Abstract
The ongoing COVID-19 pandemic has affected millions of lives adversely in the last 2 years, and the future course of the pandemic remains unknown. Initially, COVID-19 infection was thought to be an acute respiratory illness, but slowly it became clear that it is a multi-system disease involving almost all body organs. Still more alarming is the realization that this infection is not as “acute” only as previously believed but has lasting effects in various organ systems. Kidneys are no exception, not only being involved during acute COVID-19 infection in multiple ways but also are important organs, in which infection leads to chronic kidney disease with varying manifestations. This review will restrict the majority of the discussion to long-term sequelae of COVID-19 concerning kidneys.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
The ongoing COVID-19 pandemic has affected millions of lives adversely in the last 2 years, and the future course of the pandemic remains unknown. Initially, COVID-19 infection was thought to be an acute respiratory illness, but slowly it became clear that it is a multi-system disease involving almost all body organs. Still more alarming is the realization that this infection is not as “acute” only as previously believed but has lasting effects in various organ systems. Kidneys are no exception, not only being involved during acute COVID-19 infection in multiple ways but also are important organs, in which infection leads to chronic kidney disease with varying manifestations. This review will restrict the majority of the discussion to long-term sequelae of COVID-19 concerning kidneys.
9.1 Understanding Basic Kidney Syndrome
Before discussing long-term kidney consequences, it will be prudent to know common kidney syndromes, which will help understand kidney abnormalities following COVID-19.
-
A.
Acute kidney injury: This is defined as a recent onset of renal dysfunction manifested by an elevation of serum creatinine; therefore fall in estimated glomerular filtration rate (eGFR) with or without oliguria, and patients may recover in more than 80–90% of cases. However, approximately 10–20% of patients with severe acute kidney injury (AKI) remain at risk of developing chronic kidney disease (CKD) [1].
-
B.
Chronic kidney disease: It is defined as evidence of renal disease present for >3 months with or without decrease in eGFR <60 ml/min. Evidence of a renal disease is usually in the form of abnormal urinary protein loss of >30 mg/day, urinary sediment anomalies (RBC, WBC, casts, etc.), and/or radiological renal abnormality [2]. Once correctly diagnosed, CKD does not recover and tends to progress to more advanced kidney disease, a stage called end-stage kidney disease (ESKD). At the ESKD, the patient cannot be managed with only medical treatment and will need renal replacement therapy (RRT) in the form of dialysis and/or renal transplant.
-
C.
Glomerular diseases: Many renal diseases involve the glomerulus and are grouped under the heading glomerular diseases. The two most important criteria for defining glomerular diseases are significant proteinuria (> 1.0 g/day) and glomerular hematuria (dysmorphic RBCs and/or RBC casts). Based upon the degree of proteinuria, kidney dysfunction, and rapidity of onset of disease, the glomerular diseases are subdivided into clinical syndromes such as acute glomerulonephritis (AGN), nephrotic syndrome (NS), acute nephritic syndrome, rapidly progressive GN (RPGN), chronic glomerulonephritis (CGN), and asymptomatic urinary abnormalities (AUA).
-
D.
Hypertension: Most (around 90%) patients of hypertension in the community are primary hypertension, but among the 10% cases of secondary hypertension, kidney diseases remain the most common cause.
9.2 Renal Diseases and COVID-19
Kidney diseases are important risk factors for acquiring COVID-19 infection because of kidney patients’ immunocompromised status, which may be due to the intake of immunosuppressive drugs or the intrinsic nature of their disease condition. The following broad category of patients concerning kidney disease may get COVID-19 infection:
-
1.
A healthy person with normal kidney functions
-
2.
Patients with kidney diseases on immunosuppression
-
Kidney disease patients on immunosuppressive medication
-
Kidney transplant patients
-
-
3.
Patients with pre-existing CKD
-
CKD of varying severity and varying etiology
-
Kidney transplant patients with graft dysfunction
-
-
4.
Patients on dialysis: peritoneal dialysis or hemodialysis
9.3 Acute COVID-19 and Kidney Involvement
Kidney involvement in COVID-19 can range from asymptomatic urinary abnormalities, including varying degrees of proteinuria and hematuria, to kidney dysfunction presenting as AKI, which may require RRT in selected cases. AKI affects around 20–40% of critically ill COVID-19 patients. Renal replacement therapy during hospitalization is required in 5–10% of all COVID-19 patients and for 20–30% of those who are critically ill [3]. In a recently published meta-analysis, the pooled prevalence of AKI among all hospitalized COVID-19 patients was 28%, with 9% requiring RRT [3]. Patients of COVID-19 with AKI have a significantly worse outcome when compared to patients without AKI. Proteinuria is common in COVID-19 even without renal dysfunction and often remits spontaneously in a few weeks following clinical recovery. During the acute stage, proteinuria has been reported in 28–84% of COVID-19 patients [4]. The degree of proteinuria may vary depending on the type of glomerular involvement. Most patients have low-grade proteinuria, which can be explained due to defective reabsorption of filtered proteins seen with acute tubular injury.
Almost all cohort studies, systematic reviews, and meta-analyses have uniformly shown that CKD patients are more prone to develop severe complications of COVID-19 [5]. Apart from the chronic disease itself, the immunological state of CKD patients predisposes them to severe COVID-19. One meta-analysis with 1389 COVID-19-infected patients reported 3.03 times increased odds of developing the severe disease among CKD patients. Hypertension and diabetes mellitus per se are also associated with severe COVID-19 and being common causes of CKD in the general population; these comorbidities in tandem increase the risk of severe COVID-19 multifold [6]. Severe COVID-19 infection, in turn, has an adverse impact on the kidneys and worsens CKD progression.
9.4 COVID-19 and Kidneys: Pathophysiology and Development of Long COVID
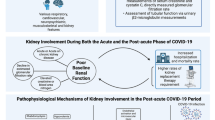
Renal insult due to COVID-19 is multifactorial (Fig. 9.1). Severe infection and critical illness accompanying hemodynamic compromise can lead to renal dysfunction similar to that in other infections. Biopsy studies, including multiple postmortem biopsy studies, have shown acute tubular injury (ATI) as the most common histological pattern. This is followed by thrombotic microangiopathy, collapsing glomerulopathy, podocytopathy, and vasculitis-like features [7]. Though not conclusive, the SARS-CoV-2 has been demonstrated in renal biopsies, and direct viral infection is also attributed as a cause of renal injury [8]. Case reports of glomerular diseases associated with COVID-19 have been reported, but it would not be possible to attribute causation [8]. A predilection for collapsing glomerulopathy, referred to as COVAN (COVID-19-associated nephropathy) in patients with APOL1 genotype, requires further characterization. The glomerular involvement with or without podocytopathy explains the proteinuria seen in COVID-19 patients.
Post-infectious syndromes and sequelae are known with several viruses such as cytomegalovirus (CMV), Ebstein-Barr virus (EBV), chikungunya, and Coxsackie virus. Chronic fatigue syndrome, neuropsychiatric manifestations, and somatic symptoms are common with these post-viral syndromes. This explains the biological plausibility of post-COVID-19 syndrome. A dysregulated immune system, severity of the acute infection, especially in the presence of comorbid illnesses, explains the severe manifestations and delayed/incomplete recovery seen in long COVID. Persistent renal inflammation triggers pro-fibrotic signaling and, thereby, progressive CKD is seen in a subset of long COVID patients.
9.5 Long COVID and Kidneys
Several studies have proposed the possible pathophysiologic mechanisms, direct and indirect mechanisms of nephrotoxicity, and patient outcomes in COVID-19 in these last 2 years. The primary focus of COVID-19 research after the peak COVID-19 waves has been on the long-term medical complications and sequelae of COVID-19 on multiple organ systems commonly referred to as “long COVID-19 syndrome” or “post-COVID-19 syndrome” or “post-acute sequelae of SARS-CoV-2 infection” (PASC) [9]. Long COVID is a continuum of acute illness pathogenesis (Fig. 9.2). Although up to 55 long-term effects involving almost all organ systems have been described following COVID-19 infection, renal sequelae are often more demanding with regard to the burden on healthcare resources and require special attention [10].
9.6 Risk Factors for Kidney Involvement in Long COVID
Not all patients who develop COVID-19 develop long-term complications. From the literature available, it appears that the risk of developing long COVID may depend on the severity of the acute illness and the presence of pre-existing renal disease. Though an in-depth assessment of the risk factors predisposing to long COVID involvement of the kidneys is not yet available, there are a few patient-related and disease-related risk factors that confer a higher risk for long COVID (Fig. 9.3).
9.7 Prevalence
The prevalence of long COVID is yet to be conclusively determined as several long-term studies on COVID-19 survivors are ongoing across multiple centers in the world. Moreover, the lack of clear diagnostic criteria for long COVID makes it difficult to diagnose this condition confidently and, hence, difficult to estimate its true prevalence.
Most follow-up studies showed a higher risk of persisting/new-onset renal dysfunction in COVID-19 survivors. Even patients with no evidence of AKI during the acute phase of hospitalization have reduced eGFR at 6 months of follow-up [11]. Though this can partly be explained due to the fallacies of using serum creatinine, it warrants further follow-up. Some reports, however, suggest a course similar to other influenza-like illnesses with good renal recovery in the majority of patients [12, 13]. The incidence of renal dysfunction was reported to be about 4% in the COVERSCAN cohort, a population in the UK deemed to be at low risk of COVID-19 mortality, with only 19% hospitalization rates [14]. A summary of the significant findings of renal outcomes is given in Table 9.1. There are no follow-up studies on proteinuria except one, wherein it was shown that proteinuria resolved in two-thirds of all patients by a median of 12 days, thereby suggesting a transient process [15].
9.8 Manifestations of Long COVID in Kidneys
The various long-term manifestations in the kidneys following COVID-19 are:
-
1.
Chronic kidney disease—As discussed above, both new-onset and pre-existing CKD present as CKD. CKD patients and critically ill patients who received dialysis during acute COVID-19 have a higher probability of rapid progression to ESKD and need life-long renal replacement therapy. The various complications can be grouped under:
-
(a)
New-onset CKD
-
(b)
Progression of pre-existing CKD to advanced stages of CKD
-
(c)
End-stage kidney disease (ESKD)
-
(a)
-
2.
Glomerular disease—Proteinuria is commonly reported in COVID-19-affected patients during the acute phase. In most cases, proteinuria is transient due to acute illness and cannot be solely attributed to SARS-CoV-2. Significant and persistent proteinuria is seen when the glomerular filtration barrier is affected and can be due to podocytopathy, COVAN (especially in African Americans with APOL1 genotype), and also thrombotic microangiopathy [4].
-
3.
Case reports of ANCA vasculitis, IgA vasculitis, lupus, and anti-GBM disease in patients with COVID-19—These are less likely to remit unless treated with directed immunosuppressive therapies and may progress to CKD [8].
9.9 COVID-19 and Renal Transplantation
Renal transplant recipients constitute a distinctive group of kidney patients who need long-term immunosuppression for stable kidney function. This makes them predisposed to COVID-19 (and severe COVID-19). The lack of a specific antiviral therapy necessitated multiple therapies like hydroxychloroquine, ivermectin, remdesivir, tocilizumab, and protease inhibitors-based anti-retroviral therapies (lopinavir/ritonavir) in the management of COVID-19 [18]. Remdesivir has been used successfully in transplant recipients without significant adverse effects [19]. Apart from the uncertain efficacy, the use of protease inhibitors which inhibit CYP3A metabolism results in significant calcineurin inhibitor (tacrolimus and cyclosporine) toxicity and precludes their use in transplant recipients [18]. Considering their immunosuppressed state with multiple comorbidities, they are expected to have delayed recovery after COVID-19. In one study involving transplant recipients, only 11.53% of COVID-19 survivors were free of clinical symptoms or laboratory abnormality during routine follow-up evaluation [20]. Also, these abnormalities depend on a history of hospitalization, presence of diabetes mellitus, and degree of renal function (eGFR) in the post-COVID-19 period in these patients. The coagulation abnormalities, in particular, were more frequent in these patients.
In addition to the above mentioned clinical outcomes, immunological outcomes are of particular concern in this patient population. COVID-19 infection necessitates modification of maintenance immunosuppressive regimen in most transplant recipients. This increases the risk of organ rejection. Furthermore, change in immunoreactivity against alloantigens due to SARS-CoV-2 and persistent immunoreactivity to the virus during follow-up is reported. The B-cell and T-cell response to antigens and immunosuppressive drugs after COVID-19 is unclear. The clinical implications of these findings about the risk of graft rejection and/or future risk of malignancy as seen with other immunomodulatory viruses like CMV, EBV, or BKV needs to be evaluated on follow-up.
9.10 Screening
All patients who have recovered from COVID-19 need to be evaluated post-discharge for features of long COVID. An ideal post-COVID-19 care clinic needs multidisciplinary collaboration between pulmonologists, general physicians, physiotherapists, specialist nurses, psychological counselors, researchers, and support groups to ensure complete recovery. One such initiative which has shown positive results is the RECOVERY program, a comprehensive post-COVID-19 center at Yale [21]. In resource-limited settings, teleconsultation can be done for recovered patients to identify those at risk for long COVID, and those patients can be subsequently evaluated in detail in the clinic. General screening of all recovered patients should include a blood pressure measurement and blood sugar estimation. New-onset hypertension and diabetes have also been reported in COVID-19 survivors, and these, in turn, increase the risk for progressive CKD [22, 23].
For patients with suspected kidney involvement, the following investigations are suggested:
-
1.
Urine routine and microscopy
-
2.
Blood urea and serum creatinine
-
3.
Urine protein/creatinine ratio
-
4.
Hemogram
-
5.
Ultrasonography of kidneys
All patients with new-onset renal dysfunction and progressive CKD need to be evaluated to rule out other common causes of worsening renal function. If clinically indicated, an autoimmune workup, serum protein electrophoresis, urine sediment evaluation, and advanced imaging studies must be performed.
Standard renal function tests are considered late markers and are deranged only after the injury is established. The search for renal troponin has been ongoing for decades. Tubular injury is most common in COVID-19, and tubular biomarkers have been evaluated for this purpose. One such marker, Dickkopf-3 (DKK-3), when expressed, increases the risk of tubulointerstitial fibrosis. Following tubular injury, urinary DKK-3 and interleukin-6 (IL-6) increase in the acute phase due to tubular injury and inflammation. Studies have shown that if they remain elevated at 6 months, or demonstrate a biphasic increase after the initial fall in levels, this indicates fibroblast activation, abnormal cytokine signaling, and propensity to progressive fibrosis [24]. Larger prospective studies of biomarkers are needed to understand their role in monitoring the AKI–CKD transition in COVID-19 survivors.
9.11 Management
The natural history of long COVID is still largely unknown. Also, with no known preventive drugs and/or strategies for long COVID, optimal medical management remains the only option. Because of limited evidence, definitive management recommendations have not been published. Most of the management protocols rely on local clinical experience and prior data from influenza-like illnesses and consensus guidelines. Renal function tests of COVID-19 survivors have to be monitored periodically after recovery. Any patient with a rapid progression, defined as a yearly eGFR decrease >5 mL/min/1.73m2, will need re-evaluation for other confounding factors. If there are no other apparent causes, general management of CKD is to be done. A comprehensive renal care plan consisting of regular monitoring of renal function in consultation with the nephrologist is essential.
Medical management is the cornerstone of therapy in CKD patients. Apart from COVID-19-related inflammation, multiple factors may affect kidney disease progression [25]. Blood pressure and glycemic control have to be ensured to slow down the progression of kidney failure. Though there were initial speculations about the harmful effects of angiotensin-converting enzyme inhibitors (ACEi) in COVID-19 patients, subsequent well-conducted randomized studies have failed to show any risk of adverse effects with the use of these drugs. ACEi and angiotensin receptor blockers (ARBs) remain the drug of choice for the management of hypertension with appropriate monitoring for hyperkalemia. The utility of steroids, anticoagulants, and/or other drugs in long COVID is being evaluated in multiple prospective and randomized trials worldwide.
Complications of kidney disease are often evident after stage 3 CKD and necessitate specific therapy. Management of anemia with iron supplementation and erythropoietin-stimulating agents (if hemoglobin <10 g/dL), bicarbonate supplementation for acidosis, and vitamin D therapy for mineral bone disease need to be optimized. Dialysis access planning in advanced CKD (stage 4/5) should be done. Patients with CKD are more susceptible to cardiovascular diseases. COVID-19 survivors also have an increased risk of cardiovascular disease. Therefore the risk factors are additive and a comprehensive cardiovascular assessment has to be performed.
9.12 Conclusion
Renal involvement in COVID-19 has been recognized from the beginning of the pandemic and our understanding has evolved significantly. Nevertheless, further research is required to identify potential modifying factors, genetic predisposition as in APOL1 genotype, biomarkers to detect and monitor the renal injury, and disease-modifying therapy for COVID-19. Collaborative studies like the National COVID Cohort Collaborative (N3C) will help us to evaluate the long-term clinical consequences comprehensively and generate COVID-19 analytics for better informed patient care and follow-up [26]. Comprehensive post-COVID-19 programs and clinics will play an important role not only for patient care but also to carry out research that will help us better manage COVID-19 survivors. As our understanding of COVID-19 evolves, our management plan for post-COVID-19 survivors is bound to change with time.
9.13 Take-Home Points
-
Kidney patients are more susceptible to acquiring COVID-19 infection and developing severe illness.
-
Case definition of long COVID-19 syndrome requires standardization to allow valid diagnosis and establish management strategies.
-
Long-COVID in kidneys can manifest as CKD, ESRD, or glomerular disease.
-
Patients with severe COVID-19, AKI, and/or proteinuria during acute COVID-19 and patients with CKD who had COVID-19 need regular monitoring of renal function after discharge.
-
Any new, persistent, or progressive renal dysfunction/proteinuria needs assessment by a nephrologist.
-
General management of hypertension, diabetes, and CKD with closer follow-up for those with ongoing renal issues is paramount.
References
Heung M, Chawla LS. Acute kidney injury: gateway to chronic kidney disease. Nephron Clin Pract. 2014 Sep 24;127(1–4):30–4.
Chapter 1: Definition and classification of CKD. Kidney Int Suppl. 2013 Jan;3(1):19–62.
Silver SA, Beaubien-Souligny W, Shah PS, et al. The prevalence of acute kidney injury in patients hospitalized with COVID-19 infection: a systematic review and meta-analysis. Kidney Med. 2021 Feb;3(1):83–98.e1.
Mohamed MMB, Velez JCQ. Proteinuria in COVID-19. Clin Kidney J. 2021 Mar 1;14(Supplement_1):i40–7.
Long JD, Strohbehn I, Sawtell R, et al. COVID-19 survival and its impact on chronic kidney disease. Transl Res J Lab Clin Med. 2021 Nov 10;S1931-5244(21):00266–8.
Callender LA, Curran M, Bates SM, et al. The impact of pre-existing co-morbidities and therapeutic interventions on COVID-19. Front Immunol. 2020;11:1991.
Sharma P, Ng JH, Bijol V, et al. Pathology of COVID-19-associated acute kidney injury. Clin Kidney J. 2021 Mar 1;14(Supplement_1):i30–9.
Shimmel A, Shaikhouni S, Mariani L. Current understanding of clinical manifestations of COVID-19 in glomerular disease. Glomerular Dis. 2021;1(4):250–64.
Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021 Apr;27(4):601–15.
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021 Aug 9;11(1):16144.
Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021 Jan 16;397(10270):220–32.
Bouquegneau A, Huart J, Lutteri L, et al. POS-196 patients with covid-19 mostly recover from tubular proteinuria and acute kidney injury after hospital discharge. Kidney Int Rep. 2021 Apr 1;6(4):S82.
Writing Committee for the COMEBAC Study Group, Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021 Apr 20;325(15):1525–34.
Dennis A, Wamil M, Alberts J, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021 Mar 30;11(3):e048391.
Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol JASN. 2020 Jun;31(6):1157–65.
Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney outcomes in Long COVID. J Am Soc Nephrol JASN. 2021 Nov;32(11):2851–62.
Nugent J, Aklilu A, Yamamoto Y, et al. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open. 2021 Mar 1;4(3):e211095.
Kataria A, Yakubu I, Winstead R, et al. COVID-19 in kidney transplantation: epidemiology, management considerations, and the impact on kidney transplant practice. Transplant Direct. 2020 Aug;6(8):e582.
Meshram HS, Kute VB, Patel H, et al. Feasibility and safety of remdesivir in SARS-CoV2 infected renal transplant recipients: a retrospective cohort from a developing nation. Transpl Infect Dis. 2021 Aug;23(4):e13629.
Basic-Jukic N, Juric I, Furic-Cunko V, et al. Follow-up of renal transplant recipients after acute COVID-19—a prospective cohort single-center study. Immun Inflamm Dis. 2021;9(4):1563–72.
Lutchmansingh DD, Knauert MP, Antin-Ozerkis DE, et al. A clinic blueprint for post-coronavirus disease 2019 recovery: learning from the past. Looking to the Future Chest. 2021 Mar;159(3):949–58.
Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021 Mar;31(372):n693.
Chen G, Li X, Gong Z, et al. Hypertension as a sequela in patients of SARS-CoV-2 infection. PLoS One. 2021 Apr 28;16(4):e0250815.
Husain-Syed F, Villa G, Wilhelm J, et al. Renal markers for monitoring acute kidney injury transition to chronic kidney disease after COVID-19. Nephrol Dial Transplant. 2021 Nov 1;36(11):2143–7.
Yende S, Parikh CR. Long COVID and kidney disease. Nat Rev Nephrol. 2021 Dec;17(12):792–3.
Haendel MA, Chute CG, Bennett TD, et al. The national COVID cohort collaborative (N3C): rationale, design, infrastructure, and deployment. J Am Med Inform Assoc. 2021 Mar 1;28(3):427–43.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Arunkumar, S., Agarwal, S.K. (2022). Renal Abnormalities Following COVID-19. In: Mohan, A., Mittal, S. (eds) Post COVID-19 Complications and Management. Springer, Singapore. https://doi.org/10.1007/978-981-19-4407-9_9
Download citation
DOI: https://doi.org/10.1007/978-981-19-4407-9_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4406-2
Online ISBN: 978-981-19-4407-9
eBook Packages: MedicineMedicine (R0)