Abstract
Microplastics (MPs) are minuscule plastic particles smaller than 5 mm in length that have become a significant threat to because of their toxicity in our natural environment and detrimental impacts on our water resources, aquatic life, and humans. Physical, chemical, ecological, and biological impacts are all possible ways of causing dangers posed by MPs. Microplastics also sorb and collect potentially toxic contaminants in aquatic environments. As a result, ingesting polluted microplastics may expose marine species and even the food chain to hazardous contaminants. However, wastewater treatment plants (WWTPs) are the primary source of microplastics that enter marine ecosystems. Microplastics in aquatic environments must be controlled to protect the environment and human health. This chapter examines the sources of microplastics in wastewater, their properties, ecotoxicity, and health risks, existing and newly developed methods for characterization of microplastics in wastewater, and for pollution prevention and control, bioremediation techniques for the removal of microplastics from wastewater have been developed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Industrial waste is the principal source of environmental pollution because of the existence of nutrients of environmental concerns, potentially toxic heavy metals, organic pollutants, and emerging contaminants that pose major ecotoxicological risks and environmental dangers (Chandel et al. 2022; Chaturvedi et al. 2021; Saxena et al. 2016, 2020a, b, c, d; Deb et al. 2020; Kumar et al. 2020; Bharagava and Saxena 2020; Mulla et al. 2019; Bharagava et al. 2017a, b, c, 2018; Goutam et al. 2018; Gautam et al. 2017; Saxena and Bharagava 2015, 2017). Among the environmental contaminants, the release of emerging contaminants along with industrial effluents is a major environmental concern. The extensive use of plastic goods in today’s world eventually leads to emission of minute plastic particles into the environment. The diameter of the microplastic particles (MPs) is less than 5 mm (GESAMP 2015). The exact level of microplastics in the environment, including unidentified microplastics, is considered to be substantially higher than what uncontrolled plastic product flows predict (Kim et al. 2015). The microplastics (MPs) amount in seawater has been continuously increasing over the last decade, with a growing trend along the shorelines (Barnes et al. 2009), MPs pollution is a relatively new issue in the world, due to the growing use of plastics in practically all aspects of human activities and there is a lack of appropriate treatment of domestic and industrial wastewater (Bui et al. 2020).
At present, with the widespread use of MPs, particularly in the marine environment, marine life is unsheltered to MPs with broad range of effects which depends on the presence toxic chemicals from plastic additives and adsorbed pollutants such as pesticides, persistent organic pollutants, or metals leaching into the environment, particularly in the marine environment (Van Emmerik et al. 2018; Fossi et al. 2014). MPs are hazardous and can also serve as pathogen reservoirs, putting marine life in danger (Kor and Mehdinia 2020). MPs are found largely in coastal habitats, and their exact influence on human health has yet to be identified. Marine life, on the other hand, is at the centre of the food chain and provides a significant portion of the nutrients consumed daily by human beings (Bui et al. 2020). The growing presence of MPs in the environment and biota has attracted the curiosity of scientists and the general public, with emerging evidence of microplastics’ detrimental effects (de Sá et al. 2015; Jeong et al. 2016). Surface runoff, wind advection, and WWTPs effluent are just a few of the ways MPs enter water bodies (Dris et al. 2015). Thousands of microplastic particles are deposited in WWTPs every day (Okoffo et al. 2019). Although there is no direct link between MPs concentrations and population density in WWTPs intake streams, agriculture and industrial activities appear to be important factors (Long et al. 2019). To determine the amount of microplastics that enter and exit WWTPs, it is essential to develop a precise and repeatable experimental approach for counting the microplastic particles in sewage influent and effluent.
MPs have been removed using a variety of processes, including grit chamber and primary sedimentation, coagulation, sand filtering, dissolved air floatation and fast (gravity) sand removal (Wang et al. 2020; Hidayaturrahman and Lee 2019; Chen et al. 2018; Bayo et al. 2020; Lares et al. 2018; Murphy et al. 2016). According to a study, microplastics concentration in the WWTP influents was found to be in between 15 and 640 particles L−1, while it was significantly lower in case of the effluent, although varied over four orders of magnitude (Kang et al. 2018). As a result, it is unclear if the discrepancies in microplastic concentrations in wastewater are related to variances in plastic pollution levels or differences in sampling and analytical procedures (Kang et al. 2018).
MPs are currently identified and/or quantified using scientific analytical techniques such as spectroscopy, microscopy, and/or thermal analysis. The most common characterization methodology mentioned in the literature is the use of spectroscopic techniques such as Raman spectroscopy (Peñalver et al. 2020) and Fourier transform infrared (FTIR). MPs have been characterized using scanning electron microscopy based techniques such as, SEM-energy dispersive X-ray spectroscopy(SEM-EDS) and other techniques like Environmental Scanning electron microscopy-EDS (ESEM-EDS) (Rocha-Santos and Duarte 2015). Microplastics thermal analysis is a new technology for MPs characterization. This method is based on identifying the polymer based on the degradation products it produces pyrolysis gas chromatography–mass spectrometry (py-GC-MS), thermogravimetry (TGA), hyphenated TGA such as TGA-differential scanning calorimetry (DSC), TGA–thermal desorption–gas chromatography–mass spectrometry (TGA-TD-GC-MS), TGA–mass spectrometry (TGA-MS), and DSC are some of the other techniques used to characterize (Peñalver et al. 2020).
MPs traversed by the stream eventually enter the sea; hence, WWTPs that discharge their effluents into rivers contribute to ocean pollution. On the other hand, river mouths are the major area for MP contamination (Leslie et al. 2017). To avoid marine MP contamination, it is critical to find effective and environmentally benign methods of removing MPs in WWTPs. Biological methods using bacteria, fungi, and lower eukaryotes have been the focus of most investigations for MPs removal (Masiá et al. 2020). It is still difficult to use living organisms in MPs bioremediation. The key issue with these microscopic creatures is containing them within WWTPs to avoid inadvertent introduction of these organisms in the ecosystem (Nuzzo et al. 2020). Larger organisms, for instance, higher eukaryotes, may be simpler to contain in theory, but their practical use in MPs bioremediation is currently a niche market (Masiá et al. 2020). This chapter examines the sources of microplastics in wastewaters, their properties, ecotoxicity, and health risks, approaches that are already in use and those that are being developed for characterization of microplastics in wastewater, and bioremediation strategies for the removal of microplastics from wastewater for pollution prevention and control.
2 Sources of Microplastics in Wastewater
Microplastics are produced from a variety of land-based sources and eventually end up in wastewater treatment plants, which are thought to be the link between contaminants and natural habitats (Rochman et al. 2015). Primary microplastics are those that have been made intentionally, whereas secondary microplastics are those that have been produced by a different type of physical, chemical, or biological degradation (Cooper and Corcoran 2010). Microplastics discovered in wastewater treatment plants primarily consist of fibres and microbeads. Microbeads with a size of 250 μm are found in around 0.5–5% of cosmetics (Bowmer and Kershaw 2010). Exfoliants and toothpaste have been shown to release 4500–95,500 microbeads and 4000 microbeads, respectively, with each use (Carr et al. 2016). Synthetic textile washing, on the other hand, releases around 35% of fibre microplastics into the oceans (Boucher and Friot 2017). A load of roughly 5–6 kg, for example, was found to release 6,000,000–700,000 fibres from polyester and acrylic fabrics, respectively (Boucher and Friot 2017). The number of liberated fibres, however, is dependent on the washing conditions, textile qualities, use, and softener and detergent type (Cesa et al. 2017).
Other domestically produced consumer goods, such as contact lens cleaners and jewellery, have also been found to leak MPs. On the other hand, non-domestic sources, have been reported to leak MPs, including (a) air blasting, (b) transportation and manufacturing, (c) Styrofoam products, (d) textile sector, and (e) dust from the drilling and cutting plastics (Prata 2018). Bayo et al. (2020) recently revealed that seasonal variability is also a significant influence, with the highest amounts of MPs seen during warmer periods, as temperature accelerates plastic degradation and fragmentation. Furthermore, due to urban runoff, large amounts of microplastics have been detected during rainy events (Masiá et al. 2020).
3 Properties of Microplastics
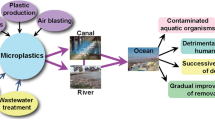
Microplastics are a polymer blend that comes in a variety of shapes. Microplastics’ form is a key criterion for classification. Microplastics in nine different shapes were identified in the influent and effluent of WWTPs: rod, fragment, film, pellet, foam, ellipse, line, and flake (McCormick et al. 2014). Pellets can be cylindrical, circular, flat, ovoid, and spheruloids, while fragments can be rounded, subrounded, subangular, and angular, to name a few. Microplastics, on the other hand, have uneven, elongated, deteriorated, rough, and broken edges as their most common morphologies. MPs in the environment are shown in terms of their sources, transport, accumulation, and fate in Fig. 7.1. MPs are non-biodegradable, water-insoluble synthetic polymers with a high proclivity for fragmentation and microbial ingestion (Beiras et al. 2018). MPs are bioaccumulated by bacteria, fungi, phytoplankton, and zooplankton in many ways in both terrestrial and marine environments (Paul-Pont et al. 2018). Bioadsorption, biouptake (cellular uptake), and biodegradation are the three main mechanisms through which MPs interact/accumulate in microorganisms (MOs) (Avio et al. 2017). MP bioaccumulation has been shown to alter the growth and metabolism of microorganisms (fungi, bacteria, phytoplankton, and zooplankton) (Xu et al. 2019; Sun et al. 2018).
Sources, transport, accumulations, and fate of MPs in the environment (adapted from Wu et al. 2019)
MP bioaccumulation is a serious concern since if swallowed, it can destroy aquatic life. Because of their minute size, microplastics are easily eaten by different marine organisms (Ferreira et al. 2016). Because microplastics are disseminated at diverse trophic levels, microplastic concentrations in the body may grow as a result of bioaccumulation at higher trophic levels. Microplastics penetrate the food chain and eventually reach humans (Nelms et al. 2018). This shows that the most serious consequences of microplastic poisoning may be experienced by people. There is currently minimal knowledge about the effects of microplastics on food webs, and no laboratory trials on bioaccumulation toxicity induced by microplastics at higher trophic levels have been conducted (Anagnosti et al. 2021). As a result, whether or if any size of plastic may be transmitted to higher trophic levels is unknown. Many persistent organic pollutants (POPs), such as dioxins, polybrominated diphenyl ethers and PCBs have been well-documented occurrences of trophic transfer within marine food webs (Ogata et al. 2009; Hu et al. 2005).
Biological availability refers to the small percentage of the total number of particles/chemicals in the environment that are accessible for absorption by an organism. Because smaller particles have a larger volume ratio, stronger penetrating power, and greater ability to be taken up by marine species, MP bioavailability is known to improve as particle size decreases (Botterell et al. 2019). Microplastics density in the water column may alter their bioavailability. Low-density plastics like PE on the sea surface, for example, are likely to come into touch with filter planktivores, feeders, and suspension feeders in the upper water column (Kooi et al. 2017). Other factors influencing microplastic bioavailability in aquatic habitats include colour, shape, ageing, and abundance (Wright et al. 2013; Crawford and Quinn 2017). The binding affinity of MPs particles with other pollutants has an impact on their bioavailability (Bhagat et al. 2020).
Bioaccessibility and bioavailability are critical principles for calculating the risks of exposure to environmental pollutants. The bioavailability of MPs affects their overall effects on organisms (Cole and Galloway 2015). MP bioavailability to be directly absorbed by a wide spectrum of species is enhanced by their small size (Law and Thompson 2014). A planktivore may confuse MPs for natural food during normal eating behaviour, since their size % is comparable to that of planktonic organisms and sediments (Wright et al. 2013). Scherer et al. (2017) discovered that C. riparius can uptake 90 µm MP particles is much lower than that of 10 µm MP particles, despite intraspecific variability in feeding rates (p < 0.01). As a result, it was found that as MP size drops, their potential bioavailability in the food chain increases.
Microplastics can function as vectors for harmful chemical pollutants, and because they are most exposed in the marine ecosystem, many marine species inadvertently consume them (Fred-Ahmadu et al. 2020). PAHs, for example, have high partition coefficients when it comes to plastics, indicating that they have a significant affinity for polymers (Fred-Ahmadu et al. 2020). Because some of the most often observed environmental plastics have a lesser density than seawater (density 1.02 g/cm3), they float in water bodies’ surface microlayers and may sorb contaminants (SML) (PerkinElmer 2019; Sundt et al. 2014). The contaminant-laden plastics floating in the water can be eaten by marine creatures and seabirds in the epipelagic zone. Even when additive effects are taken into account, polymers like PS, PVC, and PU, as well as plastics with fouling surfaces, have a higher density than seawater or freshwater. The process of “microbial fouling” aids the adsorption of various contaminants onto the surface of microplastics in confined lakes (Neto et al. 2019). As a result, contaminant-sorbed microplastics fall to the bottom of the ocean, where they are available for ingestion by benthic creatures (Teuten et al. 2007).
Chemical contaminants that have been absorbed by microplastics can desorb and biomagnify their way up the food chain, from lower trophic species to fish (Bakir et al. 2014; Rochman et al. 2013). Sorbed pollutants on microplastic particles are easily leached by digestive juices (Voparil and Mayer 2000). MPs that have been ingested for a longer period of time are more effective. remain in an organism’s intestines, the more likely pollutants may translocate into bodily tissue. Polybrominated diphenyl ethers (PBDEs) smeared on microplastics were discovered to be incorporated in the tissue of marine amphipods (Chua et al. 2014). As a result of being near to the sources and consumption of these chemicals, the adsorption of various POPs such as polychlorinated biphenyls, hexachlorobenzenes (HCBs), heavy metals and PBDEs to Hydrophobic plastic particles with a large surface area to volume ratio is more prone in freshwater ecosystem than in marine ecosystem (Dris et al. 2015). Freshwater organisms may thus be exposed to increased levels of contaminants, particularly in areas near industrial and populated areas, where increased level concentrations of hydrophobic pollutants, as well as a higher presence of microplastics, may exist, and in areas near agricultural areas, where both POPs (i.e. pesticides) and plastic products are used.
4 Ecotoxicity and Health Hazards of Microplastics
Marine animals such as zooplankton (Desforges et al. 2015), mussels (Qu et al. 2018), oysters (Leslie et al. 2017), corals (Hall et al. 2015), and microplastics in the environment may be consumed by fish (Collard et al. 2015). Once swallowed by marine species, microplastics constitute a threat to them. Health hazards posed by MPs to aquatic biota are presented in Table 7.1. Physical, chemical, ecological, and biological impacts are all possible ways of causing dangers (Provencher et al. 2018). Microplastics cause mechanical damage to organisms. Microplastics, for example, have the capacity to block the intestines and cause harm to the gut (through villi cracking and enterocyte splitting), and even affect organism filtering activity and phagocytosis, resulting in organism death (Canesi et al. 2015; Lei et al. 2018). Furthermore, MPs could build up in food web as a result of predation. Microplastics, for example, were discovered to be fed through the pelagic food web by Satlewal et al. (2008), from zooplankton to mysid shrimps. MPs were also observed to move from algae to zooplankton to goldfish, according to Cedervall et al. (2012).
Microplastics would sorb and collect contaminants in aquatic environments chemically. As a result, ingesting polluted microplastics may expose marine species and even the food chain to hazardous contaminants (Santana et al. 2017; Brennecke et al. 2016). In this case, microplastics act as conduits for hazardous pollutants (Carbery et al. 2018). However, little evidence of the influence of trophic transfer of microplastics and pollutants from the food chain on human health exists at this time, necessitating additional investigation. According to Koelmans et al. (2016), the proportion of total hydrophobic organic contaminants (HOCs) deposited on microplastics was modest in contrast to other media in marine ecosystems, and ingestion of microplastics by marine animals may not provide a HOC risk. According to Wang and Wang, PHE sorption capabilities on PE, PS, and PVC microplastics were higher than sorption capacities on sediment samples (Wang and Wang 2018).
Microplastics can also serve as a microbe’s artificial substrate in addition to serving as carriers of linked chemical burdens to aquatic species. This has sparked concerns about the biological consequences for freshwater ecosystems as they provide important advantages and services, including as habitat for a diverse range of native plants and animals, drinking water, and recreational opportunities (Meng et al. 2020). In terms of ecology, this might have a significant influence on how microplastics interact with freshwater biotas, such as colonized creatures floating over greater distances and microplastics becoming vectors for poisonous bacteria/algae, diseases, and even invading species. The taxonomic composition of bacterial assemblages colonising microplastics in a heavily urbanized river in Chicago, Illinois, differed significantly from those colonising suspended organic matter and water column, and that various taxon, such as pathogens and plastic-decomposing organisms, were more abundant on microplastics, according to McCormick et al. (2016). Several research works have looked into the impact of MPs on marine animal reproduction in the ecosystem (Sharifinia et al. 2020). Sussarellu et al. (2016) describe an emerging perspective that MPs reduce reproductive output by altering organism food consumption and energy allocation. According to Lei et al. (2018), MP particles from various sources, such as (Polyamide, Polyethylene, Polypropylene, Polyvinyl Chloride) PA, PE, PP, and PVC, considerably lower reproductive success in the nematode Caenorhabditis elegans, although only PE- and PVC-MPs had a significant impact on brood size.
Microplastics are biologically sensitive to colonization by microorganisms. Microplastics may influence microbial community evolution and gene exchange (such as antibiotic resistance genes and metal resistance genes) among bacteria (Yang et al. 2019). The antibiotic resistance gene profile is determined by the microbial community composition, according to Yang et al. (2019). Freshwater invertebrates, water fleas (Daphnia magna), and various fish species all actively feed on microplastic particles (1–100 μm), according to laboratory research, and MP particle intake causes critical immobilization of these animals (Besseling et al. 2019; Oliveira et al. 2013; Rehse et al. 2016), as well as affecting predator-prey relationships (Besseling et al. 2019; Oliveira et al. 2013; Rehse et al. 2016; Rochman et al. 2017). However, several studies have revealed that MPs have no effect on ecosystem processes (Krause et al. 2020), making predictions about ecosystem-level consequences more difficult. Furthermore, intergenerational effects on Daphnia magna revealed no impacts in the first generation, while neonates exposed to the same concentration of MPs were extinct after two generations (Martins and Guilhermino 2018). Many freshwater benthic consumers (e.g. Oligochaeta worms, Chironomidae larvae, gammaridae, and amphipods) act as ecosystem engineers in sediments and are heavily exposed to MPs, chemical additives, sorbed pollutants, and possible microbial diseases (Frère et al. 2018; McCormick et al. 2014), posing a serious risk of broad range of impacts, particularly on benthos (Izvekova and Ivova-Katchanova 1972; Ward and Ricciardi 2007). For example, lugworms (Arenicola marina) that ate MPs had less bioturbation, which decreased the primary productivity of bioturbated substrate and changed lugworm respiration (Wright et al. 2013; Green et al. 2016). PVC microplastics were found in the diet of African freshwater catfish in a new study (Iheanacho and Odo 2020a, 2020b). In this case, the microplastic caused neurotoxicity, oxidative stress, and lipid peroxidation, all of which had an impact on the fish’s physiological status. The majority of Microplastics are found in waterways surrounding big cities, particularly in poorer nations with inadequate waste management systems (Xu et al. 2020).
MPs have been found in a variety of places where, all kinds of marine life exists ranging from microscopic species (such as phytoplankton and zooplankton) to enormous predators (mammals and fish) (Anagnosti et al. 2021; Wang et al. 2020). Microplastics have been demonstrated to alter the reproduction, mortality, development, cellular response, behaviour, life span, assimilation efficiency, regeneration, oxygen consumption, egestion, metabolism, nutrition, neurotoxicity, carcinogenicity, and gene expression of aquatic creatures (Haegerbaeumer et al. 2019; Xu et al. 2020). Ingestion of microplastics has a direct impact on small creatures at the bottom of the food chain, producing malnutrition and the inability to eliminate microplastics causes mechanical stress called saturation (Cole et al. 2013; Wright 2015). Microplastic absorption by phytoplankton has been shown to disrupt photosynthesis and, as a result, organism growth (Kalčíková et al. 2017; Bhattacharya et al. 2010). MPs long-term exposure caused considerable alterations in energy stores in two sediment-dwelling bivalve species, Abra nitida and Ennucula tenuis, but did not affect burrowing activity survival, condition index (Bour et al. 2018). The size as well as number of particles were connected to the outcomes, with larger particles and higher concentrations having more severe consequences. At greater concentrations, microplastics caused oxidative stress; damage to the gut, liver, and gill tissues; increased heart rate; and impeded development and motility in goldfish larvae, resulting in oxidative stress; damage to the intestine, liver, and gill tissues; and increased heart rate (Yang et al. 2019).
Because agricultural plastic film and plastic particles are used extensively in industrial production, MPs pollution on land could be more problematic than in the marine environment (Ramos et al. 2015). MPs also represent a threat to terrestrial creatures, as well as human health, through the food chain and other channels (Sharma and Chatterjee 2017). Plastic films or irrigation water containing MPs can both introduce MPs into the soil system (Rillig 2012). MPs have been found in some studies to have an impact on soil organisms, such as altering the isotopic composition of soil collembolans and perturbing the microbiome (Zhu et al. 2018). Earthworms in the soil can be harmed by polystyrene MPs, which can even kill them (Cao et al. 2017). These findings imply that MPs pollution in soils is harmful to soil organisms and that MPs pose an ecological danger in terrestrial ecosystems. In mice, polystyrene MPs were found to cause dysbiosis of the gut microbiota, intestinal barrier failure, and metabolic problems, according to a study (Jin et al. 2019; Lu et al. 2018). MPs could be consumed by micro- and mesofaunas such as mites, collembola, and enchytraeids, accumulating in the soil detrital food web (Rillig 2012). After seeding and planting Lolium perenne (perennial ryegrass) in soils containing MP-clothing fibres, shoot lengths, dry root biomass, dry root–shoot ratio, and chlorophyll a–b ratio high-density polyethylene (HDPE), biodegradable polylactic acid (PLA), and all altered dramatically hence concluded, In the presence of MP-clothing fibres or PLA, seed germination was lower than in control soil (Boots et al. 2019).
Water and nutrient absorption by plant roots is also hampered by the presence of MPs. Plant biomass, root characteristics, tissue elemental composition and soil microbial activity have all been shown to be strongly affected by soil MPs, according to current research (de Souza Machado et al. 2018). Humans consume a wide range of plant and animal products that may include MPs, posing a variety of health hazards. Microplastics are mostly absorbed by ingestion, inhalation, and skin contact (Prata et al. 2020). Microplastics have been detected in beer (Liebezeit and Liebezeit 2014), seafood (Smith et al. 2018), honey and sugar (Liebezeit and Liebezeit 2013), sea salt (Kim et al. 2018), and drinking water (Mintenig et al. 2019). On average, humans consume 4000 MPs each year from water to drink, 37–1000 microplastics from edible sea salt (Van Cauwenberghe and Janssen 2014; Kosuth et al. 2017) and 11,000 microplastics from shellfish. Microplastics (specifically nanoplastics) might reach agricultural fruits/seeds and consequently goes inside the human body through food consumption, according to studies by Sun et al. (2020). Eventually, plant uptake of microplastics may impact human health as well as food security and safety. MPs can also be inhaled through the respiratory system. Airborne microplastics are the most common cause of respiratory exposure. According to a study (Vianello et al. 2019), humans can absorb up to 272 particles each day from indoor air.
The length of time inhaled airborne microplastics travel through the lungs is determined by their size (Enyoh et al. 2019). MPs with a diameter of less than 2.5 m will settle in the lungs first, allowing them to penetrate past the respiratory barrier. Inhalation for a long time Low-level exposure to tiny particles can potentially result in gene mutations (Kingsley et al. 2017). After 10–20 years of being exposed to polypropylene fibres, synthetic textile workers had a higher cancer incidence rate. Workers who worked with polyvinyl chloride had a higher risk of lung cancer as they got older, worked more years, and spent more time in the factories (Prata et al. 2020).
Another form of exposure is dermal touch, but this is a less important route (Prata et al. 2020). Because only particles smaller than 100 nm can be absorbed directly via the skin due to stratum corneum penetration, most microplastics are difficult to absorb (Revel et al. 2018). Microplastics are resistant to chemical breakdown in vivo when they reach the body (Wang et al. 2020). The inhibition of acetylcholinesterase by microplastics could also lead to neurotoxicity (Jeong and Choi 2019). According to a simulated digestion research, microplastics might affect lipid digestion after being consumed by humans by forming microplastics-oil droplet heteroaggregates and inhibiting digestive enzyme activity (Tan et al. 2020), providing a threat to human digestion health.
Microplastics can also be absorbed by human tissues via endocytosis (gastrointestinal tract and airway surface) and paracellular persorption, which is influenced by surface charge, microplastic size, surface functionalization, generated protein corona, and hydrophobicity, among other factors (Wright and Kelly 2017). Increased permeability of the gastrointestinal mucosa can be caused by malnutrition and diets containing high-fructose carbohydrates (due to alterations in the flora of the intestine) and high saturated fats (West-Eberhard 2019). Inhalation and ingestion of MPs in rats resulted in microplastics being discovered in the circulation as well as liver and spleen are examples of distant tissues (Eyles et al. 1995; Jani et al. 1990). A placental perfusion model in humans indicated that 240 nm particle size can cross the placental barrier (Wick et al. 2010). Damage to the DNA replication and repair machinery, as well as DNA damage caused by ROS or particle translocation into the nucleus, can all contribute to MP particle genotoxicity (Rubio et al. 2020).
Microplastics disrupts nuclear membranes, causes oxidative stress, produces damage-related molecular patterns, and activates downstream inflammatory and apoptotic/necrotic pathways in mammalian cells (Yong et al. 2020; Hwang et al. 2020). According to relevant animal model research, these MPs can be transported from living cells to the circulatory systems and lymphatic, where they can gather and harm the cells and immunity of humans (Brown et al. 2001; Browne et al. 2008). Tissue distribution in mice demonstrated MPs accumulation in the kidney, stomach, liver and also the symptoms of energy balance disruption, oxidative stress, and neurotoxicity, after oral administration of fluorescent 5 and 20 m particle sizes at 106 and 104 mg/mL, respectively (Deng and Zhang 2019). After exposure to particulate matter, in vivo neurotoxicity has been reported, possibly due to oxidative stress and activation of the brain’s microglia (immune cells) from direct contact with translocated particles or the action of circulating pro-inflammatory cytokines (from other inflammation sites), resulting in neuron damage (Mohan Kumar et al. 2008).
Several studies have linked microplastics to abnormalities in energy homeostasis. Microplastics, for example, may decrease energy intake (a) by causing a decrease in feeding activity (e.g. in crabs, marine worms, and clams) (Xu et al. 2017; Watts et al. 2015); (b) due to decreased predatory performance (e.g. in fishes) (Wen et al. 2018); and (c) due to alterations in digestive enzymes, thereby causing a loss in digestive capacity (Wen et al. 2018).
Additives and monomers from the microplastics matrix may seep into the body, leading to exposure of tissues to endocrine disruptors including phthalates and bisphenol A, which interfere with endogenous hormones even in minute amounts (Cole and Galloway 2015). Changes in the gut microbiome could have negative consequences, such as the spread of dangerous bacteria, a rise in endotoxemia and intestinal permeability (West-Eberhard 2019). Human’s inhale, ingest, and eat microplastics through the air (Gasperi et al. 2018), bottled water (Zuccarello et al. 2019), seafood and table salt (Nelms et al. 2018; Zuccarello et al. 2019). Recent studies have shown microplastics in excreta of humans (Yong et al. 2020), indicating that microplastics have been eaten. Plastic toxins were found in every human tissue analysed from Alzheimer’s patients in a recent study, which linked toxicity and neurological impairment to lifelong exposure to microplastics (Manivannan et al. 2019).
5 Factors Affecting Toxicity of Microplastics
Plastic toxicity varies depending on the polymer type. Polyurethane, PVC, polyacrylonitriles, styrene-based copolymers and epoxy resins, categorized as the most dangerous (category 1A or 1B mutagen or carcinogen) because of the hazard division of monomers (Lithner et al. 2011). It is crucial to keep in mind that the higher toxicity of smaller particles is not always apparent, since it depends on a variety of elements such as exposure time, charge, cell type, dose, and polymer type. Larger particles necessitate the use of specialist cells to phagocytose them (Alberts et al. 2002). Endocytic and passive uptake mechanisms can take up smaller particles. Particle size and toxicity are usually inversely proportional. The toxicity of 500 nm PS particles (IC50 12.6 g/mL) was found to be higher than that of 50 nm (IC50 > 100 g/mL) in NIH/3T3 and ES-D3 mouse embryo cultures, for example (Hesler et al. 2019). Because of their small size and high surface–volume ratio, MP/NP can absorb additional contaminants such as heavy metals, persistent organic pollutants (POPs), and viruses (de Souza Machado et al. 2018; Yu et al. 2019). Plastics can have persistent organic pollutants (polycyclic aromatic hydrocarbons, polychlorinated biphenyls, DDT), heavy metals (Cd, Cr, As, Hg, As, Br, Zn, Cu, Sb, Sn, Ti, Mn, Co, Ba), and pathogenic Vibrio spp. (Campanale et al. 2020; Brennecke et al. 2016; Prinz and Korez 2020; Kirstein et al. 2016; Velzeboer et al. 2014; Rodrigues et al. 2019).
The absorption, transport, and toxicity of particles can all be influenced by the surface charge of MPs (Yacobi et al. 2010; Fröhlich et al. 2012; Loos et al. 2014a, 2014b). Plasticizers, stabilizers, dyes, lubricants, and flame retardants are among the leachates/plastic additives, which account for around 4% of MPs content and potentially pose health hazards (Bouwmeester et al. 2015; Campanale et al. 2020; EFSA CONTAM Panel 2016). Hahladakis et al. (2018) show that the existence and release of additives, on the other hand, does not always imply a health risk, as toxicity is dictated by the plastic composition and the rate of leachate migration, as well as the amount and solubility of leachate in the surrounding environment. The migration of additives is in large amounts from plastics in fatty foods and when stored at high temperatures or for long periods (Hahladakis et al. 2018).
Chemical adsorption on MPs can be influenced by some circumstances. MPs type, size, environmental salinity and pH, and plastic ageing are only a few of the variables (Mammo et al. 2020). For the same size (200–250 mm), different kinds of microplastics, such as PP, PVC, polyethylene terephthalate (PET), and PE, have varied surface areas and distribution coefficients (Teuten et al. 2007). At differing pH levels, the sort of charge on microplastics surface and chemicals influences whether adsorption increases or decreases. Adsorption is enhanced when the MP surface and chemicals have opposite charges, but adsorption is reduced when the MP surface and chemicals have identical charges (Karlsson et al. 2017). According to Seidensticker et al. (2018), due to repulsion between comparably charged polar compounds and plastic surfaces, non-polar molecules have stronger sorption on PE and PS than polar compounds. The influence of salinity on chemical sorption on MPs can be assessed using changes in the partition coefficients of a chemical with a change in salinity. Log KMP-SW in saltwater and log KMP-W in the same chemical water are different, according to Wang et al. (2020), suggesting that salinity impacts chemical sorption on MPs.
Weathering or ageing of MPs has been reported to increase the rate of chemical sorption (Endo et al. 2005; Rios et al. 2007). Due to environmental interactions such as long-term exposure to the sun, which can cause photo-oxidation, aged plastics have rough surfaces. This causes plastics to degrade into smaller sizes, increasing their surface area and sorption capacity (Brennecke et al. 2016). Adsorption is linked to several sorption sites that are dependent on crystallinity (Joshi et al. 2017). Higher crystallinity produces a clean surface with fewer sorption sites, lowering adsorption. Previous research has found that the crystallinity of MPs influences HOC partitioning, which affects adsorption (Guo et al. 2012). Guo et al. (2012) found that lowering the crystallinity of PE from 59 to 26% increased the sorption of phenanthrene, naphthalene, and lindane. Liu et al. (2019) studied the differences in ciprofloxacin sorption on PVC (low crystalline) and PS (high crystalline) and found that ciprofloxacin sorption on PS was lower than that on PVC.
6 Techniques for Characterization of Microplastics in Wastewater
The sample of wastewater is the initial step in its characterization, and it has a direct impact on the MPs study’s outcomes. Filtration collection is the most popular method for taking samples from wastewater in WWTPs (Kang et al. 2020). Input and effluent samples, on the other hand, can provide information on microplastics sources, total MP removal rate, and pollutant loading. After sampling, the sample must be predigested to remove contaminants and increase extraction efficiency, as well as to minimize MP loss and damage to the greatest extent possible. Purification is another crucial stage in MPs characterization since it demands the removal of the largest amount of organic materials while causing the least amount of damage to MPs. The digestive regents utilized and their concentration, as well as reaction variables such as temperature and duration, might affect the purifying effect (Kang et al. 2020) MPs must be removed from pre-treatment samples after purification to be detected and analysed. Flotation (Imhof et al. 2013) and Elutriation (based on an upward gas or liquid flow to separate MPs) (Mahon et al. 2017) are two unique procedures that have been invented but not generally implemented. Density separation and filtration are two popular ways of extracting MPs (Kang et al. 2020).
Microplastics analysis can be bifurcated into two categories: chemical characterization and physical characterization. Chemical characterization, on the other hand, is primarily used to investigate the composition of microplastics. Several analytical techniques are currently being used to characterize microplastics, including polarized light optical microscopy (PLOM) (Sharifinia et al. 2020), energy-dispersive X-ray spectroscopy (EDS) (Li et al. 2018; Mahon et al. 2017), Raman spectroscopy, and Fourier transform infrared spectroscopy (FTIR) (Sun et al. 2019). The term “physical characterization” refers to the process of determining the distribution of size of microplastics and also other physical characteristics, for instance colour and shape. Several studies performed by authors on the detection and characterization of MPs using different analytical techniques are listed in Table 7.2.
7 Bioremediation Strategies for Microplastics
7.1 Bacterial Degradation of Microplastics
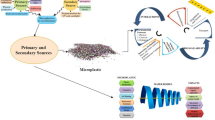
Numerous investigations on the use of microbes for MP breakdown are now underway. A list of microorganisms reported for the degradation of MPs is presented in Table 7.3. The characteristics features of candidate microbial species for bioremediation of MPs are depicted in Fig. 7.2. Pure bacterial cultures have been employed in studies on the breakdown of Microplastics by microbes in the laboratory (Yuan et al. 2020). Enrichment culturing is usually used to isolate microbial cultures from silt, sludge, and wastewater. Pure strains have the benefit of being a simple approach for examining metabolic pathways or assessing the influence of various environmental factors on MP degradation in MP degradation studies.
Characteristics features of candidate microbial species for bioremediation of MPs in WWTPs (adapted from Masiá et al. 2020)
Furthermore, the whole MP breakdown process, as well as variations in MPs, can be precisely tracked by functional bacteria (Janssen et al. 2002). Auta et al. (2018) identified two pure bacterial cultures from mangrove silt and utilized them to break down PP MPs. The weight loss of PP MPs induced by Bacillus sp. strain 27 and Rhodococcus sp. strain In MP degradation investigations, metabolic pathways or analysing the effect of various environmental conditions on MP degradation are both important. 36 was 4.0 and 6.4%, respectively, after 40 days of incubation. Following considerable study, it was revealed that the bacterium is responsible for altering the appearance of microplastics and their functional group structures and other features (Auta et al. 2018). To summarize, future studies are required to optimize techniques and enhance bacteria strains to increase their speed arbitrate the degradation process and increase the pace of MP breakdown (Yuan et al. 2020).
7.2 Fungal Degradation of Microplastics
Along with bacteria, fungi can get attached with and use Microplastics (Mitik-Dineva et al. 2009). Fungi can make MPs less hydrophobic by increasing the formation of chemical bonds like carboxyl, carbonyl, and ester functional groups. Until recently, however, there was little research on the fungal-arbitrated elimination of MPs in the literature. Using ectopic screening, the problems of obtaining fungal strains with strong MP-degrading activity were demonstrated (Yuan et al. 2020). Research into the breakdown of microplastics by fungi in various environments is still underway, but some progress has been made. Penicillium simplicissimum YK was identified by Yamada-Onodera et al. (2001) for application in PE biodegradation. Surprisingly, the aforementioned strain was able to grow better on a solid medium added with 0.5% PE after 500 h of irradiation than on unirradiated media. Dantzler et al. tested two different isolates of Pestalotiopsis microspora for their ability to degrade polyurethane (PUR) to determine if the fungus can degrade a range of MPs in another research (Russell et al. 2011). They discovered that a serine hydrolase was revealed to be the reason for the breakdown of PUR, suggesting that fungus-secreted enzymes can help with MP biodegradation. Devi identified two isolated fungus strains capable of degrading HDPE (Aspergillus tubingensis VRKPT1 and Aspergillus flavus VRKPT2) (Devi et al. 2015). Hydrolyzable polymers can also be degraded by fungi. Deguchi et al. (1997) were the first to report oxidative assault on nylon-6,6 in the white-rot fungus, IZU-154, Phanerochaete chrysosporium and Trametes versicolor. These findings indicated that these fungal strains have a high ability to break down MPs in vitro. To improve the rate of fungal-mediated MP degradation, future investigations should employ genomes and proteomics approaches.
7.3 Microalgal Degradation of Microplastics
The microalgae and plastic interaction waste can substantially alter the properties of these polymers, affecting their fate in aquatic ecosystems (Yokota et al. 2017). Among primary producers, filamentous cyanobacteria of the genus Phormidium are known to break down hydrocarbons (Oberbeckmann et al. 2016). It has been well known that species from this genus can be found on plastic surfaces. This raises the intriguing possibility that Phormidium is hydrolysing the plastic in the plastisphere actively (Yokota et al. 2017). The advantage of increased sunshine exposure on floating plastic pieces may be the real source of plastic trash enrichment as cyanobacteria are photosynthetic in nature (Roager and Sonnenschein 2019). Microalgae were exposed to high-density polyethylene microplastics at a concentration of 1 g L−1 and 400–1000 μm diameter polypropylene (Lagarde et al. 2016); 2 mm polystyrene at 3.96 g L−1 (Long et al. 2017), and microbeads from cosmetic products at around 4000 microbeads (Long et al. 2017); and 2 mm polystyrene. Long et al. (2015) explored this interaction in a lab experiment using various aggregates generated from two different algae species (the cryptophyte Rhodomonas salina, the diatom Chaetoceros neogracile, and a mix of both) and 2 m polystyrene microbeads. The experiment revealed that the microbeads were enriched in all three forms of aggregates. Once absorbed, the microbeads increased aggregate sinking rates to several hundred meters per day, a substantial increase over loose beads' sinking rate (less than 4 mm day−1). These results are evidence to the idea that the aggregates of phytoplankton can act as an MP sink. Furthermore, when an aggregate splits, more surfaces and macropores become available for microbeads to cling to, allowing microbeads to be incorporated not just at the aggregate surface or in macropores, but throughout the aggregate (Long et al. 2015).
7.4 Microbial Consortia in Microplastics Degradation
Numerous investigations have revealed that when an axenic bacterium (pure bacterial cultures) biodegrades organic substances, hazardous end products are produced that impede the growth of microbes (Dobretsov et al. 2013). Using a mixture of bacteria to establish an intact community of microbes that have the ability to assist minimization of harmful metabolite effects on microplastics-degrading bacteria when compared to axenic bacterial cultures. In addition to that, a microbe’s poisonous metabolites can frequently be used as a growing substrate of different microorganisms. Consortia bacteria have synergistic symbiotic and mutual relationships, which allows them to be more tolerable and active during pollutant treatment (Singh and Wahid 2015). Park and Kim (2019) looked at how a mesophilic mixture of bacteria generated from trash debris broke down PE MPs. Both Paenibacillus sp. and Bacillus sp. were plentiful in the mixture of bacteria and decreased the dry weight of Microplastics particles (14.7% after 60 days) as well as the mean diameter of Microplastics particle (22.8% after 60 days). Earthworms (Lumbricus terrestris) degrade LDPE, according to Huerta Lwanga et al. (2018). The earthworm’s intestinal bacteria absorbed and destroyed LDPE MPs after they were consumed. This shows that MP-degrading microbes are present in the earthworm’s digestive system. Lwanga also looked at the role of bacteria in gut of earthworms in the decomposition of microplastics. The most common isolates from the earthworm’s gut were members of the genera Firmicutes and Actinobacteria. Researchers have discovered that when isolated strains were tested for microplastics degradation, the particle size of LDPE-MPs was drastically decreased in the presence of bacteria. There are also eicosane, tricosane, and other volatile chemicals present. Only the treatments that included both LDPE-MP and bacteria produced docosane, indicating that these long-chain alkanes were produced as a result of bacterial-mediated LDPE-MPs breakdown. Due to interactions between many microorganisms and various enzymes, the breakdown and usage of microplastics by mixture of bacteria is a baffling process. As a result, it will be critical to in the future to develop in-depth research on the influencing factors (Yuan et al. 2020).
7.5 Microbial Biofilm in Microplastics Degradation
MPs are exposed to inorganic particles, organic matter, and microbes in aquatic settings (Parrish and Fahrenfeld 2019). Microorganisms of many forms and sizes, including bacteria, algae, viruses, protists, and fungi can cling at the surfaces of Microplastics as a result (Oberbeckmann et al. 2016). Biofilms, which are complex ecosystems made up primarily of microbes, organic and inorganic particles, cell secretions, and other materials, can form as a result of the colonization of these bacteria (Flemming 1998). Microplastics with rough or smooth surfaces, low or high densities, and a wide range of chemical compositions can be used as a biofilm development substrate. Biofilms change and degrade MPs’ physical qualities, according to growing research (Rummel et al. 2017). Lobelle and Cunliffe (2011) studied the development of early biofilms on PE MPs surfaces for three weeks. One week later, biofilms were easily visible on PE surfaces, and they remained intact to proliferate for the next 14 days. The total number of heterotrophic microorganisms on the lowered MPs increased as well, Week three saw an increase in cell count from 1.4 × 104 cells cm−2 to 1.2 × 105 cells cm−2. Over the course of 3 weeks, As the contact between seawater and air became more hydrophilic, the MPs began to sink. Under the influence of biofilms, there was some damage and certain changes that occurred to PE MPs. Miao et al. (2019) investigated the microbial community in various substrates using high-throughput sequencing and generated community metrics such as evenness, diversity, and species richness. Natural materials have less bacteria connected to plastic degradation than MPs, according to the researchers. MPs were shown to have higher levels of Phycisphaerales, Pirellulaceae, Roseococcus, and Cyclobacteriaceae than originally occurring substrates, demonstrating that microplastics are microbial specific. Biofilms utilizes microplastics as carbon sources, energy sources, and adhesion media in conjunction with microbes and enzymes, attacking and degrading them. Biofilms’ destruction of MPs, on the other hand, is extremely difficult and has yet to be thoroughly researched. MP breakdown products, for example, have not been effectively collected and studied. Furthermore, routine data analysis for weight loss and cosmetic alterations has aided in the present understanding of biofilms’ impacts on MPs. More controlled study and product tracking will be carried out in the future to better understand these relationships and degradation behaviour.
7.6 Bioreactor Systems for Microplastic Removal from Wastewater
The majority of microplastics were eliminated from the bioreactor system by bacterial consumption and the formation of sludge aggregates. Domesticated activated sludges, in particular, have been reported to increase microplastic build-up in WWTPs. During the subsequent secondary settling operation, microplastic-containing sludge was eliminated (Jeong et al. 2016). In WWTPs, the A2O bioreactor system is the most extensively utilized (Liu et al. 2021). Due to the sludge return, however, it has a low microplastics removal effectiveness. Microplastics that had been absorbed by the sludge would return to the aqueous phase in a small percentage (20%). Furthermore, the breakdown of microplastics in A2O is relatively sluggish (Liu et al. 2021). As a result, the typical activated sludge method of removing microplastics from WWTPs is inefficient.
Membrane bioreactor or MBR technology has lately gained popularity WWTPs treatment method. Because of the high concentration of mixed-liqueur suspended particles, it has an exceptional performance in removing microplastics (removal efficiency of 99.9%) (range from 6000 mg L−1 to 10,000 mg L−1) (Dvořák et al. 2013; Talvitie et al. 2017). Membrane separation and the classic activated sludge technique were combined in MBR technology. The bulk of microplastics were maintained on the MBR system’s biofilm carrier side. This revealed that the adsorption effect is one of the most important factors in microplastic elimination in the MBR system. The pore size of the membrane employed in MBR systems is typically 0.1 m (Atasoy et al. 2007). Biofilter technology is employed as a major technology following the bioreactor system. MPs with the small size of particles and lesser density are flooded into the biofilter treatment unit. The removal of MPs became more challenging as a result of these factors. Biofilter technology, on the other hand, has the best microplastic removal performance (Lei et al. 2018). The major strategies for removing microplastics are biofilm filtration and adsorption, and biofilter technology combined physical and biological purification processes (Liu et al. 2021). Because microplastics are considered microbe transporters, their presence will have an impact on the microbial activity and community. According to Li et al. (2020), the number of functioning units of taxonomy dropped from 1665 to 1533 after the addition of PVC. As a result, there was an increase in the number of functional units of taxonomy to 1735. As a result, the presence of microplastics PVC did not result in a significant decrease in operational taxonomic units and had no effect on microbial community structure. It is also reassuring to learn that virgin microplastics had no effect on phosphorus-accumulating organisms, nitrite-oxidizing bacteria, or ammonia-oxidizing bacteria (Liu et al. 2019). As a result, the microplastics effect on the functioning of bioreactor system must not be overestimated. On the other hand, the additives toxicity in MPs to microorganisms was unknown. The influence of microbe-containing MPs on traditional pollution remediation should be studied in the future.
8 Challenges and Future Perspectives
Multiple knowledge gaps and areas of disagreement must be addressed before the number of MPs in WWTPs can be estimated. To have a better picture of annual MP deposits into WWTPs, temporal and regional trends in MPs must be investigated. An estimate of the annual variance of MPs in wastewater and the ability of WWTPs to handle such flows have yet to be explored, but it is crucial for gaining a better grasp of worldwide MPs wastewater trends. Furthermore, nothing is known about MPs’ ability to store and transfer chemical and microbiological contaminants across the landscape (including diseases). Treatment plants contain a wide range of dangerous pollutants and pathogens, but little is known about MPs’ ability to adsorb them at all phases of the treatment process and thereafter. Existing studies of microplastics in WWTPs have some flaws that need to be addressed in future research. The establishment of standardized sampling and analysis methodologies to understand the fate of microplastics better in WWTPs or any other media of environment should be the centre of attention of future research. Standardization of reporting units for MP concentration is required. Furthermore, because size of MP particle ranges from 100 nm to 5 mm, mass units are the most accurate depiction of MP contamination within a given sample, allowing for more efficient comparisons between sampling locations. Simultaneously, additional study into specific microplastics should be prioritized, particularly in industrial zones. Hydraulic retention time, salinity, and dissolved organic matter, all of which influence the treatment processes' ability to eliminate microplastics in WWTPs, deserve further investigation. Furthermore, the microplastic removal effect of reaction intermediates, removal of contaminants and their toxicity created by the current treatment technique was unknown. Several MPs-degrading functional microbes have been identified, and several methods for characterizing them have been established. To garner a decrease in MPs, functional microbial agents must be explored and even genetically engineered. The investigation of MP degradation mediated by microbes is a significant undertaking. Greater formation of microbial potential and their use in MP treatment will be required in the future to reduce MP pollution.
On average, a total of 70% of MPs were eliminated during primary treatment. The role of dissolved air flotation (DAF) in the removal of MPs was the most evident at this stage (Bui et al. 2020). The membrane bioreactor (MBR) system is currently the most outstanding treatment technique for secondary treatment, with a success rate of over 99% (Lares et al. 2018). This study indicates that integrating primary treatment units with MBR procedures improves MP removal from wastewaters. One of these technologies’ disadvantages is that there are still few studies testing MBRs’ efficacy on MPs; the influence of MPs on the cost–benefit analysis and membrane fouling, have gotten little attention. Furthermore, the operating parameters and environmental elements of MBR had a considerable impact on MP removal efficiency. And the research has not focused on these difficulties so far. As a result, it is critical to pay more attention to future investigations on Microplastics removal in various bioreactors, particularly MBRs.
It is also worth noting that the lack of consistency in analytical investigations leads to a wide range of outcomes. Ignorance of small MPs (20 m) and lack of MPs mass estimation are just a few of the significant issues with the analysis approach. Despite purification and clean treatment, it is impossible to eliminate contaminants from MPs samples, resulting in an unspecific spectrum that is difficult to differentiate from the library’s standard spectrum. Long-term data is required for the accurate assessment of MPs concentrations in WWTPs throughout the year.
9 Conclusion and Recommendations
Despite the fact that WWTPs are not specifically designed to remove MPs, millions of MPs are discharged into the environment every day, both from treated water outflow and sewage sludge used for soil augmentation. As a result, these facilities are seen as a potential source of MPs entering aquatic environments. However, all MP study results are based on laboratory circumstances that can never match the actual environment, hence absolute choices based on laboratory research on how MPs function in an organism’s natural habitat are unreliable, and therefore, extensive future research is required. This chapter’s conclusions and recommendations are as follows:
-
a.
WWTPs should be prioritized as hotspots for preventing microplastics from entering the environment.
-
b.
More emphasis on improving and implementing advanced tertiary treatment techniques to remove more MPs from treated water is needed.
-
c.
Depending on the species, bioremediation could be a viable option for degrading or accumulating microplastics in wastewater treatment.
-
d.
Research into new methods and biotechnologies for removing MPs from sludges with high efficiency is required.
-
e.
Evaluation of candidate species’ ability to retain MPs in realistic environmental concentrations should be carried out.
References
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Transport into the cell from the plasma membrane: endocytosis. In: Molecular biology of the cell, 4th edn. Garland Science, New York
Anagnosti L, Varvaresou A, Pavlou P, Protopapa E, Carayanni V (2021) Worldwide actions against plastic pollution from microbeads and microplastics in cosmetics focusing on European policies. Has the issue been handled effectively? Mar Pollut Bull 162:111883
Atasoy E, Murat S, Baban A, Tiris M (2007) Membrane bioreactor (MBR) treatment of segregated household wastewater for reuse. CLEAN–Soil Air Water 35:465–472
Auta HS, Emenike CU, Jayanthi B, Fauziah SH (2018) Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar Pollut Bull 127:15–21
Avio CG, Cardelli LR, Gorbi S, Pellegrini D, Regoli F (2017) Microplastics pollution after the removal of the Costa Concordia wreck: first evidences from a biomonitoring case study. Environ Pollut 227:207–214
Bakir A, Rowland SJ, Thompson RC (2014) Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ Pollut 185:16–23
Barnes DK, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc B Biol Sci 364:1985–1998
Bayo J, Olmos S, López-Castellanos J (2020) Microplastics in an urban wastewater treatment plant: the influence of physicochemical parameters and environmental factors. Chemosphere 238:124593
Beiras R, Bellas J, Cachot J, Cormier B, Cousin X, Engwall M, Vidal-Liñán L (2018) Ingestion and contact with polyethylene microplastics does not cause acute toxicity on marine zooplankton. J Hazard Mater 360:452–460
Besseling E, Wegner A, Foekema EM, Van Den Heuvel-Greve MJ, Koelmans AA (2013) Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ Sci Technol 47:593–600
Besseling E, Wang B, Lürling M, Koelmans AA (2014) Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol 48:12336–12343
Besseling E, Redondo-Hasselerharm P, Foekema EM, Koelmans AA (2019) Quantifying ecological risks of aquatic micro-and nanoplastic. Crit Rev Environ Sci Technol 49:32–80
Bhagat J, Zang L, Nishimura N, Shimada Y (2020) Zebrafish: an emerging model to study microplastic and nanoplastic toxicity. Sci Total Environ 728:138707
Bharagava RN, Saxena G (2020) Progresses in bioremediation technologies for industrial waste treatment and management: challenges and future prospects. In: Bioremediation of industrial waste for environmental safety. Springer, Singapore, pp 531–538
Bharagava RN, Chowdhary P, Saxena G (2017a) Bioremediation: an ecosustainable green technology: its applications and limitations. In: Bharagava RN (ed) Environmental pollutants and their bioremediation approaches, 1st edn. CRC Press, Boca Raton, pp 1–22. https://doi.org/10.1201/9781315173351-2
Bharagava RN, Saxena G, Chowdhary P (2017b) Constructed wetlands: an emerging phytotechnology for degradation and detoxification of industrial wastewaters. In: Bharagava RN (ed) Environmental pollutants and their bioremediation approaches, 1st edn. CRC Press, Boca Raton, pp 397–426. https://doi.org/10.1201/9781315173351-15
Bharagava RN, Saxena G, Mulla SI, Patel DK (2017c) Characterization and identification of recalcitrant organic pollutants (ROPs) in tannery wastewater and its phytotoxicity evaluation for environmental safety. Arch Environ Contam Toxicol 75(2):259–272. https://doi.org/10.1007/s00244-017-0490-x
Bharagava RN, Purchase D, Saxena G, Mulla SI (2018) Applications of metagenomics in microbial bioremediation of pollutants: from genomics to environmental cleanup. In: Das S, Dash H (eds) Microbial diversity in the genomic era, 1st edn. Academic, New York. https://doi.org/10.1016/B978-0-12-814849-5.00026-5
Bhattacharya P, Lin S, Turner JP, Ke PC (2010) Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J Phys Chem C 114:16556–16561
Boots B, Russell CW, Green DS (2019) Effects of microplastics in soil ecosystems: above and below ground. Environ Sci Technol 53:11496–11506
Botterell ZL, Beaumont N, Dorrington T, Steinke M, Thompson RC, Lindeque PK (2019) Bioavailability and effects of microplastics on marine zooplankton: a review. Environ Pollut 245:98–110
Boucher J, Friot D (2017) Primary microplastics in the oceans: a global evaluation of sources, vol 10. IUCN, Gland
Bour A, Avio CG, Gorbi S, Regoli F, Hylland K (2018) Presence of microplastics in benthic and epibenthic organisms: influence of habitat, feeding mode and trophic level. Environ Pollut 243:1217–1225
Bouwmeester H, Hollman PC, Peters RJ (2015) Potential health impact of environmentally released micro-and nanoplastics in the human food production chain: experiences from nanotoxicology. Environ Sci Technol 49:8932–8947
Bowmer T, Kershaw P (2010) Proceedings of the GESAMP international workshop on microplastic particles as a vector in transporting persistent, bio-accumulating and toxic substances in the ocean 28–30 June 2010. GESAMP, Paris
Brennecke D, Duarte B, Paiva F, Caçador I, Canning-Clode J (2016) Microplastics as vector for heavy metal contamination from the marine environment. Estuar Coast Shelf Sci 178:189–195
Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K (2001) Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol 175:191–199
Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC (2008) Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ Sci Technol 42:5026–5031
Browne MA, Galloway TS, Thompson RC (2010) Spatial patterns of plastic debris along estuarine shorelines. Environ Sci Technol 44:3404–3409
Bui XT, Nguyen PT, Nguyen VT, Dao TS, Nguyen PD (2020) Microplastics pollution in wastewater: characteristics, occurrence and removal technologies. Environ Technol Innov 19:101013
Campanale C, Massarelli C, Savino I, Locaputo V, Uricchio VF (2020) A detailed review study on potential effects of microplastics and additives of concern on human health. Int J Environ Res Public Health 17:1212
Canesi L, Ciacci C, Bergami E, Monopoli MP, Dawson KA, Papa S, Corsi I (2015) Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar Environ Res 111:34–40
Cao D, Wang X, Luo X, Liu G, Zheng H (2017) Effects of polystyrene microplastics on the fitness of earthworms in an agricultural soil. In: IOP conference series: earth and environmental science, vol 61. IOP Publishing, Bristol, p 012148
Carbery M, O’Connor W, Palanisami T (2018) Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ Int 115:400–409
Carr SA, Liu J, Tesoro AG (2016) Transport and fate of microplastic particles in wastewater treatment plants. Water Res 91:174–182
Casado MP, Macken A, Byrne HJ (2013) Ecotoxicological assessment of silica and polystyrene nanoparticles assessed by a multitrophic test battery. Environ Int 51:97–105
Cedervall T, Hansson LA, Lard M, Frohm B, Linse S (2012) Food chain transport of nanoparticles affects behaviour and fat metabolism in fish. PLoS One 7:32254
Cesa FS, Turra A, Baruque-Ramos J (2017) Synthetic fibers as microplastics in the marine environment: a review from textile perspective with a focus on domestic washings. Sci Total Environ 598:1116–1129
Chandel H, Shyam K, Kumar N, Sharma G, Yadav M, Murugesan S, Thakur S, Saxena G (2022) Anaerobic ammonium oxidation (anammox) technology for nitrogen removal from wastewater: recent advances and challenges. Integr Environ Technol Wastewater Treat Sustain Dev:23–48
Chaturvedi M, Mishra A, Sharma K, Sharma G, Saxena G, Singh AK (2021) Emerging contaminants in wastewater: sources of contamination, toxicity, and removal approaches. In: Emerging treatment technologies for waste management. Springer, Singapore, pp 103–132
Chen R, Qi M, Zhang G, Yi C (2018) Comparative experiments on polymer degradation technique of produced water of polymer flooding oilfield. IOP Conf Ser 113(1):012208
Chua EM, Shimeta J, Nugegoda D, Morrison PD, Clarke BO (2014) Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes compressa. Environ Sci Technol 48:8127–8134
Claessens M, De Meester S, Van Landuyt L, De Clerck K, Janssen CR (2011) Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar Pollut Bull 62:2199–2204
Cole M, Galloway TS (2015) Ingestion of nanoplastics and microplastics by Pacific oyster larvae. Environ Sci Technol 49:14625–14632
Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS (2013) Microplastic ingestion by zooplankton. Environ Sci Technol 47:6646–6655
Collard F, Gilbert B, Eppe G, Parmentier E, Das K (2015) Detection of anthropogenic particles in fish stomachs: an isolation method adapted to identification by Raman spectroscopy. Arch Environ Contam Toxicol 69:331–339
Cooper DA, Corcoran PL (2010) Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar Pollut Bull 60:650–654
Costa MF, Ivar do Sul JA, Silva-Cavalcanti JS, Araújo MC, Spengler A, Tourinho PS (2010) On the importance of size of plastic fragments and pellets on the strandline: a snapshot of a Brazilian beach. Environ Monit Assess 168:299–304
Crawford CB, Quinn B (2017) The biological impacts and effects of contaminated microplastics. In: Microplastic pollutants. Elsevier, Kidlington, pp 159–178
Davarpanah E, Guilhermino L (2015) Single and combined effects of microplastics and copper on the population growth of the marine microalgae Tetraselmis chuii. Estuar Coast Shelf Sci 167:269–275
de Sá LC, Luís LG, Guilhermino L (2015) Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ Pollut 196:359–362
de Souza Machado AA, Kloas W, Zarfl C, Hempel S, Rillig MC (2018) Microplastics as an emerging threat to terrestrial ecosystems. Glob Chang Biol 24:1405–1416
Deb VK, Rabbani A, Upadhyay S, Bharti P, Sharma H, Rawat DS, Saxena G (2020) Microbe-assisted phytoremediation in reinstating heavy metal-contaminated sites: concepts, mechanisms, challenges, and future perspectives. In: Microbial technology for health and environment. Springer, Singapore, pp 161–189
Deguchi T, Kakezawa M, Nishida T (1997) Nylon biodegradation by lignin-degrading fungi. Appl Environ Microbiol 63:329–331
Deng Y, Zhang Y (2019) Response to uptake of microplastics and related health effects: a critical discussion of Deng et al., Scientific reports 7: 46687, 2017. Arch Toxicol 93:213–215
Desforges JPW, Galbraith M, Ross PS (2015) Ingestion of microplastics by zooplankton in the Northeast Pacific Ocean. Arch Environ Contam Toxicol 69:320–330
Devi RS, Kannan VR, Nivas D, Kannan K, Chandru S, Antony AR (2015) Biodegradation of HDPE by Aspergillus spp. from marine ecosystem of Gulf of Mannar, India. Mar Pollut Bull 96(1–2):32–40
Dobretsov S, Abed RM, Teplitski M (2013) Mini-review: inhibition of biofouling by marine microorganisms. Biofouling 29:423–441
Dris R, Gasperi J, Rocher V, Saad M, Renault N, Tassin B (2015) Microplastic contamination in an urban area: a case study in Greater Paris. Environ Chem 12:592–599
Dvořák L, Svojitka J, Wanner J, Wintgens T (2013) Nitrification performance in a membrane bioreactor treating industrial wastewater. Water Res 47:4412–4421
EFSA CONTAM Panel (2016) Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J 14:4501–4530
Endo S, Takizawa R, Okuda K, Takada H, Chiba K, Kanehiro H, Date T (2005) Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: variability among individual particles and regional differences. Mar Pollut Bull 50:1103–1114
Enyoh CE, Verla AW, Verla EN, Ibe FC, Amaobi CE (2019) Airborne microplastics: a review study on method for analysis, occurrence, movement and risks. Environ Monit Assess 191:1–17
Eyles J, Alpar HO, Field WN, Lewis DA, Keswick M (1995) The transfer of polystyrene microspheres from the gastrointestinal tract to the circulation after oral administration in the rat. J Pharm Pharmacol 47:561–565
Ferreira P, Fonte E, Soares ME, Carvalho F, Guilhermino L (2016) Effects of multi-stressors on juveniles of the marine fish Pomatoschistus microps: gold nanoparticles, microplastics and temperature. Aquat Toxicol 170:89–103
Flemming HC (1998) Relevance of biofilms for the biodeterioration of surfaces of polymeric materials. Polym Degrad Stab 59:309–315
Fok L, Cheung PK (2015) Hong Kong at the pearl river estuary: a hotspot of microplastic pollution. Mar Pollut Bull 99:112–118
Fossi MC, Coppola D, Baini M, Giannetti M, Guerranti C, Marsili L, Clò S (2014) Large filter feeding marine organisms as indicators of microplastic in the pelagic environment: the case studies of the Mediterranean basking shark (Cetorhinus maximus) and fin whale (Balaenoptera physalus). Mar Environ Res 100:17–24
Fred-Ahmadu OH, Bhagwat G, Oluyoye I, Benson NU, Ayejuyo OO, Palanisami T (2020) Interaction of chemical contaminants with microplastics: principles and perspectives. Sci Total Environ 706:135978
Frère L, Maignien L, Chalopin M, Huvet A, Rinnert E, Morrison H, Paul-Pont I (2018) Microplastic bacterial communities in the Bay of Brest: influence of polymer type and size. Environ Pollut 242:614–625
Fries E, Dekiff JH, Willmeyer J, Nuelle MT, Ebert M, Remy D (2013) Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ Sci: Processes Impacts 15:1949–1956
Fröhlich E, Meindl C, Roblegg E, Ebner B, Absenger M, Pieber TR (2012) Action of polystyrene nanoparticles of different sizes on lysosomal function and integrity. Part Fibre Toxicol 9:1–13
Gasperi J, Wright SL, Dris R, Collard F, Mandin C, Guerrouache M, Tassin B (2018) Microplastics in air: are we breathing it in? Curr Opin Environ Sci Health 1:1–5
Gautam S, Kaithwas G, Bharagava RN, Saxena G (2017) Pollutants in tannery wastewater, pharmacological effects and bioremediation approaches for human health protection and environmental safety. In: Bharagava RN (ed) Environmental pollutants and their bioremediation approaches, 1st edn. CRC Press, Boca Raton, pp 369–396. https://doi.org/10.1201/9781315173351-14
GESAMP (2015) Transport into the cell from the plasma membrane: endocytosis. In: Molecular biology of the cell, 4th edn. Garland Science, New York
Goutam SP, Saxena G, Singh V, Yadav AK, Bharagava RN (2018) Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem Eng J 336:386–396. https://doi.org/10.1016/j.cej.2017.12.029
Green DS, Boots B, Sigwart J, Jiang S, Rocha C (2016) Effects of conventional and biodegradable microplastics on a marine ecosystem engineer (Arenicola marina) and sediment nutrient cycling. Environ Pollut 208:426–434
Guo X, Wang X, Zhou X, Kong X, Tao S, Xing B (2012) Sorption of four hydrophobic organic compounds by three chemically distinct polymers: role of chemical and physical composition. Environ Sci Technol 46:7252–7259
Haegerbaeumer A, Mueller MT, Fueser H, Traunspurger W (2019) Impacts of micro-and nano-sized plastic particles on benthic invertebrates: a literature review and gap analysis. Front Environ Sci 7:17
Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P (2018) An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater 344:179–199
Hall NM, Berry KLE, Rintoul L, Hoogenboom MO (2015) Microplastic ingestion by scleractinian corals. Mar Biol 162:725–732
Heo NW, Hong SH, Han GM, Hong S, Lee J, Song YK, Shim WJ (2013) Distribution of small plastic debris in cross-section and high strandline on Heungnam beach, South Korea. Ocean Sci J 48:225–233
Hesler M, Aengenheister L, Ellinger B, Drexel R, Straskraba S, Jost C, Kohl Y (2019) Multi-endpoint toxicological assessment of polystyrene nano-and microparticles in different biological models in vitro. Toxicol In Vitro 61:104610
Hidayaturrahman H, Lee TG (2019) A study on characteristics of microplastic in wastewater of South Korea: identification, quantification, and fate of microplastics during treatment process. Mar Pollut Bull 146:696–702
Hu J, Jin F, Wan Y, Yang M, An L, An W, Tao S (2005) Trophodynamic behavior of 4-nonylphenol and nonylphenol polyethoxylate in a marine aquatic food web from Bohai Bay, North China: comparison to DDTs. Environ Sci Technol 39:4801–4807
Hwang J, Choi D, Han S, Jung SY, Choi J, Hong J (2020) Potential toxicity of polystyrene microplastic particles. Sci Rep 10:1–12
Iheanacho SC, Odo GE (2020a) Dietary exposure to polyvinyl chloride microparticles induced oxidative stress and hepatic damage in Clarias gariepinus (Burchell, 1822). Environ Sci Pollut Res 27:21159–21173
Iheanacho SC, Odo GE (2020b) Neurotoxicity, oxidative stress biomarkers and haematological responses in African catfish (Clarias gariepinus) exposed to polyvinyl chloride microparticles. Comp Biochem Physiol C Toxicol Pharmacol 232:108741
Imhof HK, Ivleva NP, Schmid J, Niessner R, Laforsch C (2013) Contamination of beach sediments of a subalpine lake with microplastic particles. Curr Biol 23:867–868
Izvekova EI, Ivova-Katchanova AA (1972) Sedimentation of suspended matter by Dreissena polymorpha Pallas and its subsequent utilization by Chironomidae larvae. Pol Arch Hydrobiol 19:203–210
Jani P, Halbert GW, Langridge J, Florence AT (1990) Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol 42:821–826
Janssen PH, Yates PS, Grinton BE, Taylor PM, Sait M (2002) Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl Environ Microbiol 68:2391–2396
Jeong J, Choi J (2019) Adverse outcome pathways potentially related to hazard identification of microplastics based on toxicity mechanisms. Chemosphere 231:249–255
Jeong CB, Won EJ, Kang HM, Lee MC, Hwang DS, Hwang UK, Lee JS (2016) Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ Sci Technol 50:8849–8857
Jin Y, Lu L, Tu W, Luo T, Fu Z (2019) Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ 649:308–317
Joshi G, Naithani S, Varshney VK, Bisht SS, Rana V (2017) Potential use of waste paper for the synthesis of cyanoethyl cellulose: a cleaner production approach towards sustainable environment management. J Clean Prod 142:3759–3768
Kalčíková G, Gotvajn AŽ, Kladnik A, Jemec A (2017) Impact of polyethylene microbeads on the floating freshwater plant duckweed Lemna minor. Environ Pollut 230:1108–1115
Kang HJ, Park HJ, Kwon OK, Lee WS, Jeong DH, Ju BK, Kwon JH (2018) Occurrence of microplastics in municipal sewage treatment plants: a review. Environ Health Toxicol 33:e2018013
Kang P, Ji B, Zhao Y, Wei T (2020) How can we trace microplastics in wastewater treatment plants: a review of the current knowledge on their analysis approaches. Sci Total Environ 745:140943
Karlsson MV, Carter LJ, Agatz A, Boxall AB (2017) Novel approach for characterizing pH-dependent uptake of ionizable chemicals in aquatic organisms. Environ Sci Technol 51:6965–6971
Kim IS, Chae DH, Kim SK, Choi S, Woo SB (2015) Factors influencing the spatial variation of microplastics on high-tidal coastal beaches in Korea. Arch Environ Contam Toxicol 69:299–309
Kim JS, Lee HJ, Kim SK, Kim HJ (2018) Global pattern of microplastics (MPs) in commercial food-grade salts: sea salt as an indicator of seawater MP pollution. Environ Sci Technol 52:12819–12828
Kingsley SL, Deyssenroth MA, Kelsey KT, Awad YA, Kloog I, Schwartz JD, Wellenius GA (2017) Maternal residential air pollution and placental imprinted gene expression. Environ Int 108:204–211
Kirstein IV, Kirmizi S, Wichels A, Garin-Fernandez A, Erler R, Löder M, Gerdts G (2016) Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar Environ Res 120:1–8
Koelmans AA, Bakir A, Burton GA, Janssen CR (2016) Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies. Environ Sci Technol 50:3315–3326
Kooi M, Nes E, Scheffer M, Koelmans AA (2017) Ups and downs in the ocean: effects of biofouling on vertical transport of microplastics. Environ Sci Technol 51:7963–7971
Kor K, Mehdinia A (2020) Neustonic microplastic pollution in the Persian Gulf. Mar Pollut Bull 150:110665
Kosuth M, Wattenberg EV, Mason SA, Tyree C, Morrison D (2017) Synthetic polymer contamination in global drinking water. Orb Media, Washington
Krause S, Baranov V, Nel HA, Drummond J, Kukkola A, Hoellein T, Lynch I (2020) Gathering at the top? Environmental controls of microplastic uptake and biomagnification in freshwater food webs. Environ Pollut 27:115750
Kumar V, Chandra R, Thakur IS, Saxena G, Shah MP (2020) Recent advances in physicochemical and biological treatment approaches. Combined application of physico-chemical & microbiological processes for industrial effluent treatment plant, p. 79
Lagarde F, Olivier O, Zanella M, Daniel P, Hiard S, Caruso A (2016) Microplastic interactions with freshwater microalgae: hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ Pollut 215:331–339
Lares M, Ncibi MC, Sillanpää M, Sillanpää M (2018) Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res 133:236–246
Law KL, Thompson RC (2014) Microplastics in the seas. Science 345:144–145
Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z, He D (2018) Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci Total Environ 619:1–8
Leslie HA, Brandsma SH, Van Velzen MJM, Vethaak AD (2017) Microplastics en route: field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ Int 101:133–142
Li X, Chen L, Mei Q, Dong B, Dai X, Ding G, Zeng EY (2018) Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res 142:75–85
Li L, Liu D, Song K, Zhou Y (2020) Performance evaluation of MBR in treating microplastics polyvinylchloride contaminated polluted surface water. Mar Pollut Bull 150:110724
Liebezeit G, Liebezeit E (2013) Non-pollen particulates in honey and sugar. Food Addit Contam Part A 30:2136–2140
Liebezeit G, Liebezeit E (2014) Synthetic particles as contaminants in German beers. Food Addit Contam Part A 31:1574–1578
Lithner, Larsson D, Dave G (2011) Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci Total Environ 409:3309–3324
Liu Z, Yu P, Cai M, Wu D, Zhang M, Huang Y, Zhao Y (2019) Polystyrene nanoplastic exposure induces immobilization, reproduction, and stress defense in the freshwater cladoceran Daphnia pulex. Chemosphere 215:74–81
Liu W, Zhang J, Liu H, Guo X, Zhang X, Yao X, Cao Z, Zhang T (2021) A review of the removal of microplastics in global wastewater treatment plants: characteristics and mechanisms. Environ Int 146:106277
Lobelle D, Cunliffe M (2011) Early microbial biofilm formation on marine plastic debris. Mar Pollut Bull 62:197–200
Long M, Moriceau B, Gallinari M, Lambert C, Huvet A, Raffray J, Soudant P (2015) Interactions between microplastics and phytoplankton aggregates: impact on their respective fates. Mar Chem 175:39–46
Long M, Paul-Pont I, Hégaret H, Moriceau B, Lambert C, Huvet A, Soudant P (2017) Interactions between polystyrene microplastics and marine phytoplankton lead to species-specific hetero-aggregation. Environ Pollut 228:454–463
Long Z, Pan Z, Wang W, Ren J, Yu X, Lin L, Jin X (2019) Microplastic abundance, characteristics, and removal in wastewater treatment plants in a coastal city of China. Water Res 155:255–265
Loos C, Syrovets T, Musyanovych A, Mailänder V, Landfester K, Nienhaus GU, Simmet T (2014a) Functionalized polystyrene nanoparticles as a platform for studying bio-nano interactions. Beilstein J Nanotechnol 5:2403–2412
Loos C, Syrovets T, Musyanovych A, Mailänder V, Landfester K, Simmet T (2014b) Amino-functionalized nanoparticles as inhibitors of mTOR and inducers of cell cycle arrest in leukemia cells. Biomaterials 35:1944–1953
Lu L, Wan Z, Luo T, Fu Z, Jin Y (2018) Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci Total Environ 631:449–458
Lwanga EH, Thapa B, Yang X, Gertsen H, Salánki T, Geissen V, Garbeva P (2018) Decay of low-density polyethylene by bacteria extracted from earthworm’s guts: a potential for soil restoration. Sci Total Environ 624:753–757
Ma Y, Huang A, Cao S, Sun F, Wang L, Guo H, Ji R (2016) Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ Pollut 219:166–173
Mahon AM, O’Connell B, Healy MG, O’Connor I, Officer R, Nash R, Morrison L (2017) Microplastics in sewage sludge: effects of treatment. Environ Sci Technol 51:810–818
Mammo FK, Amoah ID, Gani KM, Pillay L, Ratha SK, Bux F, Kumari S (2020) Microplastics in the environment: interactions with microbes and chemical contaminants. Sci Total Environ 743:140518
Manivannan B, Yegambaram M, Supowit S, Beach TG, Halden RU (2019) Assessment of persistent, bioaccumulative and toxic organic environmental pollutants in liver and adipose tissue of Alzheimer’s disease patients and age-matched controls. Curr Alzheimer Res 16:1039–1049
Mao Y, Ai H, Chen Y, Zhang Z, Zeng P, Kang L, Li H (2018) Phytoplankton response to polystyrene microplastics: perspective from an entire growth period. Chemosphere 208:59–68
Martins A, Guilhermino L (2018) Transgenerational effects and recovery of microplastics exposure in model populations of the freshwater cladoceran Daphnia magna Straus. Sci Total Environ 631:421–428
Martins J, Sobral P (2011) Plastic marine debris on the Portuguese coastline: a matter of size? Mar Pollut Bull 62:2649–2653
Masiá P, Sol D, Ardura A, Laca A, Borrell YJ, Dopico E, Garcia-Vazquez E (2020) Bioremediation as a promising strategy for microplastics removal in wastewater treatment plants. Mar Pollut Bull 156:111252
McCormick A, Hoellein TJ, Mason SA, Schluep J, Kelly JJ (2014) Microplastic is an abundant and distinct microbial habitat in an urban river. Environ Sci Technol 48:11863–11871
McCormick AR, Hoellein TJ, London MG, Hittie J, Scott JW, Kelly JJ (2016) Microplastic in surface waters of urban rivers: concentration, sources, and associated bacterial assemblages. Ecosphere 7:e01556
Meng Y, Kelly FJ, Wright SL (2020) Advances and challenges of microplastic pollution in freshwater ecosystems: a UK perspective. Environ Pollut 256:113445
Miao L, Wang P, Hou J, Yao Y, Liu Z, Liu S, Li T (2019) Distinct community structure and microbial functions of biofilms colonizing microplastics. Sci Total Environ 650:2395–2402
Mintenig SM, Löder MGJ, Primpke S, Gerdts G (2019) Low numbers of microplastics detected in drinking water from ground water sources. Sci Total Environ 648:631–635
Mitik-Dineva N, Wang J, Truong VK, Stoddart P, Malherbe F, Crawford RJ, Ivanova EP (2009) Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughness. Curr Microbiol 58:268–273
Mohan Kumar SM, Campbell A, Block M, Veronesi B (2008) Particulate matter, oxidative stress and neurotoxicity. Neurotoxicology 29:479–488
Mulla SI, Bharagava RN, Belhaj D, Saratale GD, Bagewadi ZK, Saxena G, Ninnekar HZ (2019) An overview of nitro group-containing compounds and herbicides degradation in microorganisms. Microbial Metabol Xenobiot Comp 2019:319–335
Murphy F, Ewins C, Carbonnier F, Quinn B (2016) Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ Sci Technol 50:5800–5808
Nel HA, Froneman PW (2015) A quantitative analysis of microplastic pollution along the south-eastern coastline of South Africa. Mar Pollut Bull 101:274–279
Nelms SE, Galloway TS, Godley BJ, Jarvis DS, Lindeque PK (2018) Investigating microplastic trophic transfer in marine top predators. Environ Pollut 238:999–1007
Neto JAB, Gaylarde C, Beech I, Bastos AC, da Silva Quaresma V, de Carvalho DG (2019) Microplastics and attached microorganisms in sediments of the Vitória bay estuarine system in SE Brazil. Ocean Coast Manag 169:247–253
Ng KL, Obbard JP (2006) Prevalence of microplastics in Singapore’s coastal marine environment. Mar Pollut Bull 52:761–767
Nuzzo A, Puccio S, Martina C, Pietrangeli B, Martinez GA, Bertin L, Zanaroli G (2020) Containment of a genetically modified microorganism by an activated sludge system. New Biotechnol 55:58–64
Oberbeckmann S, Osborn AM, Duhaime MB (2016) Microbes on a bottle: substrate, season and geography influence community composition of microbes colonizing marine plastic debris. PLoS One 11:e0159289
Ogata Y, Takada H, Mizukawa K, Hirai H, Iwasa S, Endo S, Thompson RC (2009) International pellet watch: global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar Pollut Bull 58:1437–1446
Okoffo ED, O’Brien S, O’Brien JW, Tscharke BJ, Thomas KV (2019) Wastewater treatment plants as a source of plastics in the environment: a review of occurrence, methods for identification, quantification and fate. Environ Sci Water Res Technol 5:1908–1931
Oliveira M, Ribeiro A, Hylland K, Guilhermino L (2013) Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common go by Pomatoschistus microps (Teleostei, Gobiidae). Ecol Indic 34:641–647
Paço A, Duarte K, da Costa JP, Santos PS, Pereira R, Pereira ME, Rocha-Santos TA (2017) Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci Total Environ 586:10–15
Park SY, Kim CG (2019) Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 222:527–533
Parrish K, Fahrenfeld NL (2019) Microplastic biofilm in fresh-and wastewater as a function of microparticle type and size class. Environ Sci Water Res Technol 5:495–505
Paul-Pont I, Tallec K, Gonzalez-Fernandez C, Lambert C, Vincent D, Mazurais D, Huvet A (2018) Constraints and priorities for conducting experimental exposures of marine organisms to microplastics. Front Mar Sci 5:252
Peñalver R, Arroyo-Manzanares N, López-García I, Hernández-Córdoba M (2020) An overview of microplastics characterization by thermal analysis. Chemosphere 242:125170
PerkinElmer (2019) Melting point, glass transition temperature and structure of common polymers. https://www.perkinelmer.com/CMSResources/Images/44-74863TCH_MPTGAndStructureOfCommonPolymers.pdf
Prata JC (2018) Microplastics in wastewater: state of the knowledge on sources, fate and solutions. Mar Pollut Bull 129:262–265
Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T (2020) Environmental exposure to microplastics: An overview on possible human health effects. Sci Total Environ 702:134455
Prinz N, Korez Š (2020) Understanding how microplastics affect marine biota on the cellular level is important for assessing ecosystem function: a review. YOUMARES 9-The Oceans: Our Research, Our Future, p. 101–120
Provencher JF, Vermaire JC, Avery-Gomm S, Braune BM, Mallory ML (2018) Garbage in guano? Microplastic debris found in faecal precursors of seabirds known to ingest plastics. Sci Total Environ 644:1477–1484
Qu X, Su L, Li H, Liang M, Shi H (2018) Assessing the relationship between the abundance and properties of microplastics in water and in mussels. Sci Total Environ 621:679–686
Ramos L, Berenstein G, Hughes EA, Zalts A, Montserrat JM (2015) Polyethylene film incorporation into the horticultural soil of small periurban production units in Argentina. Sci Total Environ 523:74–81
Reddy MS, Basha S, Adimurthy S, Ramachandraiah G (2006) Description of the small plastics fragments in marine sediments along the Alang-Sosiya ship-breaking yard, India. Estuar Coast Shelf Sci 68:656–660
Rehse S, Kloas W, Zarfl C (2016) Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna. Chemosphere 153:91–99
Revel M, Châtel A, Mouneyrac C (2018) Micro (nano) plastics: a threat to human health? Curr Opin Environ Sci Health 1:17–23
Rillig MC (2012) Microplastic in terrestrial ecosystems and the soil? Environ Sci Technol 46:6453–6454
Rios LM, Moore C, Jones PR (2007) Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar Pollut Bull 54:1230–1237
Rist SE, Assidqi K, Zamani NP, Appel D, Perschke M, Huhn M, Lenz M (2016) Suspended micro-sized PVC particles impair the performance and decrease survival in the Asian green mussel Perna viridis. Mar Pollut Bull 111:213–220
Roager L, Sonnenschein EC (2019) Bacterial candidates for colonization and degradation of marine plastic debris. Environ Sci Technol 53:11636–11643
Rocha-Santos T, Duarte AC (2015) A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. Trends Anal Chem 65:47–53
Rochman CM, Hoh E, Hentschel BT, Kaye S (2013) Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environ Sci Technol 47:1646–1654
Rochman CM, Kross SM, Armstrong JB, Bogan MT, Darling ES, Green SJ, Smyth AR, Veríssimo D (2015) Scientific evidence supports a ban on microbeads. Environ Sci Technol 49:10759–10761
Rochman CM, Parnis JM, Browne MA, Serrato S, Reiner EJ, Robson M, Teh SJ (2017) Direct and indirect effects of different types of microplastics on freshwater prey (Corbicula fluminea) and their predator (Acipenser transmontanus). PLoS One 12:e0187664
Rodrigues JP, Duarte AC, Santos-Echeandía J, Rocha-Santos T (2019) Significance of interactions between microplastics and POPs in the marine environment: a critical overview. Trends Anal Chem 111:252–260
Rubio L, Marcos R, Hernández A (2020) Potential adverse health effects of ingested micro-and nanoplastics on humans. Lessons learned from in vivo and in vitro mammalian models. J Toxicol Environ Health Part B 23:51–68
Rummel CD, Jahnke A, Gorokhova E, Kühnel D, Schmitt-Jansen M (2017) Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ Sci Technol Lett 4:258–267
Russell JR, Huang J, Anand P, Kucera K, Sandoval AG, Dantzler KW, Strobel SA (2011) Biodegradation of polyester polyurethane by endophytic fungi. Appl Environ Microbiol 77:6076–6084
Santana MFM, Moreira FT, Turra A (2017) Trophic transference of microplastics under a low exposure scenario: insights on the likelihood of particle cascading along marine food-webs. Mar Pollut Bull 121:154–159
Satlewal A, Soni R, Zaidi M, Shouche Y, Goel R (2008) Comparative biodegradation of HDPE and LDPE using an indigenously developed microbial consortium. J Microbiol Biotechnol 18:477–482
Saxena G, Bharagava RN (2015) Persistent organic pollutants and bacterial communities present during the treatment of tannery wastewater. In: Chandra R (ed) Environmental waste management, 1st edn. CRC Press, Boca Raton, pp 217–247. https://doi.org/10.1201/b19243-10
Saxena G, Bharagava RN (2017) Organic and inorganic pollutants in industrial wastes, their ecotoxicological effects, health hazards and bioremediation approaches. In: Bharagava RN (ed) Environmental pollutants and their bioremediation approaches, 1st edn. CRC Press, Boca Raton, pp 23–56. https://doi.org/10.1201/9781315173351-3
Saxena G, Chandra R, Bharagava RN (2016) Environmental pollution, toxicity profile and treatment approaches for tannery wastewater and its chemical pollutants. Rev Environ Contam Toxicol 240:31–69. https://doi.org/10.1007/398_2015_5009
Saxena G, Purchase D, Mulla SI, Bharagava RN (2020a) Degradation and detoxification of leather tannery effluent by a newly developed bacterial consortium GS-TE1310 for environmental safety. J Water Process Eng 38:101592
Saxena G, Purchase D, Mulla SI, Saratale GD, Bharagava RN (2020b) Phytoremediation of heavy metal-contaminated sites: eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev Environ Contam Toxicol 249:71–131
Saxena G, Goutam SP, Mishra A, Mulla SI, Bharagava RN (2020c) Emerging and ecofriendly technologies for the removal of organic and inorganic pollutants from industrial wastewaters. In: Bioremediation of industrial waste for environmental safety. Springer, Singapore, pp 113–126
Saxena G, Thakur IS, Kumar V, Shah MP (2020d) Electrobioremediation of contaminants: concepts, mechanisms, applications and challenges. In: Combined application of physico-chemical & microbiological processes for industrial effluent treatment plant. Springer, Singapore, pp 291–313
Scherer C, Brennholt N, Reifferscheid G, Wagner M (2017) Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Sci Rep 7:1–9
Seidensticker S, Grathwohl P, Lamprecht J, Zarfl C (2018) A combined experimental and modeling study to evaluate pH-dependent sorption of polar and non-polar compounds to polyethylene and polystyrene microplastics. Environ Sci Eur 31:1–12
Sharifinia M, Bahmanbeigloo ZA, Keshavarzifard M, Khanjani MH, Lyons BP (2020) Microplastic pollution as a grand challenge in marine research: a closer look at their adverse impacts on the immune and reproductive systems. Ecotoxicol Environ Saf 204:111109
Sharma S, Chatterjee S (2017) Microplastic pollution, a threat to marine ecosystem and human health: a short review. Environ Sci Pollut Res 24:21530–21547
Singh L, Wahid ZA (2015) Methods for enhancing bio-hydrogen production from biological process: a review. J Ind Eng Chem 21:70–80
Sjollema SB, Redondo-Hasselerharm P, Leslie HA, Kraak MH, Vethaak AD (2016) Do plastic particles affect microalgal photosynthesis and growth? Aquat Toxicol 170:259–261
Smith M, Love DC, Rochman CM, Neff RA (2018) Microplastics in seafood and the implications for human health. Curr Environ Health Rep 5:375–386
Sudhakar M, Doble M, Murthy PS, Venkatesan R (2008) Marine microbe-mediated biodegradation of low-and high-density polyethylenes. Int Biodeterior Biodegrad 61:203–213
Sun X, Liang J, Zhu M, Zhao Y, Zhang B (2018) Microplastics in seawater and zooplankton from the Yellow Sea. Environ Pollut 242:585–595
Sun J, Dai X, Wang Q, van Loosdrecht MC, Ni BJ (2019) Microplastics in wastewater treatment plants: detection, occurrence and removal. Water Res 152:21–37
Sun XD, Yuan XZ, Jia Y, Feng LJ, Zhu FP, Dong SS, Xing B (2020) Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat Nanotechnol 15:755–760
Sundt P, Schulze P, Syversen F (2014) Sources of microplastic-pollution to the marine environment project report. https://d3n8a8pro7vhmx.cloudfront.net/boomerangalliance/pages/507/attachments/original/1481155578/Norway_Sources_of_Microplastic_Pollution.pdf?1481155578
Sussarellu R, Suquet M, Thomas Y, Lambert C, Fabioux C, Pernet MEJ, Huvet A (2016) Oyster reproduction is affected by exposure to polystyrene microplastics. PNAS 113:2430–2435
Talvitie J, Mikola A, Koistinen A, Setälä O (2017) Solutions to microplastic pollution–removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res 123:401–407
Tan H, Yue T, Xu Y, Zhao J, Xing B (2020) Microplastics reduce lipid digestion in simulated human gastrointestinal system. Environ Sci Technol 54:12285–12294
Teuten EL, Rowland SJ, Galloway TS, Thompson RC (2007) Potential for plastics to transport hydrophobic contaminants. Environ Sci Technol 41:7759–7764
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AW, McGonigle D, Russell AE (2004) Lost at sea: where is all the plastic? Science 304:838
Van Cauwenberghe L, Janssen CR (2014) Microplastics in bivalves cultured for human consumption. Environ Pollut 193:65–70
Van Emmerik T, Kieu-Le TC, Loozen M, van Oeveren K, Strady E, Bui XT, Tassin B (2018) A methodology to characterize riverine macroplastic emission into the ocean. Front Mar Sci 5:372
Velzeboer I, Kwadijk CJAF, Koelmans AA (2014) Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ Sci Technol 48:4869–4876
Vianello A, Jensen RL, Liu L, Vollertsen J (2019) Simulating human exposure to indoor airborne microplastics using a breathing thermal manikin. Sci Rep 9:1–11
Volke-Sepúlveda T, Saucedo-Castañeda G, Gutiérrez-Rojas M, Manzur A, Favela-Torres E (2002) Thermally treated low density polyethylene biodegradation by Penicillium pinophilum and Aspergillus niger. J Appl Polym Sci 83:305–314
Voparil IM, Mayer LM (2000) Dissolution of sedimentary polycyclic aromatic hydrocarbons into the lugworm’s (Arenicola marina) digestive fluids. Environ Sci Technol 34:1221–1228
Wang W, Wang J (2018) Different partition of polycyclic aromatic hydrocarbon on environmental particulates in freshwater: microplastics in comparison to natural sediment. Ecotoxicol Environ Saf 147:648–655
Wang Z, Lin T, Chen W (2020) Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci Total Environ 700:134520
Ward JM, Ricciardi A (2007) Impacts of Dreissena invasions on benthic macroinvertebrate communities: a meta-analysis. Divers Distrib 13:155–165
Watts AJ, Urbina MA, Corr S, Lewis C, Galloway TS (2015) Ingestion of plastic microfibers by the crab Carcinus maenas and its effect on food consumption and energy balance. Environ Sci Technol 49:14597–14604
Welden NA, Cowie PR (2016) Long-term microplastic retention causes reduced body condition in the langoustine, Nephrops norvegicus. Environ Pollut 218:895–900
Wen B, Zhang N, Jin SR, Chen ZZ, Gao JZ, Liu Y, Xu Z (2018) Microplastics have a more profound impact than elevated temperatures on the predatory performance, digestion and energy metabolism of an Amazonian cichlid. Aquat Toxicol 195:67–76
West-Eberhard MJ (2019) Nutrition, the visceral immune system, and the evolutionary origins of pathogenic obesity. PNAS 116:723–731
Wick P, Malek A, Manser P, Meili D, Maeder-Althaus X, Diener L, von Mandach U (2010) Barrier capacity of human placenta for nanosized materials. Environ Health Perspect 118:432–436
Wright S (2015) The potential for microplastics to cause harm in the marine environment. University of Exeter, Exeter
Wright SL, Kelly FJ (2017) Plastic and human health: a micro issue? Environ Sci Technol 51:6634–6647
Wright SL, Rowe D, Thompson RC, Galloway TS (2013) Microplastic ingestion decreases energy reserves in marine worms. Curr Biol 23:1031–1033
Wu P, Huang J, Zheng Y, Yang Y, Zhang Y, He F, Gao B (2019) Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol Environ Saf 184:109612
Xu XY, Lee WT, Chan AKY, Lo HS, Shin PKS, Cheung SG (2017) Microplastic ingestion reduces energy intake in the clam Atactodea striata. Mar Pollut Bull 124:798–802
Xu JL, Thomas KV, Luo Z, Gowen AA (2019) FTIR and Raman imaging for microplastics analysis: state of the art, challenges and prospects. Trends Anal Chem 119:115629
Xu S, Ma J, Ji R, Pan K, Miao AJ (2020) Microplastics in aquatic environments: occurrence, accumulation, and biological effects. Sci Total Environ 703:134699
Yacobi NR, Malmstadt N, Fazlollahi F, DeMaio L, Marchelletta R, Hamm-Alvarez SF, Crandall ED (2010) Mechanisms of alveolar epithelial translocation of a defined population of nanoparticles. Am J Respir Cell Mol Biol 42:604–614
Yamada-Onodera K, Mukumoto H, Katsuyaya Y, Saiganji A, Tani Y (2001) Degradation of polyethylene by a fungus, Penicillium simplicissimum YK. Polym Degrad Stab 72:323–327
Yang J, Yang Y, Wu WM, Zhao J, Jiang L (2014) Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ Sci Technol 48:13776–13784
Yang Y, Yang J, Wu WM, Zhao J, Song Y, Gao L, Yang R, Jiang L (2015) Biodegradation and mineralization of polystyrene by plastic-eating mealworms: part 2. Role of gut microorganisms. Environ Sci Technol 49:12087–12093
Yang YF, Chen CY, Lu TH, Liao CM (2019) Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. J Hazard Mater 366:703–713
Yokota K, Waterfield H, Hastings C, Davidson E, Kwietniewski E, Wells B (2017) Finding the missing piece of the aquatic plastic pollution puzzle: interaction between primary producers and microplastics. Limnol Oceanogr Lett 2:91–104
Yong CQY, Valiyaveetill S, Tang BL (2020) Toxicity of microplastics and nanoplastics in mammalian systems. Int J Environ Res Public Health 17:1509
Yu F, Yang C, Zhu Z, Bai X, Ma J (2019) Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci Total Environ 694:133643
Yuan J, Ma J, Sun Y, Zhou T, Zhao Y, Yu F (2020) Microbial degradation and other environmental aspects of microplastics/plastics. Sci Total Environ 715:136968
Zbyszewski M, Corcoran PL (2011) Distribution and degradation of fresh water plastic particles along the beaches of Lake Huron, Canada. Water Air Soil Pollut 220:365–372
Zbyszewski M, Corcoran PL, Hockin A (2014) Comparison of the distribution and degradation of plastic debris along shorelines of the Great Lakes, North America. J Great Lakes Res 40:288–299
Zhang C, Chen X, Wang J, Tan L (2017) Toxic effects of microplastic on marine microalgae Skeletonema costatum: interactions between microplastic and algae. Environ Pollut 220:1282–1288
Zhu D, Chen QL, An XL, Yang XR, Christie P, Ke X, Zhu YG (2018) Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol Biochem 116:302–310
Zuccarello P, Ferrante M, Cristaldi A, Copat C, Grasso A, Sangregorio D, Conti GO (2019) Exposure to microplastics (< 10 μm) associated to plastic bottles mineral water consumption: the first quantitative study. Water Res 157:65–371
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Thakur, S. et al. (2022). Environmental Toxicity, Health Hazards, and Bioremediation Strategies for Removal of Microplastics from Wastewater. In: Kumar, V., Thakur, I.S. (eds) Omics Insights in Environmental Bioremediation. Springer, Singapore. https://doi.org/10.1007/978-981-19-4320-1_7
Download citation
DOI: https://doi.org/10.1007/978-981-19-4320-1_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4319-5
Online ISBN: 978-981-19-4320-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)