phillips JM, Dunne DW, Cooke A (2006) Parasitic worms and inflammatory diseases. Parasite Immunol 28:515. https://doi.org/10.1111/J.1365-3024.2006.00879.X
Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A (2009) Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol 39:1098–1107. https://doi.org/10.1002/EJI.200838871
Zinkernagel RM, Planz O, Ehl S et al (1999) General and specific immunosuppression caused by antiviral T-cell responses. Immunol Rev 168:305–315. https://doi.org/10.1111/J.1600-065X.1999.TB01300.X
Acknowledgments
We are thankful to Uka Tarsadia University, Maliba Campus, Tarsadi, Gujarat, India for providing the facilities needed for the preparation of this chapter.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Giri, P.S., Shoenfeld, Y., Dwivedi, M.K. (2022). The Protective Discourse Between Infections and Autoimmunity. In: Dwivedi, M.K., Sankaranarayanan, A., Kemp, E.H., Shoenfeld, Y. (eds) Role of Microorganisms in Pathogenesis and Management of Autoimmune Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-19-4800-8_4
Download citation
DOI: https://doi.org/10.1007/978-981-19-4800-8_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4799-5
Online ISBN: 978-981-19-4800-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)