Abstract
Bilateral Wilms’ tumor (BWT), labelled as stage V, is different, is more challenging, and currently has a significantly worse prognosis than unilateral Wilms’ tumor (uWT). On imaging, tumor on one side associated with a single lesion of more than 1 cm or multiple lesions of any size in the contralateral kidney is considered BWT. It may be synchronous about 3.6 to 8%, or metachronous (0.85%). It may be prudent to treat patients with contralateral nephrogenic rests or children with predisposing syndromes or germline mutations on the lines of BWT to maximize salvage of renal function in the long run. The aims of management of BWT are to improve survivals, while maximizing renal tissue preservation and thereby renal function using appropriate multimodal therapy. The currently recommended surgery is bilateral nephron sparing surgery (NSS) to maximize renal preservation and function. Neoadjuvant chemotherapy (ChT) may be based on imaging studies (SIOP and COG). SIOP recommends neoadjuvant Vincristine and Actinomycin-D for 4 weeks. However, if disease is stable or progressive, escalation with the addition of Doxorubicin, for a total duration of up to 12 weeks followed by surgery is advised. COG (AREN0534) and ICMR guidelines currently recommend administering three drugs for a maximum of 12 weeks. Postoperative management includes adjuvant ChT based on histological risk stratification and staging. XRT is indicated for patients with positive tumor margins, positive lymph nodes, and metastatic disease sites.
At the end of study of this chapter, the reader is expected to have a clear understanding of the current scenario of BWT, suspect and anticipate BWT based on predisposing factors and risks. The reader should be able to provide appropriate investigations, take rapid decisions on treatment plans, and know the intricacies of preoperative strategies, intraoperative techniques, and post-operative management. This should lead to improved renal salvage and hence renal function with better event-free survival and overall survival (OS). The need of the hour is to improve renal preservation with appropriate preoperative ChT and prevent prolonged ChT in the hope of shrinking the tumor. The future and advances in various fields including MRI diffusion studies and molecular studies vis-a-vis decision-making will also be touched upon.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bilateral Wilms’ tumor
- SIOP: AREN 0534
- Nephron sparing surgery
- Nephroblastomatosis
- Oncological outcomes
- Renal functional outcomes
24.1 Introduction
Bilateral Wilms’ tumors (BWT) though not very common, the occurrence of bilateral tumors in kidneys of children especially less than 10 years, is almost always WT. BWT with an overall incidence between 4 and 8% may either present simultaneously at presentation, i.e., synchronous (6.3%) or at a later date in the opposite kidney (metachronous 0.85%) [1, 2]. BWT differ from unilateral WT (uWT) by presenting earlier—peak incidence about 12–14 months earlier than uWT [3], having much rarer incidence (only one in 20 of all WT), being frequently associated with germline genetic or epigenetic aberrations, and having a higher association of constitutional predisposing syndromes. Associations with syndromes not only pose difficulties during current management, but also have serious significant implications for long-term management, surveillance, and predisposition for poor renal outcomes. BWT is also associated with a much poorer outcome both oncologically—a 4-year event-free survival (EFS) of 56% for BWT vs. 85% for uWT [4] and poor renal functional outcomes, i.e., with 20-year cumulative incidence of chronic kidney disease (CKD) III or above of 12% in BWT against a measly 0.6% in uWT cases. The further challenges of BWT management include the complexity of decision-making, lack of clear guidelines or confusing guidelines, and lack of high-quality multicentric trials/studies exclusive to BWT until very recently [5]. Historically, neoadjuvant chemotherapy (ChT) used to be administered for long durations expecting a favorable response and resultantly the surgical treatment used to be inordinately delayed. Prolonged ChT has its own short- and long-term morbidity. Balancing appropriate timely surgical resections to maximize renal preservation at the same time obtaining good oncological outcomes is the greatest challenge of BWT [6, 7].
24.2 Definition of BWT from a Management Point of View
WT is managed as per principles of BWT when [4, 6]:
-
1.
Tumor masses more than 1 cm are present in both kidneys simultaneously (synchronous), or a single lesion of > 1 cm or multiple lesions of any size in the contralateral kidney.
-
2.
A second tumor develops in the other kidney in a patient who has previously been treated for WT (metachronous).
-
3.
WT in one kidney with nephroblastomatosis (NBL) in the other kidney.
-
4.
Syndromic patients with an initial presentation of uWT but carrying a high probability of BWT later may also benefit from being treated similar to those patients presenting with BWT.
24.3 Molecular Genetics and Predisposing Factors
Genetically predisposed tumors are likely to occur earlier as well as bilaterally, either synchronously or metachronously. Nephrogenic rests (NRs), which signify early disruption in renal development, are also associated with bilateral lesions [3].
BWT are frequently associated with germline genetic or epigenetic aberrations and a higher association of constitutional predisposing syndromes like WAGR syndrome (17%) and Denys–Drash syndrome (DDS) (20%). BWT has been shown to develop in 17–52% of various WT1 germline alterations. About 17% of Beckwith–Wiedemann syndrome (BWS) develop BWT; the penetrance of these aberrations is lower. However, incidence of BWT in patients Perlman syndrome is about 55%.
A pertinent question could be “why don’t these syndromic patients develop bilateral WT in all cases”? This is because of the necessity of a second event (second hit) separately for each kidney prior to the development of a tumor. It is also shown that there exists a differential selection pressure for development of a second event for different mutations, case in point: DIS3L2 of Perlman’s syndrome shows a greater incidence of BWT as compared to the IGF2/H19 mutations of BWS. Also, mosaicism exists in children, i.e., different organs or tissues or even cells in tissues may or may not demonstrate the aberration. Hence, each kidney may or may not have the mutation especially if the aberration occurs later in renal development.
24.4 Epidemiology
One of the important differences is the early age of onset. It has been shown in several studies that BWT occurs predominantly in 15–42 months (3.6 years) [3, 8], almost about 12–14 months earlier than the peak incidence of uWT cases. Moreover, the younger the age at presentation, higher is the chance of syndromic association. Two groups of syndromes are commonly associated with BWT in a majority of the cases—one associated with genitourinary abnormalities and the other with overgrowth syndromes.
24.5 Clinical Features
While the usual clinical features of uWT are also seen in BWT, the differences include earlier presentation, association with typical syndromes, aniridia, hemihypertrophy, and genital abnormalities/ambiguity in patients with BWT. Isolated genitourinary anomalies (not related to syndromes) are more common in association with BWT, mostly cryptorchidism and hypospadias. Hypertension should be looked for and documented.
24.6 Investigations
The child is investigated similar to any WT; however following additional points may need to be remembered and addressed.
A contrast-enhanced computerized tomography (CECT) (Fig. 24.1) scan of the abdomen and thorax, or a magnetic resonance imaging (MRI) of the abdomen, is necessary, more so in suspected cases of BWT (Fig. 24.2). Since smaller lesions and NRs are usually isoechoic to renal parenchyma on ultrasound (US), CECT or MRI is more sensitive in picking up BWT [9]. Additional information sought include number (in multifocal tumors), size, and volume of the tumor(s) in each of the kidneys, presence of enlarged retroperitoneal lymph nodes (LNs), preoperative tumor rupture, presence of ascites, and metastatic disease in liver and thorax. The goal in management of BWT is to maximise renal preservation without compromising on adequacy of oncological clearance and the 3-D computer volume rendering images and the 3-D printing models could help the surgeon to plan and execute complex surgeries, preservation [7, 10]. Although MRI is nowadays being preferred for the abdominal examination, non-contrast CT scan of the chest is mandatory to rule out pulmonary metastases. Though CECT and MRI have shown to similar diagnostic accuracy as regard the locoregional disease, MRI has some distinct advantages in differentiating NBL. In T1 weighted images, NBL is usually hypointense compared to the cortex; however, it is hyperintense in T2 weighted images similar to the cortex [3, 11]. NR are also more lenticular or ovoid, smaller (<2 cm) and of uniform signal intensity, while WT is likely to be rounded [12]. MRI in post-ChT patients show bright lesions in T2 and Short-Tau Inversion Recovery (STIR) sequences in case of active NR/WT; inactive NRs are dark on the same sequences. MRI, however, requires specific protocols to maximize its utility so that high-spatial-resolution post-contrast images are obtained. One can take advantage of diffusion-weighted MR images to detect smaller lesions, both WT and NR. Histological risk assessment especially in the post-ChT preoperative scans using whole tumor Apparent Diffusion Coefficient (ADC) is the new kid on the block, as it can predict stromal subtype histopathology, thus having a prognostic role based on the inverse relation of ADC to the cellularity of the tumor. However, it is not shown to be useful in differentiating WT from NRs [9].
CT abdomen, pelvis, and thorax showing bilateral multiple tumor masses (white arrows-tumor masses, black arrows-normal kidney). (a) Axial sections. (b) Coronal sections showing bilateral masses, left renal pedicle sandwiched between the two masses, right side vessels stretched over the upper pole mass. (c) CT Thorax- lung window showing no metastases. (d) Sagittal sections showing right kidney in d1 and left kidney in d2
18-FDG PET-CT studies are currently not shown to have much role in the evaluation of BWT as it does not differentiate WT from NR [11].
24.7 Management of BWT
Unlike uWT, BWT has currently uniform management policy across the world. Upfront bilateral radical nephroureterectomies for BWT would render the child anephric and, hence, upfront ChT followed by conservative surgery is universally accepted as the ideal management with improved outcomes [4, 6, 13]. Neoadjuvant ChT is instituted to make bilateral nephron sparing surgery (NSS) a possibility in a majority of the cases without increasing local recurrences. Historically, two mistakes in management were done- too much/too long ChT preoperatively hoping for the tumor to shrink resulting in significant morbidity and performing too much radical a surgery resulting in unnecessary renal loss. These stand corrected today with the concerted efforts of multidisciplinary teams. Postoperative ChT and radiotherapy (XRT) is instituted appropriately keeping in mind to minimize the additional risks of morbidity including additional nephrotoxicity.
24.7.1 Neoadjuvant Therapy
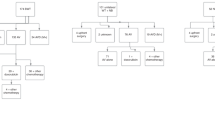
The goal of neoadjuvant therapy is to reduce the size of the lesions so that bilateral NSS can be attempted in the majority of patients. Historically, multiple drugs with varied doses were administered. The current COG protocol (AREN 0534), also endorsed by Indian Council of Medical Research (ICMR) [14], is to administer two 3-weekly cycles of 3-drug regime VAD utilizing Vincristine (VCR), Actinomycin D (AMD), and Doxorubicin (DOX). SIOP, however, still advises VCR (1.5 mg/m2) and AMD (45 μg/kg) for 4 weeks for non-metastatic BWT initially.
After 4 of 6 weeks of ChT as per the protocol being followed, the tumor response is assessed by US (SIOP) or CECT (COG) to document any decrease in size of the tumors and to assess the feasibility of NSS using RECIST criteria.
In COG protocol (AREN 0534), in case the tumor is responding to the ChT as demonstrated by a decrease in 50% volume reduction or 30% reduction in the sum of the diameters of target lesions (using Response Evaluation Criteria in Solid Tumors [RECIST]), but NSS is still not feasible, ChT can be continued for a further period of 6 weeks [4, 6]. Surgery is performed regardless of tumor status at the end of 12 weeks. The reason to avoid prolonged ChT beyond 12 weeks is that poor response may be due to unfavorable histologies. These include diffuse anaplasia (DA), non-responding blastemal predominance, which do not respond to further ChT. It may also be due to the contrasting scenario of stromal predominance, which may have adequately responded but has not shrunk in size. Rhabdomyomatous transformation does not shrink or may even increase in size; however, this is a sign of good response to ChT. There is also a concern that anaplastic transformation is associated with prolonged administration of neoadjuvant ChT [15]. For the above reasons, the true picture is revealed only on histopathological examination of the excised specimen.
However, if the initial response after 6 weeks of neoadjuvant ChT is poor, i.e., <30% reduction in tumor volume, then bilateral open wedge biopsies are advocated in these patients. If anaplasia is detected, then an intensified ChT with VCR, AMD, DOX, Cyclophosphamide (CTX), Carboplatin (CARB), and Etoposide (ETOP) is used for further 6 weeks. If blastemal predominance is detected, regimen I, i.e., VCR, AMD, DOX, CTX, and ETOP, is advocated for 6 more weeks. For all other histologies, VAD is continued for 6 more weeks. In any case, surgery is carried out after 12 weeks of ChT. Though bilateral NSS is strongly recommended, if it is not feasible, then unilateral radical nephrectomy on the worse side with NSS on the contralateral kidney is carried out.
In SIOP protocol, if the disease is stable or progressive on US review at 4 weeks of 2-drug regimen, then DOX (50 mg/m2) is added and second assessment at 8 weeks is carried out with CECT. Newer recommendations of CARB, ETOP, in lieu of DOX so as to spare the child from doxorubicin toxicity are also noted [16]. If tumor response is present, ChT is continued for a further 4 weeks and NSS is carried out. Note the avoidance of prolonged use of neoadjuvant ChT beyond 12 weeks in SIOP also, at which point, the patient would be subjected to surgery [17]. In any case, at some stage, bilateral NSS is performed either in a single stage approach or in two separate operations performed not more than two post-operative cycles apart. If staged, then the less involved kidney should be operated on first. Complete nephrectomy on one side with NSS on the opposite side is acceptable providing enough functional renal tissue can be preserved. Rarely, the patient may need to undergo bilateral nephrectomy with a planned renal transplantation a year or 2 later if complete response (CR) is achieved.
A biopsy is not indicated in either of the two protocols prior to starting neoadjuvant ChT unless there are very atypical features like age more than 10 years, unusual imaging findings like encasement of vessels, voluminous lymphadenopathy, unusual metastasis like bone(<2 years) or brain, etc. are present [12, 18].
24.7.2 Surgical Management
Several surgical options are available in the management of BWT [6, 11].
They include:
-
1.
Bilateral NSS.
-
(a)
Partial nephrectomy—ensuring a rim of normal renal tissue separating tumor from the resection margin.
-
(b)
Marginal resection—tumor along with its pseudocapsule intact; however, no normal renal tissue margin is present.
-
(c)
Longitudinal partial nephrectomy for central tumors [19].
-
(d)
Bench surgery with ex situ perfusion and autotransplantation [20].
-
(a)
-
2.
Nephroureterectomy on worse side and NSS on the contralateral side.
-
3.
Bilateral nephroureterectomy and delayed renal transplantation.
The twin goals of adequate oncological clearance with maximal renal preservation are best met by Bilateral Marginal Resections of all tumors, however, may not be feasible in all. Large series from some of the acclaimed centers reiterate that this is feasible in about 90% of cases despite of seemingly unfavorable initial imaging [6].
After administration of neoadjuvant treatment, the surgical team has to consciously decide whether decision to operate both sides simultaneously, or sequentially with a 1- to 4-week gap. SIOP recommends sequential surgery with the better side carried out first and carry out the next after 1–2 weeks for recovery [17]. However, acclaimed centers like St. Judes, Memphis, recommend simultaneous NSS citing no proven advantage of sequential surgeries [21]. Given the rarity of BWT and the duration and blood loss associated with NSS, varying levels of expertise/experience available, prudence suggests sequential surgery may be carried out until evidence from suitable studies suggest that simultaneous NSS is superior.
Radical nephroureterectomy is recommended even in BWT in certain situations, and these are the presence of DA and supra-hepatic IVC tumor thrombus not responding to ChT (incomplete regression). It is however extremely rare for both the kidneys to have DA, and hence usually NSS on the contralateral side is feasible.
Evaluation of the feasibility of NSS is usually carried out using multiphase contrast-enhanced CT scan of the abdomen and pelvis (Fig. 24.3). 3D reconstructions are also carried out. Even though predefined criteria are not available at present, polar and/or peripheral lesions, with no encasement or invasion of the renal vessels, are easy to excise. Even though the large tumors or those with proximity to renal vasculature, masses abutting the vessels, central masses, and multiple tumors may appear ominous on imaging, it may be feasible to undertake NSS safely with minimal risk of positive margins by one of the techniques mentioned above. It is to be remembered that WT grows by compressing adjacent parenchyma, which forms a pseudocapsule (or even a fibrous capsule), which lends itself to careful dissection outside the tumor margin, irrespective of the size of the tumor (Fig. 24.4). Acceptance of additional expertise or referral may save the patient from nephrectomy in some of these cases [6].
(a) Intraoperative photo after completion of marginal excision and in folding of edges sutured with pledgeted sutures (arrowhead). Renal vein is shown by white arrow and IVC shown by black arrow. (b) Tumors post bilateral NSS; CECT (volume rendered image) showing tumor remnant of the right kidney (arrowhead) with approximate reniform shape
Given the varieties of nephron sparing methods described and the different terminologies used leading to great confusion (e.g., wedge resection, partial nephrectomy, polar nephrectomy, tumorectomy, enucleation, etc.), a standardized format for reporting NSS is essential. Such a standard reporting format has been described [13, 22] with four parameters, viz., Surgical Technique (partial nephrectomy: i.e., with a rim of normal tissue or enucleation, i.e., without a rim); Surgical Resection Margin (surgeon’s description of presence of tumor breach or doubtful breach or with intact pseudocapsule); Pathological Resection Margin (i.e., intact or tumor breach present); and Remaining Renal Parenchyma (estimated by the surgeon as a percentage at end of surgery).
Use of standardized reporting will at least in the future ensure accurate comparable data to understand and apply the best possible surgical intervention.
24.7.3 Adjuvant Therapy for BWT
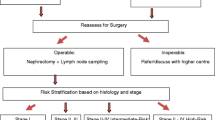
All cases of BWT require some form of adjuvant therapy. The actual adjuvant therapy depends on staging (factors including tumor margins, LN status, the occurrence of tumor rupture preoperatively or during surgery, etc.) and risk stratification based on histological criteria (anaplasia, blastemal predominance, percentage of necrosis, etc.) [23, 24].
Staging and risk stratification (according to SIOP 2001 protocol) is similar to uWT, and each side has to be staged (stage I–III) and risk-stratified separately. Treatment is based on the higher stage and risk stratification recorded. As far as SIOP recommendations are considered, the adjuvant treatment is same as that for uWT of comparable stage and risk except for stage I low risk, where ChT of stage II low risk, i.e., 27 weeks of VA, is advocated [16, 17].
AREN 0534 has recommended the following adjuvant treatment based on histological and stage criteria [4]:
-
1.
BWT with stage I and II completely necrotic tumors and stage I Intermediate Risk (IR) tumors are treated with 19 weeks of VCR and AMD.
-
2.
BWT with stage I blastemal predominant, stage III and IV completely necrotic tumor, stage II–IV IR, stage I–III focal anaplasia, and stage I diffuse anaplasia are treated with 25 weeks of VAD.
-
3.
BWT with stage II–IV blastemal predominant receive VCR, AMD, DOX, CTX, and ETOP for 28 weeks.
-
4.
Stage IV focal anaplastic tumors and stage II–IV diffuse anaplastic tumors in BWT will receive VCR, AMD, DOX, CTX, CARB, and ETOP for 31 weeks.
Significant differences in drugs used exist between SIOP and AREN0534, especially in the higher-risk groups.
In bilateral WT, paraaortic nodes cannot be accorded to the one or the other side. If only LNs are positive, then XRT is given only to paraaortic LNs. However, the local renal specimen will be staged individually and could be stage I, II, or III (positive margins, residual disease left after surgery, tumor rupture). If one or both sides are stage III (any histology) or stage II anaplastic, then accordingly unilateral or bilateral flank XRT along with XRT to paraaortic LNs would be administered. Dose to the whole kidney should not exceed 10–12 Gy (12 Gy maximum dose), even if there is unfavorable histology (UH). Brachytherapy could be given in selected cases. Whole abdominal irradiation (WAI) is reserved for large tumor spill intraoperatively involving areas outside the tumor bed as determined by the surgeon, tumor rupture before surgery, and presence of peritoneal metastases [4].
24.8 Special Circumstances
24.8.1 Completely Resolved Tumors
BWT that have completely disappeared on 6 and 12 weeks of neoadjuvant ChT are treated as per the stage of the disease, i.e., localized disease or metastatic disease before ChT. Non-metastatic CR in both kidneys is treated with a further two-drug regime of VCR and AMD for a duration of 19 weeks and metastatic disease with CR with VAD for 25 weeks; no surgery is performed [4].
24.8.2 Metastatic Disease
Metastatic disease at presentation with CR bilaterally with only neoadjuvant ChT is treated with further VAD for 25 weeks [4, 6].
24.8.3 Positive Margins
Positive margins on histology convert the disease to stage III, and the patient receives flank XRT. However, in the presence of diffuse anaplasia with positive margins, completion nephrectomy with adjuvant flank XRT should be seriously contemplated, considering the poor prognosis of patients with diffuse anaplasia.
24.9 Renal Transplantation
Children who are rendered anephric due to bilateral nephrectomy either in synchronous or metachronous disease or develop End Stage Renal Disease (ESRD) due to any reason are in requirement of renal transplantation. Traditionally, this has been delayed for 2 years of EFS before being offered as this is the duration of maximal relapse. However, newer data suggests earlier transplantation as equivalent outcomes [25]. In cases where live-related donors are available, 1-year wait period has been suggested to be sufficient.
24.10 Follow-Up and Outcomes
ICMR adapting from SIOP provided the following guidelines for follow-up of children with BWT [14]:
-
1.
Along with clinical examination including blood pressure monitoring, all children with BWT should undergo chest X-ray and ultrasound evaluation every 2 monthly for the first 2 years followed by 3 monthly for the next 2 years and annually for 10 years.
-
2.
Six-monthly evaluation for proteinuria and serum creatinine is also recommended indefinitely.
24.11 Prognosis and Long-TermOutcomes
Unlike in uWT, BWT is prognosticated against two parameters—oncological and renal functional outcome.
Oncologically speaking, metastatic disease at onset, UH including diffuse anaplasia, advanced loco-regional stage, and age at diagnosis of more than 3 years seem to be associated with poor prognosis [11]. Surprisingly, positive tumor margin in NSS does not seem to increase recurrences provided XRT is given [26].
Renal functional prognosis is related to the type of surgery performed, prolonged ChT and/or concurrent XRT, metachronous disease, associated syndromes especially WT1 related, e.g., DDS and WAGR (20-year ESRD rate of 82.7 and 43.3, respectively) and progressive disease ending in bilateral nephrectomy. Earlier age at disease (i.e., <24 months) is also associated with higher ESRD [18].
BWT has been associated with a much poorer prognosis compared to uWT, a 4-year EFS of about 56% (NWTS-5) and 10-year overall survival (OS) of only 69% (SIOP) [7]. Historically, long-term renal outcome in context of ESRD is of crucial importance and found to be 4% at 3 years in synchronous and 19.3% in metachronous BWT [14]. The same increases to 12% at 20 years and much worse for syndromic children up to 80%. Poor outcomes are multifactorial including increased anaplasia in BWT, inappropriate staging, and prolonged ChT [15].
Several single-institution studies and the recently reported multicenter trial AREN 0534 report improved outcomes with an enhanced application of NSS and better utilization of preoperative ChT. Davidoff et al. reported (about 90% NSS rate) a 3-year EFS of 64% with a 4-year OS 85.7% [1]. With a maximum follow-up of 13 years, none had estimated glomerular filtration rate (eGFR) <60 and 8.3% had CKD stage 2. AREN 0534 reported a 4-year EFS and OS of 82% and 94.9%, respectively. These remarkable results seem to stem from two interventions, i.e., decreasing the overall duration of preoperative ChT and tailoring the postoperative ChT according to post ChT histological response [4]. The utilization of 3-drug preoperative ChT which has been shown to cause greater shrinkage may also have led to greater utilization of NSS.
While the short-term renal functional outcomes of increased use of NSS bilaterally is encouraging, more long-term data with a larger number of patients will provide greater clarity.
24.12 Future Directions
While there are many unanswered questions specific to BWT, some appear more urgent than others.
The utility of three drugs vs. two drugs as preoperative ChT seems to have been established both in AREN 0534. Assessing response to neoadjuvant ChT seems to be still dependent on imaging, and current imaging techniques seem inadequate. The alternative of performing open biopsies seems too invasive. Tumor shrinkage or reduction as assessed by CECT is currently accepted. Failure of ChT to result in significant size reduction does not always mean failure of ChT for reasons mentioned previously and is currently the Achilles heel of preoperative ChT evaluation. Advanced functional imaging may be the solution. Solutions are being searched using advanced functional imaging. Apparent Diffusion Coefficient (ADC) can be calculated using diffusion weighted MRI. It has been shown that the higher the cellularity of tissues, the lower is the ADC; conversely poorly cellular areas show higher ADC value [27, 28]. This inverse relationship of ADC with cellularity of tissues can be harnessed to differentiate response to ChT.
A second area of constant debate: whether enucleation/marginal resection is adequate, or partial nephrectomy is superior. While single-center studies have tried to answer this question with small but significant numbers, large multicentric trial-based data would help surgeons globally to make informed decisions.
Thirdly, the question of evaluation and assessment of renal function in the post-operative patient. Absolute eGFR, the current standard for evaluating renal function, has been criticized as not being clinically significant in patients undergoing renal resections as it is for patients developing CKD due to medical conditions [29]. There is also considerable variability in evaluating and reporting renal outcome measures and standardizing the same will help enormously.
Epidemiological studies along with molecular genetic analysis when carried out may also be of great help not only in assessing the contribution of the various mutations to bilateral disease but will also clarify their role in the risk of developing renal failure. It may also provide clues to which patient may require NSS, thus helping in adapting and making personalized treatment plans for individual patients.
References
Davidoff AM, Interiano RB, Wynn L, Santos ND, Dome JS, Green DM, et al. Overall survival and renal function of patients with synchronous bilateral Wilms tumor undergoing surgery at a single institution. Ann Surg. 2015;262:570–6. https://doi.org/10.1097/SLA.0000000000001451.
Han Q, Li K, Dong K, Xiao X, Yao W, Liu G. Clinical features, treatment, and outcomes of bilateral Wilms’ tumor: a systematic review and meta-analysis. J Pediatr Surg. 2018;53:2465–9. https://doi.org/10.1016/j.jpedsurg.2018.08.022.
Charlton J, Irtan S, Bergeron C, Pritchard-Jones K. Bilateral Wilms tumour: a review of clinical and molecular features. Expert Rev Mol Med. 2017;19(e8):1–13. https://doi.org/10.1017/erm.2017.8.
Ehrlich PF, Chi YY, Chintagumpala MM, Hoffer FA, Perlman EJ, Kalapurakal JA, et al. Results of the first prospective multi-institutional treatment study in children with bilateral Wilms tumor (AREN0534): a report from the Children’s oncology group. Ann Surg. 2017;266:470–8. https://doi.org/10.1097/SLA.0000000000002356.
Aydın B, Akyüz C, Yalçın B, Ekinci S, Oğuz B, Akçören Z, et al. Bilateral Wilms tumors: treatment results from a single center. Turk J Pediatr. 2019;61:40–5. https://doi.org/10.24953/turkjped.2019.01.008.
Murphy AJ, Davidoff AM. Bilateral Wilms tumor: a surgical perspective. Children. 2018;5:134. https://doi.org/10.3390/children5100134.
Aldrink JH, Heaton TE, Dasgupta R, Lautz TB, Malek MM, Abdessalam SF, et al.; American Pediatric Surgical Association Cancer Committee. Update on Wilms tumor. J Pediatr Surg. 2019;54:390–7. https://doi.org/10.1016/j.jpedsurg.2018.09.005.
Oue T, Koshinaga T, Okita H, Kaneko Y, Hinotsu S, Fukuzawa M. Bilateral Wilms tumors treated according to the Japan Wilms tumor study group protocol. Pediatr Blood Cancer. 2014;61:1184–9. https://doi.org/10.1002/pbc.24979.
Servaes SE, Hoffer FA, Smith EA, Khanna G. Imaging of Wilms tumor: an update. Pediatr Radiol. 2019;49:1441–52. https://doi.org/10.1007/s00247-019-04423-3.
Girón-Vallejo Ó, García-Calderón D, Ruiz-Pruneda R, Cabello-Laureano R, Doménech-Abellán E, et al. Three-dimensional printed model of bilateral Wilms tumor: a useful tool for planning nephron sparing surgery. Pediatr Blood Cancer. 2018;65:e26894. https://doi.org/10.1002/pbc.26894.
Millar AJ, Cox S, Davidson A. Management of bilateral Wilms tumours. Pediatr Surg Int. 2017;33:461–9. https://doi.org/10.15586/codon.wt.2016.ch5.
Watson T, Oostveen M, Rogers H, Pritchard-Jones K, Olsen Ø. The role of imaging in the initial investigation of paediatric renal tumours. Lancet Child Adolesc Health. 2020;4:232–41. https://doi.org/10.1016/S2352-4642(19)30340-2.
van den Heuvel-Eibrink MM, Hol JA, Pritchard-Jones K, Van Tinteren H, Furtwängler R, Verschuur AC, et al.; International Society of Pediatric Oncology-Renal Tumor Study Group (SIOP-RTSG). Position paper: rationale for the treatment of Wilms tumour in the UMBRELLA SIOP-RTSG 2016 protocol. Nat Rev Urol. 2017;14:743–52. https://doi.org/10.1038/nrurol.2017.163.
Prasad M, Vora T, Agarwala S, Laskar S, Arora B, Bansal D, et al. Management of Wilms tumor: ICMR consensus document. Indian J Pediatr. 2017;84:437–45. https://doi.org/10.1007/s12098-017-2305-5.
Shamberger RC, Haase GM, Argani P, Perlman EJ, Cotton CA, Takashima J, et al. Bilateral Wilms’ tumors with progressive or nonresponsive disease. J Pediatr Surg. 2006;41:652–7. https://doi.org/10.1016/j.jpedsurg.2005.12.004.
Vaidya SJ, Howell L, Chowdhury T, Oostveen M, Duncan C, Powis M, et al. CCLG clinical management guidelines: renal tumours. January 2020 CCLG Renal Tumours Special Interest Group. https://www.cclg.org.uk/write/MediaUploads/Member%20area/Treatment%20guidelines/Umbrella_Clinical_Management_Guidelines_Jan_2020_FINAL.pdf
de Kraker J, Graf N, Pritchard-Jones K, Pein F. Nephroblastoma clinical trial and study SIOP 2001, protocol. SIOP RTSG. 2001. https://www.skion.nl/workspace/uploads/Protocol-SIOP-2001.pdf. Accessed 13 july 2022.
Irtan S, Ehrlich PF, Pritchard-Jones K. Wilms tumor: “state-of-the-art” update, 2016. Semin Pediatr Surg. 2016;25:250–6. https://doi.org/10.1053/j.sempedsurg.2016.09.003.
Fuchs J, Szavay P, Seitz G, Handgretinger R, Schäfer JF, Warmann SW. Nephron sparing surgery for synchronous bilateral nephroblastoma involving the renal hilus. J Urol. 2011;186:1430–6. https://doi.org/10.1016/j.juro.2011.05.068.
Harel M, Makari JH, Ferrer FA. Oncology: the role of partial nephrectomy in Wilms tumor. Curr Urol Rep. 2013;14:350–8. https://doi.org/10.1007/s11934-013-0330-0.
Davidoff AM, Giel DW, Jones DP, Jenkins JJ, Krasin MJ, Hoffer FA, et al. The feasibility and outcome of nephron-sparing surgery for children with bilateral Wilms tumor: the St. Jude Children's Research Hospital experience: 1999-2006. Cancer. 2008;112:2060–70. https://doi.org/10.1002/cncr.23406.
Godzinski J, Graf N, Audry G. Current concepts in surgery for Wilms tumor-the risk and function-adapted strategy. Eur J Pediatr Surg. 2014;24:457–60. https://doi.org/10.1055/s-0034-1396425.
Vujanić GM, Sandstedt B. The pathology of Wilms’ tumour (nephroblastoma): the International Society of Paediatric Oncology approach. J Clin Pathol. 2010;63:102–9. https://doi.org/10.1136/jcp.2009.064600.
Vujanić GM, Gessler M, Ooms AH, Collini P, Coulomb-l’Hermine A, D'Hooghe E, et al; International Society of Pediatric Oncology-Renal Tumor Study Group (SIOP-RTSG). The UMBRELLA SIOP-RTSG 2016 Wilms tumour pathology and molecular biology protocol. Nat Rev Urol. 2018;15:693–701. https://doi.org/10.1038/s41585-018-0100-3.
Grigoriev Y, Lange J, Peterson SM, Takashima JR, Ritchey ML, Ko D, et al. Treatments and outcomes for end-stage renal disease following Wilms tumor. Pediatr Nephrol. 2012;27:1325–33. https://doi.org/10.1007/s00467-012-2140-x.
Kieran K, Williams MA, Dome JS, McGregor LM, Krasin MJ, Davidoff AM. Margin status and tumor recurrence after nephron-sparing surgery for bilateral Wilms tumor. J Pediatr Surg. 2013;48:1481–5. https://doi.org/10.1016/j.jpedsurg.2013.02.033.
Humphries PD, Sebire NJ, Siegel MJ, Olsen ØE. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology. 2007;245:848–54. https://doi.org/10.1148/radiol.2452061535.
Kocaoglu M, Bulakbasi N, Sanal HT, Kismet E, Caliskan B, Akgun V, et al. Pediatric abdominal masses: diagnostic accuracy of diffusion weighted MRI. Magn Reson Imaging. 2010;28:629–36. https://doi.org/10.1016/j.mri.2010.02.010.
Ellis RJ, Cho Y, Del Vecchio SJ, McStea M, Morais C, Coombes JS, et al. Outcome measures used to report kidney function in studies investigating surgical management of kidney tumours: a systematic review. Eur Urol Focus. 2019;5:1074–84. https://doi.org/10.1016/j.euf.2018.04.012.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kumaravel, S. (2022). Bilateral Wilms’ Tumors. In: Sarin, Y.K. (eds) Wilms’ Tumor. Springer, Singapore. https://doi.org/10.1007/978-981-19-3428-5_24
Download citation
DOI: https://doi.org/10.1007/978-981-19-3428-5_24
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-3427-8
Online ISBN: 978-981-19-3428-5
eBook Packages: MedicineMedicine (R0)