Abstract
Microbes in the rumen of herbivores are responsible for effective plant biomass decomposition and digestion. Recent efforts to transform cellulosic biomass into biofuels have heightened interest in the bacterial fibrinolytic processes used by these bacteria. In ecology, plant cell wall material is used to transmit energy between the host and parasitic organisms. Herbivores eat plant material and digest it via symbiotic stomach microbiota (protozoa, fungi, and bacteria). Much anaerobic lignocellulose and hemicellulose-digesting bacteria populate the rumen. Cellulosome is a plant cell wall destroying bacteria’s strategic arsenal. Raphael Lamed identified this complex protein in 1983 in an extremophile Clostridium thermocellum. The cellulosome complex and its actions were also being studied as “Swiss knife” shape and protein complex, the cellulosome protein complex (carbohydrate-binding modules (CBM), cohesin, dockerin, enzymes, and scaffoldings. Scientists discovered these compounds in rumen microbes. A constant study has helped us learn more about rumen bacteria and how their cellulosomes break down plant cell walls. The rumen community depends on cellulolytic Ruminococcus spp. Cohesin-dockerin molecules combine to form cellulosome complexes. Designer cellulosomes are chimeric-tailored cellulosomes that function in a cell-free system. They improve the hydrolysis of cellulosic substrates to create value-added products. For this purpose, recombinant constructions and artificial self-assembling chimeric proteins are produced. The capacity of rumen microbes to digest refractory cellulose is of great industrial importance, and metagenomics research is helping to understand and determine the quantities and kinds of cellulolytic bacteria found in the bovine rumen complex ecosystem. This chapter explains the cellulosomal machinery’s extensive function in lignocellulose-degrading bacteria.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Microorganisms are the most versatile and adaptable forms of life on Earth, and they have been around for over 3.5 billion years. Bacteria governed the biosphere during the first 2 billion years of its existence, populating every biological niche from glacial ice to deep-sea hydrothermal vents. Bacteria from this period established the primary metabolic pathways that are now found in all living species, in addition to a variety of metabolic activities including nitrogen fixation, which is only seen in bacteria today. As bacteria ruled the world for so long, they reshaped the planet’s anaerobic environment into one rich in oxygen while also creating a vast quantity of organic chemicals. They eventually succeeded in creating a habitat that could support more advanced forms of life.
There are billions of years’ worth of genetic adaptations to an ever-changing world in the biochemistry and physiology of bacteria and other microbes right now. At the same time, microbes are more resistant to the ravages of human technology than larger, more sophisticated forms of life because of their physiologic and metabolic diversity and their capacity to exist in narrow niches. As a result, probably, most of the pre-human microbial species are still available for study.
A new strategy was devised by humans to identify new sources of bulk organic compounds. Plants create an enormous amount of carbohydrate-rich compounds. Lignocellulose is the primary structural component of wood; starch in grains, potatoes as well as sugars in molasses and syrup of maize which are all examples of these compounds (Lynd 1990). A mix of microbial fermentation and chemical processes might be used to transform plant materials into primary feedstock compounds in concept (Fig. 11.1). Oxygen generation, alcoholic drinks, and food generated from fermentation have all relied on the metabolic activity of particular bacteria (Lynd 1989). Direct bacterial or fungal breakdown of biomass yields a wide range of “oxy-chemicals” as a byproduct. Small molecules to complex molecules such as plastic, rubber, and solvents are all examples of feedstock chemicals that may be used to synthesize a wide variety of other chemicals. The incorporation of genes for degradative enzymes, genetic manipulations of metabolic pathways, and recombinant microorganisms with favorable growth characteristics is the tool for an applied microbiologist to obtain the most efficient conversion of the feedstock to the desired end product. Unfortunately, the accumulation of microbial cell mass represents competition for end products and frequently poses problems of disposal as well. Modern genetic engineering techniques make it possible to manipulate cells to maximize the formation of a product while permitting only a low level of cell growth.
There are hundreds of species of fungi; bacteria that can digest and use cellulose and hemicellulose in lingo-cellulose plant material. Aerobes, anaerobes as well as mesophiles and thermophiles, are among these creatures (Cavedon et al. 1990; Duong et al. 1983). They can be found in large numbers throughout the natural world. Even while many bacteria can thrive on cellulose or develop enzymes that can break down amorphous cellulose, only a handful can produce the whole complement of extracellular cellulases capable of degrading crystalline cellulose in vitro. Among the latter organisms, the most extensively studied sources of cellulolytic enzymes have been the fungi Trichoderma and Phanerochaete and the bacteria Cellulomonas (an aerobe) and Clostridium thermocellum (an anaerobe).
Every cursory perusal of current scientific literature shows cellulose hydrolysis by cellulases (EC 3.2.1.4, EC 3.2.1.91, and other enzymes) to be among the most intensively studied topics. Research and development on these enzymes and their production, properties, and applications are important if their actions are to be controlled and better utilized. By catalyzing the decay of forest and agricultural residues, these enzymes recycle nutrients and so serve to maintain soil fertility and mechanical properties.
11.2 Biomass
However, in the biotechnology context, “any organic matter that develops through photosynthetic conversion of solar energy” is widely considered to be “biomass.” Green plants, algae, and photosynthetic microorganisms turn the sun’s energy into biomass, which may be used for food, fuel, and other purposes. Over a year, photosynthesis on land and at sea generates biomass with an energy content estimated at three times that of humans on the planet.
11.2.1 Major Components of Plant Biomass
Higher land plants’ vascular tissues include cellulose fibrils embedded in an amorphous matrix of lignin and hemicelluloses in their cell walls (Table 11.1). Non-covalent forces, as well as covalent cross-links, hold these three polymers together, forming a composite substance known as lingo-cellulose, which accounts for more than 90% of the dry weight of a plant cell. The amount of each polymer varies depending on the species and age of the plant, as well as across different parts of the plant. Compared to hardwoods, softwoods often include a larger percentage of lignin. Grass has the greatest concentration of hemicellulose.
11.2.2 Cellulose
The most common carbohydrate polymer is cellulose, which is found in the walls of plant cells. While plentiful, cellulose is a tough polymer to degrade due to its inability to dissolve and the presence of hydrogen-bonded crystalline threads that are difficult to remove. Iα and Iβ are the two main cellulose isomers found in plant cellulose. When it comes to the triclinic form of cellulose (Iα), there is only one chain per unit cell, and it has a greater energy density than the more stable monoclinic Iβ form (Sugiyama and Suh 2011, Atalla and Vanderhart 1984). Plant cell wall cellulose is mostly made up of the stable Iβ form, which is more sensitive to hydrolysis than the Iα form. All of the glycosyl hydroxyl groups in cellulose are located in the equatorial plane, while the axial plane is filled by nonpolar aliphatic protons (which do not form hydrogen bonds). Because of this, the “sides” of elementary microfibrils are hydrophobic, while the “tops and bottoms” are polar and hydrogen bonding.
The cellulosome, a huge extracellular enzyme complex composed of a scaffolding protein and numerous associated cellulases, has developed in anaerobic microbes to break down plant cell walls (Bayer et al. 1985). Cellulosomes might be used in biotechnological applications such as the synthesis of ethanol or organic acids from affordable renewable resources by converting cellulosic biomass into sugars. In vitro and in vivo cellulosome research is advancing rapidly, allowing for the creation of systems that can fulfill these objectives (Levy and Shoseyov 2002; Shoseyov et al. 2003).
Microorganisms have been shown to have two distinct enzyme systems for degrading plant cell walls. Unlike cellulose, hemicellulose, pectin, and lignin are easier to break down. Endoglucanases, exoglucanases, and accessory enzymes are released by aerobic fungi and bacteria and can work together to assault plant cell walls. The glycoside hydrolases of Trichoderma reesei are the most investigated of these enzymes. Another sort of system has developed in anaerobic microbes that include the creation of a huge extracellular enzyme complex known as the “cellulosome,” which is composed of numerous bound enzymes (Warren 1996; Bayer et al. 1985; Coughlan et al. 1985).
11.2.2.1 Degradation of Cellulosic Biomass by Microorganisms
In addition to providing a sustainable source of mixed sugars, plant biomass—the most common biopolymer—has long been recognized as a possible source of alternative energy. But innovative technologies are still required to overcome the numerous obstacles to establishing premium systems for turning biomass into “fuels and chemicals” (McBee 1950; Ljungdahl and Eriksson 1985; Canganella and Wiegel 1993; Beguin and Aubert 1994; Himmel 2008; Moraïs et al. 2010). When compared to the current study strategy, we know very little about the deconstruction and conversion of plant cell walls by enzymatic hydrolysis and/or microbial hydrolysis and/or fermentation. More research is needed to fill in the gaps in our knowledge. Microorganisms play a vital part in the carbon cycle of the Earth by degrading plant cell walls. Cellulose, hemicellulose, and pectin make up 30–40% of most plant cell walls, while lignin and cellulose make up the remaining 15–40%. Oligomers and other tiny carbon molecules are broken down into glucose and other sugars by enzymatic decomposition before being converted into CO2 by the body.
Carbon and energy from plant cell wall polysaccharides may be used by a wide range of microbes, making them an important part of the carbon cycle. Sometimes free-living bacteria exploit such polysaccharides from decaying plant materials, such as compost piles and sewage sludge; in other circumstances, the microbes aid higher animals (e.g. ruminants) in the conversion of the polysaccharides into digestible parts.
When compared to aerobic bacteria, which create a large number of enzymes like cellulases and hemicellulases, anaerobe biosynthetic apparatuses are far more parsimonious in their enzyme production. When it comes to the extracellular breakdown of polymeric substrates like the plant cell wall’s refractory crystalline components, anaerobic environments are thought to be more conducive to this type of machinery’s development. It is therefore not surprising that anaerobic bacteria have developed new methods for breaking down plant stuff, and the cellulosome structure appears to be the most impressive of them. Ongoing research has shown that (Robson and Chambliss 1989; Shimada et al. 1994; Bayer et al. 2004; Valenzuela-Ortega and French 2019).
Bacteria, protozoa, fungi, and archaea all live in the gastrointestinal tract of animals (Rosenberg and Zilber-Rosenberg 2018). Most often found in the rumen system, these synergistic microbial communities (bacteria and fungus) have impressive metabolic capacities and durability. In response to this successful degradation and utilization of plant biomass, there has been a rapid increase in the development of synthetic microbial consortia for biotechnology (Minty et al. 2013). In this review, we emphasized bridging the effective strategies applied by various microorganisms and rumen microbiota to degrade the plant biomass. This context mainly focuses on cellulosome-containing microorganisms, in which first we need to discuss Hungateiclostridium thermocellum and its importance. This Hungateiclostridium thermocellum bacterium is considered one of the best laboratory grown microorganisms to study the cellulosome and its applications (Felix and Ljungdahl 1993). Bio-engineering principles approach was applied to explore the cellulosome and its strategies in rumen consortia and their mutualistic ability in efficient biodegradation of lignocellulosic feedstocks (Fontes and Gilbert 2010; Gilbert 2007).
11.3 Rumen Microbiota
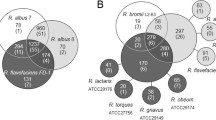
Ruminants are hoofed mammals that have a unique digestive system (rumen) that allows them to better use energy from fibrous plant material than other herbivores. The taxonomic origin of the rumen’s microbiome components based on the gene sequences encoding CAZymes implied the presence of 19 phyla of microbes. Metagenomics analysis of the rumen’s microbiome, by Gharechahi and Salekdeh (2018) exclusively identified the species belonging to the Bacteroidetes (56.3%), the Firmicutes (32.8%), the Spirochaetes (4.0%), the Fibrobacteres (2.2%), the Proteobacteria (1.4%), the Lentisphaerae (1.3%), the Euryarchaeota (0.4%) and the Verrucomicrobia (0.3%) collectively represented 98.7% of sequences (Gharechahi and Salekdeh 2018). The symbiotic organisms present in the rumen of ruminant animals facilitate the digestion of plant-based fiber. The diverse rumen microbiome contributes to the nutrition of the host animal by converting non-digestible biomass into readily absorbable compounds. This symbiosis in the rumen is particularly important for herbivores, which are unable to produce hydrolytic enzymes required for the degradation of recalcitrant lignocellulosic plant biomass endogenously that form a major component of their regular diet.
11.3.1 Physicochemical Properties of the Rumen
Ruminant herbivores such as sheep and cattle have reticulums (rumens), omasums (stomachs), and abomasums. In ruminants, fermentation takes place mostly in the rumen (Tharwat et al. 2012). Factors like pH, temperature, osmotic pressure, redox potential, and buffering capacity impact the rumen microbial community. Enzymes produced by rumen microbes help ruminants digest cellulosic material (Aschenbach et al. 2011). Due to the fermentation heat created by the rumen microbiota, ruminant temperatures range between 39 and 41 °C (Wahrmund et al. 2012), rumen’s pH is 5.5–7.0 (Krause and Oetzel 2006). Several factors affect the rumen’s pH, including the feed consumed, saliva production, and absorption of short-chain fatty acids, as well as the ruminal epithelium’s bicarbonate and phosphate exchange (Aschenbach et al. 2011). Approximately 250 mOsm/kg, the osmolality of ruminal fluid is controlled by the composition of the animal’s food, as well as the fermentative products that are produced (Lodemann and Martens 2006). Under absolutely anaerobic circumstances, a wide variety of rumen microorganisms, including bacteria, protozoa, and fungus, coexist in symbiotic relationships with ruminants (Ozutsumi et al. 2005). Bacteria are the most sensitive to the rumen’s physical and chemical features of the microbiota (McAllister et al. 1990).
11.3.2 Ruminal Bacteria
Ruminants like cattle, sheep, goats, and cervids have an enlarged gastrointestinal tract often called the forestomach. The forestomach comprises the rumen, reticulum, and omasum. The rumen of the ruminants is enriched naturally with anaerobic microorganisms like bacteria, fungi, and protists. The presence of these cellulolytic microorganisms enables rumen to function efficiently as a bioreactor for lignocellulosic conversion. Various bacterial genera are associated with the rumen ecosystem. The microbiome of rumen varies from host to host and largely depends on the diet of the animal; however the majority of the microbiome includes Prevotella, Butyrivibrio, Ruminococcus, Bacteriodales, and Clostridiales (Henderson et al. 2015). The competitive environment in the rumen is dependent on bacterial preference for certain substrates, energy requirements for maintenance, and resistance to toxic metabolic products (Russell et al. 1979). Rumen microorganisms ferment various substrates to release volatile fatty acids which in turn act as a major energy source for all the rumen microbiome. Various cellulose degradation strategies were identified which are followed by the rumen bacteria in a ruminal ecosystem (Flint 2008).
Nevertheless, the ruminant, (sheep, goats, and cervids) have an enlarged gastrointestinal tract often called the forestomach. The forestomach comprises the rumen, reticulum, and omasum. The rumen of the ruminants is enriched naturally with anaerobic microorganisms like bacteria, fungi, and protists. The presence of these cellulolytic microorganisms enables rumen to function efficiently as a bioreactor for lignocellulosic conversion. The microbiome of rumen varies from host to host and largely depends on the diet of the animal; however the majority of the microbiome includes Prevotella, Butyrivibrio, Ruminococcus, Bacteriodales, and Clostridiales (Henderson et al. 2015). Rumen microbiomes ferment various substrates to release volatile fatty acids which in turn act as a major energy source for all the rumen microbiome. Various cellulose degradation strategies were identified which are followed by the rumen bacteria in a ruminal ecosystem (Flint 2008).
Recent advancements in enzymatic studies helped find the cellulolytic functions in many bacterial species (Naas et al. 2014; Mackenzie et al. 2015; Dassa et al. 2014) including rumen bacteria. An extracellular multienzyme complex known as a cellulosome is produced by the firmicute Ruminococcus flavefaciens, one of the most researched rumen bacteria in terms of its production. The catalytic domains of this extracellular cellulosome are exposed to the substrate since it is connected to the bacterial cell surface. Dockerin-bearing enzymes often bind to numerous cohesin domains on a scaffolding subunit of a cellulosome (Bayer et al. 2004).
There are few rumen bacteria whose cellulolytic mechanism is not well understood. Cellulose depolymerization is a less understood system; it mostly involves attaching to the substrate through an unknown protein followed by cleavage of the cellulose polymer by a distinct set of cellulases (Wilson 2009; Suen et al. 2011; Ransom-Jones et al. 2014).
The rumen possesses a natural degradative environment composed of a genomically diverse set of microbiomes. However, rumen microbes are not easy to grow in pure form in the lab media, and hence the genetic information and other characteristics are not clear. Often, the underrepresented rumen microorganisms are missed in the culture, but they may contribute significantly to other surrounding microbiomes in these complex environments (Ley et al. 2008).
Despite the profound scientific research on rumen microbiota, still, a lot is unknown about their complex natural fiber utilizing engineering mechanism and their distribution. The rumen microbiome cellulolytic process has been the focus in this era. Multiple bacteria in the rumen form a complicated network that aids in the destruction and use of plant biofibers, resulting in the formation of fermentation products that are beneficial to the animal’s health and well-being (Qi et al. 2010; Mizrahi 2013; Dassa et al. 2014). Researchers found a strategic player in the rumen ecosystem, which behaves similar to the most studied complex proteins (cellulosome) found in Clostridium thermocellum. The complex protein contains scaffoldin subunit which has modular proteins like CBM, which mediate interaction with plant fibers. Mainly in anchoring to the substrate and digestion, microbes in the rumen ecosystem break down plant material by hydrolyzing polysaccharides in the local environment using specific enzymes (Flint 1997; Flint et al. 2008; Mizrahi 2013). The principal degraders of plant fiber in this environment haven’t yet been found among the few rumen plant cell wall-degrading bacteria.
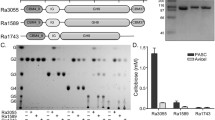
In the rumen, the most active cellulolytic Ruminococcus and Fibrobacter species degrade cellulose (Qi et al. 2010). There have been in-depth studies on two Ruminococcus species: ScaA, ScaB, ScaC, and ScaE are all found in a single gene cluster in the genomes of multiple R. flavefaciens strains, whereas only one scaffolding is found in R. albus strains 7 and SY3 and none in strain 8 (Ding et al. 2001; Rincón et al. 2004; Rincon et al. 2005). This is in contrast to only one scaffoldin in R. albus strains 7 and SY3 and none in strain 8 (Ding et al. 2001).
About 22 amino acid residues of each Ca2+-binding loop helix motifs are joined by a linker inside the dockerins, a small protein module. Bayer et al. (2004, 2013) and Haimovitz et al. (2008) provide comprehensive explanations of dockerins.
Ruminococcus species make up just a tiny percentage of the rumen ecology and are currently the only ones that transport cellulosomal components inside the rumen environment. The rumen ecology has a very effective fiber-degrading microbiome, as well as a few well-characterized cellulosome-producing bacteria (Dassa et al. 2014).
11.3.3 Rumen Protozoa
For 40–80% of the biomass-degrading microorganisms, protozoans are to be found, the majority of them belong to the Entodiniomorphida and Holotricha orders (Firkins et al. 2007; Yáñez-Ruiz et al. 2004). As most protozoa in the rumen are held in the diet, the rumen abomasum is unable to move these organisms readily (Hook et al. 2011). More than 90% of all cellulolytic protozoa come from a order known as Entodiniomorphida, which is very effective in both hydrolyzing and fermenting celluloses. The in vitro degradation of crystalline cellulose by cellulolytic protozoa of the genera Polyplastron and Eudiplodinium, and to a lesser extent by Epidinium (Fondevila and Dehority 2001), is quick and effective.
11.3.4 The Ruminal Fungi
Only 8% of the rumen microbiome is made up of rumen fungus; however, these organisms are critical to the digestion of the rumen (Nam and Garnsworthy 2007). It was discovered that some of the fungi have rhizoids that attach to the meal particles (Denman et al. 2008). Fungal populations in the rumen can be increased by feeding high-lignified fodder. When ruminants are fed a large amount of quickly fermentable carbohydrates, fungi in the duodenum, cecum, and feces are promptly eradicated (Grenet et al. 1989).
11.3.5 Cellulolytic Fungi
Hydrolytic enzymes, such as those produced by Neocallix species, Piromyces species, and orpinomyces species, are found in ruminant cellulolytic fungus. Compared to cellulolytic bacterial species, they were shown to break down structural polysaccharides more effectively in monoculture (Bernalier et al. 1992). To rapidly digest non-lignified tissues and fracture zones of lignified tissues by mechanical action, zoospores produced by cellulolytic fungi can adhere quickly to feed particles (Bernalier et al. 1992; Grenet et al. 1989). Ruminal fungi, therefore, play an important role in the digestion of lignin. Among the plant cell wall solubilizers that can open the cellulose to bacterial breakdown is the species N. frontalis (Borneman et al. 1991).
The cellulosome, a huge extracellular enzyme complex composed of a scaffolding protein and numerous associated cellulases, has developed in anaerobic microbes to break down plant cell walls. Cellulosomes might be used in biotechnological applications such as the generation of high-value goods such as ethanol or organic acids from affordable renewable resources by converting cellulosic biomass into sugars. In vitro and in vivo cellulosome research is advancing rapidly, allowing for the creation of systems that can fulfill these objectives. Another sort of system originated in anaerobic microbes and involves in the creation of a huge extracellular enzyme complex termed the “cellulosome”, which includes a scaffoldin like protein and several attached enzymes.
11.4 Cellulosome
Raphael Lamed and Ed Bayer met at Tel Aviv University in the early 1980s and began working on the cellulosome idea. The first cellulosome was identified in the anaerobic thermophilic bacteria Clostridium thermocellum (Current name: Acetivibrio thermocellus and homotypic synonyms: Clostridium thermocellum, Hungateiclostridium thermocellum, Ruminiclostridium thermocellum). This microbe Clostridium thermocellum had been isolated in 1920’s by Viljoen et al. (1926) from manure and later described by McBee (1948) and completely sequenced at the DOE Joint Genome Institute. The C. thermocellum contains a unique extracellular enzyme system capable of breaking down insoluble cellulose into ethanol which is vital for biomass energy.
Cellulose, the most prevalent organic polymer on Earth, is degraded efficiently by these enzymes. A multi-functional integrating component (named scaffoldin) organizes the numerous cellulolytic subunits (e.g., enzymes) into the complex. Multiple endoglucanases, cellobiohydrolases, xylanases, and other degradative enzymes target diverse, insoluble cellulose substrates inside a cellulosome.
Before the cellulosome was discovered, cellulase systems of cellulolytic bacteria were seen as a collection of various enzymes in a free state (Stutzenberger 1990). This idea was based on prior research on fungus cellulases. For the past 50 years, aerobic cellulolytic fungi have been widely investigated for their free cellulases. Attempts to isolate free cellulases from anaerobic bacteria have previously failed. Several cellulosome-related signature sequences (i.e., cohesins and dockerins) have been identified in diverse bacteria and fungi, but most are dockerin-tagged enzymes. The principal cellulolytic ruminal bacteria and ruminal fungus have glycoside hydrolase (GH) genes (Flint 1997; Flint and Forsberg 1995; Selinger et al. 1996).
11.4.1 Cellulosome Components
-
One or more cohesin modules can be found in the scaffoldin subunit and are linked to other functional modules. Scaffoldins may contain modules such as the CBM, dockerin, X modules of unknown function, the S-layer homology (SLH), or the sortase-anchoring motif, depending on the scaffoldin.
-
Cohesin modules are the major building blocks of scaffoldins, which are accountable for organizing the cellulolytic subunits into the multienzyme complex.
-
Dockerin modules link the catalytic enzymes to the scaffoldin, which is the primary building component of scaffoldins. Internally, the dockerin has a twofold symmetry consisting of an F-hand pattern that is repeated (a calcium-binding loop preceding a helix). Scaffoldins’ C-terminus contains dockerin as well.
Catalytic subunits
contain dockerin modules that serve to incorporate catalytic modules into the cellulosome complex. These catalytic modules include glycoside hydrolases (GH), polysaccharide lyases (PL), and carboxylesterases (CE) (https://www.weizmann.ac.il/Biomolecular_Sciences/Bayer/research-activities/cellulosome-systems; https://www.weizmann.ac.il/Biomolecular_Sciences/Bayer/research-activities/enzymes Accessed on 12 Dec 2021 Courtesy Ed Bayer’s Group, Dept. of Biomolecular Sciences, Weizmann Institute of Science; and Demain et al. 2005).
Cohesin-dockerin interactions
can be viewed as a kind of plug-and-socket mechanism in which the dockerin plugs into the cohesin socket. In general, the interaction is inter-species and intra-species (type) specific, although some cross-reactivity has been found in a few cases. The cohesin-dockerin interaction is one of the most potent protein-protein interactions known in nature, in most cases approaching the strength of high-affinity antigen-antibody interactions (Ka ~ 1011 M−1). So far, cohesins have been phylogenetically distributed into three groups according to sequence homology; type I cohesin, type II cohesin, and the recently discovered type III cohesin. The dockerins that interact with each cohesin type are, by definition, of the same type.
11.4.2 Cellulosome Systems
Cellulosomes were discovered in other cellulolytic bacteria early on (Demain et al. 2005; Beguin and Lemaire 1996) and were not exclusive to C. thermocellum. A basic cellulosome system consists of a single scaffoldin, whereas a complex cellulosome system consists of many scaffoldins that interact with each other. The structure of the cellulosome depends on the arrangement of modules on the scaffoldin subunit and the specificity of cohesins and dockerins for their modular counterparts. The dockerin-bearing subunits are directly incorporated into the cellulosome complex by primary scaffoldins, adapter scaffoldins enhance the repertory or number of components in the complex, and anchoring scaffoldins link the complex to the bacterial cell surface by anchoring scaffoldins.
Cellulosome systems may be divided into two categories based on their level of complexity (simple and complex type). There is a single carbohydrate-binding module (CBM), one or more X2 modules and up to nine cohesins in the scaffoldins of simple cellulosome systems. It is the dockerin-bearing enzymes into the complex that are integrated by these major scaffoldins. The chemical mechanism behind the cell surface association of simple cellulosomes is still a mystery in some situations. Cell wall attachment may be facilitated by the X2 module.
Different bacterial species have been shown to have complex cellulosome systems (Accessed 2020-12-12; Ding et al. 1999; Ding et al. 2000; Ding et al. 2001). The chromosome has “enzyme-linked gene clusters” that contain the genes for several cellulosome components. A complex cellulosome architecture is created when more than one scaffoldin interacts with one another in different ways. The cellulosome complex integrates enzymes directly into at least one kind of primary scaffoldin. The cellulosome complex is attached to the cell surface by a specific module or sequence of scaffoldins in each species. The “many scaffoldin gene clusters” are found on the chromosome in complicated cellulosome systems.
11.4.2.1 Regulation of Cellulosomal Genes
At the microscopic, physiological, and transcriptional levels, the variables that control cellulosomal gene expression have been studied. Polycellulosomes were found in protuberances in the early scanning electron microscopic examinations (Lamed et al. 1983). It was found that protuberances appeared in cellulose-grown cells, but not glucose-, fructose-, cellobiose-, or CMC-grown cells in research (Blair and Anderson 1999). When cells were cultured in cellulose rather than in cellobiose, engB’s relative levels of mRNA were greater, according to early transcription research (Attwood et al. 1994), indicating that the gene was transcribed as a single transcription unit.
11.4.2.2 Rumen Cellulolytic Bacteria and Fungi, Along with the Presence or Absence of Cellulosome
There are several cellulosome-producing anaerobic bacteria currently known, including Acetivibrio, Bacteroides, Clostridium acetobutylicum (a suspected but not proven cellulobacterium), Clostridium acetobutylicum (a cellulobacterium that has been shown to produce cellulosomes), Clostridium cellobioparum (a cellulobacterium that has been shown to produce cellulosomes), and Clostridium josui (a suspected but not proven cellulobacterium). If they’re not linked to bacteria’s cell wall, cellulosomes are free-floating extracellular complexes capable of degrading non-soluble substrates and transporting them to cells. C. thermocellum, C. cellulolyticum, and C. cellulovorans are some of the best-characterized cellulosomes, but their huge size and variability have hampered efforts to understand cellulosome structure and function. Others (such as Acetivibrio and Ruminococcus flavefaciens cellulosome systems) appear to be much more complex.
The following is a comprehensive list of all known cellulolytic bacterial species that utilize crystalline cellulose as their only carbon source in the process of hydrolyzing (Table 11.2). Continuous hydrolysis of at least microcrystalline cellulose like Avicel or better filter paper, cotton linters, and bacterial cellulose is required for “substantial” hydrolysis (e.g., release of reducing equivalents). A new taxonomy system published in Bergey’s Manual of Systematic Bacteriology is superimposed on the phylogenetic tree produced from 16 S rRNA sequence calculations with the ARB software package (Garrity et al. 2001 and Garrity 2001).
In rumen environments, fungi play a critical role in the conversion and consumption of plant biomass because of their powerful enzymes that degrade fibers and their invasive proliferation. Fungi that decompose cellulose in an anaerobic environment are the most common. Since the paradigm-shifting work in the 1970s concentrated mostly on rumen fungus, when he first defined anaerobic fungi, Colin Orpin discovered strange fungal phyla. According to Orpin (1975), due to the presence of the host’s food components, the rumen fungus population density increases as a result of this stimulation. Many ruminant fungi, such as Neocallimastix sp., Piromyces sp., Caecomyces sp., Orpinomyces sp., and Anaeromyces sp., are involved in the breakdown of plant biomass. Cellulosome-bearing anaerobic fungi have been extensively explored in metagenomics studies with high-quality genomic assemblies (Haitjema et al. 2017; Youssef et al. 2013).
Anaeromyces robustus, Neocallimastix californiae, Orpinomyces sp., and Piromyces finnis are the most investigated rumen fungus with cellulosome. Fungi microorganisms have a similar role in anaerobic gut environments to their aerobic counterparts in soil and water. Fungi create colonies and produce extracellular enzymes that mobilize structural plant polymers to be accessible to other microorganisms and the host, i.e. symbiosis, by holding themselves (fungi) to plant-based materials. Cellulolytic, hemicellulolytic, glycolytic, and proteolytic enzymes are all produced by anaerobic fungi, which are the microbes that use the most fiber (Ljungdahl 2008; Raghothama et al. 2001; Williams and Orpin 1987). The following sections deal with fungus cellulosomes. Fungal cellulosome enzymes and their domains (CAZyme) likely arose from bacterial enzymes via horizontal gene transfer (HGT). Similar structures in anaerobic fungi have been documented for many years by molecular biologists, which are known to assemble through sequence-divergent non-catalytic dockerin domains (NCDDs) (Haitjema et al. 2014). Many researchers are still interested in the cellulosome’s components, modular assembly method, and functional purpose.
11.4.2.2.1 Ruminococcus Cellulosome
Bacterial cellulosomes are organized employing a special type of subunit, the scaffoldin, which is composed of an array of cohesin modules. The cohesin interacts selectively and tenaciously with a complementary type of domain, the dockerin, which is borne by each of the cellulosome enzyme subunits (Fig. 11.2). The integrity of the complex is thus maintained by the cohesin-dockerin interaction (Xu et al. 2004). The scaffoldin usually contains a module termed carbohydrate-binding module (CBM) which is responsible for the binding of the complex to the substrate. All cellulosomal components which are present on the same subunit are separated by linker sequences (Doi and Kosugi 2004).
When scaffoldins were first characterized (Bayer et al. 1994), their cohesins were called type I cohesins based on sequence homology and were involved in binding cellulosomal enzymes containing dockerins known as type I dockerins (Bayer et al. 1998). Further studies showed that there were other cohesins, non-homologous to the type I cohesins, and these are known as type II and type III cohesins. Type II cohesins are found on anchoring proteins and bind to type II dockerin that are found on a scaffolding (Doi and Kosugi 2004; Leibovitz and Béguin 1996; Leibovitz et al. 1997; Lemaire et al. 1995).
The cohesin-dockerin interaction is crucial for cellulosome assembly. It mediates a set of interactions among the enzymes, scaffoldin, and anchoring protein which results in cell surface attachment of the cellulosome (Xu et al. 2003). The interaction between the cohesin and dockerin domains provides the definitive molecular mechanism that integrates the enzyme subunits into the cellulosome complex (Salamitou et al. 1994a, b; Lytle et al. 1996; Tokatlidis et al. 1993, 1991).
Cohesin and dockerin interactions within a species are not limited to specific pairings, evident from biochemical data (Gal et al. 1997; Yaron et al. 1995). It appears that the dockerins are all recognized in the same way by various cohesins. As a result, a single bacterium can produce a wide variety of cellulosomes, each with a unique makeup (depending on which enzymes are bound to the scaffolding). Likely, the increased functional display and the potential for enzyme synergism provided by this variety of cellulosomes may allow the bacterial cell to attack a wider range of substrates.
An anaerobic environment in the rumen is one of the most active places in nature for the digestion of plant cell wall components (Hungate 1966). Glycoside hydrolase genes have been discovered in the cellulolytic ruminal bacteria, ruminal fungus, and ruminal protozoa (Flint 1997; Flint and Forsberg 1995; Devillard et al. 1999). However, little is known about how ruminal microbes break down lignocellulosic material through the organization of enzyme systems. Prior biochemical and ultrastructural research has shown that Ruminococcus species have protuberances on their cells’ surfaces that mimic cellulosomes in C. thermocellum, one of the most significant ruminal bacteria in the rumen (Lamed et al. 1985; Lamed et al. 1991; Lamed et al. 1987). In enzymes from Ruminococcus, dockerin sequences similar to those reported in Clostridium spp. were discovered more recently. R. flavefaciens 17 xylanases, cellulases, and esterases (Aurilia et al. 2000; Kirby et al. 1997) and R. albus cellulases are some examples (Ohara et al. 2000a, b; Ohmiya et al. 2003; Ding et al. 2001). A scaffolding-like protein and/or anchoring proteins may be present in Ruminococcus species that arrange enzyme subunits into such complexes, as evidenced by the discovery of dockerins in these enzymes.
Microbes in the rumen use specific enzymes to hydrolyze polysaccharides in the plant cell wall, breaking them down (Flint 1997; Flint et al. 2008; Mizrahi 2013). Rumen cell wall-degrading bacteria have only been found to be a secondary plant fiber degrader a few times. In the rumen, the most active cellulose-degrading bacteria are the cellulolytic Ruminococcus and Fibrobacter spp. (Qi et al. 2010). Researchers have studied R. flavefaciens and R. albus genomes in depth, finding that R. flavefaciens strains FD-1, 007c, 17, and others encode numerous interacting scaffoldins, such as ScaA, ScaB, ScaC, and ScaE, which are contained in a single gene cluster, whereas R. albus strains 7 and SY3 encode only one scaffolding and strain 8 encodes none (Ding et al. 2001; Rincón et al. 2004; Rincon et al. 2005; Jindou et al. 2006; Miller et al. 2009; Dassa et al. 2014). Only a tiny portion of the rumen ecology is occupied by these species, and they are presently thought to be the only ones that transport cellulosomal components inside the rumen habitat.
11.4.2.3 Fungal Cellulosome
To decompose lignocellulose, bacteria, and fungi that are anaerobic produces cellulosomes, which are a protein scaffold bonded together by numerous enzymes working in concert (cohesin and dockerin). Large herbivorous mammals generally have anaerobic fungus in their rumens and hindguts, where up to 30% of their CAZymes are cellulosome-associated CAZymes (Henske et al. 2017).
To further understand cellulose depolymerization, I’m turning to fungus to see if they have cellulosomes that are similar to those seen in bacteria. To put it in perspective, the anaerobic fungal cellulosome is more comprehended than the bacterial one, which is why questions about its composition, design, and method for tethering enzymes persist to this day. Cellulosomes formed by bacteria and fungi are examined by Gilmore et al. (2015), who compare the present state of knowledge in this area, as well as their use in synthetic enzyme-tethered systems for tunneled biocatalysis. There are still many unanswered questions about the potential of fungal cellulosome-inspired systems, which have been emphasized by Gilmore et al. (2015) and Haitjema et al. (2017).
A family of repeat-rich, non-catalytic scaffolds in the genomes of five anaerobic fungi has recently been discovered by comparative genomic and proteomic confirmation (Haitjema et al. 2017). Gene sequences encoding components of fungal cellulosomes differ greatly from those of bacteria (Sunna et al. 2000; Haitjema et al. 2017; Gilmore et al. 2015); hence the structure of these domains is likewise different from that of bacterial cellulosomes. The predominant colonizers of plant material in the rumen microbiome are anaerobic gut fungus; however, they are rarely investigated due to a dearth of defined isolates in the field. Although most gut fungi have large rhizoidal networks, which are likely involved in the breakdown of plant cell walls, fungi of the Caecomyces genus lack these rhizoids. When it comes to plant cell wall hydrolysis, Caecomyces churrovis is one of the most diversified CAZyme-producing fungal isolates known to date, according to Henske and co-authors in a recent study (Henske et al. 2017; Gilmore et al. 2015).
Elucidating the type and location of fungal dockerins is essential for designing synthetic enzymes or synthetic fungal cellulosomes, as fungal dockerins exist at either the N- or C-terminus of proteins and in tandem repeats. In addition, compared to bacterial cellulosomes, fungal native cellulosomes offer a much wider variety of cellulases with dockerin domains, including GH3, GH6, and GH45 (Haitjema et al. 2017). As a result, the incorporation of novel enzymes into cellulosomes, such as those that increase activity and thermal stability, might be achieved by using fungal cellulosomes as templates for chimeric enzymes. The protease inhibitory effect of serpins led to their discovery as a protein superfamily with homologous structures throughout all kingdoms of life. There are several different types of extracellular protease inhibitors, some of which have never before been seen in the Dikarya (such as serpins, which have been found in eukaryotic metazoa, but not previously in fungi) (Youssef et al. 2013). Serpins with dockerin domains have been found, confirming their cellulosomal location and their possible involvement in fighting plant proteases, as previously suggested. Piromyces sp. strain E2 cellulosome contains celpin (538 amino acids), a serpin protein with an unclear function (Steenbakkers et al. 2008). The fungal serpin is likely to have a role in protecting the cellulosome from plant proteinases because of the cellulosome’s restricted location inside the plant tissue and the rumen’s auto-proteolysis of plant material. Piromyces sp. strain E2’s celpin protein is the first non-structural, non-hydrolytic component of a fungus cellulosome. In addition, the celpin protein of Piromyces sp. strain E2 is the first fungus to possess a serine proteinase inhibitor (Steenbakkers et al. 2008). Cellulosomes are structures extracellularly produced by an anaerobic fungus, which include scaffoldin-bound extracellular enzymes (Orpin 1994). The fungal dockerin domain (FDD), which is similar in structure to the carbohydrate-binding module family 10 (CBM10), is found in anaerobic fungi’s cellulosome-bound genes (CBM10). According to Ljungdahl, incorporating GH3, GH6, and GH45 enzymes into anaerobic fungal cellulosomes increases their biocatalytic activity (Ljungdahl 2008). Fungal cellulosomes may be an evolutionary chimeric structure—a fungal complex that co-opts helpful functions from its bacterial neighbors in the gut microbiome. Fungal cellulosomes may directly convert cellulose to fermentable monosaccharides thanks to the extra β-glucosidase provided by GH3, whereas clostridial cellulosomes create low-molecular-weight oligosaccharides (Steenbakkers et al. 2003). Fungal cellulosomes may therefore be a mixture of enzymes from a variety of gastrointestinal fungi in their natural habitats (e.g., the microbial community of the herbivore rumen). This contrasts sharply with the great species specificity of bacterial cellulosomes (Bayer et al. 2004).
11.5 Metagenomic Approach for Biomass Utilization
By analyzing genetic material from environmental samples, metagenomics may access this biogenetic diversity without the need to culture cells. As a result, biotopes with high turnover rates of recalcitrant biomass, like lignocellulosic plant cell walls, have become a major resource for bioprospecting; further, this material is a major asset in the quest for alternative biocatalytic (enzymes) for various industrial processes, such as the production of biofuels from plant feed stocks. Metagenomics technologies have made significant contributions as a result of the identification of novel enzymes, although this young venture still needs a lot of work. These are the two most common strategies for screening metagenomes: function-based and sequence-based.
By cloning a gene and over-expressing it in a suitable host, scientists have traditionally found organisms that exhibit the required activity and then used that organism to find new enzyme diversity. Genomics has opened up a new avenue of research (Ferrer et al. 2009). More and more people realize that the microbial world holds the most biodiversity in the biosphere; hence it will be microorganisms that will supply most of our enzyme diversity and novel uses. Microbes are notorious for their inability to be cultured (Amann et al. 1990; Zengler et al. 2002) which limits the use of standard methods for enzyme discovery. The “metagenomics” or “environmental genomics” techniques have been developed in response to the predicted rich enzymatic selections from the uncultured microbial community because they are based on novel genomics-based discovery methodologies. It is common for these methods to be referred to as “culture-independent” and “mined” organisms.
The race is on to discover the full extent of biocatalytic variety and usefulness. Accessing natural enzyme diversity, exploring enzymes’ wider catalytic potential, and customizing and fine-tuning promising activity for applications are all part of this process (Ferrer et al. 2009).
Biocatalysts with improved performance and lower cost are needed in industrial processes to efficiently break down resistant plant biomass into fermentable sugars. In the metagenomes of natural microbial biomass decay communities, there may be enzymes that can break down biomass. For the discovery of new enzymes that degrade biomass and the evaluation of cellulolytic enzyme activity, metagenomics is a useful tool. Research into novel glycoside hydrolases (GHases) from microbial biomass breakdown communities, particularly those from previously unknown or farmed microorganisms, is becoming increasingly common (Edwards et al. 2006; Breitbart et al. 2003; Breitbart et al. 2002; Wegley et al. 2007; Angly et al. 2006; Breitbart and Rohwer 2005; Fierer et al. 2007).
Genome sequences from an actively decomposing biomass may be used to generate a metagenome expression library, which can then be used to test for new and promising GHases. The cloning that was efficiently expressed in E. coli was the outcome of these joint efforts. The cloned GHases that were effective and selective might be useful in the breakdown of biomass.
Cellulosome and glycoside hydrolase genes were compared and found that early colonization of fiber in the rumen microbiome appears to be driven by organisms that contain enzymes that target the more resistant main chains of complex plant polysaccharides, particularly cellulose. It is also possible that a diet-dependent differential in glycoside hydrolase content exists between the bovine rumen (forages and legumes) and the termite hindgut microbiota in terms of glycoside hydrolase concentration (wood). It has been shown that the fiber-adherent rumen microbiota of the bovine shows forage-specific glycoside hydrolases (Brulc et al. 2009).
Biomass degradation genes and genomes were discovered in cow rumen microbiota using 268 GB of metagenomic sequencing data produced in recent investigations. Biomass-degrading genes and genomes have been discovered in the rumens of cows (Jami and Mizrahi 2012; Hess et al. 2011). To maintain the rumen microbiome’s equilibrium, each of the rumen’s viral communities has a specific role. Rumen viral communities, on the other hand, are less well understood. Because of their sheer abundance (an estimated 1031 viral particles per square kilometer on our globe), viruses often go unnoticed despite their potentially devastating effect on human health (Breitbart and Rohwer 2005; Rohwer and Thurber 2009).
11.6 Future Perspectives and Conclusions
Amorphous and crystallized topologies of cellulose can be found, even if its chemical makeup is simple. Native cellulose is a difficult substrate for enzymatic hydrolysis because of its insolubility and variability. Altogether more than 71 genes code for cellulosomal components in the genome of C. thermocellum, almost all containing catalytic modules with some exceptions of proteins with structural or unknown functions. Most of the genes can be assigned a putative function. The list includes cellulases (both endo- and exoglucanases), xylanases/xyloglucanases, mannanases, pectinases, pectate lyases (PL), carbohydrate-esterases (CE), glycosidases, chitinase, and a mixed-linkage β-glucanase (Zverlov et al. 2006; Lynd and Zhang 2002).
A high number of endo-xylanases, xyloglucanase, putative β-xylosidases, α-arabinofuranosidases, and glucuronidase could be responsible for the effective degradation of the hemicellulose enwrapping the cellulosic crystals. Depolymerization is supported by esterases and debranching glycosidases located in the cellulosome. The structure of the genes shows some regularity, and almost half of the putative components bear a carbohydrate-binding module (CBM). Cellulose crystals are hydrolyzed by the synergistic action of processive and nonprocessive β-glucanases of GH families 5, 8, 9, and 48. Unexpectedly Cel8A seems to play a key role in cellulose hydrolysis (Schwarz et al. 1995; Zverlov et al. 2006).
To some extent, the rumen bacteria’s ability to convert biomass into ethanol can alleviate some of the world’s dependency on petroleum. Cellulolytic and saccharolytic Clostridium species bacteria may be co-cultured with agricultural and industrial waste to provide alternative energy sources that are both environmentally friendly and economically viable. When Reddy and colleagues experimented with agricultural leftovers, particularly banana waste with newly identified Clostridium sp. (CT2) and co-culture with the anaerobic bacteria in 2010, they were able to support this method. An ethanol-tolerant cellulolytic mesophilic strain was found in a decomposing paper by the author’s team as well. There are many ways to maximize yields of bio-compounds and ethanol from rumen microbiota, and this chapter focuses on the cellulases of rumen microbiota, their presence in extracellular complexes or organelles (the cellulosomes), the binding of the cellulosome to cellulose, cellulosome genetics, regulation of their synthesis and co-culture, and other methodologies.
The nature’s most abundant carbohydrate polymer—cellulose—is found in plant cell walls. Hydrogen-bonded crystalline fibers make it incredibly difficult to break down, although it is plentiful. The cellulosome, a huge extracellular enzyme complex composed of a scaffolding protein and numerous associated cellulases, has developed in anaerobic microbes to break down plant cell walls. There are several biotechnological uses for cellulosomes, including the manufacture of high-value products like ethanol or organic acids from cheap renewable resources via cellulosome-mediated sugar conversion (Carreira and Ljungdahl 1993). To attain these objectives, new in vitro and in vivo systems are being developed thanks to rapid advancements in cellulosome research.
Our understanding of the fundamental structure and function of the cellulosome system is now at the point where cellulosome researchers may make rapid steps toward the creation of several valuable biotechnological applications for cellulosomes. Plant fiber breakdown, which is a key function of the cellulosome machinery, opens up several possibilities for the development of novel recombinant molecules that may be used to create value-added products. Several factors have contributed to our understanding of cellulosomal mechanisms, including advanced biotechnological techniques, metagenomics data, and new database management systems. These factors, combined with the rumen microbiota’s mutualism, have led to a paradigm shift in the study of the plant biomass utilization process in ruminants (Tringe and Rubin 2005).
Cellulosome-producing bacteria have been extensively investigated in this rumen habitat, which has a fiber-degrading microbiome. In this ecosystem, all of the modular proteins were allocated and participated in catabolic functions, as well as microbial interactions to a certain level. As the microbiomes store a great deal of information on mutualism and the use of refractory cellulose-based biomass, this sort of study is helpful to the next generation of researchers trying to understand evolutionary changes.
To date, studies have shown that the cellulosome has a wide range of complementary parts, all of which can interact and be involved in many extracellular functional activities in the rumen ecosystem. There is still much to learn about how these enzymes interact with other proteins from a physiological perspective in the targeted ecological niche. The rumen microbiome’s operation can be better understood by looking at the role played by the cellulosomal machinery utilized by the rumen microorganisms. Fungal cellulosomes may have a selection advantage over bacteria in these conditions because of their plasticity, which suggests that fungal cellulosomes have numerous scaffoldins. As a result of this fundamental understanding of these unique components, biotechnological cellulosomes may be designed with greater efficiency.
References
Amann RJ, Binder BL, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA targeted oligonucleotide probes with flow-cemetry for analysing mixed microbial populations. Appl Environ Microbiol 56:1910–1925
Anderson KL, Blair BG (1996) Regulation of the cellulolytic activity of Eubacterium cellulosolvens 5494: a review. SAAS Bull Biochem Biotechnol 9:57–62
Angly F et al (2006) The marine viromes of four oceanic regions. PLoS Biol 4:e368
Aschenbach JR, Penner GB, Stumpff F, Gäbel G (2011) Ruminant nutrition symposium: role of fermentation acid absorption in the regulation of ruminal pH. J Anim Sci 89:1092–1107
Atalla RH, Vanderhart DL (1984) Native cellulose: a composite of two distinct crystalline forms. Science 223(4633):283–285
Attwood GT, Blaschek HP, White BA (1994) Transcriptional analysis of the Clostridium cellulovorans endoglucanase gene, engB. FEMS Microbiol Lett 124:277–284
Aurilia V, Martin JC, McCrae SI, Scott KP, Rincon MT, Flint HJ (2000) Three multidomain esterases from the cellulolytic rumen anaerobe Ruminococcus flavefaciens 17 that carry divergent dockerin sequences. Microbiology 146:1391–1397
Bayer EA, Setter E, Lamed R (1985) Organization and distribution of the cellulosome in Clostridium thermocellum. J Bacteriol 163:552–559
Bayer EA, Morag E, Lamed R (1994) The cellulosome—a treasure trove for biotechnology. Trends Biotechnol 12:379–386
Bayer EA, Chanzy H, Lamed R, Shoham Y (1998) Cellulose, cellulases and cellulosomes. Curr Opin Struct Biol 8:548–557
Bayer EA, Belaich J-P, Shoham Y, Lamed R (2004) The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annu Rev Microbiol 58:521–554
Bayer EA, Shoham Y, Lamed R (2013) Lignocellulose-decomposing bacteria and their enzyme systems. In: The prokaryotes. Springer, New York, NY, pp 215–266
Beguin P, Aubert JP (1994) The biological degradation of cellulose. FEMS Microbiol Rev 13:25–58
Beguin P, Lemaire M (1996) The cellulosome: an exocellular, multiprotein complex specialized in cellulose degradation. Crit Rev Biochem Mol Biol 31:201–236
Berger E, Jones WA, Jones DT, Woods DR (1990) Sequencing and expression of a cellodextrinase (ced1) gene from Butyrivibrio fibrisolvens H17c cloned in Escherichia coli. Mol Gen Genet 223:310–318
Bernalier A, Fonty G, Bonnemoy F, Gouet P (1992) Degradation and fermentation of cellulose by the rumen anaerobic fungi in axenic cultures or in association with cellulolytic bacteria. Curr Microbiol 25:143–148
Blair BG, Anderson KL (1999) Regulation of cellulose inducible structures of Clostridium cellulovorans. Can J Microbiol 45:242–249
Borneman WS, Ljungdahl LG, Hartley RD, Akin DE (1991) Isolation and characterization of p-coumaroyl esterase from the anaerobic fungus Neocallimastix strain MC-2. Appl Environ Microbiol 57:2337–2344
Breitbart M, Rohwer F (2005) Method for discovering novel DNA viruses in blood using viral particle selection and shotgun sequencing. BioTechniques 39:729–736
Breitbart M et al (2002) Genomic analysis of uncultured marine viral communities. Proc Natl Acad Sci U S A 99:14250–14255
Breitbart M et al (2003) Metagenomic analyses of an uncultured viral community from human feces. J Bacteriol 185:6220–6223
Brulc JM, Antonopoulos DA, Berg Miller ME, Wilson MK, Yannarell AC, Dinsdale EA, Edwards RE, Frank ED, Emerson JB, Wacklin P, Coutinho PM, Henrissat B, Nelson KE, White BA (2009) Proc Natl Acad Sci U S A 106(6):1948–1953. https://doi.org/10.1073/pnas.0806191105)
Canganella F, Wiegel J (1993) The potential of thermophilic clostridia in biotechnology. In: Woods DR (ed) The clostridia and biotechnology. Butterworth-Heinemann, Boston, MA, pp 393–429
Carreira LH, Ljungdahl LG (1993) Production of ethanol from biomass using anaerobic thermophilic bacteria. In: Wise DL (ed) Liquid fuel developments. CRC Press, Boca Raton, FL, pp 1–28
Cavedon K, Leschine SB, Canale-Parola E (1990) Cellulase system of a free-living, mesophilic Clostridium (strain C7). J Bacteriol 172:4222–4230
Coughlan MP, Hon-Nami K, Hon-Nami H, Ljungdahl LG, Paulin JJ, Rigsby WE (1985) The cellulolytic enzyme complex of Clostridium thermocellum is very large. Biochem Biophys Res Commun 3:904–909
Dassa B, Borovok I, Ruimy-Israeli V, Lamed R, Flint HJ, Duncan SH et al (2014) Rumen cellulosomics: divergent fiber-degrading strategies revealed by comparative genome-wide analysis of six ruminococcal strains. PLoS One 9:e99221
Demain AL, Newcomb M, Wu JH (2005) Cellulase, clostridia, and ethanol. Microbiol Mol Biol Rev 69(1):124. https://doi.org/10.1128/MMBR.69.1.124-154.2005
Denman SE, Nicholson MJ, Brookman JL, Theodorou MK, McSweeney CS (2008) Detection and monitoring of anaerobic rumen fungi using an ARISA method. Lett Appl Microbiol 47:492–499
Devillard E, Newbold CJ, Scott KP, Forano E, Wallace RJ, Jouany J-P, Flint HJ (1999) A xylanase produced by the rumen anaerobic protozoan Polyplastron multivesiculatum shows close sequence similarity to family 11 xylanases from gram-positive bacteria. FEMS Microbiol Lett 181:6720–6729
Ding SY, Bayer EA, Steiner D, Shoham Y, Lamed R (1999) A novel cellulosomal scaffoldin from Acetivibrio cellulolyticus that contains a family 9 glycosyl hydrolase. J Bacteriol 181(21):6720–6729
Ding SY, Bayer EA, Steiner D, Shoham Y, Lamed R (2000) A scaffoldin of the Bacteroides cellulosolvens cellulosome that contains 11 type II cohesins. J Bacteriol 182(17):4915–4925
Ding SY, Rincon MT, Lamed R, Martin JC, McCrae SI, Aurilia V, Shoham Y, Bayer EA, Flint HJ (2001) Cellulosomal scaffoldin-like proteins from Ruminococcus flavefaciens. J Bacteriol 183(6):1945–1953. https://doi.org/10.1128/JB.183.6.1945-1953.2001
Doi HR, Kosugi A (2004) Cellulosomes: plant-cell-wall-degrading enzyme complexes. Nat Rev Microbiol 2(7):541–551. https://doi.org/10.1038/nrmicro925
Duong CTV, Johnson EA, Demain AL (1983) Thermophilic, anaerobic and cellulolytic bacteria. Enzyme Ferm Biotechnol 7:156–195
Edwards RA et al (2006) Using pyrosequencing to shed light on deep mine microbial ecology. BMC Genomics 7:57
Felix CR, Ljungdahl LG (1993) The cellulosome: the extracellular organelle of Clostridium. Annu Rev Microbiol 47:791–819
Ferrer M, Beloqui A, Timmis KN, Golyshin PN (2009) Metagenomics for mining new genetic resources of microbial communities. J Mol Microbiol Biotechnol 16(1–2):109–123
Fields MW, Mallik S, Russell JB (2000) Fibrobacter succinogenes S85 ferments ball-milled cellulose as fast as cellobiose until cellulose surface area is limiting. Appl Microbiol Biotechnol 54:570–574
Fierer N et al (2007) Metagenomic and small-subunit rRNA analyses reveal the genetic diversity of Bacteria, Archaea, Fungi, and viruses in soil. Appl Environ Microbiol 73:7059–7066
Firkins JL, Yu Z, Morrison M (2007) Ruminal nitrogen me[1]tabolism: perspectives for integration of microbiology and nutrition for dairy. J Dairy Sci 90(E. Suppl):E1–E16. https://doi.org/10.3168/jds.2006-518
Flint HJ (1997) The rumen microbial ecosystem—some recent developments. Trends Microbiol 5:483–488
Flint HJ (2008) Cellulase systems of anaerobic microorganisms from the rumen and large intestine. In: Biomass recalcitrance. Blackwell Publishing Ltd., Oxford, pp 393–406
Flint HJ, Forsberg CW (1995) Polysaccharide degradation in the rumen: biochemistry and genetics. In: Engelhardt WV, Leonard-Marek S, Breves G, Giesecke D (eds) Ruminant physiology, digestion, metabolism, growth and reproduction. Proceedings of the Eighth International Symposium on Ruminant Physiology. Ferdinand Enke Verlag, Stuttgart, pp 43–70
Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA (2008) Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol 6:121–131
Fondevila M, Dehority BA (2001) In vitro growth and starch digestion by Entodinium exiguum as influenced by the presence or absence of live bacteria. J Anim Sci 79:2465–2471
Fontes CM, Gilbert HJ (2010) Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu Rev Biochem 79:655–681. https://doi.org/10.1146/annurev-biochem-091208-085603
Gal L, Page’s S, Gaudin C, Bélaïch A, Reverbel-Leroy C, Tardif C, Bélaïch J-P (1997) Characterization of the cellulolytic complex (cellulosome) produced by Clostridium cellulolyticum. Appl Environ Microbiol 63:903–909
Garrity GM (ed) (2001) Bergey’s manual of systematic bacteriology, 2nd edn. Springer, New York, NY
Garrity GM, Winters M, Kuo AW, Searles DB (2001) Taxonomic outline of the procaryotes. Release 1.0. Bergey’s manual of systematic bacteriology, 2nd edn. Springer, New York, NY, p 320
Gharechahi J, Salekdeh GH (2018) A metagenomic analysis of the camel rumen’s microbiome identifies the major microbes responsible for lignocellulose degradation and fermentation. Biotechnol Biofuels 11:216. https://doi.org/10.1186/s13068-018-1214-9
Gilbert HJ (2007) Cellulosomes: microbial nanomachines that display plasticity in quaternary structure. Mol Microbiol 63(6):1568–1576. https://doi.org/10.1111/j.1365-2958.2007.05640.x
Gilmore SP, Henske JK, O’Malley MA (2015) Driving biomass breakdown through engineered cellulosomes. Bioengineered 6(4):204–208. https://doi.org/10.1080/21655979.2015.1060379
Grenet E, Breton A, Barry P, Fonty G (1989) Rumen anaerobic fungi and plant substrate colonization as affected by diet composition. Anim Feed Sci Technol 26:55–70
Haimovitz R, Barak Y, Morag E, Voronov-Goldman M, Shoham Y, Lamed R, Bayer EA (2008) Cohesin-dockerin microarray: diverse specificities between two complementary families of interacting protein modules. Proteomics 8:968–979
Haitjema CH, Solomon KV, Henske JK, Theodorou MK, O’Malley MA (2014) Anaerobic gut fungi: advances in isolation, culture, and cellulolytic enzyme discovery for biofuel production. Biotechnol Bioeng 111:1471–1482
Haitjema CH et al (2017) A parts list for fungal cellulosomes revealed by comparative genomics. Nat Microbiol 2:17087
Henderson G, Cox F, Ganesh S, Jonker A, Young W, Global Rumen Census Collaborators, Janssen PH (2015) Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep 5:14567
Henske JK, Gilmore SP, Knop D, Cunningham FJ, Sexton JA, Smallwood CR et al (2017) Transcriptomic characterization of Caecomyces churrovis: a novel, non-rhizoid-forming lingo-cellulolytic anaerobic fungus. Biotechnol Biofuels 10:305
Hess M, Sczyrba A, Egan R, Kim T-W, Chokhawala H, Schroth G, Luo S, Clark DS, Chen F, Zhang T, Mackie RI, Pennacchio LA, Tringe SG, Visel A, Woyke T, Wang Z, Rubin EM (2011) Science 331(6016):463–467. https://doi.org/10.1126/science.1200387
Himmel ME (2008) Biomass recalcitrance – deconstructing the plant cell wall for bioenergy. Blackwell Publishing, Oxford
Hook SE, Steele MA, Northwood KS, Dijkstra J, France J, Wright ADG et al (2011) Impact of subacute ruminal acidosis (SARA) adaptation and recovery on the density and diversity of bacteria in the rumen of dairy cows. FEMS Microbiol Ecol 78:275–284. https://doi.org/10.1111/j.1574-6941.2011.01154.x
Hungate RE (1966) The rumen and its microbes. Academic Press, New York, NY
Jami E, Mizrahi I (2012) Similarity of the ruminal bacteria across individual lactating cows. Anaerobe 18:338–343
Jindou S, Borovok I, Rincon MT, Flint HJ, Antonopoulos DA, Berg ME et al (2006) Conservation and divergence in cellulosome architecture between two strains of Ruminococcus flavefaciens. J Bacteriol 188:7971–7976
Kelly WJ, Asmundson RV, Hopcroft DH (1987) Isolation and characterization of a strictly anaerobic, cellulolytic spore former: Clostridium chartatabidum sp. nov. Arch Microbiol 147:169–173
Kirby J, Martin JC, Daniel AS, Flint HJ (1997) Dockerin-like sequences in cellulases and xylanases from the rumen cellulolytic bacterium Ruminococcus flavefaciens. FEMS Microbiol Lett 149(2):213–219
Krause KM, Oetzel GR (2006) Understanding and preventing subacute ruminal acidosis in dairy herds: a review. Anim Feed Sci Technol 126:215–236
Lamed R, Setter E, Bayer EA (1983) Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J Bacteriol 156(2):828–836
Lamed R, Kenig R, Setter E, Bayer EA (1985) Major characteristic of the cellulolytic system of Clostridium thermocellum coincide with those of the purified cellulosome. Enzym Microb Technol 7:37–41
Lamed R, Naimark J, Morgenstern E, Bayer EA (1987) Specialized cell surface structures in cellulolytic bacteria. J Bacteriol 169(8):3792–3800
Lamed R, Morag E, Moryosef O, Bayer EA (1991) Cellulosome-like entities in Bacteroides cellulosolvens. Curr Microbiol 22:27–34
Leibovitz E, Béguin P (1996) A new type of cohesin domain that specifically binds the dockerin domain of the clostridium thermocellum cellulosome-integrating protein CipA. J Bacteriol 178(11):3077–3084. https://doi.org/10.1128/jb.178.11.3077-3084.1996. Erratum in: J Bacteriol 1996 Sep;178(17):5335. PMID: 8655483; PMCID: PMC178055
Leibovitz E, Ohayon H, Gounon P, Béguin P (1997) Characterization and subcellular localization of the clostridium thermocellum scaffoldin dockerin binding protein SdbA. J Bacteriol 179(8):2519–2523. https://doi.org/10.1128/jb.179.8.2519-2523.1997. PMID: 9098047; PMCID: PMC178998
Lemaire M, Ohayon H, Gounon P, Fujino T, Beguin P (1995) OlpB, a new outer layer protein of Clostridium thermocellum, and binding of its S-layer-like domains to components of the cell envelope. J Bacteriol 177:2451–2459
Levy I, Shoseyov O (2002) Cellulose-binding domains: biotechnological applications. Biotechnol Adv 20:191–213
Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS et al (2008) Evolution of mammals and their gut microbes. Science 320:1647–1651
Ljungdahl LG (2008) The cellulase/hemicellulase system of the anaerobic fungus Orpinomyces PC-2 and aspects of its use. Ann N Y Acad Sci 1125:308–321
Ljungdahl LG, Eriksson K-E (1985) Ecology of microbial cellulose degradation. In: Marshall KC (ed) Advances in microbial ecology, vol 8. Plenum, New York, NY, pp 237–299
Lodemann U, Martens H (2006) Effects of diet and osmotic pressure on Na+ transport and tissue conductance of sheep isolated rumen epithelium. Exp Physiol 91:539–550
Lynd LR (1989) Production of ethanol from lignocellulosic material using thermophilic bacteria: critical evaluation of potential and review. Adv Biochem Eng Biotechnol 38:1–52
Lynd LR (1990) Large-scale fuel ethanol from lignocellulose. Potential, economics, and research priorities. Appl Biochem Biotechnol 24(25):695–719
Lynd LR, Zhang Y (2002) Quantitative determination of cellulase concentration as distinct from cell concentration in studies of microbial cellulose utilization: analytical framework and methodological approach. Biotechnol Bioeng 77:467–475
Lytle B, Myers C, Kruus K, Wu JH (1996) Interactions of the CelS binding ligand with various receptor domains of the Clostridium thermocellum cellulosomal scaffolding protein CipA. J Bacteriol 178:1200–1203
Mackenzie AK, Naas AE, Kracun SK, Schückel J, Fangel JU, Agger JW et al (2015) A polysaccharide utilization locus from an uncultured bacteroidetes phylotype suggests ecological adaptation and substrate versatility. Appl Environ Microbiol 81:187–195
McAllister TA, Rode LM, Major DJ, Cheng KJ, Buchanan-Smith JG (1990) Effect of ruminal microbial colonization on cereal grain digestion. Can J Anim Sci 70:571–579
McBee RH (1948) The culture and physiology of a thermophilic cellulose fermenting bacterium. J Bacteriol 56:653–663
McBee RH (1950) The anaerobic thermophilic cellulolytic bacteria. Bacteriol Rev 14:51–63
Miller MB, Antonopoulos DA, Rincon MT, Band M, Bari A, Akraiko T et al (2009) Diversity and strain specificity of plant cell wall degrading enzymes revealed by the draft genome of Ruminococcus flavefaciens FD-1. PLoS One 4:e6650–e6650
Minty JJ, Singer ME, Scholz SA, Bae CH, Ahn JH, Foster CE, Liao JC, Lin XN (2013) Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc Natl Acad Sci U S A 110(36):14592–14597. https://doi.org/10.1073/pnas.1218447110. PMID: 23959872; PMCID: PMC3767521
Mizrahi I (2013) Rumen symbioses. In: The prokaryotes. Springer, Berlin, pp 533–544
Moraïs S, Barak Y, Caspi J, Hadar Y, Lamed R et al (2010) Cellulase-xylanase synergy in designer cellulosomes for enhanced degradation of a complex cellulosic substrate. mBio 1(5):e00285–e00210. https://doi.org/10.1128/mBio.00285-10
Naas AE, Mackenzie AK, Mravec J, Schückel J, Willats WGT, Eijsink VGH et al (2014) Do rumen Bacteroidetes utilize an alternative mechanism for cellulose degradation? MBio 5:e01401–e01414
Nam IS, Garnsworthy PC (2007) Biohydrogenation of linoleic acid by rumen fungi compared with rumen bacteria. J Appl Microbiol 103:551–556
Ohara H, Karita S, Kimura T, Sakka K, Ohmiya K (2000a) Characterization of the cellulolytic complex (cellulosome) from Ruminococcus albus. Biosci Biotechnol Biochem 64:254–260
Ohara H, Noguchi J, Karita S, Kimura T, Sakka K, Ohmiya K (2000b) Sequence of egV and properties of EgV, a Ruminococcus albus endoglucanase containing a dockerin domain. Biosci Biotechnol Biochem 64:80–88
Ohmiya K, Sakka K, Kimura T, Morimoto K (2003) Application of microbial genes to recalcitrant biomass utilization and environmental conversation. J Biosci Bioeng 95:549–551
Orpin CG (1975) Studies on the rumen flagellate Neocallimastix frontalis. J Gen Microbiol 91:249–262. https://doi.org/10.1099/00221287-91-2-249]
Orpin CG (1994) Anaerobic fungi: taxonomy, biology, and distribution in nature. In: Mountfort DO, Orpin CG (eds) Anaerobic fungi: biology, ecology, and function. Marcel Dekker, Inc, New York, NY, pp 1–45
Ozutsumi Y, Tajima K, Takenaka A, Itabashi H (2005) The effect of protozoa on the composition of rumen bacteria in cattle using 16S rRNA gene clone libraries. Biosci Biotechnol Biochem 69:499–506
Qi M, Jakober K, McAllister T (2010) Rumen microbiology. In: Animal and plant productivity. Encyclopedia of Life Support Systems, Oxford, pp 161–176
Raghothama S, Eberhardt RY, Simpson P, Wigelsworth D, White P, Hazlewood GP, Nagy T, Gilbert HJ, Williamson MP (2001) Characterization of a cellulosome dockerin domain from the anaerobic fungus Piromyces equi. Nat Struct Biol 8:775–778
Ransom-Jones E, Jones DL, Edwards A, McDonald JE (2014) Distribution and diversity of members of the bacterial phylum Fibrobacteres in environments where cellulose degradation occurs. Syst Appl Microbiol 37:502–509
Reddy YHK, Srijana M, Harikrishna N, Reddy DM, Reddy G (2010a) Ethanol tolerant anaerobic cellulolytic ethanologenic bacteria isolated from decomposed paper. Curr Trends Biotechnol Pharm 4(4):947–956. ISSN 0973-8916
Reddy YHK, Srijana M, Reddy DM, Reddy G (2010b) Coculture fermentation of banana agro-waste to ethanol by cellulolytic thermophilic Clostridium thermocellum CT2. Afr J Biotechnol 9(13):1926–1934
Rincón MT, Martin JC, Aurilia V, McCrae SI, Rucklidge GJ, Reid MD et al (2004) ScaC, an adaptor protein carrying a novel cohesin that expands the dockerin-binding repertoire of the Ruminococcus flavefaciens 17 cellulosome. J Bacteriol 186:2576–2585
Rincon MT, Cepeljnik T, Martin JC, Lamed R, Barak Y, Bayer EA, Flint HJ (2005) Unconventional mode of attachment of the Ruminococcus flavefaciens cellulosome to the cell surface. J Bacteriol 187:7569–7578
Robson LM, Chambliss GH (1989) Cellulases of bacterial origin. Enzym Microb Technol 11:626–644
Rohwer F, Thurber RV (2009) Viruses manipulate the marine environment. Nature 459:207–212. https://doi.org/10.1038/nature08060
Rosenberg E, Zilber-Rosenberg I (2018) The hologenome concept of evolution after 10 years. Microbiome 6:78. https://doi.org/10.1186/s40168-018-0457-9
Russell JB, Sharp WM, Baldwin RL (1979) The effect of pH on maximum bacterial growth rate and its possible role as a determinant of bacterial competition in the rumen. J Anim Sci 48:251–255
Salamitou S, Lemaire M, Fujino T, Ohayon H, Gounon P, Béguin P, Aubert J-P (1994a) Subcellular localization of Clostridium thermocellum ORF3p, a protein carrying a receptor for the docking sequence borne by the catalytic components of the cellulosome. J Bacteriol 176:2828–2834
Salamitou S, Raynaud O, Lemaire M, Coughlan M, Béguin P, Aubert J-P (1994b) Recognition specificity of the duplicated segments present in Clostridium thermocellum endoglucanase CelD and in the cellulosome-integrating protein CipA. J Bacteriol 176:2822–2827
Schellhorn HE, Forsberg CW (1984) Multiplicity of extracellular β-(1,4)-endoglucanases of Bacteroides succinogenes S85. Can J Microbiol 30:930–937
Schwarz WH, Bronnenmeier K, Landmann B, Wanner G, Staudenbauer WL, Kurose N, Takayama T (1995) Molecular characterization of four strains of the cellulolytic thermophile clostridium stercorarium. Biosci Biotechnol Biochem 59:1661–1665
Selinger LB, Forsberg CW, Cheng KJ (1996) The rumen: a unique source of enzymes for enhancing livestock production. Anaerobe 2:263–284
Shimada K, Karita S, Sakka K, Ohmiya K (1994) Cellulases, xylanases, and their genes from bacteria. Bioprocess Technol 19:395–429
Shoseyov O, Levy I, Shani Z, Mansfield SD (2003) Modulation of wood fibers and paper by cellulose binding domains. In: Mansfield SD, Saddler JN (eds) Applications of enzymes to lignocellulosics. American Chemical Society, Washington, DC, pp 116–131
Steenbakkers PJM et al (2003) Beta-Glucosidase in cellulosome of the anaerobic fungus Piromyces sp. strain E2 is a family 3 glycoside hydrolase. Biochem J 370:963–970
Steenbakkers PJM, Irving JA, Harhangi HR, Swinkels WJC, Akhmanova A, Dijkerman R, Jetten MSM, van der Drift C, Whisstock JC, Op den Camp HJM (2008) A serpin in the cellulosome of the anaerobic fungus Piromyces sp. strain E2. Mycol Res 112:999–1006
Stutzenberger F (1990) Bacterial cellulases. In: Fogarty WM, Kelly CT (eds) Microbial enzymes and biotechnology. Elsevier Applied Science, London, pp 37–70
Suen G, Weimer PJ, Stevenson DM, Aylward FO, Boyum J, Deneke J et al (2011) The complete genome sequence of Fibrobacter succinogenes S85 reveals a cellulolytic and metabolic specialist. PLoS One 6:e18814
Sugiyama J, Suh S-O (2011) Chapter 158 - Sympodiomycopsis Sugiyama, Tokuoka & Komagata (1991). In: The yeasts, 5th edn, pp 1995–1997
Sunna A, Gibbs MD, Chin CW, Nelson PJ, Bergquist PL (2000) A gene encoding a novel multidomain beta-1,4-mannanase from Caldibacillus cellulovorans and action of the recombinant enzyme on kraft pulp. Appl Environ Microbiol 66:664–670
Tharwat M, Al-Sobayil F, Ali A, Buczinski S (2012) Transabdominal ultrasonographic appearance of the gastrointestinal viscera of healthy camels (Camelus dromedaries). Res Vet Sci 93:1015–1020
Tokatlidis K, Salamitou S, Beguin P, Dhurjati P, Aubcrt JP (1991) Interaction of the duplicated segment carried by Clostridium thermocellum cellulases with cellulosome components. FERS Lett 291:185–188
Tokatlidis K, Dhurjati P, Beguin P (1993) Properties conferred on Closlridium thermoocellum endoglucanase CclC by grafting the duplicated segment of endoglucanasc CclD. Prot Eng 6:947–952
Tringe SG, Rubin EM (2005) Metagenomics: DNA sequencing of environmental samples. Nat Rev Genet 6:805–814
Valenzuela-Ortega M, French CE (2019) Engineering of industrially important microorganisms for assimilation of cellulosic biomass: towards consolidated bioprocessing. Biochem Soc Trans 47(6):1781–1794. https://doi.org/10.1042/BST20190293. PMID: 31845725
Viljoen JA, Fred EB, Peterson WH (1926) The fermentation of cellulose by thermophilic bacteria. J Agric Sci 16(1):1–17
Wahrmund JL, Ronchesel JR, Krehbiel CR, Goad CL, Trost SM, Richards CJ (2012) Ruminal acidosis challenge impact on ruminal temperature in feedlot cattle. J Anim Sci 90:2794–2801
Warren RAJ (1996) Microbial hydrolysis of polysaccharides. Annu Rev Microbiol 50:183–212
Wegley L, Breitbart M, Edwards RA, Rohwer F (2007) Metagenomic analysis of the microbial community associated with the coral Porites astreoides. Environ Microbiol 9:2707–2719
Williams AG, Orpin CG (1987) Polysaccharide-degrading enzymes formed by three species of anaerobic rumen fungi grown on a range of carbohydrate substrates. Can J Microbiol 33:418–426
Wilson DB (2009) Evidence for a novel mechanism of microbial cellulose degradation. Cellulose 16:723–727
Xu Q et al (2003) The cellulosome system of Acetivibrio cellulolyticus includes a novel type of adaptor protein and a cell surface anchoring protein. J Bacteriol 185:4548–4557
Xu Q et al (2004) Architecture of the Bacteroides cellulosolvens cellulosome: description of a cell surface-anchoring scaffoldin and a family 48 cellulase. J Bacteriol 186:968–977
Yáñez-Ruiz DR, Moumen A, Martín García AI, Alcaide EM (2004) Ruminal fermentation and degradation patterns, protozoa population, and urinary purine derivatives excretion in goats and wethers fed diets based on two-stage olive cake: effect of PEG supply. J Anim Sci 82:2023–2032
Yaron S, Morag E, Bayer EA, Lamed R, Shoham Y (1995) Expression, purification and subunit-binding properties of cohesins 2 and 3 of the Clostridium thermocellum cellulosome. FEBS Lett 360:121–124
Youssef NH et al (2013) The genome of the anaerobic fungus Orpinomyces sp. Strain C1A reveals the unique evolutionary history of a remarkable plant biomass degrader. Appl Environ Microbiol 79:4620–4634
Zengler K, Toledo G, Rappe M, Elkins J, Mathur EJ, Short JM, Keller M (2002) Cultivating the uncultured. Proc Natl Acad Sci U S A 99:15681–15686. https://doi.org/10.1073/pnas.252630999
Zverlov VV, Schwarz WH (2006) The C. thermocellum Cellulosome: novel components and insights from the genomic sequence. In: Uversky V, Kataeva IA (eds) Cellulosome. Nova Science Publishers, Inc, New York, NY, pp 119–151
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mukkala, S., Bramhachari, P.V., Reddy, Y.H.K. (2022). The Cellulosome: A Fiber-Degrading Strategist of the Rumen Microbiome. In: Veera Bramhachari, P. (eds) Understanding the Microbiome Interactions in Agriculture and the Environment. Springer, Singapore. https://doi.org/10.1007/978-981-19-3696-8_11
Download citation
DOI: https://doi.org/10.1007/978-981-19-3696-8_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-3695-1
Online ISBN: 978-981-19-3696-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)