Abstract
Plants as sessile organisms are exposed to persistently changing stress factors. The primary stresses such as drought, salinity, cold and heat are interconnected in their effects on plants. These factors cause damage to the plant cell and lead to secondary stresses such as osmotic and oxidative stresses. Plants cannot avoid exposure to these factors but adapt morphologically and physiologically by some other mechanisms. Almost all stresses induce the production of a group of proteins called heat shock proteins (Hsps) or stress-induced proteins. The induction of transcription factors of these proteins is a common phenomenon in all living things. These proteins are grouped in plants into five classes according to their approximate molecular weight: (1) Hsp100, (2) Hsp90, (3) Hsp70, (4) Hsp60, and (5) small heat shock proteins (sHsps). Higher plants have at least 20 sHsps and there might be 40 kinds of these sHsps in one plant species. The diversification of these proteins reflects an adaptation to tolerate the heat stress. Transcription of heat shock protein genes is controlled by regulatory proteins called heat stress transcription factors (Hsfs). Plants show at least 21 Hsfs with each one having its role in regulation, but they also cooperate in all phases of periodical heat stress responses (triggering, maintenance, and recovery). There are more than 52 plant species (including crop ones) that have been genetically engineered for different traits such as yield, herbicide and insecticide resistance, and some metabolic changes. In conclusion, major heat shock proteins have some kind of related roles in solving the problem of misfolding and aggregation, as well as their role as chaperones.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
The constant flow of energy through all biological organisms provides the dynamic driving force for the maintenance of biological processes such as cellular biosynthesis and transport. The maintenance of steady-state results in a metastable condition called homeostasis. Any undesired modulation disrupting homeostasis is known as biological stress. Plants as sessile organisms are exposed to persistently changing environmental stress factors. Biological stress in plants is divided into two categories: abiotic and biotic stress. Abiotic stress is a physical stress (e.g., temperature, drought, chemical, light, and salt) that the environment may impose on the plant. Biotic stress is a biological insult (e.g., insects, pests, and pathogens) to which a plant may be exposed during its lifetime.
Abiotic stresses, especially heat, drought, and salinity stresses are the major problems in agriculture. They significantly affect the growth of plants and productivity of crops. It is considered the major cause of >50% reduction in average yield of major crops. Heat stress is turning out to be a major problem in the cultivation of various crops like wheat. Of late, a drastic decrease in the total seed setting and yield has been observed in many wheat growing regions of India mainly due to the terminal heat stress. The problem of heat stress is likely to exacerbate with global climate change adding to the exasperation of the stakeholders. Heat stress has been shown to influence photosynthesis, cellular and subcellular membrane components, seed setting, protein content, and antioxidant enzyme activity; thereby significantly limiting crop productivity (Georgieva 1999). Besides mitigating heat stress, crop productivity under the stress may be enhanced by adaptation strategies.
Numerous heat-responsive proteins have been identified from different crop species. However, the expression patterns of these genes and proteins under heat stress are still not clear. Different stress-associated proteins have been identified from crops like rice, maize, and Arabidopsis and their characterization has also been carried out in response to different stresses.
8.2 Mechanism of Heat Stress
The primary stresses such as high temperature, drought, salinity, cold, and chemicals are interconnected in their effects on plants. These factors cause damage to the plant cell and lead to secondary stresses such as osmotic and oxidative stresses. Plants cannot avoid the exposure to these factors but adapt morphologically and physiologically by some other mechanisms. Heat stress as well as other stresses can trigger some mechanisms of defense such as the expression of stress-associated chaperones, the heat shock proteins (HSPs), which was not expressed under “normal” conditions (Kumar et al. 2016). Almost all stresses induce the production of a group of proteins called heat shock proteins (HSPs) or Stress-induced proteins. Heat stress/shock response is a universal phenomenon and heat shock proteins (HSPs) form the most crucial defense system in all living systems at the cellular level (Katschinski 2004). The cytoprotective effects of HSPs were attributed primarily to their chaperone activities, which minimize the proteotoxicity induced by the accumulation of unfolded or denatured proteins upon stress (Katschinski 2004). HSP synthesis is tightly regulated by different members of heat shock transcription factors (Hsfs) at transcriptional level (Morimoto 1998). Hsfs alone can function in the maintenance of cellular homeostasis that include regulation of cell cycle, cell proliferation, redox homeostasis, and cell death mechanisms (Katschinski 2004; Sreedhar et al. 2006).
8.3 Heat Shock Proteins
During evolution, plants have developed sophisticated mechanisms to sense the subtle changes in growth conditions, and trigger signal transduction cascades, which in turn activate stress-responsive genes and ultimately lead to changes at the physiological and biochemical levels. Abiotic stress especially thermal stress adversely affects the functioning of cellular and metabolic pathways in plants. One of the main effects is on functioning of normal cellular proteins. Under thermal stress there is aggregation and misfolding of important cellular proteins occurred. Plants have developed different defense mechanisms to adapt to these adverse conditions. Under the course of defense mechanisms at molecular level, transcription and translation of a special set of proteins like Heat Shock Proteins (HSPs) occur (Kotak et al. 2007; Kumar et al. 2016). Diversification in HSPs may reflect an adaptation to tolerate heat stress. These molecular chaperones assist in protein refolding under stress conditions, protects plants against stress by re-establishing normal protein conformation, and thus cellular homeostasis.
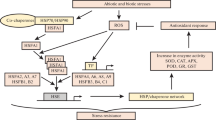
Under stressful conditions, cell response triggered the production of heat shock proteins (HSP). They were named heat shock protein as first described in relation to heat shock, but are now also known to be expressed during other stresses like exposure to cold, UV light, during wound healing, or tissue remodeling. Many HSPs also functions as chaperone by stabilizing new proteins or by helping the refolding of damaged proteins of the cell due to stress (Fig. 8.1). This increase in the expression of HSPs are transcriptionally regulated and the dramatic upregulation of the heat shock proteins is a key heat shock response and is induced primarily by heat shock factors (Hsfs) that are located in the cytoplasm in an inactive state. These factors are considered as transcriptional activators for heat shock (Baniwal et al. 2004; Hu et al. 2009). HSPs are found in virtually all living organisms, from bacteria to plants and humans.
8.4 Thermal Stability of HSPs
Incorrect protein folding into cells can cause several conformational disorders and in order to prevent such structural misfolding and to maintain homeostasis, cells have evolved an efficient protein quality control system (PQC) as an endogenous process. This PQC system needed molecular chaperones (including all HSP families) and their main function is to prevent inappropriate interactions, avoiding protein aggregation by assisting their correct folding and if protein correction is not possible, guiding them to cell degradation system. To maintain the thermal stability of proteins, the chaperone system changes from a folding to a storing function at heat shock temperatures. The temperature at which this change occurs depends on the presence of a thermosensor in at least one of the components of the chaperone systems. One of the most important chaperones is the Heat shock protein 90 kDa (HSP90), which is responsible for the correct folding of a wide range of proteins. In the folding process, it is essential that HSP90 form complexes with co-chaperones, and providing a cooperative action during the maturation cycle of client proteins.
8.5 Classification of Heat Shock Proteins
The expression of heat shock protein gene was first observed by Italian Scientist F. Ritossa in the chromosome puffing of Drosophila melanogaster in response to heat shock. An increase in protein synthesis was observed that occurred also by the use of other stress factors such as azide, salicylate, and 2,4-dinitrophenol (Ritossa 1962). After that report, these proteins were identified and named as heat shock protein (HSP) (Tissieres et al. 1974). Thereafter, various studies were started to find out the relationship between the synthesis of these proteins and the tolerance of stresses. On the other hand, Lin et al. (1984) reported that the exposure of Glycine max seedlings to heat shock (from 28 to 45 °C) for 10 min (longer periods killed the seedlings) induce the synthesis of HSPs at the cost of other proteins synthesis.
Several types of heat shock proteins have been identified in almost all organisms (Bharti and Nover 2002). HSPs are mainly characterized on the basis of the presence of a carboxylic terminal called heat shock domain (Helm et al. 1993). HSPs having molecular weights ranging from 10 to 200 kDa are characterized as chaperones where they participate in the induction of the signal during heat stress (Schoffl et al. 1998). Heat shock proteins of archaea have been classified on the basis of their approximate molecular weight as (1) Heat shock protein of molecular weight 100 kDa: HSP100, (2) HSP90, (3) HSP70, (4) HSP60, and small heat shock proteins (sHSPs) where the molecular weight ranges from 15 to 42 kDa (Trent 1996). Schlesinger (1990) reported that in eukaryotic organisms, the principle heat shock proteins of human beings do not differ from those of bacteria except for the presence of HSP33. Later, the HSPs of human beings were grouped into five families (Kregel 2002) as in Table 8.1.
In plants, according to molecular weight, amino acid sequence homologies and functions, five classes of HSPs are characterized: (1) HSP 100, (2) HSP 90, (3) HSP 70, (4) HSP 60, and (5) small heat shock proteins (sHSPs) (Kotak et al. 2007; Gupta et al. 2010).
The high molecular weight HSPs are characterized as molecular chaperones. Higher plants have at least 20 sHSPs and there might be 40 kinds of these sHSPs in one plant species. The name of HSPs in bacteria differs from those in eukaryotic cells as given below but the nomenclature for sHSPs are same in both organisms (Kotak et al. 2007).
Escherichia coli | Eukaryotic cells |
|---|---|
ClpB | HSP100 |
HtpG | HSP90 |
Dnak | HSP70 |
GroEL | HSP60 |
8.6 Role of Different HSPs
Under thermal stress, the general role of HSPs is to act as molecular chaperones and regulate the protein folding, accumulation, localization, and degradation of proteins in all plants and animal species (Hu et al. 2009; Gupta et al. 2010), indicated that HSPs protect the cells from injury and facilitate recovery and survival after a return to normal growth conditions. On the other hand, under nonthermal stress, their function could be different: as it may protect the protein from damage and maintain the correct protein structure (Timperio et al. 2008). As chaperones, these proteins prevent the irreversible aggregation of other proteins and under heat stress, they participate in refolding of proteins (Tripp et al. 2009). Each group of these HSPs has a unique mechanism and the role of each is as follows.
8.6.1 Class: HSP 100
This class of proteins is responsible for the reactivation of aggregated proteins (Parsell and Lindquist 1993). They basically re-solubilize the nonfunctional protein aggregates and help to degrade irreversibly damaged polypeptides (Kim et al. 2007). This class HSPs function is not restricted only to acclimation to high temperatures, but they also provide housekeeping functions, essential for chloroplast development (Lee et al. 2006), and facilitating the normal situation of the organism after severe stress (Gurley 2000).
8.6.2 Class: HSP 90
HSP90 can bind with HSP70 to form chaperone complexes and act as molecular chaperones, playing important role in signaling protein function and trafficking (Pratt and Toft 2003; Kumar et al. 2012), regulating the cellular signals such as the regulation of glucocorticoid receptor (GR) activity (Pratt et al. 2004). Cytoplasmic HSP 90 reacts with resistance protein (R), the signal receptor from the pathogen, and participates in providing resistance from pathogens. Thus, HSP90 is considered the essential component of innate immune response and pathogenic resistance in rice (Thao et al. 2007). Yamada et al. (2007) reported that in A. thaliana, in the absence of heat stress, cytoplasmic HSP90 negatively inhibits the Hsf, but under heat stress this role is temporarily supressed, so that Hsf is active.
8.6.3 Class: HSP 70
The HSP 70 plays role as a chaperone for newly translated proteins and prevents their accumulations as aggregates, helps in their proper folding, protein import and translocation, and proteolytic degradation of unstable proteins by targeting the proteins to lysosomes or proteasomes (Su and Li 2008). HSP 70 along with sHSPs play a crucial role in protecting plant cells from the detrimental effects of heat stress (Rouch et al. 2004; Kumar et al. 2016). HSP 70B is present in the stroma of chloroplasts, also involved in photo-protection and repairing of photosystem II during and after photoinhibition (Schroda et al. 1999). A study on A. thaliana reported that HSP70 was found in the stroma of chloroplast involved in the differentiation of germinating seeds (Su and Li 2008). Structurally, HSP70 consists of a highly conserved N-terminal ATPase domain of 44 kDa and a C-terminal peptide-binding domain of 25 kDa. HSP70 family chaperones are considered to be the most highly conserved HSPs, with, 50% identical residues between the Escherichia coli homolog DnaK and the eukaryotic HSP70.
8.6.4 Class: HSP 60
A well-known chaperonin, responsible for assisting plastid proteins is Rubisco (Wang et al. 2004). This class of HSPs participates in folding, aggregation, and transport of many mitochondrial and chloroplast proteins (Lubben et al. 1989). HSP60 prevents the aggregation of newly transcribed protein before their folding (Parsell and Lindquist 1993). Functionally, plant chaperonins are limited and stromal chaperones (HSP 70 and HSP 60) are involved in attaining functional conformation of newly imported proteins to the chloroplast (Jackson-Constan et al. 2001).
8.6.5 Class: HSP 40
HSP40 proteins regulate complex formation between polypeptides and HSP70 by different mechanisms. First, HSP40 interacts with HSP70-polypeptide to stimulate its ATPase activity (Cyr et al. 1992). Second, HSP40 proteins have polypeptide-binding domains (PPDs) that bind and deliver specific proteins to HSP70 (Cheetham and Caplan 1998). Third, within the same cellular compartment, specialized members of the HSP40 family are localized to different sites, which facilitate the interaction of different HSP70–HSP40 complexes to bind unique proteins at that site (Shen et al. 2002). This class of protein is also known as J-domain-containing protein (J-protein). It acts as a co-chaperone component of the HSP70 system, increasing HSP70 affinity for proteins (Kampinga and Craig 2010). It has a conserved 70-amino acid J-domain that interacts with the nucleotide-binding domain (NBD) of HSP70 and participates in various virus–plant interactions. Similar to HSP70, the function of HSP40 in viral pathogenesis has been well established. For example, the coat protein of Potato virus Y interacts with DnaJ-like protein (HSP40), which is important for cell-to-cell movement (Hofius et al. 2007). The functions of HSP70 and HSP40 in plant immunity have been generally identified as chaperones in microbial pathogenesis, particularly, in viral movement. Several HSP70 and HSP40 were demonstrated as positive regulators in plant immunity. Overexpression or knockdown of these HSPs enhance resistance and susceptibility to pathogen infections respectively, although the mechanisms remain unclear.
8.6.6 Class: sHSPs (Small HSPs)
The genes encode for small HSPs, their expression is limited in the absence of environmental stress and occurs in some stages of growth and development of plants such as embryogenesis, germination, development of pollen grains, and fruit ripening (Sun et al. 2002). Structurally these proteins have a common alpha-crystalline domain of 80–100 amino acid residues in the C-terminal region (Seo et al. 2006; Kumar et al. 2013). Functionally, these proteins are responsible for the degradation of the proteins having unsuitable folding. The representative protein of this class of HSPs is the enzyme-bound ubiquitin (molecular weight is 8.5 kDa) (Ferguson et al. 1990). Unlike chaperones, these proteins have ATP-independent activity (Miernyk 1999). sHSPs can bind to partially folded or denatured proteins, preventing irreversible unfolding or wrong protein aggregation but they cannot refold the non-native proteins (Sun et al. 2002). Nakamoto and Vigh (2007) concluded that under stress conditions, small heat shock proteins play an important role in controlling the membrane quality and maintaining membrane integrity.
8.7 HSPs/Chaperones Network
In the protective mechanism of HSPs/chaperones, many chaperones act in concert with the chaperone machinery network. During stress, several enzymes and structural proteins undergo detrimental structural and functional changes. Therefore, maintaining proteins in their functional conformations, preventing from aggregation of non-native proteins, refolding of denatured proteins to regain their functional conformation, and removal of nonfunctional but potentially harmful polypeptides (arising from aggregation, misfolding, or denaturation) are particularly important for cell survival under stress. Therefore, the different classes of HSPs/chaperones cooperate in cellular protection and play complementary and sometimes overlapping roles in the protection of proteins from stress. Small HSPs (sHSPs) bind to non-native proteins and prevent their aggregation, thus providing a reservoir of substrates for subsequent refolding by members of the HSP70/HSP100 chaperone families. The Chaperone/HSPs network under stress, how they regulate different proteins’ stability/degradation is presented in Fig. 8.2.
The response of plants to heat shock resulted in changes in the level of enzymes, cellular membrane structure, photosynthesis activity, and protein metabolism (Singla et al. 1997). It has been reported that high temperature changed the properties of membranes of nucleus, endoplasmic reticulum, mitochondria, and chloroplasts of rice plant, O. sativa (Pareek et al. 1998). Lipids in the thylakoid membranes of the chloroplast are very important to improve photosynthesis and hence stress tolerance.
The transcription of these genes is controlled by regulatory proteins called heat shock transcription factors (Hsfs) located in the cytoplasm in an inactive state. So these factors are considered transcriptional activators for heat shock (Baniwal et al. 2004; Hu et al. 2009). Plants are characterized by a large number of transcriptional factors (Baniwal et al. 2007). These factors have been classified (Tripp et al. 2009) into three classes according to the structural differences in their aggregation in triples, i.e., oligomerization domains as follows:
-
Plant HsfA such as HsfA1 and HsfA2 in L. esculentum
-
Plant HsfB such as HsfB1 in L. esculentum
-
Plant HsfC
The synthesis of HSPs depends upon activity of special class of transcription factors called Heat Shock Factors (Hsfs). Hsfs are modular transcription factors encoded by a large gene family in plants. Hsfs have three highly conserved features: the amino terminal DNA binding domain of approximately 100 amino acids (Harrison et al. 1994) and a domain having three leucine zippers mediating multimerization (Wu et al. 1994; Swamynathan 1995) and an additional leucine zipper motif at the carboxy terminus. Hsfs trimerizes via the formation of a triple-stranded α-helical coiled coil, involving the three conserved leucine zippers next to the DNA binding domain (Peteranderl and Nelson 1993). Hsfs bind to heat shock elements (HSE) in a sequence-specific and reversible manner, leading to the activation of transcription of heat shock proteins (Morimoto et al. 1994; Goswami et al. 2016; Fig. 8.3).
8.8 Genetically Modified Plants for Heat Stress Tolerance
Several plant species (more than 52) have been genetically modified for different traits including crop plants like tomato, potato, soybean, maize, rice, and cotton. Other non-crop transgenic plants were also developed for different abiotic stress tolerance in laboratory. High-temperature stress is one of the major abiotic stresses for crop plants. The plant reaction to high-temperature stress resulted in changes in cell membrane stability, photosynthesis activity, enzyme denatured, and protein synthesis (Goswami et al. 2015). High temperature also changes the properties of membranes of mitochondria, chloroplast, endoplasmic reticulum, and nucleus. Lipids in the thylakoid membrane of chloroplast are important for membrane stability and also for photosynthetic efficiency which may be disturbed due to very high or very low temperatures. By increasing the expression of glycerol 3-phosphate acyltransferase enzyme in tobacco plant, the degree of lipid unsaturation was increased which makes the plants cold tolerant. An increase in the degree of saturation of membrane lipids may lead to an increase in the heat tolerance of the plants. Other ways to develop thermotolerance of plants, by changing the level of HSPs expression, Hsfs expression, increase in the synthesis of osmolytes in the cells, modifying the endogenous genes of crop plants such as rubisco activase, oxygen-evolving enhancer proteins, signaling molecules like calcium-dependent protein kinase (CDPK), mitogen-activated protein kinases (MAPK), and genes involved in starch biosynthesis pathways through site-directed mutagenesis and make them thermotolerant. Some examples of the attempts taken for developing thermotolerant crop plants are given in Table 8.2.
8.9 Conclusion
Although, many attempts have been made in the past to develop genetically modified plants for stress tolerance, but with limited sucess. Most attempts were for one trait, while in nature the prevailing conditions is quite complex and require cohorted effort to protect the plant from the vageries of stress. Heat shock proteins basically works as catalytic chaperones preventing the heat stress from inducing protein aggregation/ denaturation and helps in maintaining different metabolic reactions under extreme condition. Out of all the HSPs, sHSPs showed very high-fold increase in the expression in response to heat stress and has been observed to store unfolded proteins. Few HSPs also acts as proteases protecting the cell from damage under abnormal condition. The HSPs has been reported to facilitate the restoration of normal cell function by assisting the refolding of denatured/ aggregated proteins, along with protection of nascent proteins. It also helps in removing the irreparable proteins from the cells. The HSP90 family has been observed to play very important role in signalling as well as defense against biotic and abiotic stresses. The HSP70 family represents one of the most highly conserved classes of heat shock proteins. HSPs ensure proper protein folding and their transfer to final location. There is a need to explore the gene-protein networks of HSPs operating inside the cells and their correlation with the signalling pathways in order to understand the mechanism underlying heat stress tolerance in plants. The information generated will pave the way for the development of climate-smart crop.
References
Baniwal SK, Bharti K, Chan KY, Fauth M, Ganguli A, Kotak S, Mishra SK, Nover L, Port M, Scharf K, Tripp L, Weber C, Zielinski D, von Koskull-Doring P (2004) Heat stress response in plants: a complex game with chaperones and more than 20 heat stress transcription factors. J Biosci 29:471–487
Baniwal SK, Chan KY, Scharf KD, Nover L (2007) Role of heat stress transcription factor HsfA5 as specific repressor of HsfA4. J Biol Chem 282(6):3605–3613
Bharti K, Nover L (2002) Heat stress-induced signaling. In: Scheel D, Wasternack C (eds) Plant signal transduction: Frontiers in molecular biology. Oxford University Press, UK, Oxford, pp 74–115
Cheetham ME, Caplan AJ (1998) Structure function and evolution of DnaJ: conservation and adaptation of chaperone function. Cell Stress Chaperones 3:28–36
Cyr DM, Lu X, Douglas MG (1992) Regulation of HSP70 function by a eukaryotic DnaJ homolog. J Biol Chem 267:20927–20931
Ferguson DL, Guikema JA, Paulsen GM (1990) Ubiquitin pool modulation and protein degradation in wheat roots during high temperature stress. Plant Physiol 92:740–746
Georgieva K (1999) Some mechanisms of damage and acclimation of the photosynthetic apparatus due to high temperature. Bulg J Plant Physiol 25:89–99
Goswami S, Kumar RR, Dubey K, Singh JP, Tiwari S, Kumar A, Smita S, Mishra DC, Kumar S, Grover M, Padaria JC (2016) SSH analysis of endosperm transcripts and characterization of heat stress regulated expressed sequence tags in bread wheat. Front Plant Sci 7
Goswami S, Kumar RR, Sharma SK, Kala YK, Singh K, Gupta R, Dhawan G, Rai GK, Singh GP, Pathak H, Rai RD (2015) Calcium trigger protein kinases induced signal transduction for augmenting the thermotolerance of developing wheat grain under heat stress. J Plant Biochem Biotechnol 24:441–452
Gupta SC, Sharma A, Mishra M, Mishra R, Chowdhuri DK (2010) Heat shock proteins in toxicology: how close and how far? Life Sci 86:377–384
Gurley WB (2000) HSP101: a key component for the acquisition of thermotolerance in plants. Plant Cell 12:457–460
Harrison CJ, Bohm AA, Nelson HCM (1994) Crystal structure of the DNA binding domain of the heat shock transcription factor. Science 263:224–227
Helm KW, Lafayete PR, Nago RT, Key JL, Vierling E (1993) Localization of small heat shock proteins to the higher plant endomembrane system. Mol Cell Biol 13:238–247
Hofius D, Maier AT, Dietrich C, Jungkunz I, Bornke F, Maiss E, Sonnewald U (2007) Capsid protein-mediat.Ed recruitment of host DnaJ-like proteins is required for potato virus Y infection in tobacco plants. J Virol 81:11870–11880
Hu W, Hu G, Han B (2009) Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. Plant Sci 176:583–590
Jackson-Constan D, Akita M, Keegstra K (2001) Molecular chaperones involved in chloroplast protein import. Biochim Biophys Acta 1541:102–113
Kampinga HH, Craig EA (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11:579–592
Katschinski DM (2004) On heat and cells and proteins. News Physiol Sci 19:11–15
Kim HJ, Hwang NR, Lee KJ (2007) Heat shock responses for understanding diseases of protein denaturation. Mol Cells 23:123–131
Kotak S, Larkindale J, Lee U, von Koskull-Doring P, Vierling E, Scharf KD (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10:310–316
Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92:2177–2186
Kumar RR, Goswami S, Gupta R et al (2016) The stress of suicide: temporal and spatial expression of putative heat shock protein 70 protect the cells from heat injury in wheat (Triticum aestivum). J Plant Growth Regul 35:65–82
Kumar RR, Goswami S, Sharma SK, Pathak H, Rai GK, Rai RD (2012) Genome wide identification of target heat shock protein 90 genes and their differential expression against heat stress in wheat. Int J Biochem Res Rev 2:15–30
Kumar RR, Sharma SK, Goswami S, Singh R, Pathak H, Rai RD (2013) Characterization of differentially expressed stress-associated proteins in starch granule development under heat stress in wheat (Triticum aestivum L.). Indian J Biochem Biophys 50:126–138
Lee U, Rioflorido I, Hong SW, Larkindale J, Waters ER, Vierling E (2006) The Arabidopsis ClpB/HSP100 family of proteins: chaperones for stress and chloroplast development. Plant J 49:115–127
Lin CY, Roberts JK, Key JL (1984) Acquisition of thermotolerance in soybean seedlings: synthesis and accumulation of heat shock proteins and their cellular localization. Plant Physiol 74:152–160
Lubben TH, Donaldson GK, Viitanen PV, Gatenby AA (1989) Severa1 proteins imported into chloroplasts form stable complexes with the GroEL-related chloroplast molecular chaperone. Plant Cell 1:1223–1230
Miernyk JA (1999) Protein folding in the plant cell. Plant Physiol 121:695–703
Morimoto RI (1998) Regulation of heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12:3788–3796
Morimoto RI, Jurivich DA, Kroeger PE, Mathur SK, Murphy SP, Nakai A, Sarge K, Abravaya K, Sistonen LT (1994) In: Morimoto RI, Tissieres A, Georgopulos C (eds) Regulation of heat shock gene transcription by a family of heat shock factors; in the biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, New York, pp 417–455
Nakamoto H, Vigh L (2007) The small heat shock proteins and their clients. Cell Mol Life Sci 64:294–306
Pareek A, Singla SL, Grover A (1998) Plant HSP90 family with special reference to rice. J Biosci 23:361–367
Parsell PA, Lindquist S (1993) The function of heat-shock proteins in stress tolerance, degradation and reactivation of damaged proteins. Annu Rev Genet 27:437–496
Peteranderl R, Nelson HCM (1993) Trimerization of the heat shock transcription factor by a triple stranded alpha helical coiled coil. Biochemistry 31:12272–12276
Pratt WB, Galigniana MD, Harrell JM, Deranco DB (2004) Role of HSP90 and the HSP90-binding immunophilins in signalling protein movement. Cell Signal 16:857–872
Pratt WB, Toft DO (2003) Regulation of signaling protein function and trafficking by the HSP90/HSP70-based chaperone machinery. Exp Biol Med 228:111–133
Ritossa F (1962) A new puffing pattern induced by heat shock and DNP in drosophila. Experientia 18:571–573
Rouch JM, Bingham SE, Sommerfeld MR (2004) Protein expression during heat stress in thermo-intolerance and thermotolerance diatoms. J Exp Mar Biol Ecol 306:231–243
Schlesinger MJ (1990) Heat shock proteins. J Biol Chem 265:12111–12114
Schoffl F, Prandl R, Reindl A (1998) Regulation of the heat shock response. Plant Physiol 117:1135–1141
Schroda M, Vallon V, Wollman F, Beck CF (1999) A chloroplast-targeted heat shock protein 70 (HSP70) contributes to the photoprotection and repair of photosystem II during and after photoinhibition. Plant Cell 11:11165–11178
Seo JS, Park TJ, Lee YM, Park HG, Yoon YD, Lee JS (2006) Small heat shock protein 20 gene (HSP20) of the intertidal copepod Tigriopus japonicus as a possible biomarker for exposure to endocrine disruptors. Bulletin of Environmental Contamination & Toxicology 76(4)
Shen Y, Meunier L, Hendershot LM (2002) Identification and characterization of a novel endoplasmic reticulum (ER) DnaJ homologue which stimulates ATPase activity of BiP in vitro and is induced by ER stress. J Biol Chem 277:15947–15956
Singla SL, Preek A, Grover A (1997) High temperature. In: Prasad MNV (ed) Plant ecophysiology. John Wiley, New York, pp 101–127
Sreedhar AS, Deepu O, Abhishek A, Srinivas UK (2006) Heat shock transcription factors: a comprehensive review. In: Stress response: a molecular biology approach. (eds) as (Research Signpost ISBN), vol 81, pp 308–0109-4
Su PH, Li HM (2008) Arabidopsis stromal 70-kDa heat shock proteins are essential for plant development and important for thermotolerance of germinating seeds. Plant Physiol 146:1231–1241
Sun W, Motangu MV, Verbruggen N (2002) Small heat shock proteins and stress tolerance in plants. Biochim Biophys Acta 1577:1–9
Sung DY, Kaplan F, Lee KJ, Guy CL (2003) Acquired tolerance to temperature extremes. Trends Plant Sci 8:179–187
Swamynathan SK (1995) Heat shock response in higher eukaryotes: cloning of heat shock transcription factor 1 (rHsf1) and mechanism of heat induction of albumin, PH D. Thesis, Jawaharlal Nehru University, New Delhi
Thao NP, Chen L, Nakashima A, Hara S, Umemura K, Takahashi A, Shirasu K, Kawasaki T, Shimamoto K (2007) RAR1 and HSP90 form a complex with Rac/Rop GTPase and function2 in innate-immune responses in rice. Plant Cell 19:4035–4045
Timperio AM, Egidi MG, Zolla L (2008) Proteomics applied on plant abiotic stresses: role of heat shock proteins (HSP). J Proteome 71:391–411
Tissieres A, Mitchell HK, Tracy UM (1974) Protein synthesis in salivary glands of D. Melanogaster. Relation to chromosome puffs. J Mol Biol 84:389–398
Trent JD (1996) A review of acquired thermotolerance, heat-shock proteins and molecular chaperones in archaea. FEMS Microbiol Rev 18:249–258
Tripp J, Mishra SK, Scharf KD (2009) Functional dissection of the cytosolic chaperone network in tomato mesophyll protoplasts. Plant Cell Environ 32:123–133
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Wu C, Clos J, Giorgi G, Haroun RI, Kim SJ, Rabindran SK, Westwood JT, Wisniewski J, Yim G (1994) In: Morimoto RI, Tissieres A, Georgopulos C (eds) Structure and regulation of heat shock transcription factor; in the biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, New York, pp 395–416
Yamada K, Fukao Y, Hayashi M, Fukazawa M, Suzuki I, Nishimura M (2007) Cytosolic HSP90 regulates the heat shock response that is responsible for heat acclimation in Arabidopsis thaliana. J Biol Chem 282:37794–37804
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Dahuja, A., Goswami, S., Kumar, R.R., T, V., Praveen, S. (2022). Heat Shock Proteins: Catalytic Chaperones Involved in Modulating Thermotolerance in Plants. In: Kumar, R.R., Praveen, S., Rai, G.K. (eds) Thermotolerance in Crop Plants. Springer, Singapore. https://doi.org/10.1007/978-981-19-3800-9_8
Download citation
DOI: https://doi.org/10.1007/978-981-19-3800-9_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-3799-6
Online ISBN: 978-981-19-3800-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)